Cytotoxicity and Mineralization Potential of Four Calcium Silicate-Based Cements on Human Gingiva-Derived Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Gingiva-Derived Stem Cells
2.2. Experimental Disks of Four Calcium Silicate-Based Cements
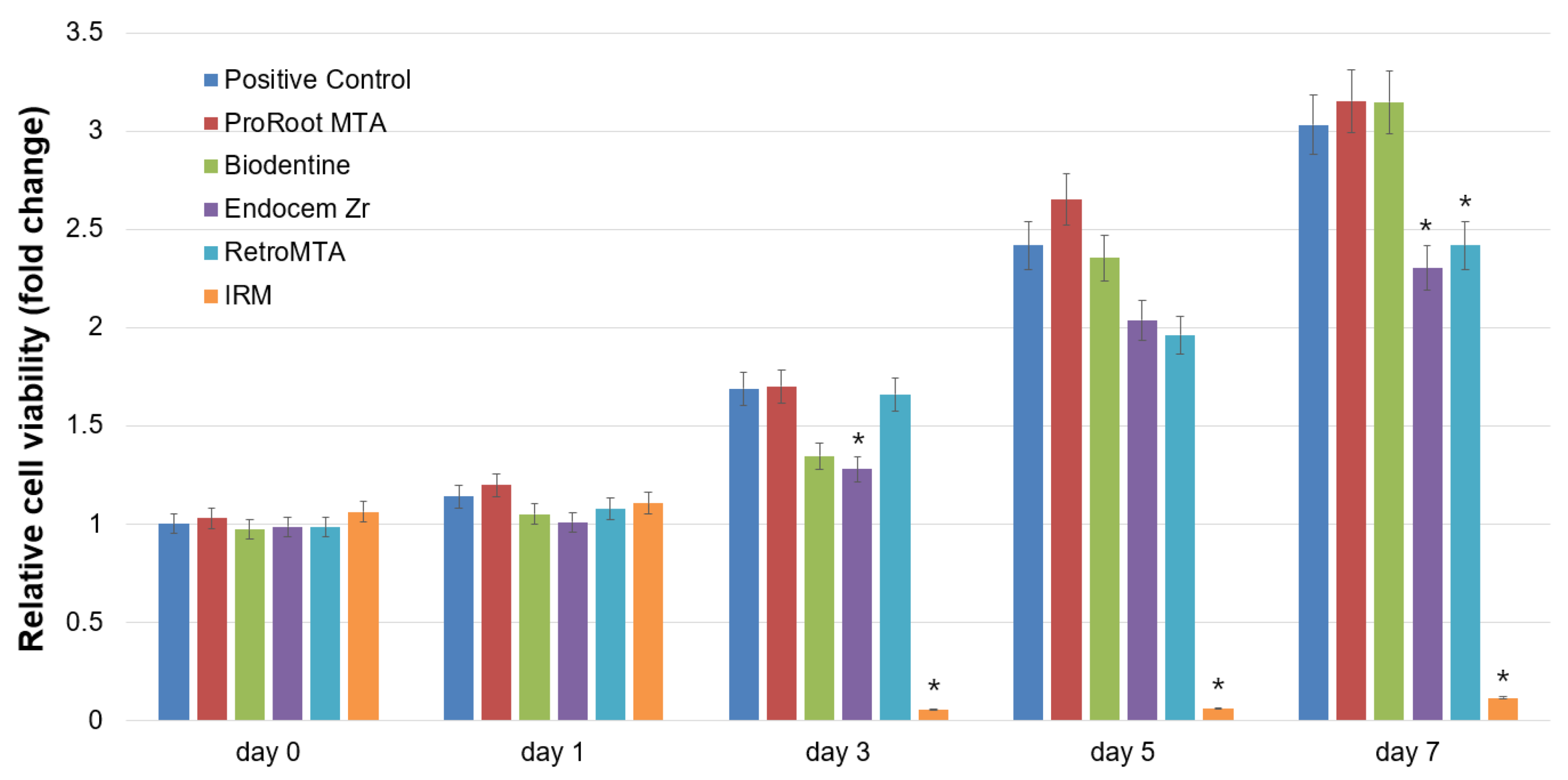
2.3. Cell Viability Assay
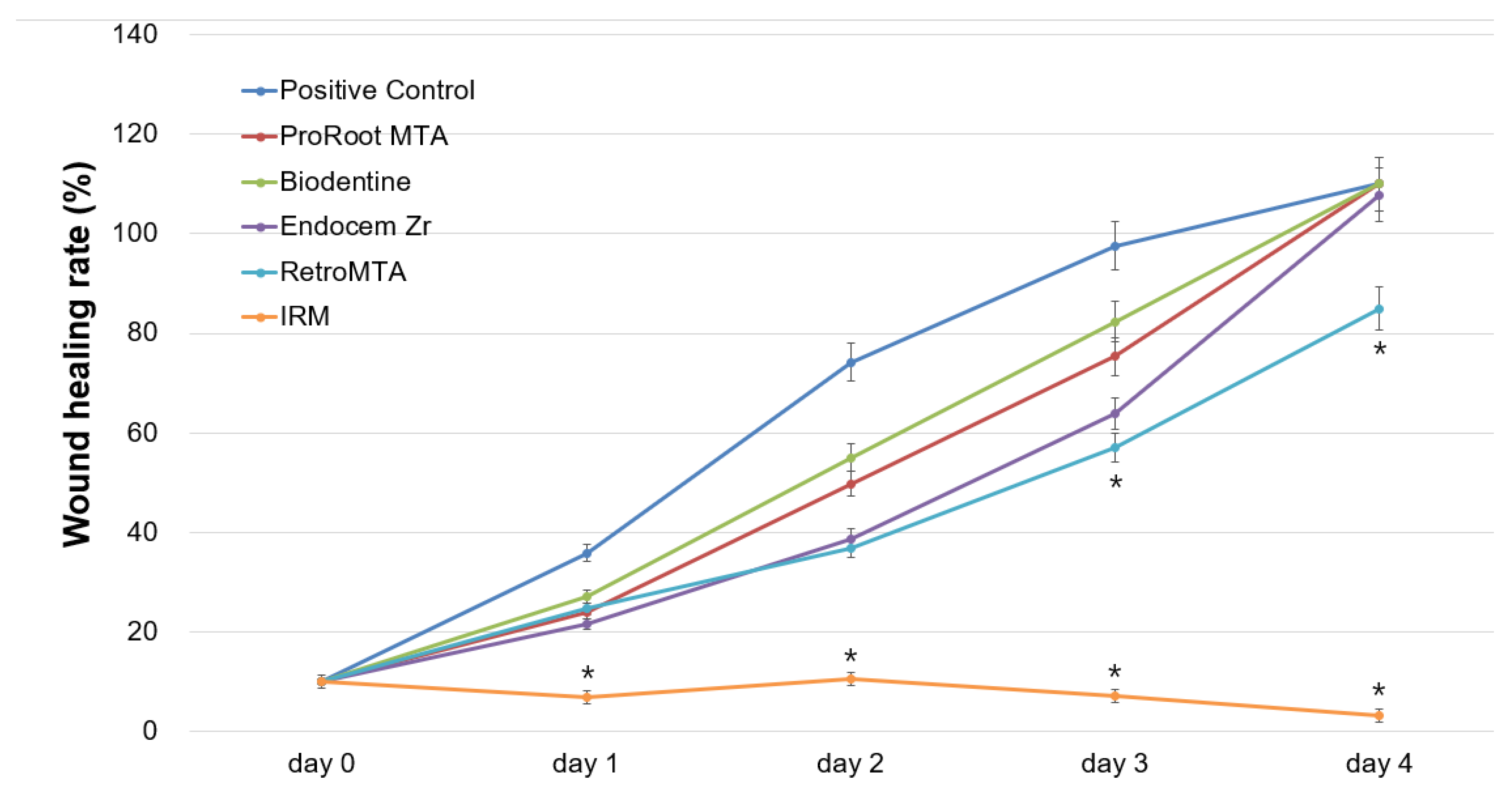
2.4. Cell Migration Assay
2.5. Live/Dead Staining Assay
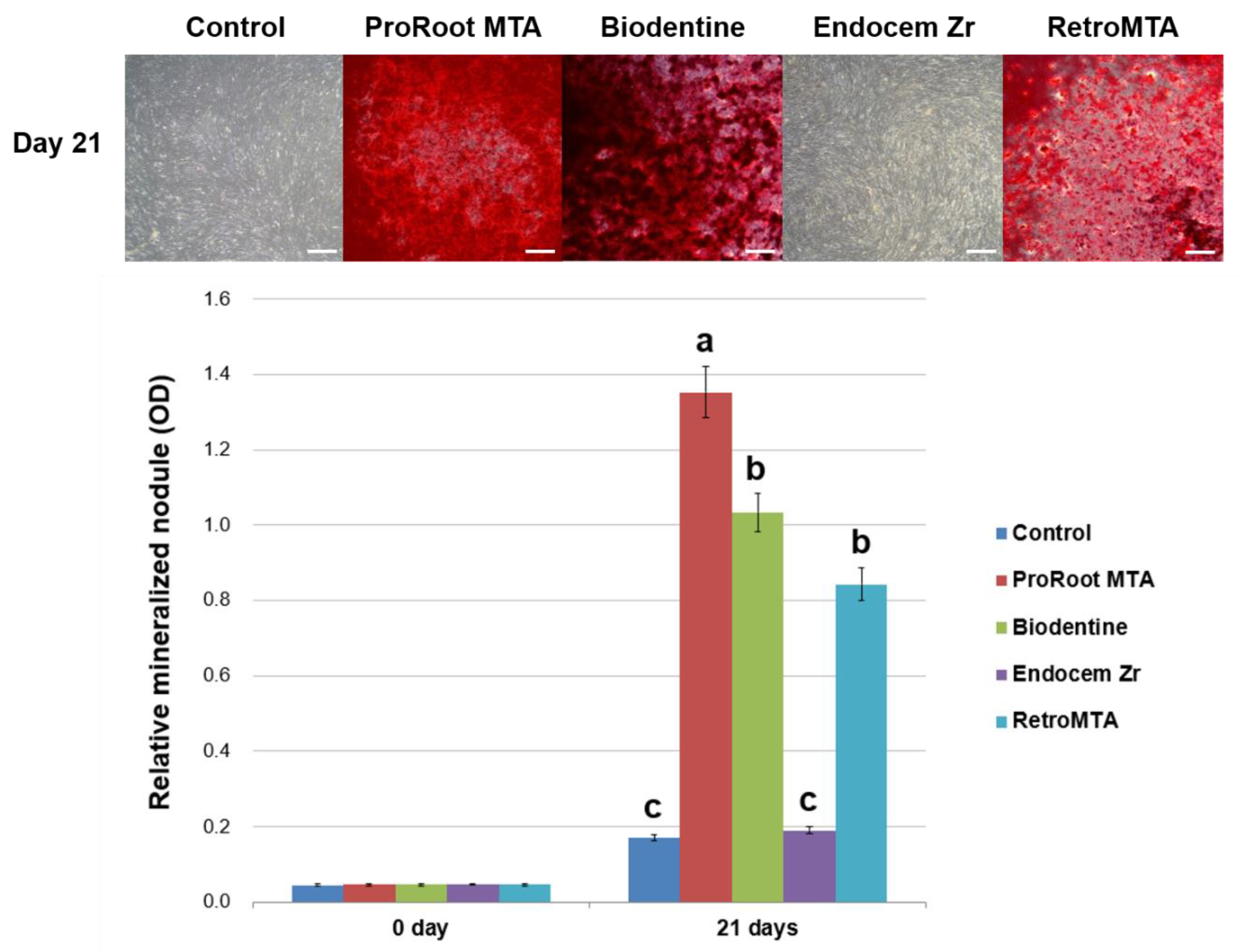
2.6. Alizarin Red S (ARS) Staining Assay
2.7. pH Measurement
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fuss, Z.; Tsesis, I.; Lin, S. Root resorption—Diagnosis, classification and treatment choices based on stimulation factors. Dent. Traumatol. 2003, 19, 175–182. [Google Scholar] [CrossRef]
- Heithersay, G.S. Management of tooth resorption. Aust. Dent. J. 2007, 52, S105–S121. [Google Scholar] [CrossRef] [Green Version]
- Karypidou, A.; Chatzinikolaou, I.D.; Kouros, P.; Koulaouzidou, E.; Economides, N. Management of bilateral invasive cervical resorption lesions in maxillary incisors using a novel calcium silicate-based cement: A case report. Quintessence Int. 2016, 47, 637–642. [Google Scholar] [CrossRef]
- Duarte, M.A.H.; Marciano, M.A.; Vivan, R.R.; Tanomaru Filho, M.; Tanomaru, J.M.G.; Camilleri, J. Tricalcium silicate-based cements: Properties and modifications. Braz. Oral Res. 2018, 32, e70. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, J. Characterization and hydration kinetics of tricalcium silicate cement for use as a dental biomaterial. Dent. Mater. 2011, 27, 836–844. [Google Scholar] [CrossRef]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef]
- Peng, W.; Liu, W.; Zhai, W.; Jiang, L.; Li, L.; Chang, J.; Zhu, Y. Effect of tricalcium silicate on the proliferation and odontogenic differentiation of human dental pulp cells. J. Endod. 2011, 37, 1240–1246. [Google Scholar] [CrossRef]
- Du, R.; Wu, T.; Liu, W.; Li, L.; Jiang, L.; Peng, W.; Chang, J.; Zhu, Y. Role of the extracellular signal-regulated kinase 1/2 pathway in driving tricalcium silicate-induced proliferation and biomineralization of human dental pulp cells in vitro. J. Endod. 2013, 39, 1023–1029. [Google Scholar] [CrossRef]
- Rathinam, E.; Rajasekharan, S.; Chitturi, R.T.; Martens, L.; De Coster, P. Gene expression profiling and molecular signaling of dental pulp cells in response to tricalcium silicate cements: A systematic review. J. Endod. 2015, 41, 1805–1817. [Google Scholar] [CrossRef]
- Marciano, M.A.; Camilleri, J.; Costa, R.M.; Matsumoto, M.A.; Guimaraes, B.M.; Duarte, M.A.H. Zinc oxide inhibits dental discoloration caused by white mineral trioxide aggregate Angelus. J. Endod. 2017, 43, 1001–1007. [Google Scholar] [CrossRef] [Green Version]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: Composition, properties, and clinical applications. J. Investig. Clin. Dent. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Loison-Robert, L.S.; Tassin, M.; Bonte, E.; Berbar, T.; Isaac, J.; Berdal, A.; Simon, S.; Fournier, B.P.J. In vitro effects of two silicate-based materials, Biodentine and BioRoot RCS, on dental pulp stem cells in models of reactionary and reparative dentinogenesis. PLoS ONE 2018, 13, e0190014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Hurt, A.P.; Coleman, N.J. The application of (29)Si NMR spectroscopy to the analysis of calcium silicate-based cement using Biodentine™ as an example. J. Funct. Biomater. 2019, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Setbon, H.M.; Devaux, J.; Iserentant, A.; Leloup, G.; Leprince, J.G. Influence of composition on setting kinetics of new injectable and/or fast setting tricalcium silicate cements. Dent. Mater. 2014, 30, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Laurent, P.; About, I. Hydration of Biodentine, Theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp capping in entire tooth cultures. J. Endod. 2014, 40, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Zanini, M.; Sautier, J.M.; Berdal, A.; Simon, S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J. Endod. 2012, 38, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Shin, Y.S.; Lee, H.S.; Kim, S.O.; Shin, Y.; Jung, I.Y.; Song, J.S. Color changes of teeth after treatment with various mineral trioxide aggregate-based materials: An ex vivo study. J. Endod. 2015, 41, 737–741. [Google Scholar] [CrossRef]
- Choi, Y.; Park, S.J.; Lee, S.H.; Hwang, Y.C.; Yu, M.K.; Min, K.S. Biological effects and washout resistance of a newly developed fast-setting pozzolan cement. J. Endod. 2013, 39, 467–472. [Google Scholar] [CrossRef]
- Pornamazeh, T.; Yadegari, Z.; Ghasemi, A.; Sheykh-Al-Eslamian, S.M.; Shojaeian, S. In vitro cytotoxicity and setting time assessment of calcium-enriched mixture cement, Retro mineral trioxide aggregate and mineral trioxide aggregate. Iran. Endod. J. 2017, 12, 488–492. [Google Scholar] [CrossRef]
- Collado-Gonzalez, M.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Ortolani-Seltenerich, P.S.; Alvarez-Muro, T.; Lozano, A.; Forner, L.; Llena, C.; Moraleda, J.M.; Rodriguez-Lozano, F.J. Cytotoxicity and bioactivity of various pulpotomy materials on stem cells from human exfoliated primary teeth. Int. Endod. J. 2017, 50, e19–e30. [Google Scholar] [CrossRef]
- Jin, S.H.; Lee, J.E.; Yun, J.H.; Kim, I.; Ko, Y.; Park, J.B. Isolation and characterization of human mesenchymal stem cells from gingival connective tissue. J. Periodontal Res. 2015, 50, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Biocompatibility of new pulp-capping materials NeoMTA Plus, MTA Repair HP, and Biodentine on human dental pulp stem cells. J. Endod. 2018, 44, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, L.C.D.; Yadlapati, M.; Dorn, S.O.; Silva, R.; Letra, A. Analysis of radiopacity, pH and cytotoxicity of a new bioceramic material. J. Appl. Oral Sci. 2015, 23, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, A.R.; Emara, R.; Taher, M.M.; Al-Allaf, F.A.; Almalki, M.; Almasri, M.A.; Siddiqui, S.S. Effects of mineral trioxide aggregate, calcium hydroxide, Biodentine and Emdogain on osteogenesis, odontogenesis, angiogenesis and cell viability of dental pulp stem cells. BMC Oral Health 2019, 19, 133. [Google Scholar] [CrossRef]
- Kim, H.S.; Zheng, M.; Kim, D.K.; Lee, W.P.; Yu, S.J.; Kim, B.O. Effects of 1,25-dihydroxyvitamin D3 on the differentiation of MC3T3-E1 osteoblast-like cells. J. Periodontal Implant Sci. 2018, 48, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.H.; Oh, S.; Kim, S. Improvement of the osteogenic potential of ErhBMP-2-/EGCG-coated biphasic calcium phosphate bone substitute: In vitro and in vivo activity. J. Periodontal Implant Sci. 2019, 49, 114–126. [Google Scholar] [CrossRef]
- Widbiller, M.; Lindner, S.R.; Buchalla, W.; Eidt, A.; Hiller, K.A.; Schmalz, G.; Galler, K.M. Three-dimensional culture of dental pulp stem cells in direct contact to tricalcium silicate cements. Clin. Oral Investig. 2016, 20, 237–246. [Google Scholar] [CrossRef]
- Sequeira, D.B.; Seabra, C.M.; Palma, P.J.; Cardoso, A.L.; Peça, J.; Santos, J.M. Effects of a new bioceramic material on human apical papilla cells. J. Funct. Biomater. 2018, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Li, D.; Kohli, M.R.; Yu, Q.; Kim, S.; He, W.-X. Effect of Biodentine™ on the proliferation, migration and adhesion of human dental pulp stem cells. J. Dent. 2014, 42, 490–497. [Google Scholar] [CrossRef]
- Lee, M.; Kang, C.M.; Song, J.S.; Shin, Y.; Kim, S.; Kim, S.O.; Choi, H.J. Biological efficacy of two mineral trioxide aggregate (MTA)-based materials in a canine model of pulpotomy. Dent. Mater. J. 2017, 36, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Kodama, S.; Okiji, T. Evaluation of calcium-releasing and apatite-forming abilities of fast-setting calcium silicate-based endodontic materials. Int. Endod. J. 2015, 48, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Deacon, A.D.; Coleman, N.J. The impact of zirconium oxide nanoparticles on the hydration chemistry and biocompatibility of white Portland cement. Dent. Mater. J. 2013, 32, 808–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.J.; Kim, E.; Song, M.; Park, J.W.; Shin, S.J. Effects of two fast-setting calcium-silicate cements on cell viability and angiogenic factor release in human pulp-derived cells. Odontology 2016, 104, 143–151. [Google Scholar] [CrossRef]
- Margunato, S.; Tasli, P.N.; Aydin, S.; Karapinar Kazandag, M.; Sahin, F. In vitro evaluation of ProRoot MTA, Biodentine, and MM-MTA on human alveolar bone marrow stem cells in terms of biocompatibility and mineralization. J. Endod. 2015, 41, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Singh, M.; Nawal, R.R.; Chaudhry, S.; Yadav, S.; Mohanty, S.; Talwar, S. Evaluation of biocompatibility and osteogenic potential of tricalcium silicate-based cements using human bone marrow-derived mesenchymal stem cells. J. Endod. 2018, 44, 446–451. [Google Scholar] [CrossRef]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlinska, J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Wongwatanasanti, N.; Jantarat, J.; Sritanaudomchai, H.; Hargreaves, K.M. Effect of bioceramic materials on proliferation and odontoblast differentiation of human stem cells from the apical papilla. J. Endod. 2018, 44, 1270–1275. [Google Scholar] [CrossRef]
- Wattanapakkavong, K.; Srisuwan, T. Release of transforming growth factor beta 1 from human tooth dentin after application of either ProRoot MTA or Biodentine as a coronal barrier. J. Endod. 2019, 45, 701–705. [Google Scholar] [CrossRef]
- Khan, S.; Kaleem, M.; Fareed, M.A.; Habib, A.; Iqbal, K.; Aslam, A.; Ud Din, S. Chemical and morphological characteristics of mineral trioxide aggregate and Portland cements. Dent. Mater. J. 2016, 35, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Formosa, L.M.; Mallia, B.; Bull, T.; Camilleri, J. The microstructure and surface morphology of radiopaque tricalcium silicate cement exposed to different curing conditions. Dent. Mater. 2012, 28, 584–595. [Google Scholar] [CrossRef]
- Kim, M.; Yang, W.; Kim, H.; Ko, H. Comparison of the biological properties of ProRoot MTA, OrthoMTA, and Endocem MTA cements. J. Endod. 2014, 40, 1649–1653. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part I: Chemical, physical, and antibacterial properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef] [PubMed]


| Material | Manufacturer | Composition | Batch Number |
|---|---|---|---|
| ProRoot MTA | Dentsply Tulsa Dental Specialties, Tulsa, OK, USA | Portland cement (tricalcium silicate, dicalcium silicate, and tricalcium aluminate) 75% Calcium sulfate dihydrate (gypsum) 5% Bismuth oxide 20% | 0000186484 |
| Biodentine | Septodont, Saint-Maur-des-Fossés, France | Tricalcium silicate, dicalcium silicate, calcium carbonate, calcium oxide, and zirconium oxide in its powder form Water, calcium chloride, and soluble polymer as an aqueous liquid | B24553 |
| Endocem Zr | Maruchi, Wonju, Korea | Calcium oxide 27%–37% Silicon dioxide 7%–11% Aluminum oxide 3%–5% Magnesium oxide 1.7%–2.5% Ferrous oxide 1.3%–2.3% Zirconium dioxide 43%–46% | ZF7812231228 |
| RetroMTA | BioMTA, Seoul, Korea | Calcium carbonate 60%–80% Silicon dioxide 5%–15% Aluminum oxide 5%–10% Calcium zirconia complex 20%–30% | RM1810D14 |
| Material | pH (7 days) | |||||||
|---|---|---|---|---|---|---|---|---|
| Osteogenic Media | ddH2O | |||||||
| Mean | Mean | |||||||
| Control | 7.56 | 7.59 | 7.50 | 7.55 | 7.02 | 7.06 | 7.08 | 7.05 |
| ProRoot MTA | 8.58 | 8.63 | 8.67 | 8.63 | 11.33 | 11.34 | 11.29 | 11.32 |
| Biodentine | 9.65 | 9.71 | 9.67 | 9.68 | 11.31 | 11.30 | 11.34 | 11.32 |
| Endocem Zr | 8.61 | 8.69 | 8.74 | 8.68 | 10.77 | 10.79 | 10.82 | 10.79 |
| RetroMTA | 8.55 | 8.57 | 8.58 | 8.57 | 11.32 | 11.35 | 11.32 | 11.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Park, J.-B.; Song, D.; Kim, H.-M.; Kim, S.-Y. Cytotoxicity and Mineralization Potential of Four Calcium Silicate-Based Cements on Human Gingiva-Derived Stem Cells. Coatings 2020, 10, 279. https://doi.org/10.3390/coatings10030279
Lee D, Park J-B, Song D, Kim H-M, Kim S-Y. Cytotoxicity and Mineralization Potential of Four Calcium Silicate-Based Cements on Human Gingiva-Derived Stem Cells. Coatings. 2020; 10(3):279. https://doi.org/10.3390/coatings10030279
Chicago/Turabian StyleLee, Donghee, Jun-Beom Park, Dani Song, Hye-Min Kim, and Sin-Young Kim. 2020. "Cytotoxicity and Mineralization Potential of Four Calcium Silicate-Based Cements on Human Gingiva-Derived Stem Cells" Coatings 10, no. 3: 279. https://doi.org/10.3390/coatings10030279





