Recent Advances in Protective Coatings for Cultural Heritage–An Overview
Abstract
:1. Introduction
2. Metallic Objects
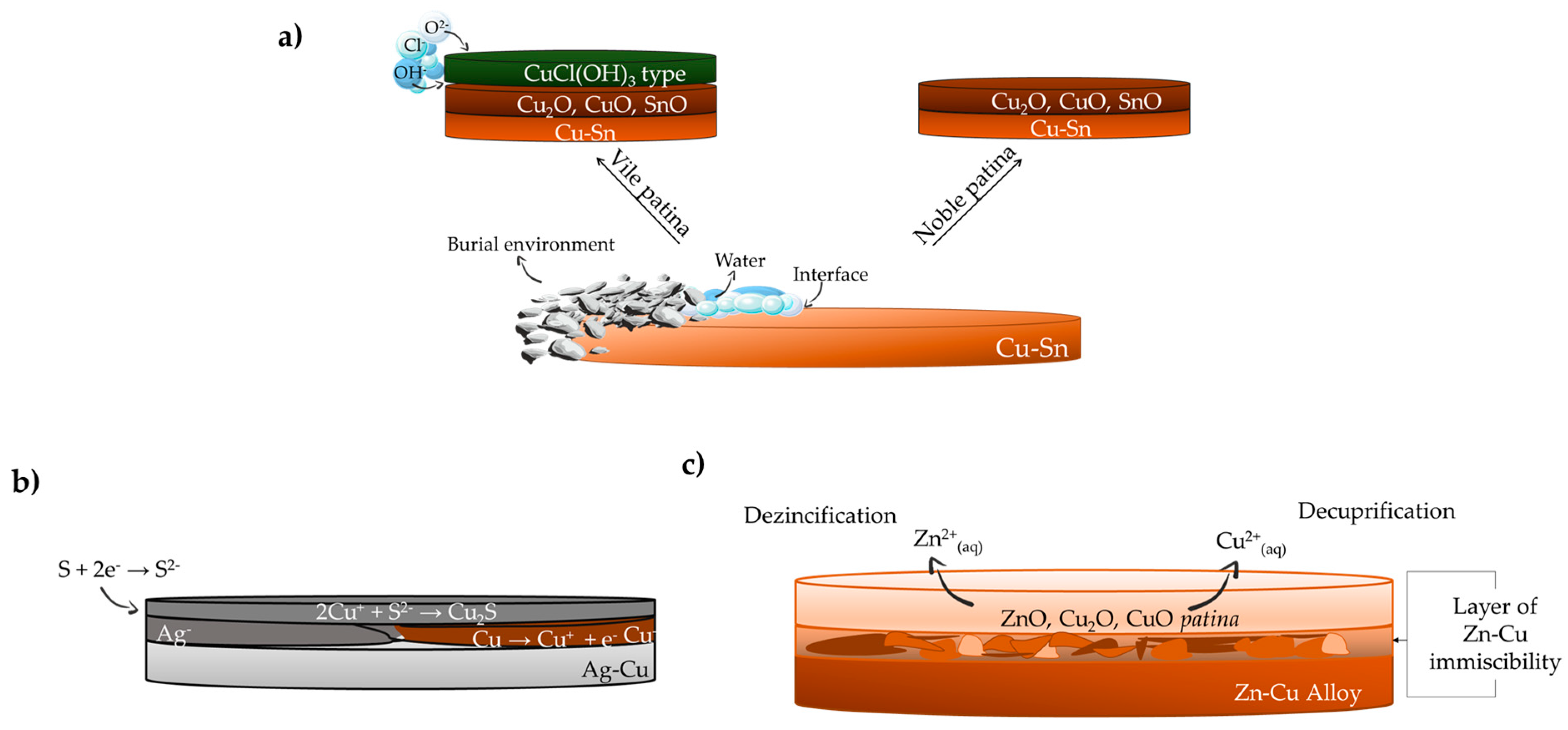
2.1. Copper and Bronzes
2.1.1. Triazole Derivatives
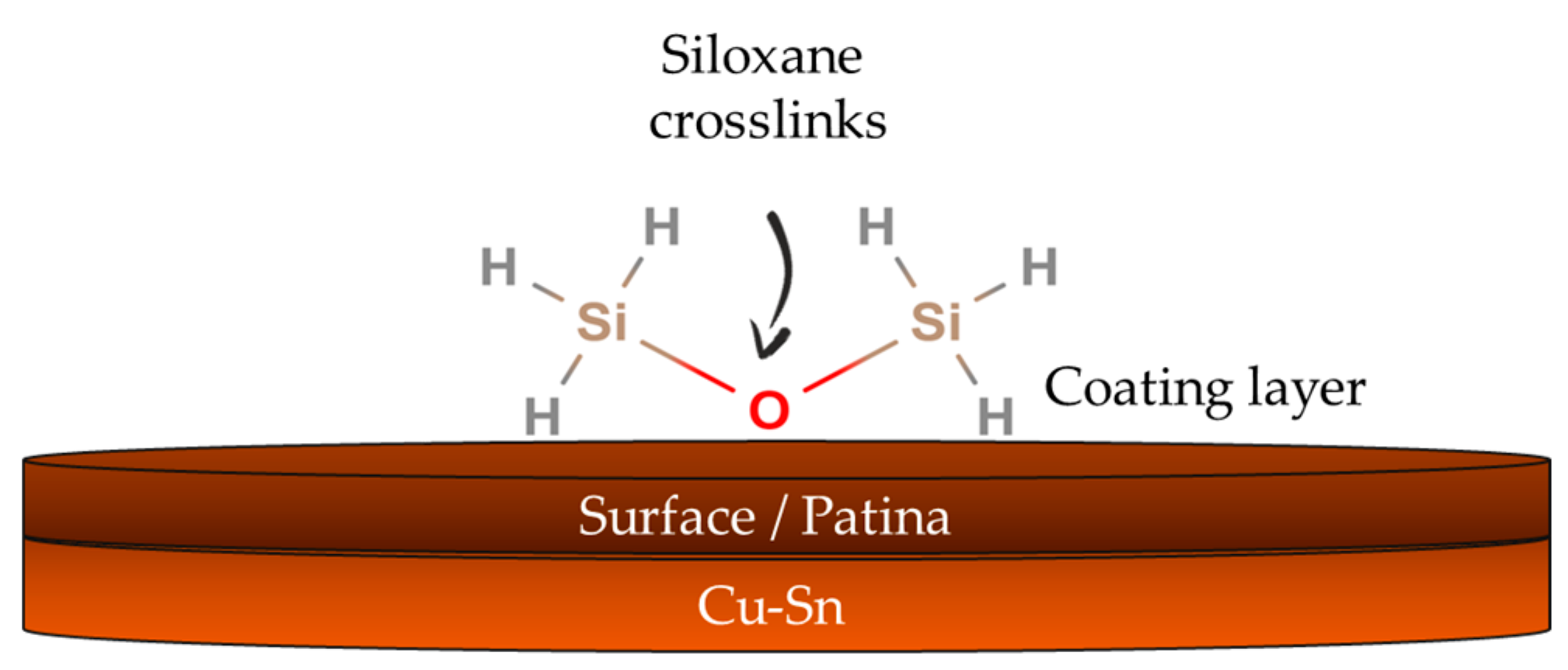
2.1.2. Silanes and Fluoropolymers
2.1.3. Nano-Composites
2.1.4. Biofilms and Biopolymers
2.2. Silver and Iron
3. Glass
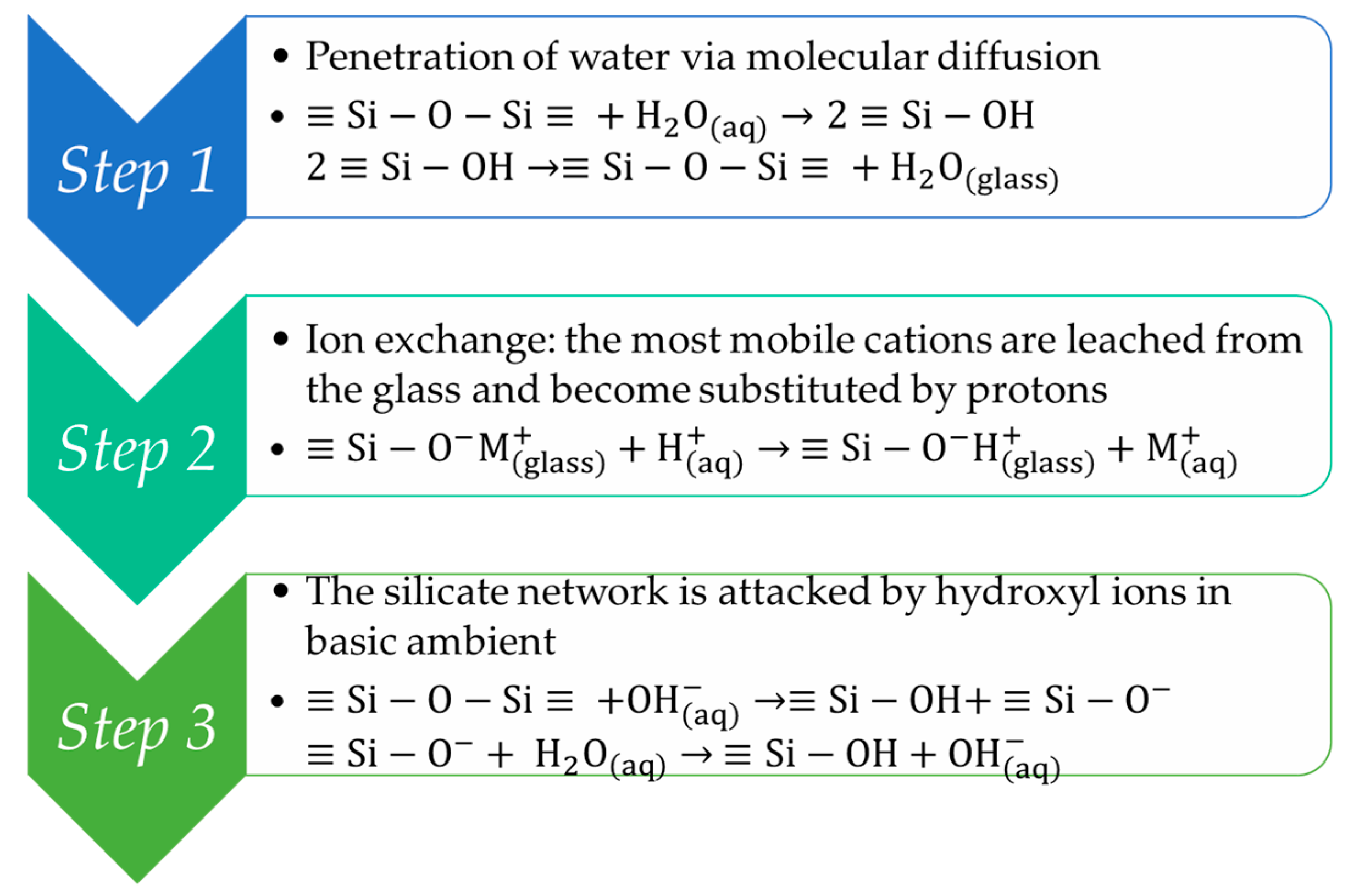
3.1. Sol-Gel Films
3.2. Hybrid Sol-Gel
4. Limestone
4.1. Acrylic Polymers
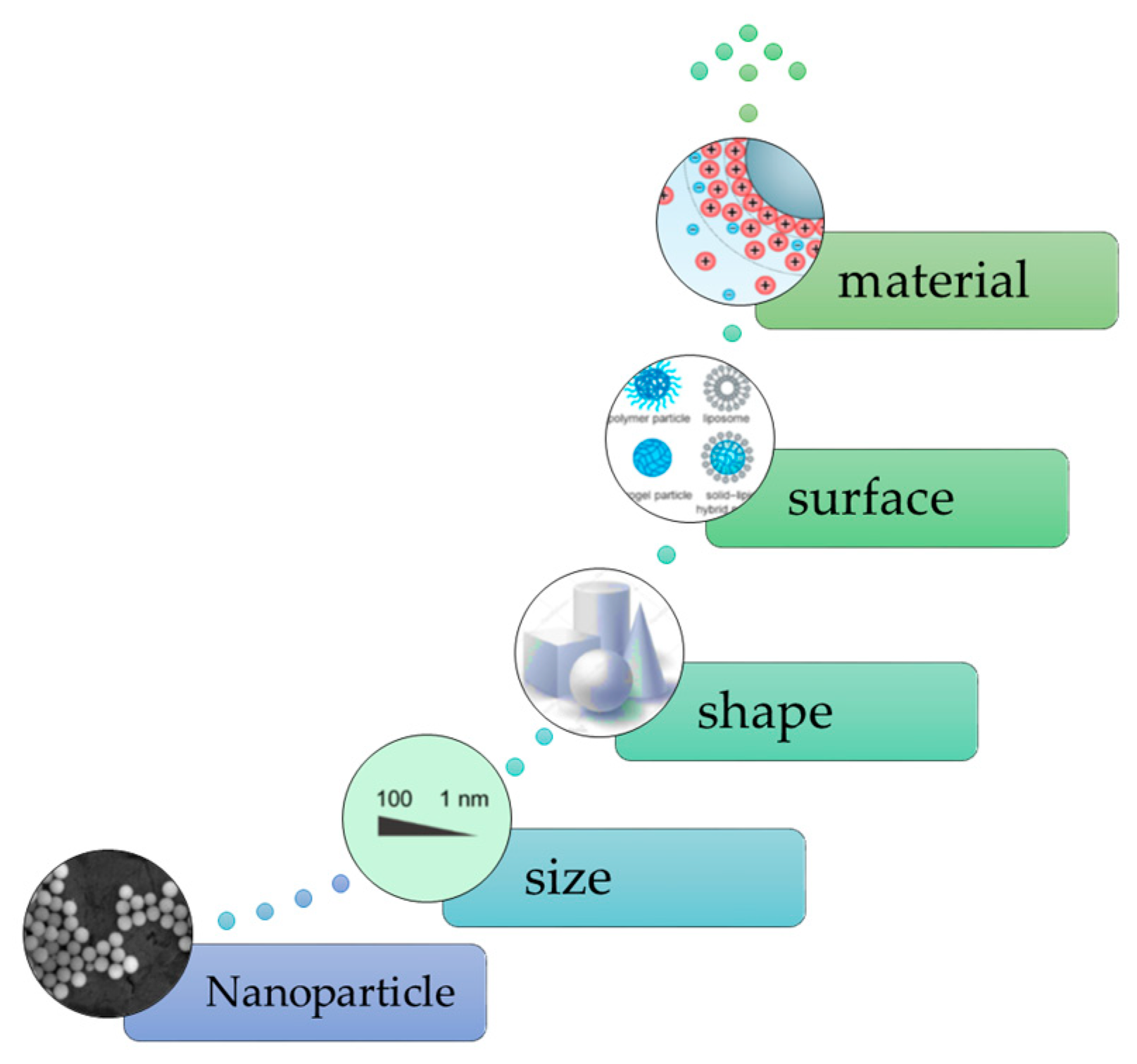
4.2. Nano-Composites
4.3. Bio-Films
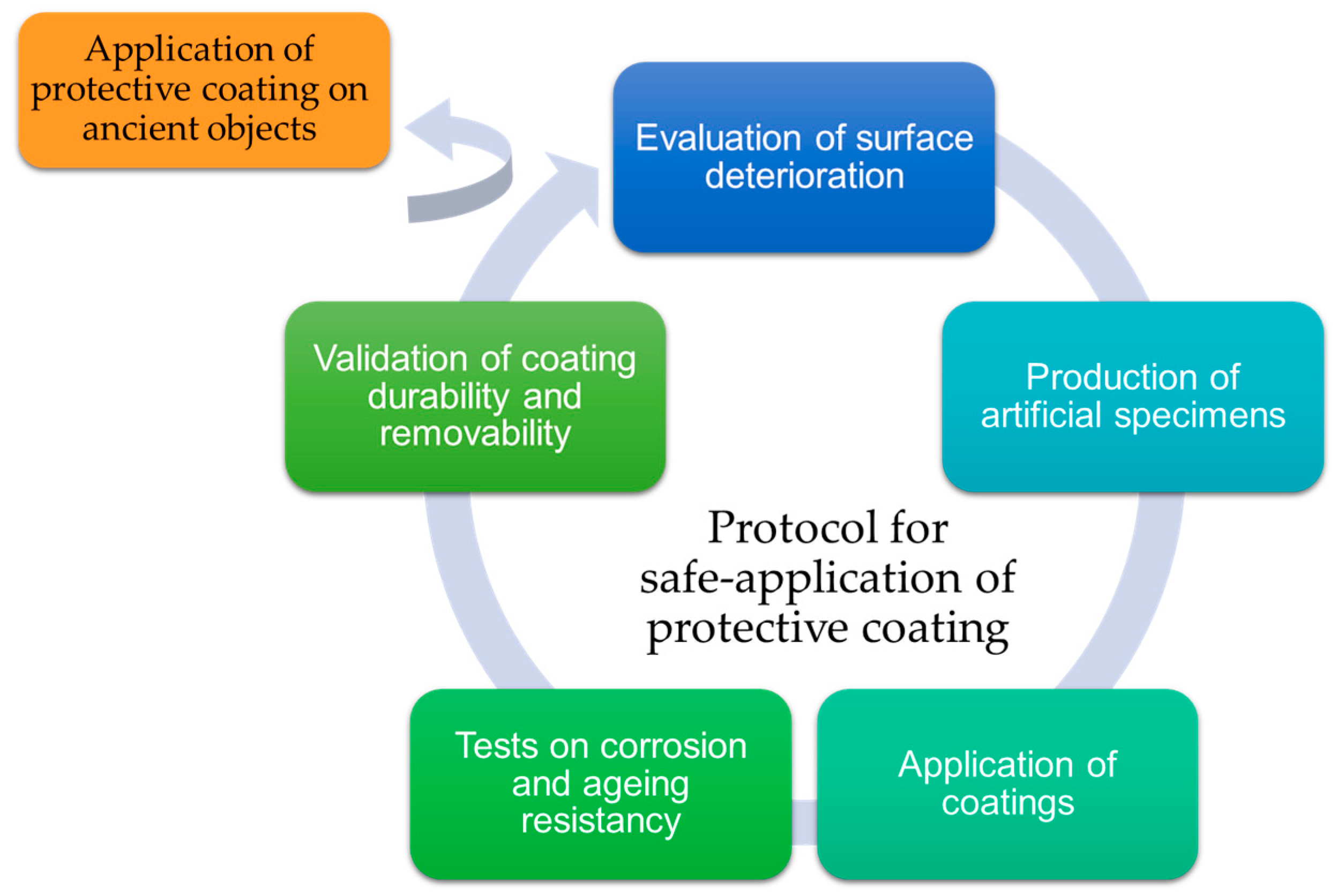
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brandi, C. Il Restauro. Teoria e Pratica (1939–1986); Saggi Arte; Editori Riuniti: Roma, Italy, 2009. [Google Scholar]
- Godoi, R.H.M.; Kontozova, V.; van Grieken, R. The shielding effect of the protective glazing of historical stained glass windows from an atmospheric chemistry perspective: Case study Sainte Chapelle, Paris. Atmos. Environ. 2006. [Google Scholar] [CrossRef]
- De Ferri, L.; Lottici, P.P.; Lorenzi, A.; Montenero, A.; Vezzalini, G. Hybrid sol-gel based coatings for the protection of historical window glass. J. Sol-Gel Sci. Technol. 2013, 66, 253–263. [Google Scholar] [CrossRef]
- Davison, S. A review of adhesives and consolidants used on glass antiquities. Stud. Conserv. 2014. [Google Scholar] [CrossRef]
- Revie, R.W. An Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Ivaskova, M.; Kotes, P.; Brodnan, M. Air pollution as an important factor in construction materials deterioration in Slovak Republic. Procedia Eng. 2015, 108, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Di Turo, F.; Proietti, C.; Screpanti, A.; Fornasier, M.F.; Cionni, I.; Favero, G.; De Marco, A. Impacts of air pollution on cultural heritage corrosion at European level: What has been achieved and what are the future scenarios. Environ. Pollut. 2016, 218, 1–9. [Google Scholar] [CrossRef]
- Bernardi, E.; Chiavari, C.; Lenza, B.; Martini, C.; Morselli, L.; Ospitali, F.; Robbiola, L. The atmospheric corrosion of quaternary bronzes: The leaching action of acid rain. Corros. Sci. 2009, 51, 159–170. [Google Scholar] [CrossRef]
- Robbiola, L.; Blengino, J.M.; Fiaud, C. Morphology and mechanisms of formation of natural patinas on archaeological Cu-Sn alloys. Corros. Sci. 1998, 40, 2083–2111. [Google Scholar] [CrossRef]
- Fabrizi, L.; Di Turo, F.; Medeghini, L.; Di Fazio, M.; Catalli, F.; De Vito, C. The application of non-destructive techniques for the study of corrosion patinas of ten Roman silver coins: The case of the medieval Grosso Romanino. Microchem. J. 2019, 145, 419–427. [Google Scholar] [CrossRef]
- Doménech-Carbò, A.; Doménech-Carbó, M.T.; Pasies, T.; Bouzas, M.d. Modeling Corrosion of Archaeological Silver-Copper Coins Using the Voltammetry of Immobilized Particles. Electroanalysis 2012, 24, 1945–1955. [Google Scholar] [CrossRef]
- Di Fazio, M.; Felici, A.C.; Catalli, F.; De Vito, C. Microstructure and chemical composition of Roman orichalcum coins emitted after the monetary reform of Augustus (23 B.C.). Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulou, O.; Vassiliou, P.; Grassini, S.; Angelini, E.; Gouda, V. Soil-induced corrosion of ancient Roman brass—A case study. Mater. Corros. 2016, 67, 160–169. [Google Scholar] [CrossRef]
- Zhou, P.; Hutchison, M.J.; Erning, J.W.; Scully, J.R.; Ogle, K. An in situ kinetic study of brass dezincification and corrosion. Electrochim. Acta 2017, 229, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Qiu, P.; Leygraf, C. Initial oxidation of brass induced by humidified air. Appl. Surf. Sci. 2011, 258, 1235–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, T.L.; Hackenberg, J.J. Determination of the onset of the dezincification of .alpha.-brass using x-ray photoelectron (ESCA) spectroscopy. J. Am. Chem. Soc. 1982, 104, 5390–5394. [Google Scholar] [CrossRef]
- Refait, P.; Abdelmoula, M.; Génin, J.M.R. Mechanisms of formation and structure of green rust one in aqueous corrosion of iron in the presence of chloride ions. Corros. Sci. 1998, 40, 1547–1560. [Google Scholar] [CrossRef]
- Dillmann, P.; Mazaudier, F.; Hœrlé, S. Advances in understanding atmospheric corrosion of iron. I. Rust characterisation of ancient ferrous artefacts exposed to indoor atmospheric corrosion. Corros. Sci. 2004, 46, 1401–1429. [Google Scholar] [CrossRef]
- Doménech, A.; Lastras, M.; Rodríguez, F.; Osete, L. Mapping of corrosion products of highly altered archeological iron using voltammetry of microparticles. Microchem. J. 2013, 106, 41–50. [Google Scholar] [CrossRef]
- Neff, D.; Dillmann, P.; Bellot-Gurlet, L.; Beranger, G. Corrosion of iron archaeological artefacts in soil: Characterisation of the corrosion system. Corros. Sci. 2005, 47, 515–535. [Google Scholar] [CrossRef]
- Ashkenazi, D.; Nusbaum, I.; Shacham-Diamand, Y.; Cvikel, D.; Kahanov, Y.; Inberg, A. A method of conserving ancient iron artefacts retrieved from shipwrecks using a combination of silane self-assembled monolayers and wax coating. Corros. Sci. 2017, 123, 88–102. [Google Scholar] [CrossRef]
- Johnson, R. The removal of microcrystalline wax from archaeological ironwork. Stud. Conserv. 1984, 29, 107–109. [Google Scholar] [CrossRef]
- Favre-quattropani, L.; Groening, P.; Ramseyer, D.; Schlapbach, L. The protection of metallic archaeological objects using plasma polymer coatings. Surf. Coat. Technol. 2000, 125, 377–382. [Google Scholar] [CrossRef]
- Klages, C.P.; Dietz, A.; Höing, T.; Thyen, R.; Weber, A.; Willich, P. Deposition and properties of carbon-based amorphous protective coatings. Surf. Coat. Technol. 1996, 80, 121–128. [Google Scholar] [CrossRef]
- Kosec, T.; Kek, D.; Milošev, I. Impedance and XPS study of benzotriazole films formed on copper, copper–zinc alloys and zinc in chloride solution. Corros. Sci. 2008, 50, 1987–1997. [Google Scholar] [CrossRef]
- Bierwagen, G.; Shedlosky, T.J.; Stanek, K. Developing and testing a new generation of protective coatings for outdoor bronze sculpture. Prog. Org. Coat. 2003, 48, 289–296. [Google Scholar] [CrossRef]
- Kosec, T.; Legat, A.; Miloev, I. The comparison of organic protective layers on bronze and copper. Prog. Org. Coat. 2010, 69, 199–206. [Google Scholar] [CrossRef]
- Rahmouni, K.; Takenouti, H.; Hajjaji, N.; Srhiri, A.; Robbiola, L. Protection of ancient and historic bronzes by triazole derivatives. Electrochim. Acta 2009, 54, 5206–5215. [Google Scholar] [CrossRef]
- Finšgar, M.; Milošev, I. Inhibition of copper corrosion by 1,2,3-benzotriazole: A review. Corros. Sci. 2010, 52, 2737–2749. [Google Scholar] [CrossRef]
- Faltermeier, R.B. A corrosion inhibitor test for copper-based artifacts. Stud. Conserv. 1999, 44, 121–128. [Google Scholar] [CrossRef]
- Brunoro, G.; Frignani, A.; Colledan, A.; Chiavari, C. Organic films for protection of copper and bronze against acid rain corrosion. Corros. Sci. 2003, 45, 2219–2231. [Google Scholar] [CrossRef]
- Galtayries, A.; Mongiatti, A.; Marcus, P.; Chiavari, C. Surface characterisation of corrosion inhibitors on bronzes for artistic casting. In Corrosion of Metallic Heritage Artefacts; Woodhead Publishing: Sawston, UK, 2007; pp. 335–351. [Google Scholar] [CrossRef]
- Balbo, A.; Chiavari, C.; Martini, C.; Monticelli, C. Effectiveness of corrosion inhibitor films for the conservation of bronzes and gilded bronzes. Corros. Sci. 2012, 59, 204–212. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, F. Structure and growth of self-assembling monolayers. Prog. Surf. Sci. 2000, 65, 151–257. [Google Scholar] [CrossRef]
- Varvara, S.; Muresan, L.M.; Rahmouni, K.; Takenouti, H. Evaluation of some non-toxic thiadiazole derivatives as bronze corrosion inhibitors in aqueous solution. Corros. Sci. 2008, 50, 2596–2604. [Google Scholar] [CrossRef]
- Muresan, L.; Varvara, S.; Stupnišek-Lisac, E.; Otmačić, H.; Marušić, K.; Horvat-Kurbegović, S.; Takenouti, H. Protection of bronze covered with patina by innoxious organic substances. Electrochim. Acta 2007, 52, 7770–7779. [Google Scholar] [CrossRef]
- Muresan, L.; Oniciu, L.; Froment, M.; Maurin, G. Inhibition of lead electrocrystallization by organic additives. Electrochim. Acta 1992, 37, 2249–2254. [Google Scholar] [CrossRef]
- Chiavari, C.; Balbo, A.; Bernardi, E.; Martini, C.; Bignozzi, M.C.; Abbottoni, M.; Monticelli, C. Protective silane treatment for patinated bronze exposed to simulated natural environments. Mater. Chem. Phys. 2013, 141, 502–511. [Google Scholar] [CrossRef]
- Masi, G.; Josse, C.; Esvan, J.; Chiavari, C.; Bernardi, E.; Martini, C.; Fabjan, E.S. Evaluation of the protectiveness of an organosilane coating on patinated Cu-Si-Mn bronze for contemporary art. Prog. Org. Coat. 2019, 127, 286–299. [Google Scholar] [CrossRef] [Green Version]
- Aufray, M.; Josse, C.; Balbo, A.; Monticelli, C.; Fabjan, E.Š.; Škrlep, L.; Chiavari, C. Practical adhesion measurements of protective coatings on bronze by three-point bending test. J. Coat. Technol. Res. 2019, 16, 1465–1477. [Google Scholar] [CrossRef] [Green Version]
- Sadat-Shojai, M.; Ershad-Langroudi, A. Polymeric coatings for protection of historic monuments: Opportunities and challenges. J. Appl. Polym. Sci. 2009, 112, 2535–2551. [Google Scholar] [CrossRef]
- Kosec, T.; Škrlep, L.; Fabjan, E.Š.; Škapin, A.S.; Masi, G.; Bernardi, E.; Robbiola, L. Development of multi-component fluoropolymer based coating on simulated outdoor patina on quaternary bronze. Prog. Org. Coat. 2019, 131, 27–35. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M.; Pipoli, M.; Morelli, A.; Frigione, M. Anti-Graffiti Behavior of Oleo/Hydrophobic Nano-Filled Coatings Applied on Natural Stone Materials. Coatings 2019, 9, 740. [Google Scholar] [CrossRef] [Green Version]
- Frigione, M.; Lettieri, M. Novel Attribute of Organic–Inorganic Hybrid Coatings for Protection and Preservation of Materials (Stone and Wood) Belonging to Cultural Heritage. Coatings 2018, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Bescher, E.; Mackenzie, J.D. Sol-gel coatings for the protection of brass and bronze. J. Sol. Gel. Sci. Technol. 2003, 26, 1223–1226. [Google Scholar] [CrossRef]
- Meng, B.; Mueller, U.; Garcia, O.; Malaga, K. Performance of a New Anti-Graffiti Agent Used for Immovable Cultural Heritage Objects. Int. J. Archit. Herit. 2014, 8, 820–834. [Google Scholar] [CrossRef]
- Ling, H.; Maiqian, N.; Guozheng, L. Preparation and feasibility analysis of fluoropolymer to the sandstone protection. Prog. Org. Coat. 2008, 62, 206–213. [Google Scholar] [CrossRef]
- Faraldi, F.; Cortese, B.; Caschera, D.; Di Carlo, G.; Riccucci, C.; De Caro, T.; Ingo, G.M. Smart conservation methodology for the preservation of copper-based objects against the hazardous corrosion. Thin Solid Film. 2017, 622, 130–135. [Google Scholar] [CrossRef]
- Salzano de Luna, M.; Buonocore, G.G.; Giuliani, C.; Messina, E.; Di Carlo, G.; Lavorgna, M.; Ingo, G.M. Long-Lasting Efficacy of Coatings for Bronze Artwork Conservation: The Key Role of Layered Double Hydroxide Nanocarriers in Protecting Corrosion Inhibitors from Photodegradation. Angew. Chem. Int. Ed. 2018, 57, 7380–7384. [Google Scholar] [CrossRef]
- Kiele, E.; Lukseniene, J.; Griguceviciene, A.; Selskis, A.; Senvaitiene, J.; Ramanauskas, R.; Kareiva, A. Methyl-modified hybrid organic-inorganic coatings for the conservation of copper. J. Cult. Herit. 2014, 15, 242–249. [Google Scholar] [CrossRef]
- El-Haddad, M.N. Chitosan as a green inhibitor for copper corrosion in acidic medium. Int. J. Biol. Macromol. 2013, 55, 142–149. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Wu, Y. The inhibition effect and mechanism of l-cysteine on the corrosion of bronze covered with a CuCl patina. Corros. Sci. 2015, 97, 89–99. [Google Scholar] [CrossRef]
- Badawy, W.A.; Ismail, K.M.; Medany, S.S. Optimization of the electropolymerization of 1-amino-9,10-anthraquinone conducting films from aqueous media. Electrochim. Acta 2006, 51, 6353–6360. [Google Scholar] [CrossRef]
- Ismail, K.M. Evaluation of cysteine as environmentally friendly corrosion inhibitor for copper in neutral and acidic chloride solutions. Electrochim. Acta 2007, 52, 7811–7819. [Google Scholar] [CrossRef]
- Albini, M.; Letardi, P.; Mathys, L.; Brambilla, L.; Schröter, J.; Junier, P.; Joseph, E. Comparison of a bio-based corrosion inhibitor versus benzotriazole on corroded copper surfaces. Corros. Sci. 2018, 143, 84–92. [Google Scholar] [CrossRef]
- Giuntoli, G.; Rosi, L.; Frediani, M.; Sacchi, B.; Salvadori, B.; Porcinai, S.; Frediani, P. Novel coatings from renewable resources for the protection of bronzes. Prog. Org. Coat. 2014, 77, 892–903. [Google Scholar] [CrossRef]
- Pedna, A.; Giuntoli, G.; Frediani, M.; Frediani, P.; Rosi, L. Synthesis of functionalized polyolefins with novel applications as protective coatings for stone Cultural Heritage. Prog. Org. Coat. 2013, 76, 1600–1607. [Google Scholar] [CrossRef]
- Giuliani, C.; Pascucci, M.; Riccucci, C.; Messina, E.; de Luna, M.S.; Lavorgna, M.; Di Carlo, G. Chitosan-based coatings for corrosion protection of copper-based alloys: A promising more sustainable approach for cultural heritage applications. Prog. Org. Coat. 2018, 122, 138–146. [Google Scholar] [CrossRef]
- Grassini, S.; Angelini, E.; Mao, Y.; Novakovic, J.; Vassiliou, P. Aesthetic coatings for silver based alloys with improved protection efficiency. Prog. Org. Coat. 2011, 72, 131–137. [Google Scholar] [CrossRef]
- Milanesi, C.; Baldi, F.; Borin, S.; Brusetti, L.; Ciampolini, F.; Iacopini, F.; Cresti, M. Deterioration of medieval painting in the chapel of the Holy Nail, Siena (Italy) partially treated with Paraloid B72. Int. Biodeterior. Biodegrad. 2009, 63, 844–850. [Google Scholar] [CrossRef]
- Scalarone, D.; Lazzari, M.; Chiantore, O. Acrylic protective coatings modified with titanium dioxide nanoparticles: Comparative study of stability under irradiation. Polym. Degrad. Stab. 2012. [Google Scholar] [CrossRef]
- Cano, E.; Bastidas, D.M.; Argyropoulos, V.; Fajardo, S.; Siatou, A.; Bastidas, J.M.; Degrigny, C. Electrochemical characterization of organic coatings for protection of historic steel artefacts. J. Solid State Electrochem. 2010, 14, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Barrera, P.R.; Gómez, F.R.; Ochoa, E.G. Assessing of New Coatings for Iron Artifacts Conservation by Recurrence Plots Analysis. Coatings 2018, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- de Bardi, M.; Hutter, H.; Schreiner, M.; Bertoncello, R. Sol-gel silica coating for potash-lime-silica stained glass: Applicability and protective effect. J. Non. Cryst. Solids 2014. [Google Scholar] [CrossRef]
- Melcher, M.; Wiesinger, R.; Schreiner, M. Degradation of glass artifacts: Application of modern surface analytical techniques. Acc. Chem. Res. 2010. [Google Scholar] [CrossRef] [PubMed]
- de Bardi, M.; Hutter, H.; Schreiner, M.; Bertoncello, R. Potash-lime-silica glass: Protection from weathering. Herit. Sci. 2015. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, A.; Becherini, F.; Bassato, G.; Bellio, M. Condensation on ancient stained glass windows and efficiency of protective glazing systems: Two French case studies, Sainte-Chapelle (Paris) and Saint-Urbain Basilica (Troyes). J. Cult. Herit. 2006. [Google Scholar] [CrossRef]
- Klein, L.; Aparicio, M.; Jitianu, A. Handbook of Sol-Gel Science and Technology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Lazzari, M.; Chiantore, O. Drying and oxidative degradation of linseed oil. Polym. Degrad. Stab. 1999, 65, 303–313. [Google Scholar] [CrossRef]
- Abd-Allah, R. Stabilization and treatment of corroded glass objects displayed in the museum of Jordanian heritage. Mediterr. Archaeol. Archaeom. 2007, 7, 19–28. [Google Scholar]
- Abd-Allah, R. In situ glass conservation: A case study from the archaeological site of Barsinia, Jordan. Adumatu J. 2007, 16, 25–36. [Google Scholar]
- Bougault, V. La Restauration des Vitraux Anciens; Laboratoire de Recherche des Monuments Historiques: Champs-sur-Marne, France, 2004. [Google Scholar]
- Wheeler, G. Sol-gel science and cultural heritage. In Handbook of Sol-Gel Science and Technology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Carmona, N.; Villegas, M.A.; Navarro, J.M.F. Protective silica thin coatings for historical glasses. Thin Solid Film. 2004. [Google Scholar] [CrossRef]
- Monti, M.; Bianco, B.D.; Bertoncello, R.; Voltolina, S. New protective coatings for ancient glass: Silica thin-films from perhydropolysilazane. J. Cult. Herit. 2008. [Google Scholar] [CrossRef]
- Bertoncello, R.; Monti, M.; Sada, C. Silica thin-films from perhydropolysilazane for the protection of ancient glass. Mater. Sci. 2016, 106–113. [Google Scholar] [CrossRef]
- Bertoncello, R.; Milanese, L.; Dran, J.C.; Bouquillon, A.; Sada, C. Sol-gel deposition of silica films on silicate glasses: Influence of the presence of lead in the glass or in precursor solutions. J. Non. Cryst. Solids 2006. [Google Scholar] [CrossRef]
- Bianco, B.D.; Bertoncello, R. Sol-gel silica coatings for the protection of cultural heritage glass. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2008. [Google Scholar] [CrossRef]
- Carmona, N.; Villegas, M.A.; Navarro, J.M.F. Sol-gel coatings in the ZrO2-SiO2 system for protection of historical works of glass. Thin Solid Film. 2006. [Google Scholar] [CrossRef]
- Holubová, B.; Cílová, Z.Z.; Kučerová, I.; Zlámal, M. Weatherability of hybrid organic-inorganic silica protective coatings on glass. Prog. Org. Coat. 2015. [Google Scholar] [CrossRef]
- Tsakalof, A.; Manoudis, P.; Karapanagiotis, I.; Chryssoulakis, I.; Panayiotou, C. Assessment of synthetic polymeric coatings for the protection and preservation of stone monuments. J. Cult. Herit. 2007, 8, 69–72. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Grossi, C.M. Millennium-long damage to building materials in London. Sci. Total Environ. 2009, 407, 1354–1361. [Google Scholar] [CrossRef]
- Ghedini, N.; Ozga, I.; Bonazza, A.; Dilillo, M.; Cachier, H.; Sabbioni, C. Atmospheric aerosol monitoring as a strategy for the preventive conservation of urban monumental heritage: The Florence Baptistery. Atmos. Environ. 2011, 45, 5979–5987. [Google Scholar] [CrossRef]
- Belfiore, C.M.; Barca, D.; Bonazza, A.; Comite, V.; La Russa, M.F.; Pezzino, A.; Sabbioni, C. Application of spectrometric analysis to the identification of pollution sources causing cultural heritage damage. Environ. Sci. Pollut. Res. 2013, 20, 8848–8859. [Google Scholar] [CrossRef]
- Grossi, C.M.; Murray, M. Characteristics of carbonate building stones that influence the dry deposition of acidic gases. Constr. Build. Mater. 1999, 13, 101–108. [Google Scholar] [CrossRef]
- Mosca, S.; Artesani, A.; Gulotta, D.; Nevin, A.; Goidanich, S.; Valentini, G.; Comelli, D. Raman mapping and time-resolved photoluminescence imaging for the analysis of a cross-section from a modern gypsum sculpture. Microchem. J. 2018, 139, 500–505. [Google Scholar] [CrossRef]
- Orecchio, S. Analytical method, pattern and sources of polycyclic aromatic hydrocarbons (PAHs) in the stone of the Temples of Agrigento (Italy). J. Hazard. Mater. 2010, 176, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Searle, D.E.; Mitchell, D.J. The effect of coal and diesel particulates on the weathering loss of Portland Limestone in an urban environment. Sci. Total Environ. 2006, 370, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Massey, S.W. The effects of ozone and NO(x) on the deterioration of calcareous stone. Sci. Total Environ. 1999, 227, 109–121. [Google Scholar] [CrossRef]
- Cultrone, G.; Sebastián, E. Laboratory simulation showing the influence of salt efflorescence on the weathering of composite building materials. Environ. Geol. 2008, 56, 729–740. [Google Scholar] [CrossRef]
- Nuhoglu, Y.; Oguz, E.; Uslu, H.; Ozbek, A.; Ipekoglu, B.; Ocak, I.; Hasenekoglu, I. The accelerating effects of the microorganisms on biodeterioration of stone monuments under air pollution and continental-cold climatic conditions in Erzurum, Turkey. Sci. Total Environ. 2006, 364, 272–283. [Google Scholar] [CrossRef] [Green Version]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Zanardini, E.; Abbruscato, P.; Ghedini, N.; Realini, M.; Sorlini, C. Influence of atmospheric pollutants on the biodeterioration of stone. Int. Biodeterior. Biodegrad. 2000, 45, 35–42. [Google Scholar] [CrossRef]
- Garcia-Vallés, M.; Topal, T.; Vendrell-Saz, M. Lichenic growth as a factor in the physical deterioration or protection of Cappadocian monuments. Environ. Geol. 2003, 43, 776–781. [Google Scholar] [CrossRef]
- Herrera, L.K.; Arroyave, C.; Guiamet, P.; de Saravia, S.G.; Videla, H. Biodeterioration of peridotite and other constructional materials in a building of the Colombian cultural heritage. Int. Biodeterior. Biodegrad. 2004, 135–141. [Google Scholar] [CrossRef]
- Diakumaku, E.; Gorbushina, A.A.; Krumbein, W.E.; Panina, L.; Soukharjevski, S. Black fungi in marble and limestones—An aesthetical, chemical and physical problem for the conservation of monuments. Sci. Total Environ. 1995, 167, 295–304. [Google Scholar] [CrossRef]
- Feller, R.L. New solvent-type varnishes. Stud. Conserv. 1961. [Google Scholar] [CrossRef]
- Melo, M.J.; Bracci, S.; Camaiti, M.; Chiantore, O.; Piacenti, F. Photodegradation of acrylic resins used in the conservation of stone. Polym. Degrad. Stab. 1999. [Google Scholar] [CrossRef]
- Favaro, M.; Mendichi, R.; Ossola, F.; Russo, U.; Simon, S.; Tomasin, P.; Vigato, P.A. Evaluation of polymers for conservation treatments of outdoor exposed stone monuments. Part I: Photo-oxidative weathering. Polym. Degrad. Stab. 2006. [Google Scholar] [CrossRef]
- Chiantore, O.; Lazzari, M. Photo-oxidative stability of paraloid acrylic protective polymers. Polymer 2001. [Google Scholar] [CrossRef]
- Baer, N.S.; Feller, R.L. Accelerated Aging: Photochemical and Thermal Aspects. J. Am. Inst. Conserv. 1996. [Google Scholar] [CrossRef]
- Alessandrini, G.; Aglietto, M.; Castelvetro, V.; Ciardelli, F.; Peruzzi, R.; Toniolo, L. Comparative evaluation of fluorinated and unfluorinated acrylic copolymers as water-repellent coating materials for stone. J. Appl. Polym. Sci. 2000. [Google Scholar] [CrossRef]
- Sabatini, V.; Cattò, C.; Cappelletti, G.; Cappitelli, F.; Antenucci, S.; Farina, H.; Di Silvestro, G. Protective features, durability and biodegration study of acrylic and methacrylic fluorinated polymer coatings for marble protection. Prog. Org. Coat. 2018. [Google Scholar] [CrossRef]
- Poli, T.; Toniolo, L. Protection efficacy of fluorinated acrylic copolymers applied on historical Italian marbles. Mater. Res. Soc. Symp. Proc. 2005. [Google Scholar] [CrossRef]
- Benedetti, E.; D’Alessio, A.; Zini, M.F.; Bramanti, E.; Tirelli, N.; Vergamini, P.; Moggi, G. Characterization of acrylic resins and fluoroelastomer blends as potential materials in stone protection. Polym. Int. 2000. [Google Scholar] [CrossRef]
- Toniolo, L.; della Volpe, C.; Brugnara, M.; Poli, T. Partially fluorinated acrylic copolymers as coatings for stone protection: Characterization and surface properties. Mater. Res. Soc. Symp. Proc. 2002. [Google Scholar] [CrossRef]
- Jensen, A.A.; Poulsen, P.B.; Bossi, R.; Miljøundersøgelser, D.; FORCE Technology. Survey and Environmental/Health Assessment of Fluorinated Substances in Impregnated Consumer Products and Impregnating Agents; Danish Environmental Protection Agency: Copenhagen, Denmark, 2008. [Google Scholar]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Watanabe, T. Photogeneration of highly amphiphilic TiO2 surfaces. Adv. Mater. 1998. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015. [Google Scholar] [CrossRef]
- Nano-Cathedral. Available online: http://www.nanocathedral.eu (accessed on 21 January 2020).
- Boostani, H.; Modirrousta, S. Review of Nanocoatings for Building Application. Procedia Eng. 2016, 145, 1541–1548. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. A Review on Superhydrophobic Polymer Nanocoatings: Recent Development and Applications. Ind. Eng. Chem. Res. 2018, 57, 2727–2745. [Google Scholar] [CrossRef]
- Aldoasri, M.; Darwish, S.; Adam, M.; Elmarzugi, N.; Ahmed, S. Protecting of Marble Stone Facades of Historic Buildings Using Multifunctional TiO2 Nanocoatings. Sustainability 2017, 9, 2002. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Lampakis, D. Waterborne superhydrophobic and superoleophobic coatings for the protection of marble and sandstone. Materials 2018, 11, 585. [Google Scholar] [CrossRef] [Green Version]
- Karapanagiotis, I.; Pavlou, A.; Manoudis, P.N.; Aifantis, K.E. Water repellent ORMOSIL films for the protection of stone and other materials. Mater. Lett. 2014, 131, 276–279. [Google Scholar] [CrossRef]
- Ntelia, E.; Karapanagiotis, I. Superhydrophobic Paraloid B72. Prog. Org. Coat. 2020, 139. [Google Scholar] [CrossRef]
- Miliani, C.; Velo-Simpson, M.L.; Scherer, G.W. Particle-modified consolidants: A study on the effect of particles on sol-gel properties and consolidation effectiveness. J. Cult. Herit. 2007. [Google Scholar] [CrossRef]
- Gherardi, F.; Colombo, A.; Goidanich, S.; Simonutti, R.; Toniolo, L. Innovative Nano-TiO2 Particles for Self-cleaning Treatments of Historic Architecture and Sculptures. Restor. Build. Mouments 2014. [Google Scholar] [CrossRef]
- Bergamonti, L.; Predieri, G.; Paz, Y.; Fornasini, L.; Lottici, P.P.; Bondioli, F. Enhanced self-cleaning properties of N-doped TiO2 coating for Cultural Heritage. Microchem. J. 2017. [Google Scholar] [CrossRef]
- Kapridaki, C.; Verganelaki, A.; Dimitriadou, P.; Maravelaki-Kalaitzaki, P. Conservation of monuments by a three-layered compatible treatment of TEOS-Nano-Calcium Oxalate consolidant and TEOS-PDMS-TiO2 hydrophobic/photoactive hybrid nanomaterials. Materials 2018, 11, 684. [Google Scholar] [CrossRef] [Green Version]
- Innovaconcrete. Available online: http://www.innovaconcrete.eu (accessed on 21 January 2020).
- Ruffolo, S.A.; la Russa, M.F.; Malagodi, M.; Rossi, C.O.; Palermo, A.M.; Crisci, G.M. ZnO and ZnTiO3 nanopowders for antimicrobial stone coating. Appl. Phys. A Mater. Sci. Process. 2010. [Google Scholar] [CrossRef]
- Aldosari, M.A.; Darwish, S.S.; Adam, M.A.; Elmarzugi, N.A.; Ahmed, S.M. Using ZnO nanoparticles in fungal inhibition and self-protection of exposed marble columns in historic sites. Archaeol. Anthropol. Sci. 2019. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; la Russa, M.F. Nanostructured coatings for stone protection: An overview. Front. Mater. 2019. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.; Elhaddad, F.; Facio, D.S.; Mosquera, M.J. A novel TiO2-SiO2 nanocomposite converts a very friable stone into a self-cleaning building material. Appl. Surf. Sci. 2013. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Kolinkeová, B.; Panayiotou, C. Superhydrophobic films for the protection of outdoor cultural heritage assets. Appl. Phys. A Mater. Sci. Process. 2009. [Google Scholar] [CrossRef]
- de Ferri, L.; Lottici, P.P.; Lorenzi, A.; Montenero, A.; Salvioli-Mariani, E. Study of silica nanoparticles—polysiloxane hydrophobic treatments for stone-based monument protection. J. Cult. Herit. 2011. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Tuning the wetting properties of siloxane-nanoparticle coatings to induce superhydrophobicity and superoleophobicity for stone protection. Mater. Des. 2016, 108, 736–744. [Google Scholar] [CrossRef]
- Ferrari, A.M.; Pini, M.; Neri, P.; Bondioli, F. Nano-TiO2 Coatings for Limestone: Which Sustainability for Cultural Heritage? Coatings 2015, 5, 232–245. [Google Scholar] [CrossRef]
- Quagliarini, E.; Graziani, L.; Diso, D.; Licciulli, A.; D’Orazio, M. Is nano-TiO2 alone an effective strategy for the maintenance of stones in Cultural Heritage? J. Cult. Herit. 2018. [Google Scholar] [CrossRef]
- Goffredo, G.B.; Accoroni, S.; Totti, C.; Romagnoli, T.; Valentini, L.; Munafò, P. Titanium dioxide based nanotreatments to inhibit microalgal fouling on building stone surfaces. Build. Environ. 2017. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Osimani, A.; Aquilanti, L.; Clementi, F.; D’Orazio, M. The influence of clay brick substratum on the inhibitory efficiency of TiO2 nanocoating against biofouling. Build. Environ. 2014. [Google Scholar] [CrossRef]
- Carter, N.E.A.; Viles, H.A. Bioprotection explored: The story of a little known earth surface process. Geomorphology 2005, 67, 273–281. [Google Scholar] [CrossRef]
- Fidanza, M.R.; Caneva, G. Natural biocides for the conservation of stone cultural heritage: A review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Rodriguez-Gallego, M.; Chekroun, K.B.; Gonzalez-Munoz, M.T. Conservation of ornamental stone by Myxococcus xanthus-induced carbonate biomineralization. Appl. Environ. Microbiol. 2003, 69, 2182–2193. [Google Scholar] [CrossRef] [Green Version]
- Jroundi, F.; Fernández-Vivas, A.; Rodriguez-Navarro, C.; Bedmar, E.J.; González-Muñoz, M.T. Bioconservation of deteriorated monumental calcarenite stone and identification of bacteria with carbonatogenic activity. Microb. Ecol. 2010, 60, 39–54. [Google Scholar] [CrossRef]
- Sternberg, T.; Viles, H.; Cathersides, A. Evaluating the role of ivy (Hedera helix) in moderating wall surface microclimates and contributing to the bioprotection of historic buildings. Build. Environ. 2011, 46, 293–297. [Google Scholar] [CrossRef]
- de la Rosa, J.P.M.I.; Warke, P.A.; Smith, B.J. Lichen-induced biomodification of calcareous surfaces: Bioprotection versus biodeterioration. Prog. Phys. Geogr. 2013, 37, 325–351. [Google Scholar] [CrossRef]
- Ariño, X.; Ortega-Calvo, J.J.; Gomez-Bolea, A.; Saiz-Jimenez, C. Lichen colonization of the Roman pavement at Baelo Claudia (Cadiz, Spain): Biodeterioration vs. bioprotection. Sci. Total Environ. 1995, 167, 353–363. [Google Scholar] [CrossRef] [Green Version]
- de los Ríos, A.; Ascaso, C. Contributions of in situ microscopy to the current understanding of stone biodeterioration. Int. Microbiol. 2005, 8, 181–188. [Google Scholar] [PubMed]
- Ranalli, G.; Lustrato, G.; Alfano, G.; Andreotti, A. Long term microbial monitoring of bio-cleaned wall paintings at Pisa. Ann. Microbiol. 2009, 59, 19–29. Available online: www.annmicro.unimi.it (accessed on 8 January 2020).





| Element | Chemical Formula | Mineralogical Name |
|---|---|---|
| Cu | Cu2O | Cuprite |
| CuO | Tenorite | |
| CuCO3Cu(OH)2 | Malachite | |
| 2CuCO3Cu(OH)2 | Azurite | |
| Cu/Cl | CuCl | Nantokite |
| Cu2(OH)3Cl | Atacamite and polymorphs | |
| Cu/S | Cu2S | Chalcocite |
| CuS | Covellite | |
| CuSO4 2Cu(OH)2 | Brochantite | |
| Sn | SnO | Tin(II) oxide, stannous oxide |
| SnO2 | Cassiterite | |
| Pb | PbO | Litharge/massicot |
| Ag | Ag2S | Acanthite |
| AgCl | Chlorargyrite | |
| Fe | Fe3O4 | Magnetite |
| (Fe3+,Ni2+)8(OH,O)16Cl1.25 · nH2O | Akaganeite | |
| Zn | ZnO | Zincite |
| Bare Bronze | Brown Patina | Green Patina | |
|---|---|---|---|
| 3% BTA + ethanol | ⇓ | ⇓ | No improvement |
| Paraloid B44 + 3% BTA + carnauba wax | ⇓ | ⇓ | Protection only in the anodic part |
| Bare Bronze | Brown Patina | Green Patina | |
|---|---|---|---|
| 3% BTA + ethanol | Protection enhanced of factor 10 | Protection enhanced of factor 6 | No protection |
| Paraloid B44 + 3% BTA + carnauba wax | Protection at the initial stage | Similar to 3% BTA + ethanol | Protection at the initial stage |
| Polymer | IUPAC Name | Molecule Structure |
|---|---|---|
| PMI | 1-phenyl-4-methyl imidazole |  |
| TMI | 1-p(tolyl)-4-methyl imidazole |  |
| MAcT | 2-mercapto-5-R-acetylamino-1,3,4-thiadiazole |  |
| MAT | 2-mercapto-5-R-amino-1,3,4-thiadiazole |  |
| BTA-24 h | BTA-14 Days | Bio-Based | |
|---|---|---|---|
| Thickness (μm) | 15 | 33 | 15 |
| Blackening | ✓✓ | ✓✓ | ✓ |
| Copper oxalates | ✓ | ✓ | ✓✓ |
| Atacamite | ✓ | ✓ | ↓ |
| BTA-Cu | ↓ | ↓ | ✗ |
| Cause | Effect |
|---|---|
| Pollution | Black crusts Gypsum formation Erosion |
| Water | Saline efflorescence Mechanical stress Microorganism growth |
| Microorganisms | Colour changes Erosion |
| Plants | Mechanical stress |
| Polymer | IUPAC name | Molecule Structure |
|---|---|---|
| MA | Methyl acrylate | 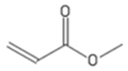 |
| EMA | Ethyl methacrylate | 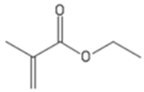 |
| MMA | Methyl methacrylate |  |
| BuMA | tert-Butyl methacrylate |  |
| iBuMA | Isobutyl methacrylate | 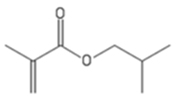 |
| XFDM | 1H,1H,2H,2H-Perfluorodecylmethacrylate | 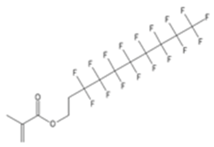 |
| HFIM | 1,1,1,3,3,3-Hexafluoroisopropylmethacrylate | 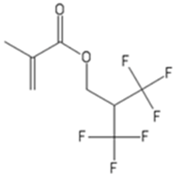 |
| TFEM | 2,2,2-Trifluoroethyl methacrylate |  |
| POMA | 3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluoro-octyl methacrylate |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artesani, A.; Di Turo, F.; Zucchelli, M.; Traviglia, A. Recent Advances in Protective Coatings for Cultural Heritage–An Overview. Coatings 2020, 10, 217. https://doi.org/10.3390/coatings10030217
Artesani A, Di Turo F, Zucchelli M, Traviglia A. Recent Advances in Protective Coatings for Cultural Heritage–An Overview. Coatings. 2020; 10(3):217. https://doi.org/10.3390/coatings10030217
Chicago/Turabian StyleArtesani, Alessia, Francesca Di Turo, Margherita Zucchelli, and Arianna Traviglia. 2020. "Recent Advances in Protective Coatings for Cultural Heritage–An Overview" Coatings 10, no. 3: 217. https://doi.org/10.3390/coatings10030217
APA StyleArtesani, A., Di Turo, F., Zucchelli, M., & Traviglia, A. (2020). Recent Advances in Protective Coatings for Cultural Heritage–An Overview. Coatings, 10(3), 217. https://doi.org/10.3390/coatings10030217





