Electrophoretic Deposition and Characteristics of Chitosan–Nanosilver Composite Coatings on a Nanotubular TiO2 Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Specimens
2.2. Electrochemical Oxidation of Ti13Zr13Nb Alloy
2.3. Electrophoretic Deposition of Chitosan—Nanosilver Coatings
2.4. Structure and Morphology of Composite TiO2—Chitosan—Nanosilver Coatings
2.5. Silver Release in Simulated Body Fluid (SBF) Solution
2.6. Mechanical Studies—Nanoindentation and Nanoscratch Tests
2.7. Contact Angle Studies
2.8. Statistical Analysis
3. Results and Discussion
3.1. Structure and Morphology of Composite TiO2–Chitosan–Nanosilver Coatings
3.2. Silver Release in Simulated Body Fluid (SBF) Solution
3.3. Mechanical Studies—Nanoindentation and Nanoscratch Tests
3.4. Measurements of the Contact Angle
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dinu, M.; Franchi, S.; Pruna, V.; Cotrut, C.M.; Secchi, V.; Santi, M.; Titorencu, I.; Battocchio, C.; Iucci, G.; Vladescu, A. Ti-Nb-Zr system and its surface biofunctionalization for biomedical applications. In Titanium in Medical and Dental Applications; Froes, F.H., Qian, M., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 175–200. ISBN 978-0-12-812456-7. [Google Scholar]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Piątkowski, M.; Radwan-Pragłowska, J.; Janus, Ł.; Bogdał, D.; Matysek, D.; Cablik, V. Microwave-assisted synthesis and characterization of chitosan aerogels doped with Au-NPs for skin regeneration. Polym. Test. 2019, 73, 366–376. [Google Scholar] [CrossRef]
- Möhler, J.S.; Sim, W.; Blaskovich, M.A.T.; Cooper, M.A.; Ziora, Z.M. Silver bullets: A new lustre on an old antimicrobial agent. Biotechnol. Adv. 2018, 36, 1391–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wekwejt, M.; Moritz, N.; Świeczko-Żurek, B.; Pałubicka, A. Biomechanical testing of bioactive bone cements—A comparison of the impact of modifiers: Antibiotics and nanometals. Polym. Test. 2018, 70, 234–243. [Google Scholar] [CrossRef]
- Volova, T.G.; Shumilova, A.A.; Shidlovskiy, I.P.; Nikolaeva, E.D.; Sukovatiy, A.G.; Vasiliev, A.D.; Shishatskaya, E.I. Antibacterial properties of films of cellulose composites with silver nanoparticles and antibiotics. Polym. Test. 2018, 65, 54–68. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Gilabert-Chirivella, E.; Pérez-Feito, R.; Ribeiro, C.; Ribeiro, S.; Correia, D.M.; González-Martín, M.L.; Manero, J.M.; Lanceros-Méndez, S.; Ferrer, G.G.; Gómez-Ribelles, J.L. Chitosan patterning on titanium implants. Prog. Org. Coat. 2017, 111, 23–28. [Google Scholar] [CrossRef]
- Sani, I.K.; Pirsa, S.; Tağı, Ş. Preparation of chitosan/zinc oxide/Melissa officinalis essential oil nano-composite film and evaluation of physical, mechanical and antimicrobial properties by response surface method. Polym. Test. 2019, 79, 106004. [Google Scholar] [CrossRef]
- Urbanek, O.; Sajkiewicz, P.; Pierini, F. The effect of polarity in the electrospinning process on PCL/chitosan nanofibres’ structure, properties and efficiency of surface modification. Polymer 2017, 124, 168–175. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Olad, A.; Hagh, H.B.K. Graphene oxide and amin-modified graphene oxide incorporated chitosan-gelatin scaffolds as promising materials for tissue engineering. Compos. Part B Eng. 2019, 162, 692–702. [Google Scholar] [CrossRef]
- Divakar, D.D.; Jastaniyah, N.T.; Altamimi, H.G.; Alnakhli, Y.O.; Muzaheed; Alkheraif, A.A.; Haleem, S. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. Int. J. Biol. Macromol. 2018, 108, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Praxedes, A.P.P.; Webler, G.D.; Souza, S.T.; Ribeiro, A.S.; Fonseca, E.J.S.; Oliveira, I.N. De Non-monotonic wetting behavior of chitosan films induced by silver nanoparticles. Appl. Surf. Sci. 2016, 370, 25–31. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Li, C.; Huang, Y.; Ding, Q.; Pang, X. Preparation and characterization of chitosan-silver/hydroxyapatite composite coatings on TiO 2 nanotube for biomedical applications. Appl. Surf. Sci. 2015, 332, 62–69. [Google Scholar] [CrossRef]
- Jennings, J.A.; Velasquez Pulgarin, D.A.; Kunwar, D.L.; Babu, J.; Mishra, S.; Bumgardner, J. Bacterial inhibition by chitosan coatings loaded with silver-decorated calcium phosphate microspheres. Thin Solid Film. 2015, 596, 83–86. [Google Scholar] [CrossRef]
- Kishore, R.; Awasthi, S.; Dhayalan, A.; Ferreira, J.M.F.; Kannan, S. Deposition, structure, physical and in-vitro characteristics of Ag-doped β-Ca3( PO4)2/chitosan hybrid composite coatings on Titanium metal. Mater. Sci. Eng. C 2016, 62, 692–701. [Google Scholar]
- Lin, S.; Chen, L.; Huang, L.; Cao, S.; Luo, X.; Liu, K. Novel antimicrobial chitosan-cellulose composite films bioconjugated with silver nanoparticles. Ind. Crops Prod. 2015, 70, 395–403. [Google Scholar] [CrossRef]
- Mishra, S.K.; Ferreira, J.M.F.; Kannan, S. Mechanically stable antimicrobial chitosan-PVA-silver nanocomposite coatings deposited on titanium implants. Carbohydr. Polym. 2015, 121, 37–48. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Pan, R.; Han, D.; Chen, T.; Geng, Z.; Xiong, Y.; Chen, Y. Electrodeposition of chitosan/gelatin/nanosilver: A new method for constructing biopolymer/nanoparticle composite films with conductivity and antibacterial activity. Mater. Sci. Eng. C 2015, 53, 222–228. [Google Scholar] [CrossRef]
- Arjunan, N.; Kumari, H.L.J.; Singaravelu, C.M.; Kandasamy, R.; Kandasamy, J. Physicochemical investigations of biogenic chitosan-silver nanocomposite as antimicrobial and anticancer agent. Int. J. Biol. Macromol. 2016, 92, 77–87. [Google Scholar] [CrossRef]
- Dananjaya, S.H.S.; Erandani, W.K.C.U.; Kim, C.H.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Comparative study on antifungal activities of chitosan nanoparticles and chitosan silver nano composites against Fusarium oxysporum species complex. Int. J. Biol. Macromol. 2017, 105, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, R.; Maruthupandy, M.; Muneeswaran, T.; Hameedha Beevi, A.; Anand, M.; Ramakritinan, C.M.; Kumaraguru, A.K. Synthesis of chitosan mediated silver nanoparticles (Ag NPs) for potential antimicrobial applications. Front. Lab. Med. 2018, 2, 30–35. [Google Scholar] [CrossRef]
- Wongpreecha, J.; Polpanich, D.; Suteewong, T.; Kaewsaneha, C. One-pot, large-scale green synthesis of silver nanoparticles-chitosan with enhanced antibacterial activity and low cytotoxicity. Carbohydr. Polym. 2018, 199, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liao, X.; Zhang, J.; Yang, F.; Fan, Z. Novel chitosan hydrogels reinforced by silver nanoparticles with ultrahigh mechanical and high antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 2018, 119, 402–412. [Google Scholar] [CrossRef]
- Rinaldi, F.; Del Favero, E.; Moeller, J.; Hanieh, P.N.; Passeri, D.; Rossi, M.; Angeloni, L.; Venditti, I.; Marianecci, C.; Carafa, M.; et al. Hydrophilic silver nanoparticles loaded into niosomes: Physical-chemical characterization in view of biological applications. Nanomaterials 2019, 9, 1177. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lenhart, J.J.; Walker, H.W. Aggregation kinetics and dissolution of coated silver nanoparticles. Langmuir 2012, 28, 1095–1104. [Google Scholar] [CrossRef]
- Cavalieri, F.; Tortora, M.; Stringaro, A.; Colone, M.; Baldassarri, L. Nanomedicines for antimicrobial interventions. J. Hosp. Infect. 2014, 88, 183–190. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Lotfy, T.M.R.; Shawir, S.M.S. Preparation and antibacterial activity of chitosan-silver nanoparticles for application in preservation of minced meat. Bull. Natl. Res. Cent. 2019, 43, 83. [Google Scholar] [CrossRef]
- Chowdappa, P.; Shivakumar, G.; Chethana, C.S.; Madhur, S. Antifungal activity of chitosan-silver nanoparticle composite against Colletotrichum gloeosporioides associated with mango anthracnose. Afr. J. Microbiol. Res. 2014, 8, 1803–1812. [Google Scholar]
- Ziani, K.; Oses, J.; Coma, V.; Maté, J.I. Effect of the presence of glycerol and Tween 20 on the chemical and physical properties of films based on chitosan with different degree of deacetylation. LWT Food Sci. Technol. 2008, 41, 2159–2165. [Google Scholar] [CrossRef]
- Casariego, A.; Souza, B.W.S.; Vicente, A.A.; Teixeira, J.A.; Cruz, L.; Díaz, R. Chitosan coating surface properties as affected by plasticizer, surfactant and polymer concentrations in relation to the surface properties of tomato and carrot. Food Hydrocoll. 2008, 22, 1452–1459. [Google Scholar] [CrossRef] [Green Version]
- Mohan, L.; Durgalakshmi, D.; Geetha, M.; Sankara Narayanan, T.S.N.; Asokamani, R. Electrophoretic deposition of nanocomposite (HAp + TiO 2) on titanium alloy for biomedical applications. Ceram. Int. 2012, 38, 3435–3443. [Google Scholar] [CrossRef]
- Kodama, A.; Bauer, S.; Komatsu, A.; Asoh, H.; Ono, S.; Schmuki, P. Bioactivation of titanium surfaces using coatings of TiO2 nanotubes rapidly pre-loaded with synthetic hydroxyapatite. Acta Biomater. 2009, 5, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Ossowska, A.; Sobieszczyk, S.; Supernak, M.; Zielinski, A. Morphology and properties of nanotubular oxide layer on the “Ti-13Zr-13Nb” alloy. Surf. Coat. Technol. 2014, 258, 1239–1248. [Google Scholar] [CrossRef]
- Zielinski, A.; Antoniuk, P.; Krzysztofowicz, K. Nanotubular oxide layers and hydroxyapatite coatings on ‘Ti-13Zr013Nb’ alloy. Surf. Sci. 2014, 30, 643–649. [Google Scholar]
- Chrzanowski, W.; Szewczenko, J.; Tyrlik-Held, J.; Marciniak, J.; Zak, J. Influence of the anodic oxidation on the physicochemical properties of the Ti6Al4V ELI alloy. J. Mater. Process. Technol. 2005, 162–163, 163–168. [Google Scholar] [CrossRef]
- Gebhardt, F.; Seuss, S.; Turhan, M.C.; Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Characterization of electrophoretic chitosan coatings on stainless steel. Mater. Lett. 2012, 66, 302–304. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite hydroxyapatite-chitosan coatings. Mater. Charact. 2007, 58, 339–348. [Google Scholar] [CrossRef]
- Guo, L.; Yuan, W.; Lu, Z.; Li, C.M. Polymer/nanosilver composite coatings for antibacterial applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Surface-coating-dependent dissolution, aggregation, and reactive oxygen species (ROS) generation of silver nanoparticles under different irradiation conditions. Environ. Sci. Technol. 2013, 47, 10293–10301. [Google Scholar] [CrossRef]
- Ozaltin, K.; Panigrahi, A.; Chrominski, W.; Bulutsuz, A.G.; Kulczyk, M.; Zehetbauer, M.J.; Lewandowska, M. Microstructure and Texture Evolutions of Biomedical Ti-13Nb-13Zr Alloy Processed by Hydrostatic Extrusion. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 5747–5755. [Google Scholar] [CrossRef] [Green Version]
- Goodarzi, S.; Moztarzadeh, F.; Nezafati, N.; Omidvar, H. Titanium dioxide nanotube arrays: A novel approach into periodontal tissue regeneration on the surface of titanium implants. Adv. Mater. Lett. 2016, 7, 209–215. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J. Physiochemical and optical study of chitosan-terephthaldehyde derivative for biomedical applications. Int. J. Biol. Macromol. 2012, 51, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Rieppo, J.; Rieppo, L.; Saarakkala, S.; Jurvelin, J.S. Fourier Transform Infrared Imaging Spectroscopy in Biomedicine-Important Things to Consider When Planning a New Experiment. In Fourier Transforms-New Analytical Approaches and FTIR Strategies; Nikolic, G., Ed.; Intech Open: London, UK, 2011; pp. 1–14. [Google Scholar]
- Paluszkiewicz, C.; Stodolak, E.; Hasik, M.; Blazewicz, M. FT-IR study of montmorillonite–chitosan nanocomposite materials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2014, 112, 1–9. [Google Scholar] [CrossRef]
- Dimzon, I.K.D.; Knepper, T.P. International Journal of Biological Macromolecules Degree of deacetylation of chitosan by infrared spectroscopy and partial least squares. Int. J. Biol. Macromol. 2015, 72, 939–945. [Google Scholar] [CrossRef]
- Silva, S.M.L.; Braga, C.R.C.; Fook, M.V.L.; Raposo, C.M.O.; Carvalho, L.H.; Canedo, E.L. Application of Infrared Spectroscopy to Analysis of Chitosan/Clay Nanocomposites. In Infrared Spectroscopy; Theophanides, T., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Fan, M.; Dai, D.; Huang, B. Fourier Transform Infrared Spectroscopy for Natural Fibres. In Fourier Transform; Salih, S.M., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Mano, J.F.; Koniarova, D.; Reis, R.L. Thermal properties of thermoplastic starch/synthetic polymer blends with potential biomedical applicability. J. Mater. Sci. Mater. Med. 2003, 14, 127–135. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Ravenelle, F.; Zhu, X.X. (Eds.) Polysaccharides for Drug Delivery and Pharmaceutical Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2006; Volume 934, ISBN 0-8412-3960-6. [Google Scholar]
- Geng, Z.; Wang, R.; Zhuo, X.; Li, Z.; Huang, Y.; Ma, L.; Cui, Z.; Zhu, S.; Liang, Y.; Liu, Y.; et al. Incorporation of silver and strontium in hydroxyapatite coating on titanium surface for enhanced antibacterial and biological properties. Mater. Sci. Eng. C 2017, 71, 852–861. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Judith Vijaya, J.; John Kennedy, L.; Priadharsini, K.; Palani, P.; Louis, R.; Muthumary, J.; Silver, B.; Bright, P.; Dichlorotriazine, R. Antibacterial activity of silver nanoparticles synthesized from serine. Mater. Sci. Eng. C 2014, 49, 316–322. [Google Scholar] [CrossRef]
- Sanpui, P.; Murugadoss, A.; Prasad, P.V.D.; Ghosh, S.S.; Chattopadhyay, A. The antibacterial properties of a novel chitosan-Ag-nanoparticle composite. Int. J. Food Microbiol. 2008, 124, 142–146. [Google Scholar] [CrossRef]
- National Center for Environmental Assessment. Integrated Risk Information System (IRIS) Chemical Assessment Summary: Silver; CASRN 7440-22-4; National Center for Environmental Assessment: Oakbrook Terrace, IL, USA, 2003. [Google Scholar]
- Rtimi, S.; Kiwi, J.; Karimi, A.; Sanjinés, R. Innovative Ti1–xNbxN–Ag Films Inducing Bacterial Disinfection by Visible Light/Thermal Treatment. Acs Appl. Mater. Interfaces 2018, 10, 12021–12030. [Google Scholar] [CrossRef] [PubMed]
- Hajjaji, A.; Elabidi, M.; Trabelsi, K.; Assadi, A.A.; Bessais, B.; Rtimi, S. Bacterial adhesion and inactivation on Ag decorated TiO2-nanotubes under visible light: Effect of the nanotubes geometry on the photocatalytic activity. Colloids Surf. B Biointerfaces 2018, 170, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Nadtochenko, V.; Khmel, I.; Bensimon, M.; Kiwi, J. First unambiguous evidence for distinct ionic and surface-contact effects during photocatalytic bacterial inactivation on Cu–Ag films: Kinetics, mechanism and energetics. Mater. Today Chem. 2017, 6, 62–74. [Google Scholar] [CrossRef]
- Chernozem, R.V.; Surmeneva, M.A.; Krause, B.; Baumbach, T.; Ignatov, V.P.; Tyurin, A.I.; Loza, K.; Epple, M.; Surmenev, R.A. Hybrid biocomposites based on titania nanotubes and a hydroxyapatite coating deposited by RF-magnetron sputtering: Surface topography, structure, and mechanical properties. Appl. Surf. Sci. 2017, 426, 229–237. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Crawford, G.A.; Chawla, N.; Das, K.; Bose, S.; Bandyopadhyay, A. Microstructure and deformation behavior of biocompatible TiO2 nanotubes on titanium substrate. Acta Biomater. 2007, 3, 359–367. [Google Scholar] [CrossRef]
- Manoj Kumar, R.; Kuntal, K.K.; Singh, S.; Gupta, P.; Bhushan, B.; Gopinath, P.; Lahiri, D. Electrophoretic deposition of hydroxyapatite coating on Mg-3Zn alloy for orthopaedic application. Surf. Coat. Technol. 2016, 287, 82–92. [Google Scholar] [CrossRef]
- Hang, Y.; Liu, G.; Huang, K.; Jin, W. Mechanical properties and interfacial adhesion of composite membranes probed by in-situ nano-indentation/scratch technique. J. Membr. Sci. 2015, 494, 205–215. [Google Scholar] [CrossRef]
- Mali, S.; Misra, R.D.K.; Somani, M.C.; Karjalainen, L.P. Biomimetic nanostructured coatings on nano-grained/ultrafine-grained substrate: Microstructure, surface adhesion strength, and biosolubility. Mater. Sci. Eng. C 2009, 29, 2417–2427. [Google Scholar] [CrossRef]
- Bartmanski, M.; Cieslik, B.; Glodowska, J.; Kalka, P. Electrophoretic deposition (EPD) of nanohydroxyapatite—Nanosilver coatings on Ti13Zr13Nb alloy. Ceram. Int. 2017, 43, 11820–11829. [Google Scholar] [CrossRef]
- Tozar, A.; Karahan, İ.H. A comparative study on the effect of collagen and h-BN reinforcement of hydroxyapatite/chitosan biocomposite coatings electrophoretically deposited on Ti-6Al-4V biomedical implants. Surf. Coat. Technol. 2018, 340, 167–176. [Google Scholar] [CrossRef]
- Tozar, A.; Karahan, İ.H. A comprehensive study on electrophoretic deposition of a novel type of collagen and hexagonal boron nitride reinforced hydroxyapatite/chitosan biocomposite coating. Appl. Surf. Sci. 2018, 452, 322–336. [Google Scholar] [CrossRef]
- Cordero-Arias, L.; Cabanas-Polo, S.; Gao, H.; Gilabert, J.; Sanchez, E.; Roether, J.A.; Schubert, D.W.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition of nanostructured-TiO2/chitosan composite coatings on stainless steel. RSC Adv. 2013, 3, 11247–11254. [Google Scholar] [CrossRef] [Green Version]
- Kasraei, S.; Azarsina, M. Addition of silver nanoparticles reduces the wettability of methacrylate and silorane-based composites. Braz. Oral Res. 2012, 26, 505–510. [Google Scholar] [CrossRef]




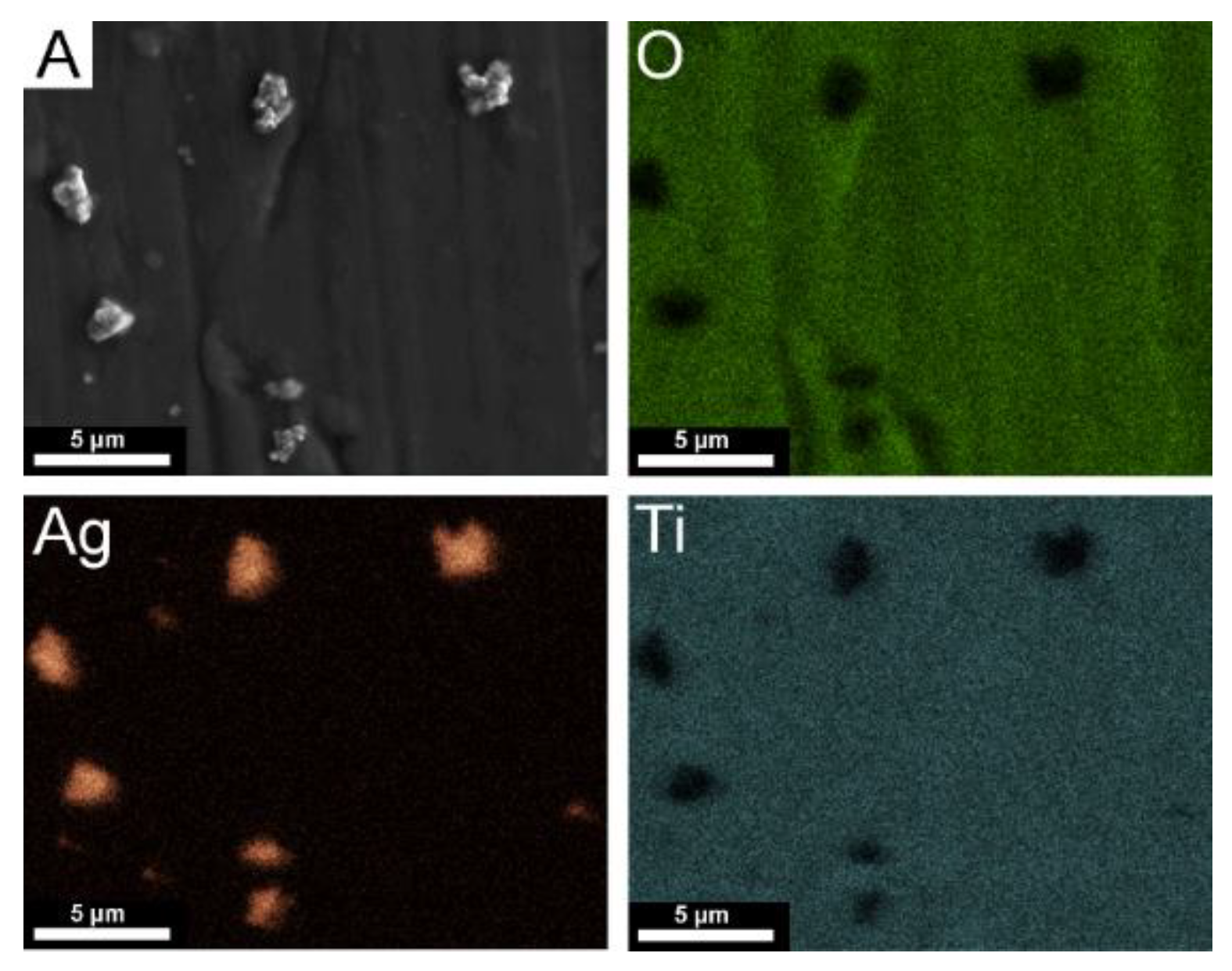
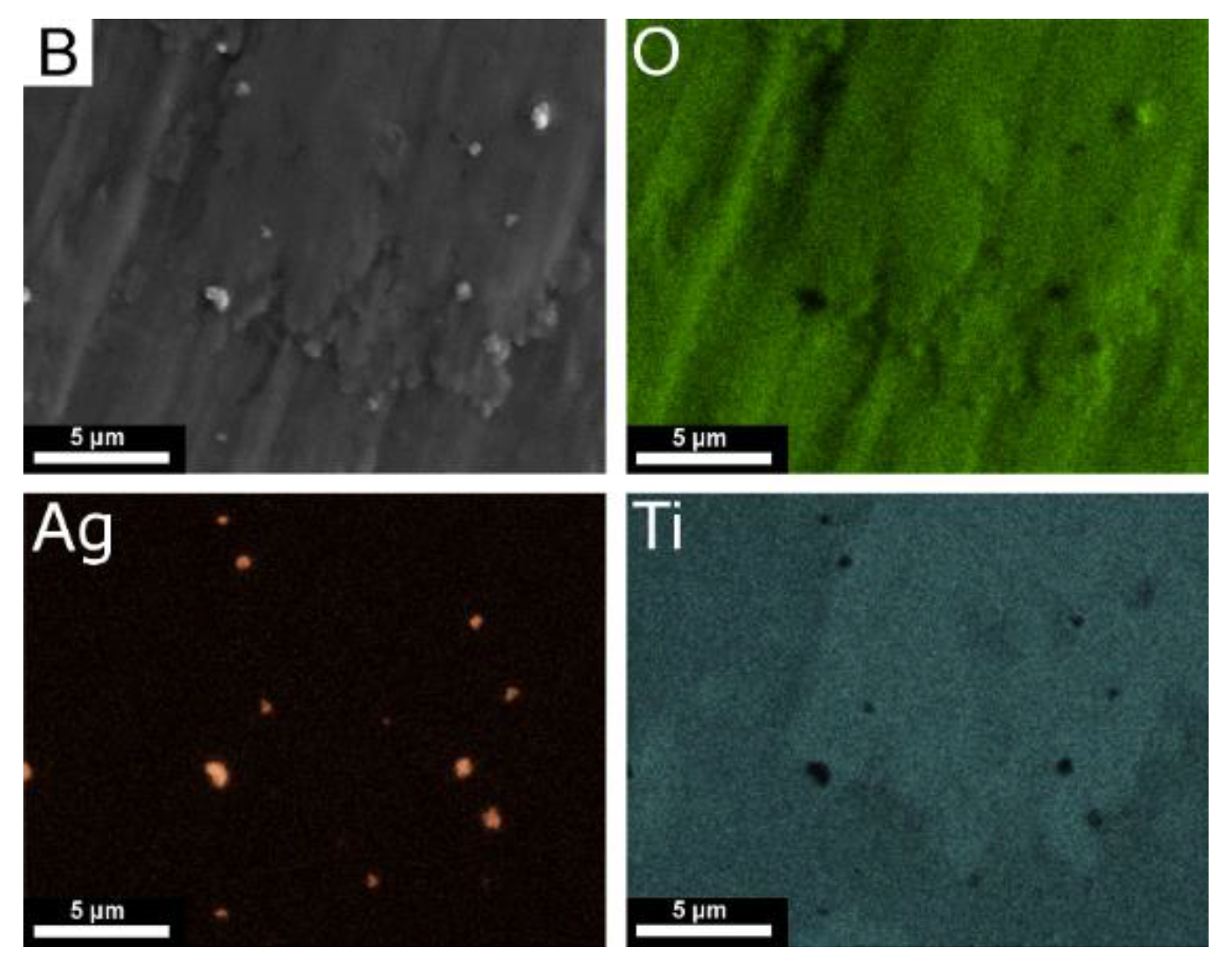





| Element | Nb | Zr | Fe | C | N | O | Ti |
|---|---|---|---|---|---|---|---|
| wt.% | 13.0 | 13.0 | 0.05 | 0.04 | 0.019 | 0.11 | remainder |
| Specimen | Properties of Electrophoretic Deposition | ||||
|---|---|---|---|---|---|
| Chitosan Content (g) | Silver Nanoparticle Content (g) | Polysorbate 20 Content (ml) | Voltage of Deposition (V) | Time of Deposition (min) | |
| 1 L of 1% (v/v) Acetic Acid | |||||
| A | 1 | 0.05 | - | 10 | 1 |
| B | 1 | 0.05 | 1 | 10 | 1 |
| C | 1 | 0.05 | 1 | 20 | 1 |
| Properties | ||
|---|---|---|
| Specimen | Sa Parameter (µm) | Thickness (µm); (mean ± SD; n = 10) |
| TiO2 layer on Ti13Zr13Nb alloy | 0.07 | 0.78 ± 0.10 |
| A | 0.10 | 0.78 ± 0.08 |
| B | 0.08 | 0.22 ± 0.04* |
| C | 0.11 | 3.18 ± 0.18* |
| reference Ti13Zr13Nb alloy | 0.15 | - |
| Nanoscratch Test Properties | ||
|---|---|---|
| Specimen | Critical Friction (mN) | Critical Load (mN) |
| TiO2 layer on Ti13Zr13Nb alloy | 58.22 ± 20.43 | 85.95 ± 28.00 |
| A | 100.72 ± 9.31* | 152.59 ± 13.93* |
| B | 101.58 ± 41.76* | 79.91 ± 25.44# |
| C | 106.65 ± 28.97* | 85.48 ± 30.34# |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartmański, M.; Pawłowski, Ł.; Zieliński, A.; Mielewczyk-Gryń, A.; Strugała, G.; Cieślik, B. Electrophoretic Deposition and Characteristics of Chitosan–Nanosilver Composite Coatings on a Nanotubular TiO2 Layer. Coatings 2020, 10, 245. https://doi.org/10.3390/coatings10030245
Bartmański M, Pawłowski Ł, Zieliński A, Mielewczyk-Gryń A, Strugała G, Cieślik B. Electrophoretic Deposition and Characteristics of Chitosan–Nanosilver Composite Coatings on a Nanotubular TiO2 Layer. Coatings. 2020; 10(3):245. https://doi.org/10.3390/coatings10030245
Chicago/Turabian StyleBartmański, Michał, Łukasz Pawłowski, Andrzej Zieliński, Aleksandra Mielewczyk-Gryń, Gabriel Strugała, and Bartłomiej Cieślik. 2020. "Electrophoretic Deposition and Characteristics of Chitosan–Nanosilver Composite Coatings on a Nanotubular TiO2 Layer" Coatings 10, no. 3: 245. https://doi.org/10.3390/coatings10030245
APA StyleBartmański, M., Pawłowski, Ł., Zieliński, A., Mielewczyk-Gryń, A., Strugała, G., & Cieślik, B. (2020). Electrophoretic Deposition and Characteristics of Chitosan–Nanosilver Composite Coatings on a Nanotubular TiO2 Layer. Coatings, 10(3), 245. https://doi.org/10.3390/coatings10030245









