Effects of Ti, Ni, and Dual Ti/Ni Plasma Immersion Ion Implantation on the Corrosion and Wear Properties of Magnesium Alloy
Abstract
:1. Introduction
2. Experimental Details and Theoretical Calculation
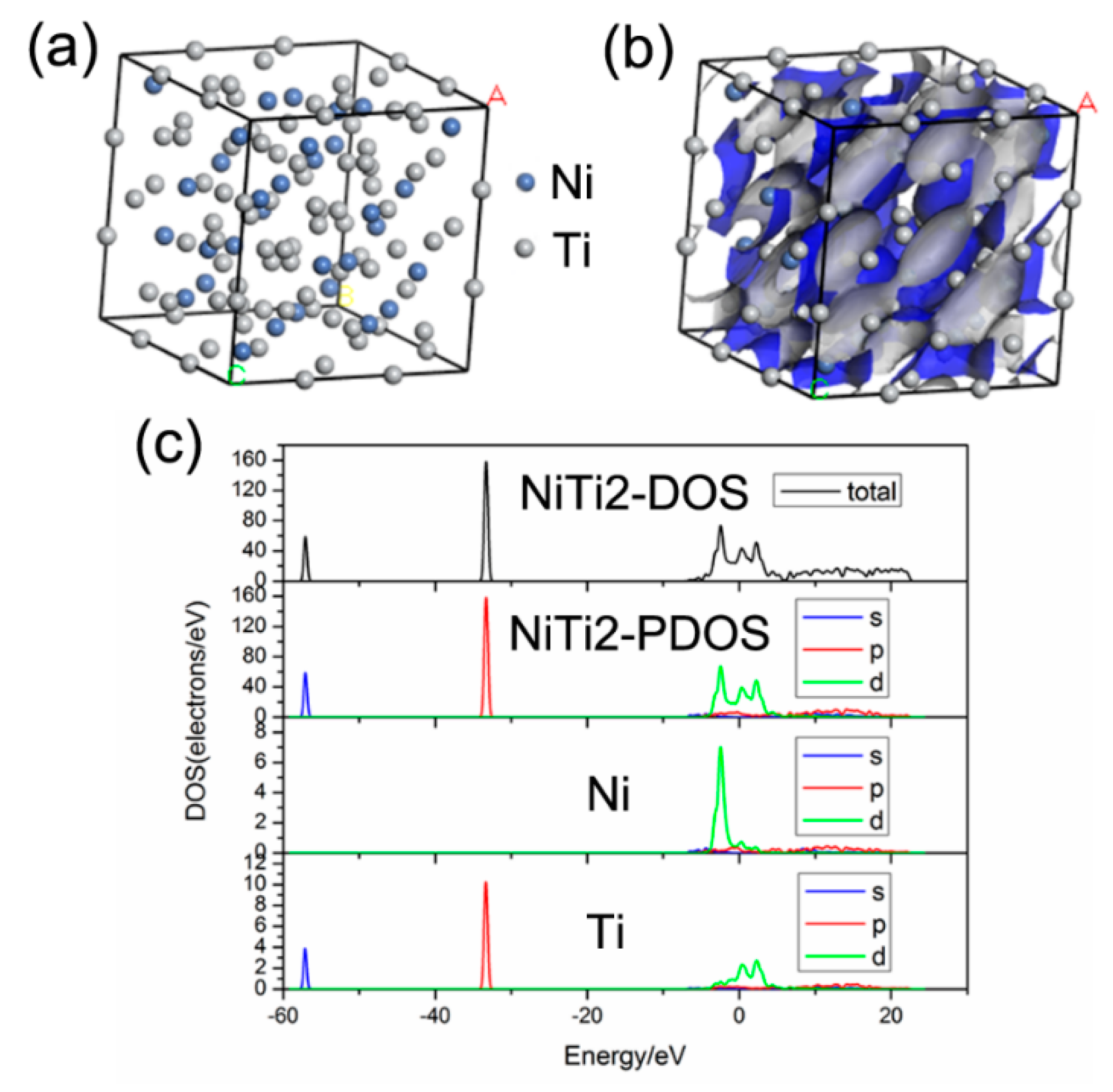
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaha, S.K.; Dayani, S.B.; Xue, Y.N. Improving corrosion and corrosion-fatigue resistance of AZ31B cast mg alloy using combined cold spray and top coatings. Coatings 2018, 8, 443. [Google Scholar] [CrossRef] [Green Version]
- Polmear, I.J. Magnesium alloys and applications. Mater. Sci. Technol. 1994, 10, 1–16. [Google Scholar] [CrossRef]
- Mukai, T.; Yamanoi, M.; Watanabe, H.; Higashi, K. Ductility enhancement in AZ31 magnesium alloy by controlling its grain structure. Scr. Mater. 2001, 45, 89–94. [Google Scholar] [CrossRef]
- Rahmati, M.; Raeissi, K.; Toroghinejad, M.R. Effect of pulse current mode on microstructure, composition and corrosion performance of the coatings produced by plasma electrolytic oxidation on AZ31 Mg alloy. Coatings 2019, 9, 688. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Li, C.; Liang, N.N.; Yang, F.L.; Jiang, J.H.; Sun, J.P.; Wu, G.S.; Ma, A.; Ma, X.L. Simultaneously improving corrosion resistance and mechanical properties of a magnesium alloy via equal-channel angular pressing and post water annealing. Mater. Des. 2019, 166, 107621. [Google Scholar] [CrossRef]
- Jian, S.Y.; Ho, M.L.; Shih, B.C. Evaluation of the corrosion resistance and cytocompatibility of a bioactive micro-arc oxidation coating on AZ31 Mg alloy. Coatings 2019, 9, 396. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.J.; Lin, X.Z.; Liu, C.H.; Yang, R.S.; Zheng, X.W.; Gong, M. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coating on AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–412. [Google Scholar] [CrossRef]
- Ignat, S.; Sallamand, P.; Grevey, D.; Lambertin, M. Magnesium alloys laser (Nd: YAG) cladding and alloying with side injection of aluminium powder. Appl. Surf. Sci. 2004, 225, 124–134. [Google Scholar] [CrossRef]
- Liu, J.L.; Yu, H.J.; Chen, C.Z.; Weng, F.; Dai, J.J. Research and development status of laser cladding on magnesium alloys: A review. Opt. Lasers. Eng. 2017, 93, 195–210. [Google Scholar] [CrossRef]
- Luca, P.; Leonardo, B.C.; Rachele, B.; Alessio, G.S.; Katya, B.; Marjorie, O.; Dabalà, M. Corrosion and mechanical properties of plasma electrolytic oxidation-coated AZ80 magnesium alloy. Mater. Corros. 2019, 70, 2103–2112. [Google Scholar]
- Luca, P.; Michele, R.; Alessandro, M.; Katya, B.; Manuele, D. Plasma Electrolytic Oxidation (PEO) as pre-treatment for sol-gel coating on aluminum and magnesium alloys. Surf. Coat. Technol. 2019, 366, 114–123. [Google Scholar]
- Wu, G.S.; Jamesh, M.I.; Chu, P.K. Surface design of biodegradable magnesium alloys—A review. Surf. Coat. Technol. 2013, 233, 2–12. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Wu, G.S.; Zhao, Y.; McKenzie, D.R.; Bilek, M.M.; Chu, P.K. Electrochemical corrosion behavior of biodegradable Mg-Y-RE and Mg-Zn-Zr alloys in Ringer’s solution and simulated body fluid. Corros. Sci. 2015, 91, 160–184. [Google Scholar] [CrossRef]
- Feliu, S.; Maffiotte, C.; Samaniego, A.; Galvan, J.C.; Barranco, V. Effect of the chemistry and structure of the native oxide surface film on the corrosion properties of commercial AZ31 and AZ61 alloys. Appl. Surf. Sci. 2011, 257, 8558–8568. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhang, L.B.; Bi, Y.Z. Corrosion behavior of Fe/Zr composite coating on ZK60 Mg alloy by Ion implantation and deposition. Coatings 2018, 8, 261. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zheng, Y.; Bi, Y.; Li, Y.; Zheng, Y.F. Improved cytocompatibility of Mg-1Ca alloy modified by Zn ion implantation and deposition. Mater. Lett. 2017, 205, 87–89. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Xiong, G.Y.; Luo, H.L.; He, F.; Huang, Y.; Wang, Y.L. Influence of zinc ion implantation on surface nanomechanical performance and corrosion resistance of biomedical magnesium–calcium alloys. Appl. Surf. Sci. 2008, 254, 5514–5516. [Google Scholar] [CrossRef]
- Ba, Z.X.; Jia, Y.Q.; Dong, Q.S.; Li, Z.Z.; Kuang, J. Effects of Zr Ion implantation on surface mechanical properties and corrosion resistance of pure magnesium. J. Mater. Eng. Perform. 2019, 28, 2543–2551. [Google Scholar] [CrossRef]
- Jin, W.H.; Wu, G.S.; Feng, H.Q.; Wang, W.H.; Zhang, X.M.; Chu, P.K. Improvement of corrosion resistance and biocompatibility of rare-earth WE43 magnesium alloy by neodymium self-ion implantation. Corros. Sci. 2015, 94, 142–155. [Google Scholar] [CrossRef]
- Li, Z.C.; Shang, Z.Z.; Wei, X.; Zhao, Q. Corrosion resistance and cytotoxicity of AZ31 magnesium alloy with N+ ion implantation. Mater. Technol. 2019, 34, 730–736. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Wu, G.S.; Zhao, Y.; Chu, P.K. Effects of silicon plasma ion implantation on electrochemical corrosion behavior of biodegradable Mg–Y–RE Alloy. Corros. Sci. 2013, 69, 158–163. [Google Scholar] [CrossRef]
- Husak, Y.; Solodovnyk, O.; Yanovska, A. Degradation and in vivo response of hydroxyapatite-coated Mg alloy. Coatings 2018, 8, 375. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.X.; Xu, Q.; Jiang, Y.H.; Wang, C.Q.; Zhang, X.W. Corrosion resistance and mechanical property of AZ31 magnesium alloy by N/Ti duplex ion implantation. Surf. Coat. Technol. 2013, 228, S538–S543. [Google Scholar] [CrossRef]
- Wu, G.S.; Ding, K.J.; Zeng, X.Q.; Wang, X.M.; Yao, S.S. Improving corrosion resistance of titanium-coated magnesium alloy by modifying surface characteristics of magnesium alloy prior to titanium coating deposition. Scr. Mater. 2009, 61, 269–272. [Google Scholar] [CrossRef]
- Xie, Z.W.; Chen, Q.; Chen, T.; Gao, X.; Yu, X.G.; Song, H.; Feng, Y.J. Microstructure and properties of nitrogen ion implantation/AlN/CrAlN/MoS2-phenolic resin duplex coatings on magnesium alloys. Mater. Chem. Phys. 2015, 160, 212–220. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Wu, G.S.; Zhao, Y.; Jin, W.H.; McKenzie, D.R.; Bilek, M.M.; Chu, P.K. Effects of zirconium and nitrogen plasma immersion ion implantation on the electrochemical corrosion behavior of Mg–Y–RE alloy in simulated body fluid and cell culture medium. Corros. Sci. 2014, 86, 239–251. [Google Scholar] [CrossRef]
- Xu, R.Z.; Wu, G.S.; Yang, X.B.; Zhang, X.M.; Wu, Z.W.; Sun, G.Y.; Li, G.Y.; Chu, P.K. Corrosion behavior of chromium and oxygen plasma-modified magnesium in sulfate solution and simulated body fluid. Appl. Surf. Sci. 2012, 258, 8273–8278. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, D.; Geng, H. Characterization of M-class genome segments of Muscovy duck reovirus S14. Virus Res. 2007, 125, 42–53. [Google Scholar] [CrossRef]
- Zhang, B.W.; Liao, S.Z. Progress on the theories of solid solubility of alloy. Shanghai Met. 1999, 21, 3–9. (In Chinese) [Google Scholar]
- Liu, Y.F.; Zhao, H.Y.; Wang, H.M.; Liu, G.P. Microstructure and wear resistance of laser clad Ti5Si3/(NiTi2 + β + Ti5Si3) composite coating. Mater. Sci. Technol. 2002, 338, 126–132. [Google Scholar]
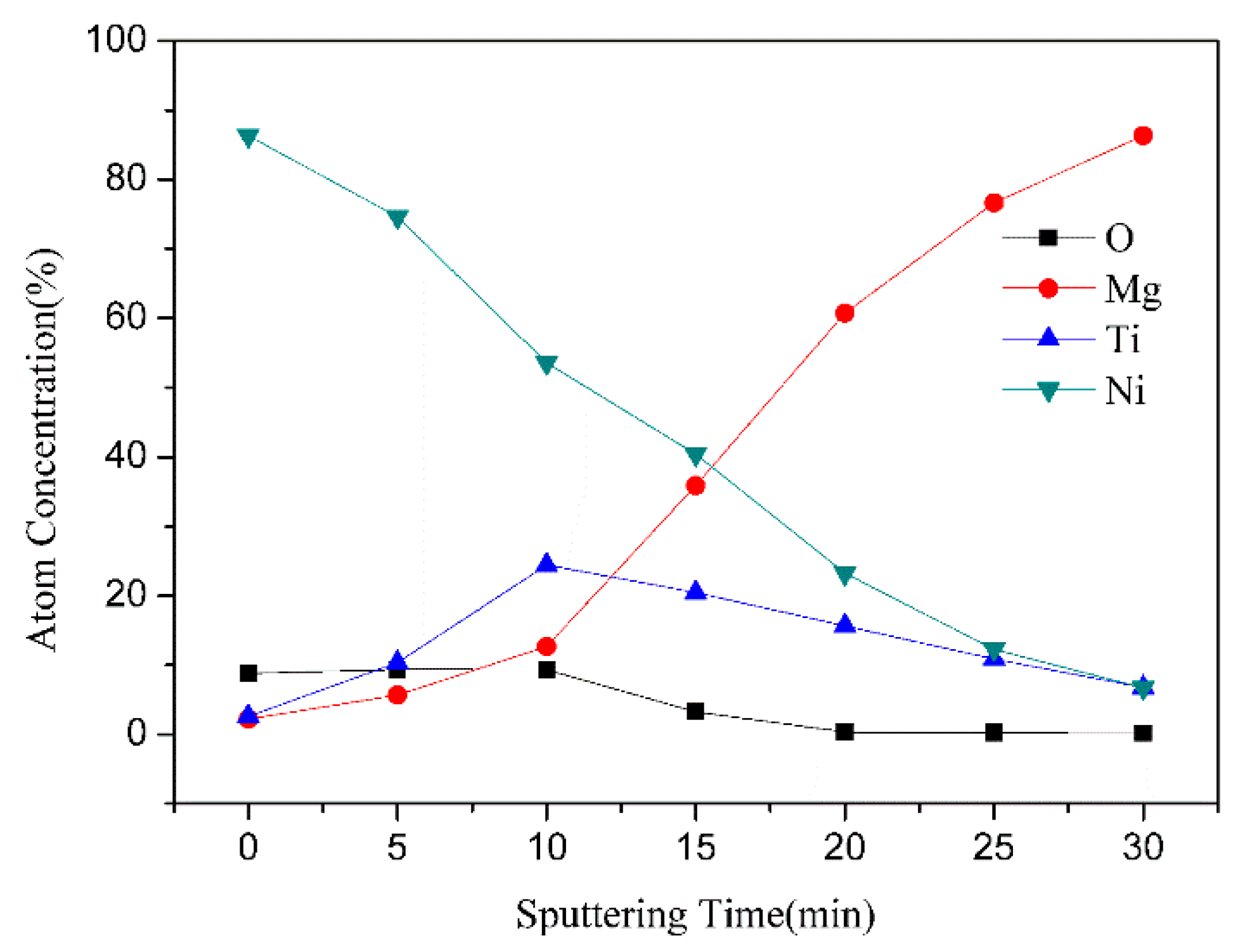

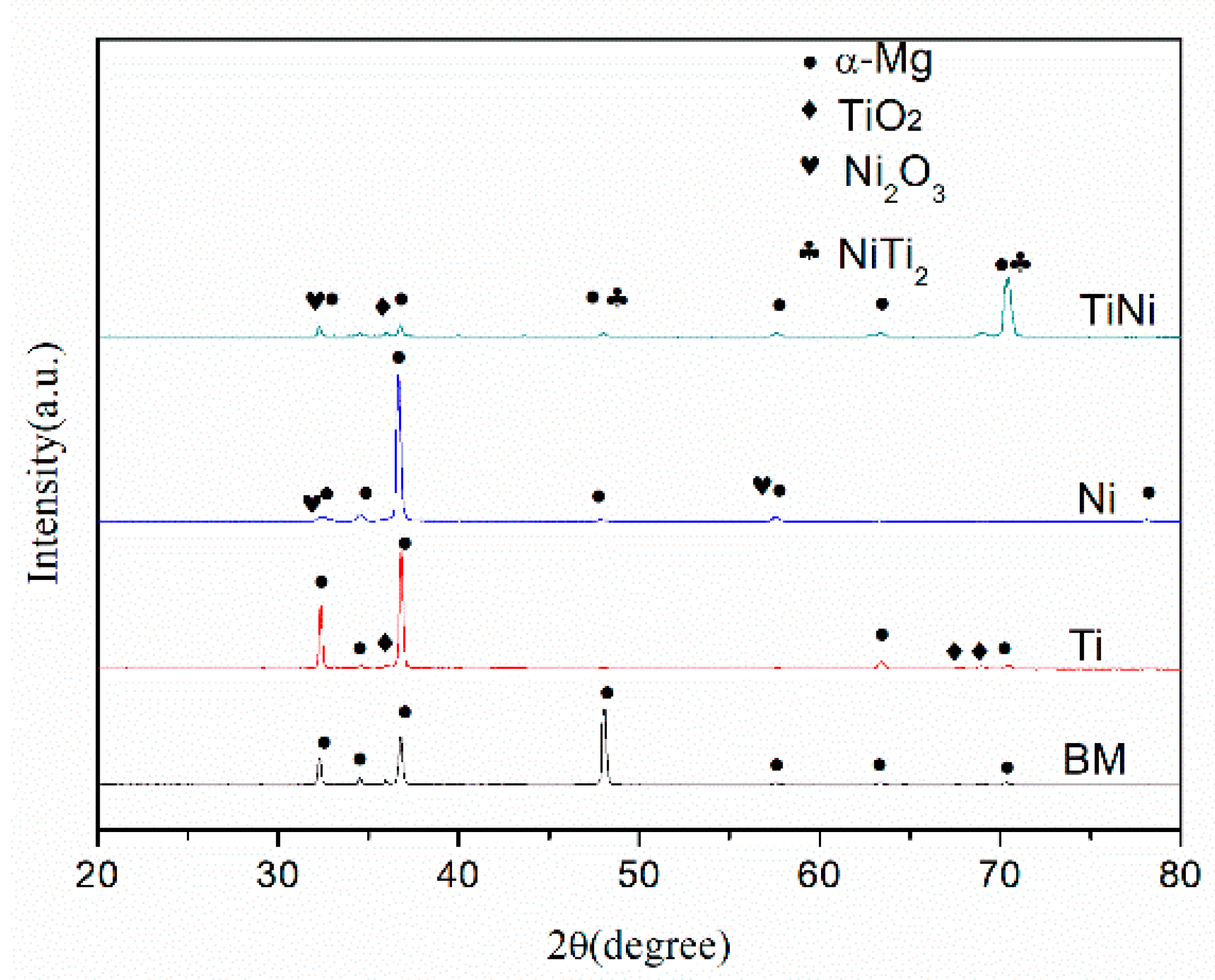
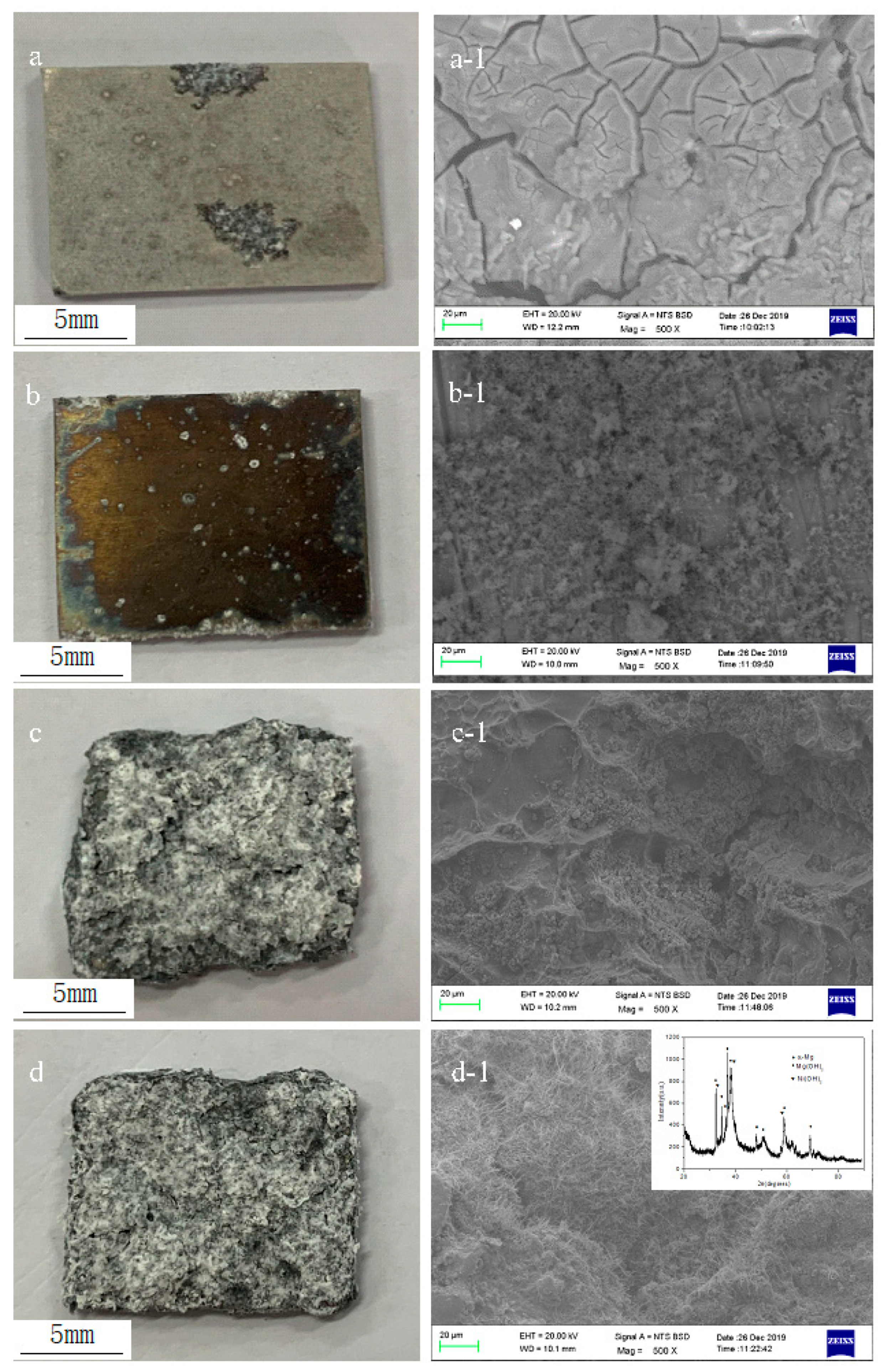
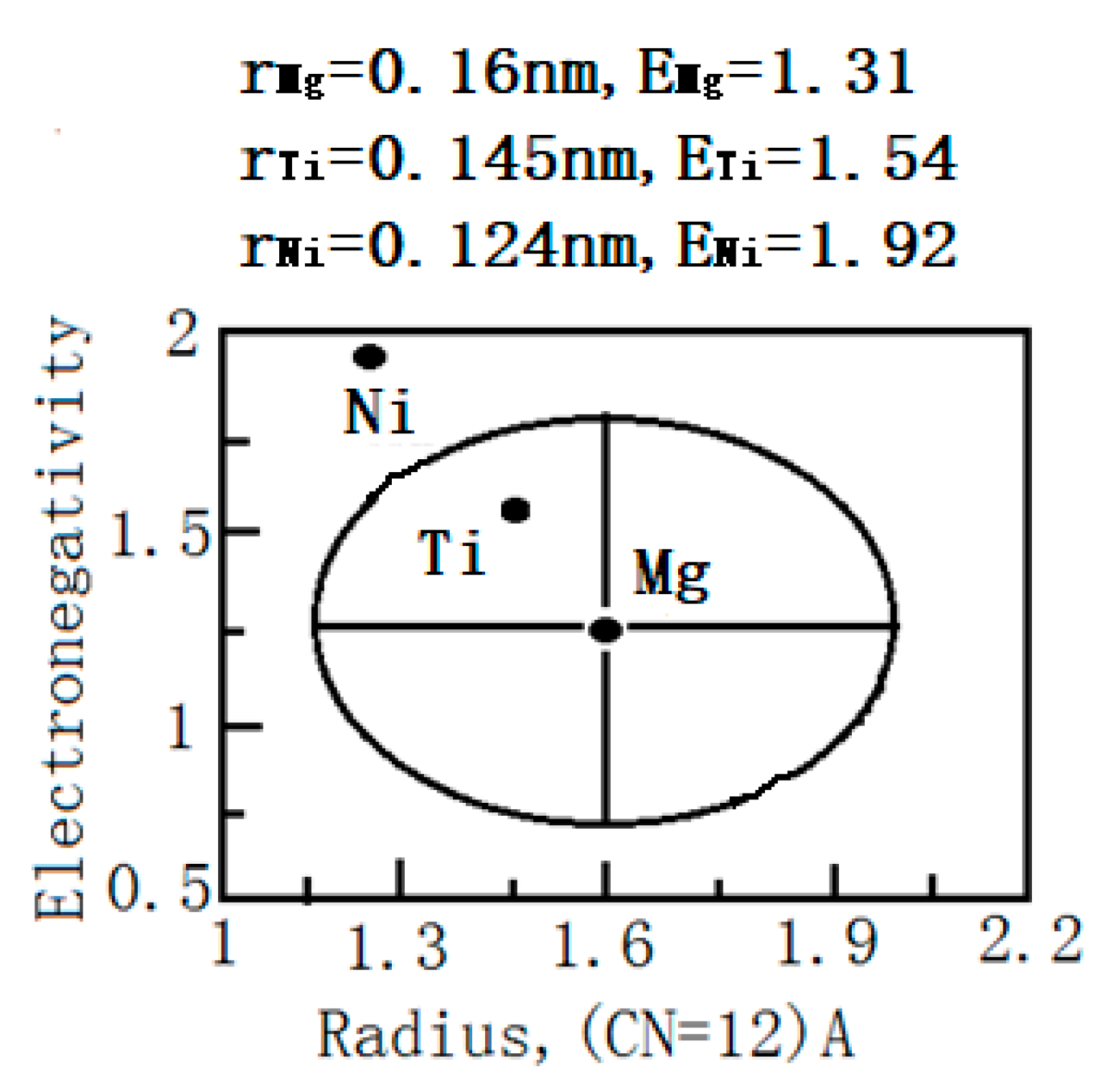

| Samples | Width (mm) | Depth (μm) | Worn Volume (10−6 mm3·m−1·N−1) |
|---|---|---|---|
| BM | 0.62 | 100.03 | 2583 |
| Ti | 0.54 | 52.63 | 702 |
| Ni | 0.44 | 40.56 | 440 |
| TiNi | 0.31 | 20.26 | 155 |
| Crystal | Space Group | Lattice Parameters | Elements | Atomic Coordinates | ||||
|---|---|---|---|---|---|---|---|---|
| a | b | c | X | Y | Z | |||
| NiTi2 | 227 | 11.3193 | 11.3193 | 11.3193 | Ni (I) | 0.912 | 0.912 | 0.912 |
| Ti (II) | 0.311 | 0 | 0 | |||||
| Ti (II) | 0.125 | 0.125 | 0.125 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Liu, Z.; Yu, B.; Ruan, Q.; Chu, P.K. Effects of Ti, Ni, and Dual Ti/Ni Plasma Immersion Ion Implantation on the Corrosion and Wear Properties of Magnesium Alloy. Coatings 2020, 10, 313. https://doi.org/10.3390/coatings10040313
Dai J, Liu Z, Yu B, Ruan Q, Chu PK. Effects of Ti, Ni, and Dual Ti/Ni Plasma Immersion Ion Implantation on the Corrosion and Wear Properties of Magnesium Alloy. Coatings. 2020; 10(4):313. https://doi.org/10.3390/coatings10040313
Chicago/Turabian StyleDai, Jun, Zheng Liu, Banglong Yu, Qingdong Ruan, and Paul K. Chu. 2020. "Effects of Ti, Ni, and Dual Ti/Ni Plasma Immersion Ion Implantation on the Corrosion and Wear Properties of Magnesium Alloy" Coatings 10, no. 4: 313. https://doi.org/10.3390/coatings10040313






