Materials for Orthopedic Bioimplants: Modulating Degradation and Surface Modification Using Integrated Nanomaterials
Abstract
:1. Introduction
2. Materials for Orthopedic Bioimplants
2.1. Titanium (Ti) and Ti-Alloys
2.2. Stainless Steel (SS)
2.3. Cobalt (Co) Alloy
2.4. Biodegradable Metals
3. Degradation of Orthopedic Bioimplants
3.1. Metallic Bioimplant Degradation: Role of Biological Factors
3.2. Time Dependent Degradation Effects
3.3. Degradation Mechanism
3.4. Metal Self-Induced Biological Responses
4. Surface Modification Effects
5. Improving the Surface Properties of Bioimplants Using Integrated Nanomaterials
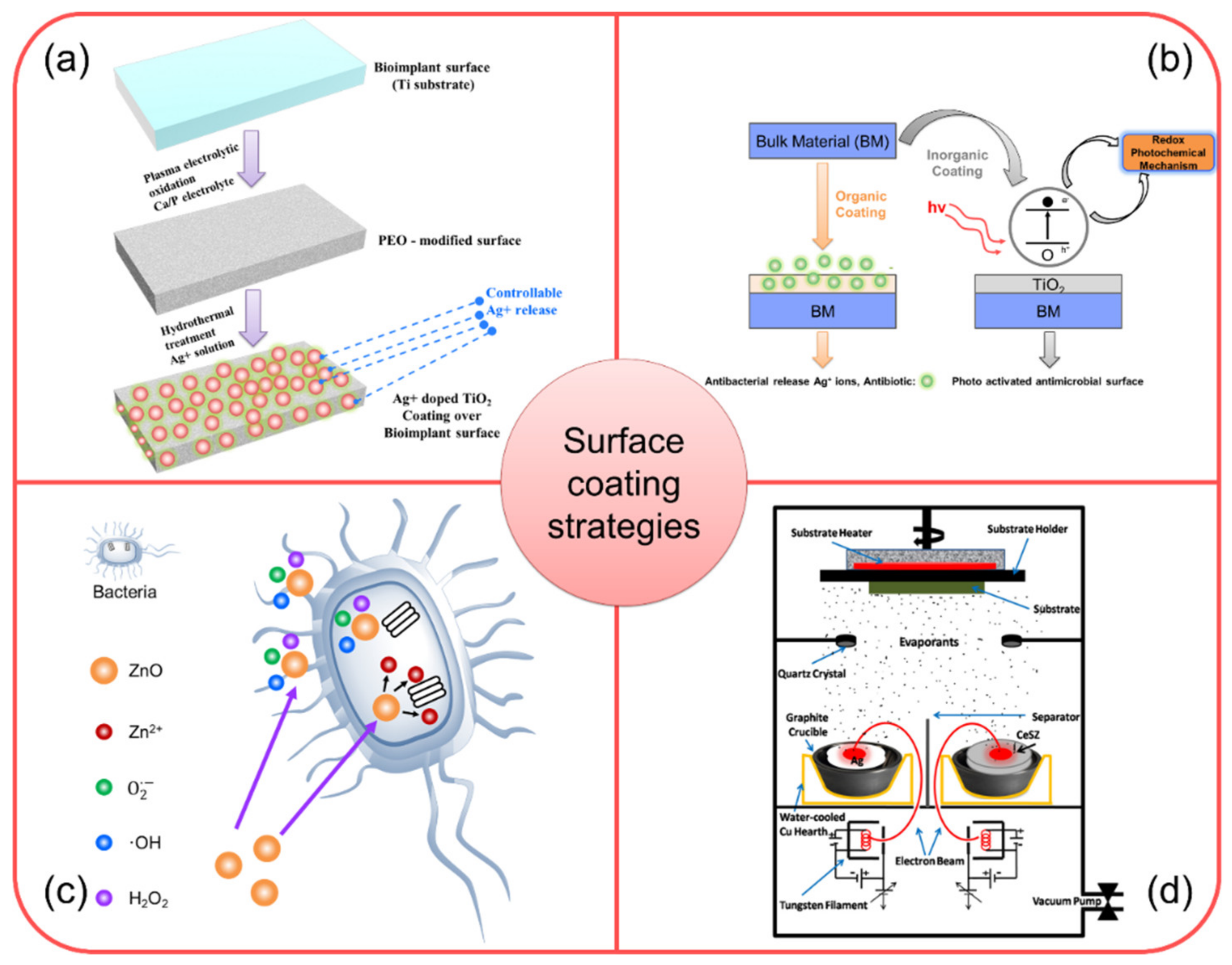
5.1. Surface Coating Using Ag-Based Nanocomposites
5.2. Surface Coating Using Nano-TiO2 and TiO2-Based Metal Nanocomposites
5.3. Surface Coating Using ZnO-Based Nanocomposite
5.4. Surface Coating Using Ag-CeSZ Nanocomposite
5.5. Surface Coating Using Ti/SiC Metal Matrix Nanocomposite
6. Conclusions and Future Recommendation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, C.-W.; Fang, F.-Z. State of the art of bioimplants manufacturing: Part I. Adv. Manuf. 2018, 6, 20–40. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, H.S.; Nakamoto, T.; Chen, S.; Cai, R.; Kawazoe, N.; Chen, G. Collagen microgel-assisted dexamethasone release from PLLA-collagen hybrid scaffolds of controlled pore structure for osteogenic differentiation of mesenchymal stem cells. J. Biomater. Sci. Polym. Ed. 2014, 25, 1374–1386. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, C.; Kim, J.-J.; Lee, J.-H.; Jin, G.-Z.; Knowles, J.C.; Kim, H.-W. Differential chondro- and osteo-stimulation in three-dimensional porous scaffolds with different topological surfaces provides a design strategy for biphasic osteochondral engineering. J. Tissue Eng. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, M.S.; Hall, C.; Murphy, P. Surface treatments for controlling corrosion rate of biodegradable Mg and Mg-based alloy implants. Sci. Technol. Adv. Mater. 2015, 16, 053501. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Holinka, M.; Moucha, C.S. Antibacterial surface treatment for orthopaedic implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, C.G.; Clanton, T.O. Bioabsorbable implants: Review of clinical experience in orthopedic surgery. Ann. Biomed. Eng. 2004, 32, 171–177. [Google Scholar] [CrossRef]
- Carpintero, P.; Caeiro, J.R.; Carpintero, R.; Morales, A.; Silva, S.; Mesa, M. Complications of hip fractures: A review. World J. Orthop. 2014, 5, 402–411. [Google Scholar] [CrossRef]
- Wilson, J.M.; Jones, N.; Jin, L.; Shin, Y.C. Laser deposited coatings of Co-Cr-Mo onto Ti-6Al-4V and SS316L substrates for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1124–1132. [Google Scholar] [CrossRef]
- Dhanopia, A.; Bhargava, M. Finite Element Analysis of Human Fractured Femur Bone Implantation with PMMA Thermoplastic Prosthetic Plate. Procedia Eng. 2017, 173, 1658–1665. [Google Scholar] [CrossRef]
- Abdul Khadar, S.D. Mechanical Strength Evaluation Analysis of Stainless Steel and Titanium Locking Plate for Femur Bone Fracture. Eng. Sci. Technol. Int. J. 2012, 3, 381–388. [Google Scholar]
- Ridzwan, M.I.Z.; Shuib, S.; Hassan, A.Y.; Shokri, A.A.; Ibrahim, M.N.M. Problem of Stress Shielding and Improvement to the Hip Implant Designs: A Review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Balamurugan, A.; Rajeswari, S.; Balossier, G.; Rebelo, A.H.S.; Ferreira, J.M.F. Corrosion aspects of metallic implants—An overview. Mater. Corros. 2008, 59, 855–869. [Google Scholar] [CrossRef]
- Kamachimudali, U.; Sridhar, T.M.; Raj, B. Corrosion of bio implants. Sadhana 2003, 28, 601–637. [Google Scholar] [CrossRef]
- Wooley, P.H.; Nasser, S.; Fitzgerald, R.H., Jr. The immune response to implant materials in humans. Clin. Orthop. Relat. Res. 1996, 63–70. [Google Scholar] [CrossRef]
- Kargozar, S.; Ramakrishna, S.; Mozafari, M. Chemistry of Biomaterials: Future Prospects. Curr. Opin. Biomed. Eng. 2019, 10, 181–190. [Google Scholar] [CrossRef]
- Min, D.I.; Monaco, A.P. Complications Associated with Immunosuppressive Therapy and Their Management. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1991, 11, 119S–125S. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants-A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Tran, P.A.; Sarin, L.; Hurt, R.H.; Webster, T.J. Opportunities for nanotechnology-enabled bioactive bone implants. J. Mater. Chem. 2009, 19, 2653–2659. [Google Scholar] [CrossRef]
- Sekhar Nanda, H.; Chandra Mishra, N. “Amphotericin B” Loaded Natural Biodegradable Nanofibers as a Potential Drug Delivery System against Leishmaniasis. Curr. Nanosci. 2011, 7, 943–949. [Google Scholar] [CrossRef]
- Wen, C. Surface Coating and Modification of Metallic Biomaterials; Woodhead Publishing: Sawston/Cambridge, UK, 2015; pp. 1–431. [Google Scholar]
- Nethi, S.K.; Nanda, H.S.; Steele, T.W.; Patra, C.R. Functionalized nanoceria exhibit improved angiogenic properties. J. Mater. Chem. B 2017, 5, 9371–9383. [Google Scholar] [CrossRef]
- Mahajan, A.; Sidhu, S.S. Surface modification of metallic biomaterials for enhanced functionality: A review. Mater. Technol. 2018, 33, 93–105. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Nanotechnology-based biomaterials for orthopaedic applications: Recent advances and future prospects. Mater. Sci. Eng. C 2020, 106, 110154. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef] [PubMed]
- Christenson, E.M.; Anseth, K.S.; van den Beucken, J.J.; Chan, C.K.; Ercan, B.; Jansen, J.A.; Laurencin, C.T.; Li, W.J.; Murugan, R.; Nair, L.S. Nanobiomaterial applications in orthopedics. J. Orthop. Res. 2007, 25, 11–22. [Google Scholar] [CrossRef]
- Moura, C.C.G.; Souza, M.A.; Dechichi, P.; Zanetta-Barbosa, D.; Teixeira, C.C.; Coelho, P.G. The effect of a nanothickness coating on rough titanium substrate in the osteogenic properties of human bone cells. J. Biomed. Mater. Res. Part A 2010, 94A, 103–111. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Ramalingam, M.; Kumar, T.S.; Soboyejo, W.O. Biomaterials: A Nano Approach; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Kiran, A.; Kumar, T.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Antibacterial and bioactive surface modifications of titanium implants by PCL/TiO2 nanocomposite coatings. Nanomaterials 2018, 8, 860. [Google Scholar] [CrossRef] [Green Version]
- Kiran, A.S.K.; Kumar, T.S.; Perumal, G.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Dual nanofibrous bioactive coating and antimicrobial surface treatment for infection resistant titanium implants. Prog. Org. Coat. 2018, 121, 112–119. [Google Scholar] [CrossRef]
- Jayaraman, P.; Gandhimathi, C.; Venugopal, J.R.; Becker, D.L.; Ramakrishna, S.; Srinivasan, D.K. Controlled release of drugs in electrosprayed nanoparticles for bone tissue engineering. Adv. Drug Deliv. Rev. 2015, 94, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Kiran, A.S.K.; Kizhakeyil, A.; Ramalingam, R.; Verma, N.K.; Lakshminarayanan, R.; Kumar, T.S.; Doble, M.; Ramakrishna, S. Drug loaded electrospun polymer/ceramic composite nanofibrous coatings on titanium for implant related infections. Ceram. Int. 2019, 45, 18710–18720. [Google Scholar] [CrossRef]
- Daugaard, H.; Elmengaard, B.; Bechtold, J.E.; Soballe, K. Bone growth enhancement in vivo on press-fit titanium alloy implants with acid etched microtexture. J. Biomed. Mater. Res. Part A 2008, 87, 434–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohan Ehrenfest, D.M.; Coelho, P.G.; Kang, B.-S.; Sul, Y.-T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Prodanov, L.; Lamers, E.; Wolke, J.; Huiberts, R.; Jansen, J.A.; Walboomers, X.F. In vivo comparison between laser-treated and grit blasted/acid etched titanium. Clin. Oral Implant. Res. 2014, 25, 234–239. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; Lázaro, P.; Vicente Rios, J.; Lluch, S.; Marqués, M.; Guillem-Martí, J.; Gil, F.J. Influence of acid-etching after grit-blasted on osseointegration of titanium dental implants: In vitro and in vivo studies. J. Mater. Sci. Mater. Med. 2013, 24, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Lieblich, M.; Barriuso, S.; Ibáñez, J.; Ruiz-de-Lara, L.; Díaz, M.; Ocaña, J.; Alberdi, A.; González-Carrasco, J.L. On the fatigue behavior of medical Ti6Al4V roughened by grit blasting and abrasiveless waterjet peening. J. Mech. Behav. Biomed. Mater. 2016, 63, 390–398. [Google Scholar] [CrossRef]
- Jayaprakash, K. Surface coating of implants—A review. Int. J. Dent. Clin. 2012, 4, 32–35. [Google Scholar]
- Bosco, R.; Van Den Beucken, J.; Leeuwenburgh, S.; Jansen, J. Surface engineering for bone implants: A trend from passive to active surfaces. Coatings 2012, 2, 95–119. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-W. Surface Modification of Ti Dental Implants by Grit-Blasting and Micro-Arc Oxidation. Mater. Manuf. Process. 2010, 25, 307–310. [Google Scholar] [CrossRef]
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.; Cardaropoli, G.; Thompson, V.P.; Lemons, J.E. Basic research methods and current trends of dental implant surfaces. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2009, 88, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Casentini, P.; Zaniboni, M.; Corsi, E.; Anello, T. Titanium-zirconium alloy narrow-diameter implants (Straumann Roxolid((R))) for the rehabilitation of horizontally deficient edentulous ridges: Prospective study on 18 consecutive patients. Clin. Oral Implant. Res. 2012, 23, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Pecora, G.E.; Ceccarelli, R.; Bonelli, M.; Alexander, H.; Ricci, J.L. Clinical evaluation of laser microtexturing for soft tissue and bone attachment to dental implants. Implant Dent. 2009, 18, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazor, Z.; Cohen, D.K. Preliminary 3-dimensional surface texture measurement and early loading results with a microtextured implant surface. Int. J. Oral Maxillofac. Implant. 2003, 18, 729–738. [Google Scholar]
- Liu, R.; Lei, T.; Dusevich, V.; Yao, X.; Liu, Y.; Walker, M.P.; Wang, Y.; Ye, L. Surface characteristics and cell adhesion: A comparative study of four commercial dental implants. J. Prosthodont. 2013, 22, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, K.-H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process—An alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef]
- Lemons, J.E.; Niemann, K.M.; Weiss, A.B. Biocompatibility studies on surgical-grade titanium-, cobalt-, and iron-base alloys. J. Biomed. Mater. Res. 1976, 10, 549–553. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lu, W.; Qin, J.; Zhang, F.; Zhang, D. Microstructure and mechanical properties of cold-rolled TiNbTaZr biomedical β titanium alloy. Mater. Sci. Eng. A 2008, 490, 421–426. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, L.; Fu, Y.; Qin, J.; Lu, W.; Zhang, D. Influence of oxygen content on microstructure and mechanical properties of Ti–Nb–Ta–Zr alloy. Mater. Des. 2011, 32, 2934–2939. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, D.; Cheng, M.; Lu, W.; Wang, L.; Zhang, X. The bone tissue compatibility of a new Ti35Nb2Ta3Zr alloy with a low Young’s modulus. Int. J. Mol. Med. 2013, 31, 689–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azadmanjiri, J.; Berndt, C.C.; Kapoor, A.; Wen, C. Development of Surface Nano-Crystallization in Alloys by Surface Mechanical Attrition Treatment (SMAT). Crit. Rev. Solid State Mater. Sci. 2015, 40, 164–181. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.; Chen, L.; Meng, Q.; Zhang, L.-C.; Qin, J.; Zhang, D.; Lu, W. Investigation of deformation mechanisms in β-type Ti-35Nb-2Ta-3Zr alloy via FSP leading to surface strengthening. Metall. Mater. Trans. A 2015, 46, 4813–4818. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Lv, Y.; Zhang, L.-C.; Chen, L.; Meng, Q.; Qu, J.; Zhang, D.; Lu, W. Microstructure evolution and superelastic behavior in Ti-35Nb-2Ta-3Zr alloy processed by friction stir processing. Acta Mater. 2017, 131, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Rabadia, C.; Liu, Y.J.; Wang, L.; Sun, H.; Zhang, L. Laves phase precipitation in Ti-Zr-Fe-Cr alloys with high strength and large plasticity. Mater. Des. 2018, 154, 228–238. [Google Scholar] [CrossRef]
- Acharya, S.; Panicker, A.G.; Laxmi, D.V.; Suwas, S.; Chatterjee, K. Study of the influence of Zr on the mechanical properties and functional response of Ti-Nb-Ta-Zr-O alloy for orthopedic applications. Mater. Des. 2019, 164, 107555. [Google Scholar] [CrossRef]
- Rabadia, C.D.; Liu, Y.; Chen, L.-Y.; Jawed, S.F.; Wang, L.; Sun, H.; Zhang, L. Deformation and strength characteristics of Laves phases in titanium alloys. Mater. Des. 2019, 179, 107891. [Google Scholar] [CrossRef]
- Weldon, L.M.; McHugh, P.E.; Carroll, W.; Costello, E.; O’Bradaigh, C. The influence of passivation and electropolishing on the performance of medical grade stainless steels in static and fatigue loading. J. Mater. Sci. Mater. Med. 2005, 16, 107–117. [Google Scholar] [CrossRef]
- Assadian, M.; Jafari, H.; Ghaffarishahri, S.; Idris, M.; Gholampour, B. Corrosion resistance of EPD nanohydroxyapatite coated 316L stainless steel. Surf. Eng. 2014, 30, 806–813. [Google Scholar] [CrossRef]
- Marti, A. Cobalt-base alloys used in bone surgery. Injury 2000, 31, D18–D21. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Hu, X.; Dong, H. Surface modification of a medical grade Co-Cr-Mo alloy by low-temperature plasma surface alloying with nitrogen and carbon. Surf. Coat. Technol. 2013, 232, 906–911. [Google Scholar] [CrossRef] [Green Version]
- Brar, H.; Platt, M.; Sarntinoranont, M.; Martin, P.; Manuel, M. Magnesium as a biodegradable and bioabsorbable material for medical Implants. JOM J. Miner. Met. Mater. Soc. 2009, 61, 31–34. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Staiger, M.P.; Dias, G.J.; Woodfield, T.B. A novel manufacturing route for fabrication of topologically-ordered porous magnesium scaffolds. Adv. Eng. Mater. 2011, 13, 872–881. [Google Scholar] [CrossRef]
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application: A Review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Manivasagam, G.; Suwas, S. Biodegradable Mg and Mg based alloys for biomedical implants. Mater. Sci. Technol. 2014, 30, 515–520. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Razavi, M.; Vashaee, D.; Moharamzadeh, K.; Boccaccini, A.R.; Tayebi, L. Porous magnesium-based scaffolds for tissue engineering. Mater. Sci. Eng. C 2017, 71, 1253–1266. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-Y.; Cui, L.-Y.; Zeng, R.-C.; Li, S.-Q.; Chen, X.-B.; Zheng, Y.; Kannan, M.B. Advances in functionalized polymer coatings on biodegradable magnesium alloys—A review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Properties and applications of titanium alloys: A brief review. Rev. Adv. Mater. Sci. 2012, 32, 133–148. [Google Scholar]
- Marsh, A.C.; Chamorro, N.P.; Chatzistavrou, X. 15—Long-term performance and failure of orthopedic devices. In Bone Repair Biomaterials, 2nd ed.; Pawelec, K.M., Planell, J.A., Eds.; Woodhead Publishing: Sawston/Cambridge, UK, 2019; pp. 379–410. [Google Scholar]
- Khadija, G.; Saleem, A.; Akhtar, Z.; Naqvi, Z.; Gull, M.; Masood, M.; Mukhtar, S.; Batool, M.; Saleem, N.; Rasheed, T. Short term exposure to titanium, aluminum and vanadium (Ti 6Al 4V) alloy powder drastically affects behavior and antioxidant metabolites in vital organs of male albino mice. Toxicol. Rep. 2018, 5, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.; Puleo, D. Ti-6Al-4V ion solution inhibition of osteogenic cell phenotype as a function of differentiation timecourse in vitro. Biomaterials 1996, 17, 1949–1954. [Google Scholar] [CrossRef]
- Choubey, A.; Balasubramaniam, R.; Basu, B. Effect of replacement of V by Nb and Fe on the electrochemical and corrosion behavior of Ti–6Al–4V in simulated physiological environment. J. Alloys Compd. 2004, 381, 288–294. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Chen, L.-Y.; Wang, L. Surface Modification of Titanium and Titanium Alloys: Technologies, Developments and Future Interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar]
- Liu, W.; Cheng, M.; Wahafu, T.; Zhao, Y.; Qin, H.; Wang, J.; Zhang, X.; Wang, L. The in vitro and in vivo performance of a strontium-containing coating on the low-modulus Ti35Nb2Ta3Zr alloy formed by micro-arc oxidation. J. Mater. Sci. Mater. Med. 2015, 26, 203. [Google Scholar] [CrossRef]
- Zembic, A.; Sailer, I.; Jung, R.E.; Hämmerle, C.H.F. Randomized-controlled clinical trial of customized zirconia and titanium implant abutments for single-tooth implants in canine and posterior regions: 3-year results. Clin. Oral Implant. Res. 2009, 20, 802–808. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in biomedical applications—properties and fabrication: A review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Syrett, B.C.; Davis, E.E. In vivo evaluation of a high-strength, high-ductility stainless steel for use in surgical implants. J. Biomed. Mater. Res. 1979, 13, 543–556. [Google Scholar] [CrossRef]
- Sutha, S.; Karunakaran, G.; Rajendran, V. Enhancement of antimicrobial and long-term biostability of the zinc-incorporated hydroxyapatite coated 316L stainless steel implant for biomedical application. Ceram. Int. 2013, 39, 5205–5212. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Kapila, S.; Sachdeva, R. Mechanical properties and clinical applications of orthodontic wires. Am. J. Orthod. Dentofac. Orthop. 1989, 96, 100–109. [Google Scholar] [CrossRef]
- Gurappa, I. Development of appropriate thickness ceramic coatings on 316 L stainless steel for biomedical applications. Surf. Coat. Technol. 2002, 161, 70–78. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477. [Google Scholar] [CrossRef]
- Yoshioka, T.; Tsuru, K.; Hayakawa, S.; Osaka, A. Preparation of alginic acid layers on stainless-steel substrates for biomedical applications. Biomaterials 2003, 24, 2889–2894. [Google Scholar] [CrossRef]
- Hayes, J.; Richards, R. The use of titanium and stainless steel in fracture fixation. Expert Rev. Med. Devices 2010, 7, 843–853. [Google Scholar] [CrossRef]
- Hosseinalipour, S.; Ershad-Langroudi, A.; Hayati, A.N.; Nabizade-Haghighi, A. Characterization of sol–gel coated 316L stainless steel for biomedical applications. Prog. Org. Coat. 2010, 67, 371–374. [Google Scholar] [CrossRef]
- Katti, K.S. Biomaterials in total joint replacement. Colloids Surf. B Biointerfaces 2004, 39, 133–142. [Google Scholar] [CrossRef]
- Goldhaber, S.B. Trace element risk assessment: Essentiality vs. toxicity. Regul. Toxicol. Pharmacol. 2003, 38, 232–242. [Google Scholar] [CrossRef]
- Nie, J.F.; Muddle, B.C. Characterisation of strengthening precipitate phases in a Mg–Y–Nd alloy. Acta Mater. 2000, 48, 1691–1703. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Brunello, G.; Sivolella, S.; Meneghello, R.; Ferroni, L.; Gardin, C.; Piattelli, A.; Zavan, B.; Bressan, E. Powder-based 3D printing for bone tissue engineering. Biotechnol. Adv. 2016, 34, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Nanda, H.S.; Chen, S.; Zhang, Q.; Kawazoe, N.; Chen, G. Collagen scaffolds with controlled insulin release and controlled pore structure for cartilage tissue engineering. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Nanda, H.S.; Kawazoe, N.; Zhang, Q.; Chen, S.; Chen, G. Preparation of collagen porous scaffolds with controlled and sustained release of bioactive insulin. J. Bioact. Compat. Polym. 2014, 29, 95–109. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, Y.S. Role of proteins in the degradation of relatively inert alloys in the human body. NPJ Mater. Degrad. 2018, 2, 26. [Google Scholar] [CrossRef]
- Johnson, I.; Jiang, W.; Liu, H. The Effects of Serum Proteins on Magnesium Alloy Degradation in Vitro. Sci. Rep. 2017, 7, 14335. [Google Scholar] [CrossRef]
- Amerstorfer, F.; Fischerauer, S.F.; Fischer, L.; Eichler, J.; Draxler, J.; Zitek, A.; Meischel, M.; Martinelli, E.; Kraus, T.; Hann, S.; et al. Long-term in vivo degradation behavior and near-implant distribution of resorbed elements for magnesium alloys WZ21 and ZX50. Acta Biomater. 2016, 42, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Leung, C.-H.; Lin, S.; Zhong, H.-J.; Ma, D.-L. Metal complexes as potential modulators of inflammatory and autoimmune responses. Chem. Sci. 2015, 6, 871–884. [Google Scholar] [CrossRef] [Green Version]
- Tengvall, P.; Lundström, I. Physico-chemical considerations of titanium as a biomaterial. Clin. Mater. 1992, 9, 115–134. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Q.; Shi, L.; Chen, Q.; Wang, J. Recent advances in oxidation and degradation mechanisms of ultrathin 2D materials under ambient conditions and their passivation strategies. J. Mater. Chem. A 2019, 7, 4291–4312. [Google Scholar] [CrossRef]
- Bennett, T.D.; Cheetham, A.K. Amorphous Metal–Organic Frameworks. Acc. Chem. Res. 2014, 47, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Barbosa, C.; Monteiro, M.; Abud, I.; Caminha, I.; Roesler, C. Fretting corrosion tests on orthopedic plates and screws made of ASTM F138 stainless steel. Rev. Bras. Eng. Biomed. 2015, 31, 169–175. [Google Scholar] [CrossRef]
- Eliaz, N.; Baldev, R. Biomaterials and Corrosion; Narosa Publishing House: New Delhi, India, 2008; pp. 356–397. [Google Scholar]
- Jimbo, R.; Ivarsson, M.; Koskela, A.; Sul, Y.-T.; Johansson, C.B. Protein adsorption to surface chemistry and crystal structure modification of titanium surfaces. J. Oral Maxillofac. Res. 2010, 1, e3. [Google Scholar] [CrossRef]
- Lehtovirta, L.; Reito, A.; Parkkinen, J.; Peräniemi, S.; Vepsäläinen, J.; Eskelinen, A. Association between periprosthetic tissue metal content, whole blood and synovial fluid metal ion levels and histopathological findings in patients with failed metal-on-metal hip replacement. PLoS ONE 2018, 13, e0197614. [Google Scholar] [CrossRef] [Green Version]
- Goodman, S.B. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials 2007, 28, 5044–5048. [Google Scholar] [CrossRef] [Green Version]
- Erdmann, N.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Thorey, F.; Bormann, D.; Meyer-Lindenberg, A. Biomechanical testing and degradation analysis of MgCa0.8 alloy screws: A comparative in vivo study in rabbits. Acta Biomater. 2011, 7, 1421–1428. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B. In vivo corrosion of CoCrMo alloy and biological responses: A review. Mater. Technol. 2018, 33, 127–134. [Google Scholar] [CrossRef]
- Nanda, H.S. Surface modification of promising cerium oxide nanoparticles for nanomedicine applications. RSC Adv. 2016, 6, 111889–111894. [Google Scholar] [CrossRef]
- Nanda, H.S. Preparation and Biocompatible Surface Modification of Redox Altered Cerium Oxide Nanoparticle Promising for Nanobiology and Medicine. Bioengineering 2016, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Geng, H.; Poologasundarampillai, G.; Todd, N.; Devlin-Mullin, A.; Moore, K.L.; Golrokhi, Z.; Gilchrist, J.B.; Jones, E.; Potter, R.J.; Sutcliffe, C.; et al. Biotransformation of Silver Released from Nanoparticle Coated Titanium Implants Revealed in Regenerating Bone. ACS Appl. Mater. Interfaces 2017, 9, 21169–21180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.-M.; Lu, X.; Wang, K.-F.; Meng, F.-Z.; Jiang, O.; Zhang, H.-P.; Zhi, W.; Fang, L.-M. Silver Nanoparticles and Growth Factors Incorporated Hydroxyapatite Coatings on Metallic Implant Surfaces for Enhancement of Osteoinductivity and Antibacterial Properties. ACS Appl. Mater. Interfaces 2014, 6, 8580–8589. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dubey, A.K. Various Biomaterials and Techniques for Improving Antibacterial Response. ACS Appl. Biol. Mater. 2018, 1, 3–20. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced Antibacterial Activity of Nanocrystalline ZnO Due to Increased ROS-Mediated Cell Injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Alagarsamy, K.; Vishwakarma, V.; Saravanan, G.; Kamalan kirubaharan, A.M. Silver-ceria stabilized zirconia composite coatings on titanium for potential implant applications. Surf. Coat. Technol. 2019, 368, 224–231. [Google Scholar]
- Wang, J.; Li, J.; Guo, G.; Wang, Q.; Tang, J.; Zhao, Y.; Qin, H.; Wahafu, T.; Shen, H.; Liu, X.; et al. Silver-nanoparticles-modified biomaterial surface resistant to staphylococcus: New insight into the antimicrobial action of silver. Sci. Rep. 2016, 6, 32699. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, C.S.; Iconaru, S.L.; Chifiriuc, M.C.; Costescu, A.; Le Coustumer, P.; Predoi, D. Synthesis and antimicrobial activity of silver-doped hydroxyapatite nanoparticles. Biomed. Res. Int. 2013, 2013, 916218. [Google Scholar] [CrossRef] [Green Version]
- Visai, L.; De Nardo, L.; Punta, C.; Melone, L.; Cigada, A.; Imbriani, M.; Arciola, C.R. Titanium oxide antibacterial surfaces in biomedical devices. Int. J. Artif. Organs 2011, 34, 929–946. [Google Scholar] [CrossRef]
- Kargupta, R.; Bok, S.; Darr, C.M.; Crist, B.D.; Gangopadhyay, K.; Gangopadhyay, S.; Sengupta, S. Coatings and surface modifications imparting antimicrobial activity to orthopedic implants. Wires Nanomed. Nanobiotechnology 2014, 6, 475–495. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 °C. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnology 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, A.; Hervella, P.; Leroux, J.-C.; Pratsinis, S.E.; Sotiriou, G.A. Toxicity of Silver Nanoparticles in Macrophages. Small 2013, 9, 2576–2584. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gu, H.; Sha, G.; Lu, W.; Yu, W.; Zhang, W.; Fu, Y.; Wang, K.; Wang, L. TC4/Ag metal matrix nanocomposites modified by friction stir processing: Surface characterization, antibacterial property, and cytotoxicity in vitro. ACS Appl. Mater. Interfaces 2018, 10, 41155–41166. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Fan, Q.; Wang, L. A Review on Friction Stir Processing of Titanium Alloy: Characterization, Method, Microstructure, Properties. Metall. Mater. Trans. B 2019, 50, 2134–2162. [Google Scholar] [CrossRef]
- Wu, S.; Weng, Z.; Liu, X.; Yeung, K.W.K.; Chu, P.K. Functionalized TiO2 Based Nanomaterials for Biomedical Applications. Adv. Funct. Mater. 2014, 24, 5464–5481. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Z.; Xie, L.; Zhang, L.-C.; Wu, L.; Fu, Y.; Wang, L.; Lu, W. Electrochemical and in vitro behavior of the nanosized composites of Ti-6Al-4V and TiO2 fabricated by friction stir process. Appl. Surf. Sci. 2017, 423, 331–339. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, C.; Xie, L.; Zhang, L.-C.; Wang, L.; Lu, W. Effects of friction stir processing on the phase transformation and microstructure of TiO 2-compounded Ti-6Al-4V alloy. Metall. Mater. Trans. A 2016, 47, 5675–5679. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Vishwakarma, V.; Kirubaharan, K.; Dharini, T.; Ramachandran, D.; Muthaiah, B. Corrosion and biocompatibility behaviour of zirconia coating by EBPVD for biomedical applications. Surf. Coat. Technol. 2018, 334, 336–343. [Google Scholar] [CrossRef]
- Jingyu, W.; Lin, W.; Yong, G.; Jinsong, Z.; Cuicui, Z. Experimental study on the osseointegration of foam TiC/Ti composites. Biomed. Mater. 2013, 8, 045001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Lv, Y.; Qian, C.; Qian, H.; Jiao, T.; Wang, L.; Zhang, F. Proliferation and osteogenic differentiation of rat BMSCs on a novel Ti/SiC metal matrix nanocomposite modified by friction stir processing. Sci. Rep. 2016, 6, 38875. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Biomedical Metals | Properties | Applications | Ref. |
|---|---|---|---|
| Ti and its alloys | Biocompatible, high fatigue strength, flexible, low Young’s modulus, expensive, low wear and corrosion resistance, good tensile strength, reduced Stress shielding, good osteointegration | total knee replacement (TKR), total hip replacement (THR), bone screws and plates for bone fracture fixation, screws/plates for maxillofacial application in the cranio-facial and mandibular areas. | [7,49,50,51,52,53,54,55,56,57,58,59,60,61] |
| SS 316L | Biocompatible, low fatigue strength, high Young’s modulus, cheaper, high tensile strength, Stress shielding | Bone plates, medullary nails, screws, pins, sutures and steel threads used in fixation of fractures. | [10,12,13,49,62,63] |
| Co-Cr alloy | Biocompatible, high tensile strength, high Young’s modulus, stress shielding | Orthopedic prostheses for the knee, shoulder, ankle and hip as well as fracture fixation devices | [10,13,49,64,65] |
| Magnesium (Mg) and its alloys | Biocompatible, good osteointegration, lower Young’s modulus, high strength, biodegradable, no stress shielding | Used as a mesh cage for segmental defect in long bone, 3-D scaffold design (tissue engineering) for bone regeneration | [20,66,67,68,69,70,71,72] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahirwar, H.; Zhou, Y.; Mahapatra, C.; Ramakrishna, S.; Kumar, P.; Nanda, H.S. Materials for Orthopedic Bioimplants: Modulating Degradation and Surface Modification Using Integrated Nanomaterials. Coatings 2020, 10, 264. https://doi.org/10.3390/coatings10030264
Ahirwar H, Zhou Y, Mahapatra C, Ramakrishna S, Kumar P, Nanda HS. Materials for Orthopedic Bioimplants: Modulating Degradation and Surface Modification Using Integrated Nanomaterials. Coatings. 2020; 10(3):264. https://doi.org/10.3390/coatings10030264
Chicago/Turabian StyleAhirwar, Harbhajan, Yubin Zhou, Chinmaya Mahapatra, Seeram Ramakrishna, Prasoon Kumar, and Himansu Sekhar Nanda. 2020. "Materials for Orthopedic Bioimplants: Modulating Degradation and Surface Modification Using Integrated Nanomaterials" Coatings 10, no. 3: 264. https://doi.org/10.3390/coatings10030264
APA StyleAhirwar, H., Zhou, Y., Mahapatra, C., Ramakrishna, S., Kumar, P., & Nanda, H. S. (2020). Materials for Orthopedic Bioimplants: Modulating Degradation and Surface Modification Using Integrated Nanomaterials. Coatings, 10(3), 264. https://doi.org/10.3390/coatings10030264









