Investigation of the Effects of Various Organic Solvents on the PCBM Electron Transport Layer of Perovskite Solar Cells
Abstract
:1. Introduction
2. Experiments
2.1. Preparation of Perovskite Precursor Solution and ETL Solution
2.2. Fabrication of the PSC Devices
2.3. Characterization of the ETLs and PSCs
3. Results and Discussion
3.1. Characterization of the ETLs
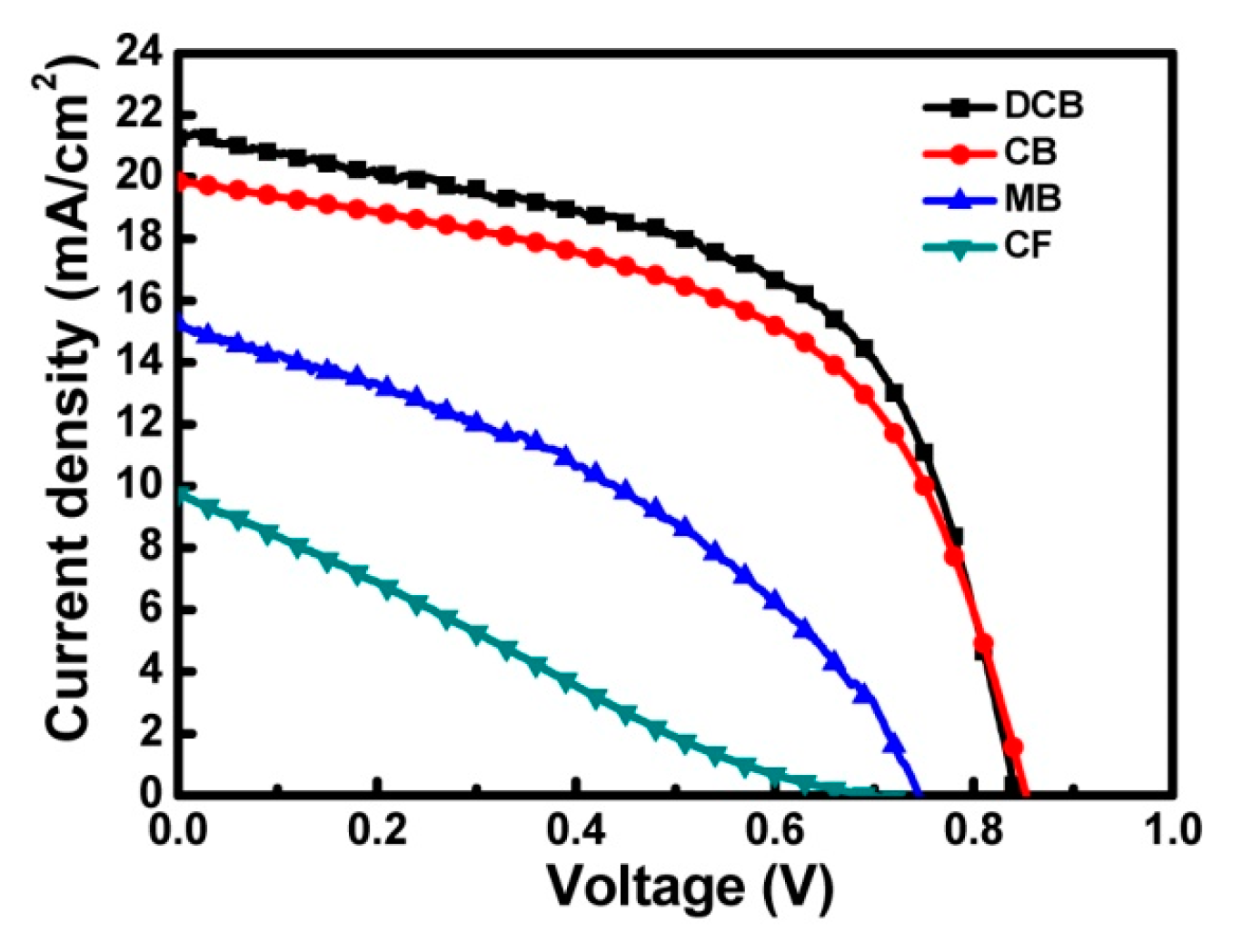
3.2. Characterization of the PSC Devices
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goetzberger, A.; Hebling, C.; Schock, H.W. Photovoltaic materials, history, status and outlook. Mater. Sci. Eng. R Rep. 2003, 40, 1–46. [Google Scholar] [CrossRef]
- Rovira, P.I.; Ferlauto, A.S.; Koh, J.; Wronski, C.R.; Collins, R.W. Optics of textured amorphous silicon surfaces. J. Non-Cryst. Solids 2000, 266, 279–283. [Google Scholar] [CrossRef]
- Hubbard, S.M.; Cress, C.D.; Bailey, C.G.; Raffaelle, R.P.; Bailey, S.G.; Wilt, D.M. Effect of strain compensation on quantum dot enhanced GaAs solar cells. Appl. Phys. Lett. 2008, 92, 123512. [Google Scholar] [CrossRef]
- Qiao, S.; Liu, J.; Fu, G.; Ren, K.; Li, Z.; Wang, S.; Pan, C. ZnO nanowire based CIGS solar cell and its efficiency enhancement by the piezo-phototronic effect. Nano Energy 2018, 49, 508–514. [Google Scholar] [CrossRef]
- Fraga, D.; Barrachina, E.; Calvet, I.; Stoyanova, T.; Carda, J.B. Developing CIGS solar cells on glass-ceramic substrates. Mater. Lett. 2018, 221, 104–106. [Google Scholar] [CrossRef]
- Tang, C.W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 1986, 48, 183. [Google Scholar] [CrossRef]
- Lami, V.; Weu, A.; Zhang, J.; Chen, Y.; Fei, Z.; Heeney, M.; Friend, R.H.; Vaynzof, Y. Visualizing the Vertical Energetic Landscape in Organic Photovoltaics. Joule 2019, 3, 2513–2534. [Google Scholar] [CrossRef]
- Tsai, C.H.; Shiu, S.L.; Lin, W.C.; Chou, Y.R.; Yu, Y.H. Synthesis of reduced graphene oxide/macrocyclic ytterbium complex nanocomposites and their application in the counter electrodes of dye-sensitized solar cells. Org. Electron. 2019, 64, 166–175. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Im, J.H.; Lee, C.R.; Lee, J.W.; Park, S.W.; Park, N.G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.B.; Duan, H.S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Koh, T.M.; Febriansyah, B.; Bruno, A.; Mathews, N.; Mhaisalkar, S.G.; Soci, C. Mixed-dimensional naphthylmethylammoinium-methylammonium lead iodide perovskites with improved thermal stability. Sci. Rep. 2020, 10, 429. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.H.; Li, B.T.; Lee, C.F.; Huang, Z.H.; Huang, Y.C.; Su, W.F. High-efficiency perovskite solar cell using cobalt doped nickel oxide hole transport layer fabricated by NIR process. Sol. Energy Mater. Sol. Cells 2020, 208, 110352. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Li, Y.; Yan, W.; Li, Y.; Wang, S.; Wang, W.; Bian, Z.; Xiao, L.; Gong, Q. Direct observation of long electron-hole diffusion distance in CH3NH3PbI3 perovskite thin film. Sci. Rep. 2015, 5, 14485. [Google Scholar] [CrossRef]
- Yang, G.; Tao, H.; Qin, P.; Ke, W.; Fang, G. Recent progress in electron transport layers for efficient perovskite solar cells. J. Mater. Chem. A 2016, 4, 3970–3990. [Google Scholar] [CrossRef]
- Cao, K.; Zuo, Z.; Cui, J.; Shen, Y.; Moehl, T.; Zakeeruddin, S.M.; Grätzel, M.; Wang, M. Efficient screen printed perovskite solar cells based on mesoscopic TiO2/Al2O3/NiO/carbon architecture. Nano Energy 2015, 17, 171–179. [Google Scholar] [CrossRef]
- Huang, L.; Li, C.; Sun, X.; Xu, R.; Du, Y.; Ni, J.; Cai, H.; Li, J.; Hu, Z.; Zhang, J. Efficient and hysteresis-less pseudo-planar heterojunction perovskite solar cells fabricated by a facile and solution-saving one-step dip-coating method. Org. Electron. 2017, 40, 13–23. [Google Scholar] [CrossRef]
- Das, S.; Yang, B.; Gu, G.; Joshi, P.C.; Ivanov, I.N.; Rouleau, C.M.; Aytug, T.; Geohegan, D.B.; Xiao, K. High-performance flexible perovskite solar cells by using a combination of ultrasonic spray-coating and low thermal budget photonic curing. ACS Photonics 2015, 2, 680–686. [Google Scholar] [CrossRef]
- Oku, T.; Matsumoto, T.; Suzuki, A.; Suzuki, K. Fabrication and characterization of a perovskite-type solar cell with a substrate size of 70 mm. Coatings 2015, 5, 646–655. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Q.; Liu, T.; Zhao, L.; Luo, D.; Wu, J.; Zhang, Y.; Zhang, W.; Liu, F.; Russell, T.P.; et al. Charge-carrier balance for highly efficient inverted planar heterojunction perovskite solar cells. Adv. Mater. 2016, 28, 10718–60724. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lee, E.C. Solvent engineering of the electron transport layer using 1,8-diiodooctane for improving the performance of perovskite solar cells. Org. Electron. 2015, 24, 101–105. [Google Scholar] [CrossRef]
- Shao, Y.; Yuan, Y.; Huang, J. Correlation of energy disorder and open-circuit voltage in hybrid perovskite solar cells. Nat. Energy 2016, 1, 15001. [Google Scholar] [CrossRef]
- Tsai, C.H.; Lin, C.M.; Kuei, C.H. Improving the performance of perovskite solar cells by adding 1,8-diiodooctane in the CH3NH3PbI3 perovskite layer. Sol. Energy 2018, 176, 178–185. [Google Scholar] [CrossRef]
- Huang, J.; Gao, C.; Zhang, D.; Tian, Q.; Zhang, F.; Liu, S.F. Influence of film quality on power conversion efficiency in perovskite solar cells. Coatings 2019, 9, 622. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.; Prochowicz, D.; Saliba, M.; Boix, P.P.; Zakeeruddin, S.M.; Grätzel, M. Interfacial kinetics of efficient perovskite solar cells. Crystals 2017, 7, 252. [Google Scholar] [CrossRef]
- Gorman, W.G.; Hall, G.D. Dielectric constant correlations with solubility and solubility parameters. J. Pharm. Sci. 1964, 53, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chung, T.; Bae, S.; Lee, S.W.; Lee, K.D.; Kim, H.; Lee, S.; Kang, Y.; Lee, H.S.; Kim, D. Improved performance and thermal stability of perovskite solar cells prepared via a modified sequential deposition process. Org. Electron. 2017, 41, 266–273. [Google Scholar] [CrossRef]
- Tsai, C.H.; Huang, W.C.; Wang, W.S.; Shih, C.J.; Chi, W.F.; Hu, Y.C.; Yu, Y.H. Reduced graphene oxide/macrocyclic iron complex hybrid materials as counter electrodes for dye-sensitized solar cells. J. Colloid Interface Sci. 2017, 495, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, Y.; Yuan, J.; Hong, Q.; Shi, G.; Yuan, D.; Wei, J.; Huang, C.; Tang, J.; Fung, M.K. Improved performance of inverted planar perovskite solar cells with F4-TCNQ doped PEDOT:PSS hole transport layers. J. Mater. Chem. A 2017, 5, 5701–5708. [Google Scholar] [CrossRef]




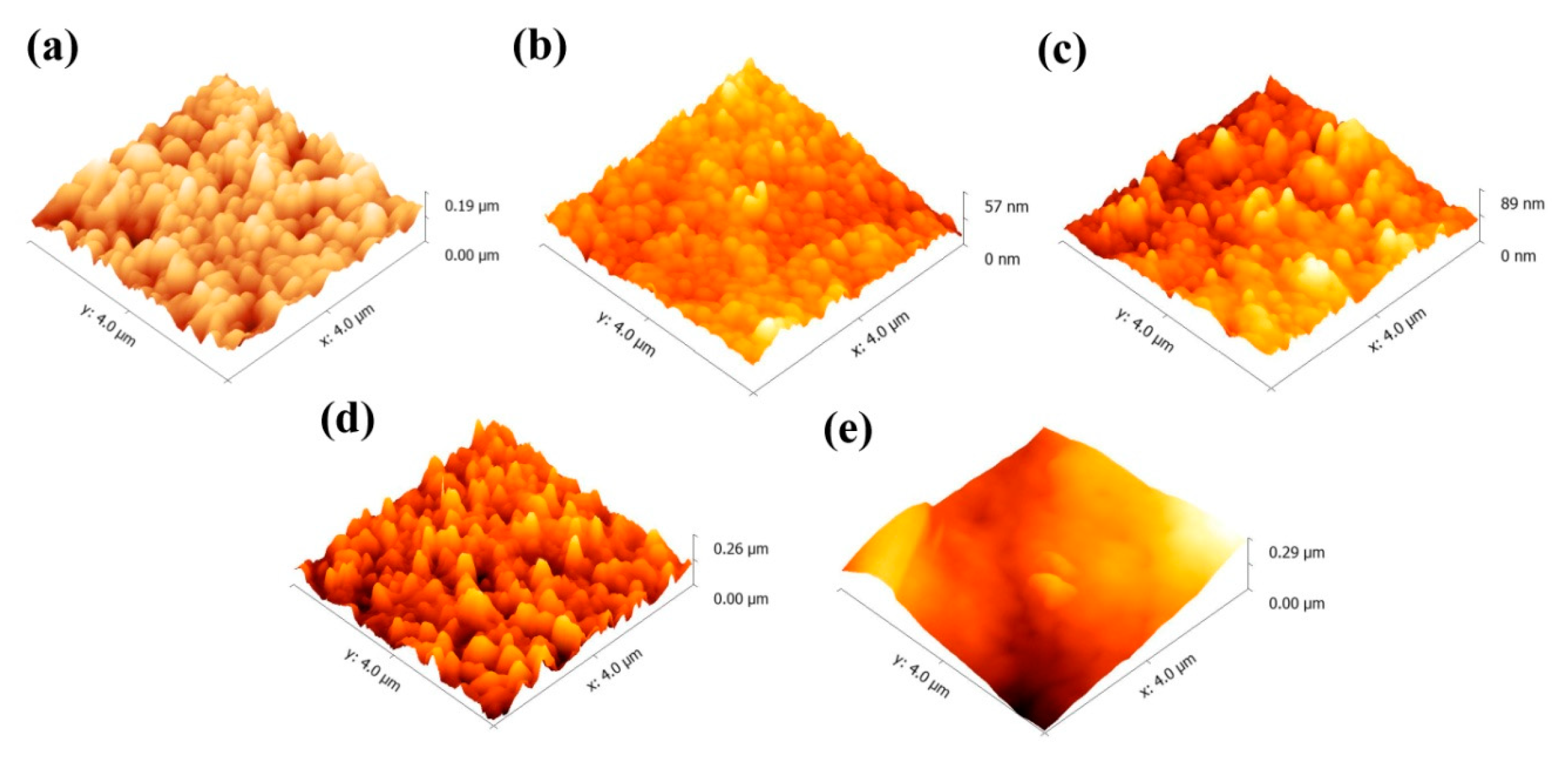
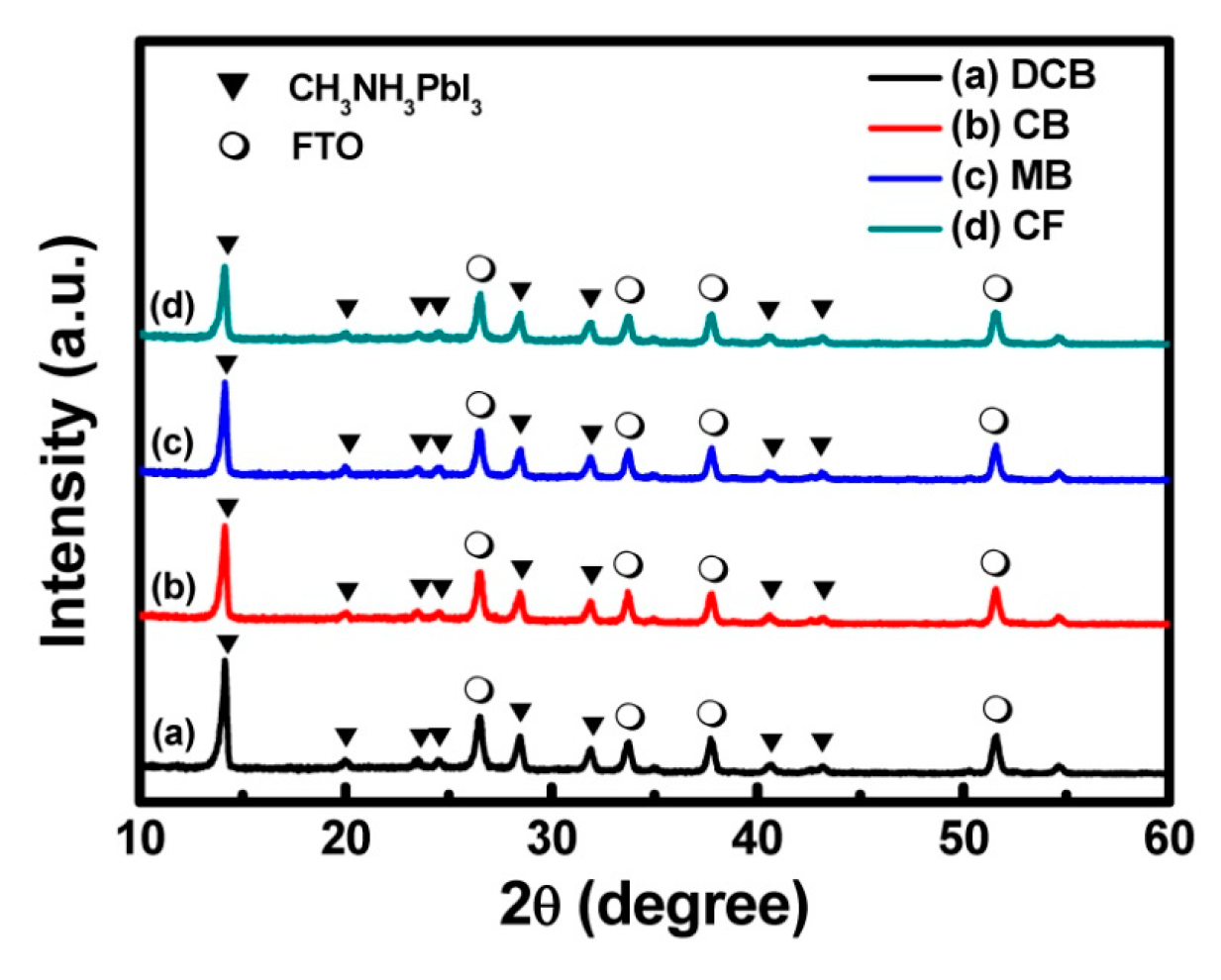


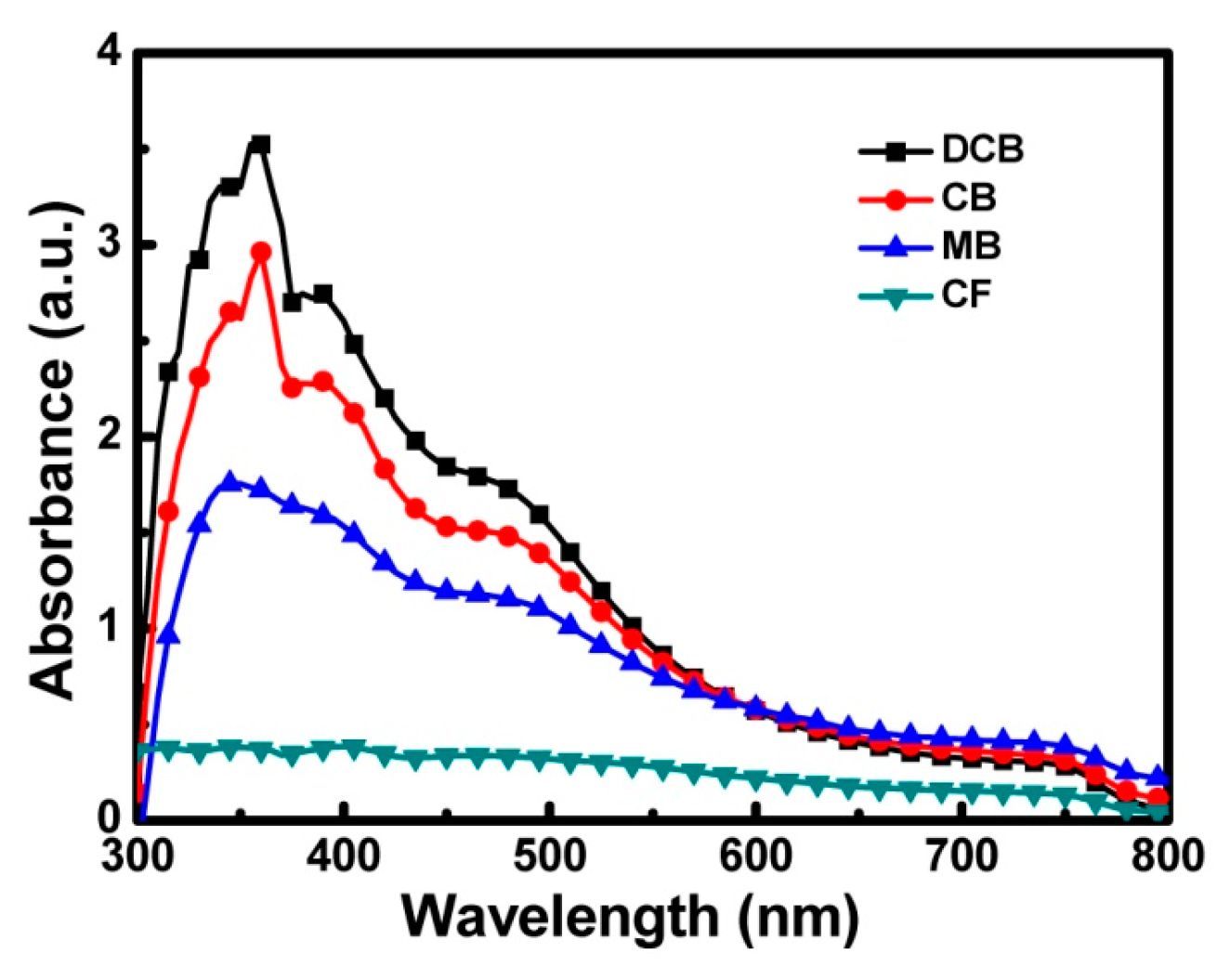



| Solvent | DCB | CB | MB | CF |
|---|---|---|---|---|
| Formula | C6H4Cl2 | C6H5Cl | C7H8 | CHCl3 |
| Boiling point (°C) | 180.5 | 131 | 111 | 61.15 |
| Vapor pressure (kPa) | 0.16 | 1.2 | 2.8 | 25.9 |
| Dielectric Constant | 9.93 | 5.62 | 2.38 | 4.81 |
| Solvent | Jsc (mA/cm2) | Voc (V) | Fill Factor | PCE (%) |
|---|---|---|---|---|
| DCB | 21.26 | 0.84 | 0.62 | 11.07 |
| CB | 19.85 | 0.85 | 0.60 | 10.12 |
| MB | 15.24 | 0.74 | 0.42 | 4.74 |
| CF | 9.76 | 0.70 | 0.26 | 1.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-H.; Lin, C.-M.; Kuei, C.-H. Investigation of the Effects of Various Organic Solvents on the PCBM Electron Transport Layer of Perovskite Solar Cells. Coatings 2020, 10, 237. https://doi.org/10.3390/coatings10030237
Tsai C-H, Lin C-M, Kuei C-H. Investigation of the Effects of Various Organic Solvents on the PCBM Electron Transport Layer of Perovskite Solar Cells. Coatings. 2020; 10(3):237. https://doi.org/10.3390/coatings10030237
Chicago/Turabian StyleTsai, Chih-Hung, Chia-Ming Lin, and Cheng-Hao Kuei. 2020. "Investigation of the Effects of Various Organic Solvents on the PCBM Electron Transport Layer of Perovskite Solar Cells" Coatings 10, no. 3: 237. https://doi.org/10.3390/coatings10030237
APA StyleTsai, C.-H., Lin, C.-M., & Kuei, C.-H. (2020). Investigation of the Effects of Various Organic Solvents on the PCBM Electron Transport Layer of Perovskite Solar Cells. Coatings, 10(3), 237. https://doi.org/10.3390/coatings10030237




