Edible Films Based on Black Chia (Salvia hispanica L.) Seed Mucilage Containing Rhus microphylla Fruit Phenolic Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Collection and Sample Preparation
Preparation of Rhus microphylla (Rm) Fruit Extracts
2.3. Characterization and Bioactivity of Rm Fruit Extracts
2.3.1. Total Phenolic Content (TPC)
2.3.2. Phenolic Profile by Ultra High-Performance Liquid Chromatography (UHPLC)
2.3.3. Antioxidant Activity
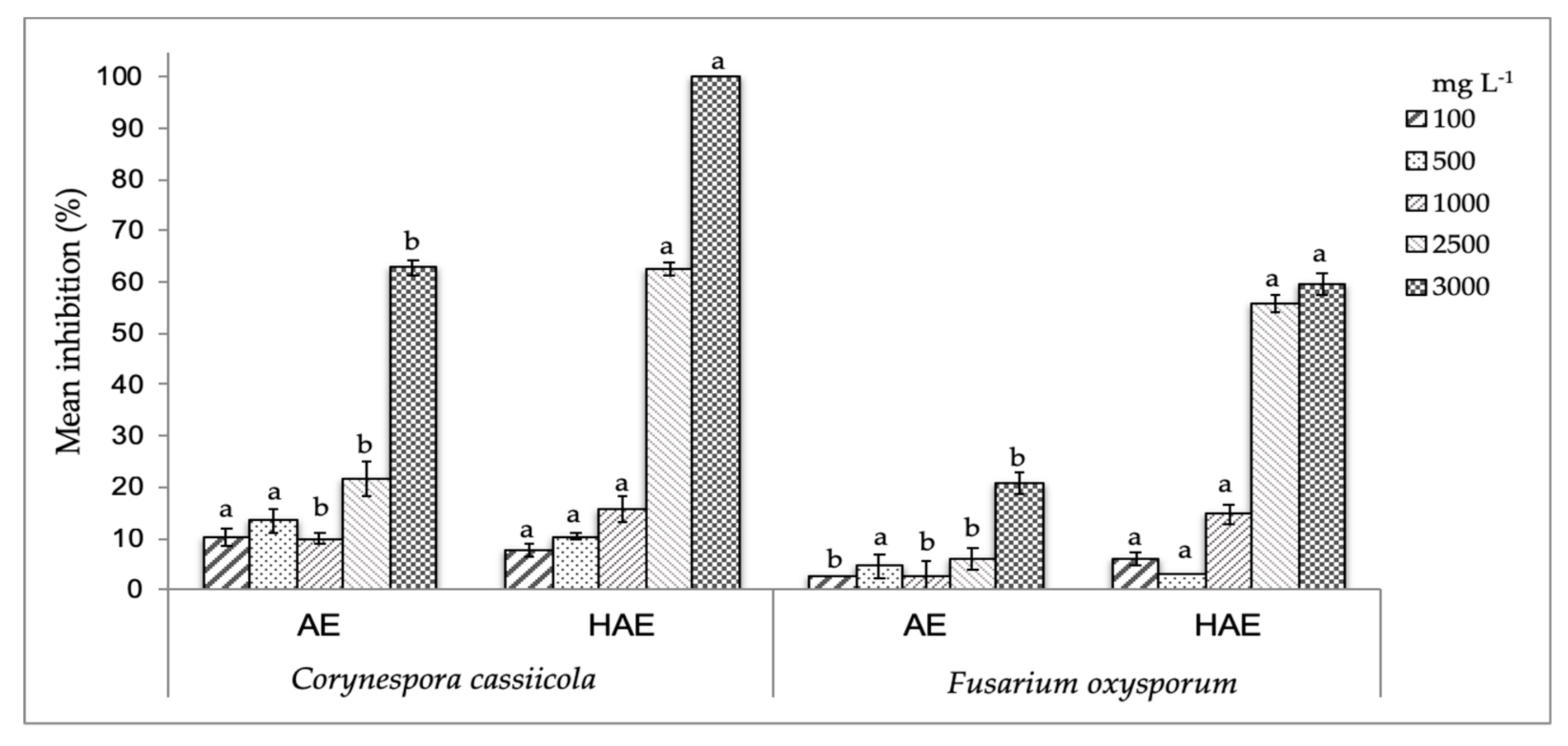
2.3.4. Antifungal Activity in vitro
2.4. Black Chia Seed Mucilage (BCm) Extraction
2.5. Film Preparation
2.6. Film Characterization
2.6.1. Surface Tension
2.6.2. Zeta Potential (Zp)
2.6.3. Film Thickness
2.6.4. Water Vapor Permeability (WVP)
2.6.5. Moisture Content and Water Solubility
2.6.6. Optical Properties
2.6.7. Mechanical Properties
2.6.8. Rheology Behavior
2.7. Statistical Analyses
3. Results and Discussion
3.1. Characterization and Bioactivity of Rm Fruit Extracts
3.2. Film Characterization
3.2.1. Surface Tension and Zeta Potential
3.2.2. Water Vapor Permeability (WVP)
3.2.3. Moisture Content and Water Solubility
3.2.4. Optical Properties
3.2.5. Film Thickness and Mechanical Properties
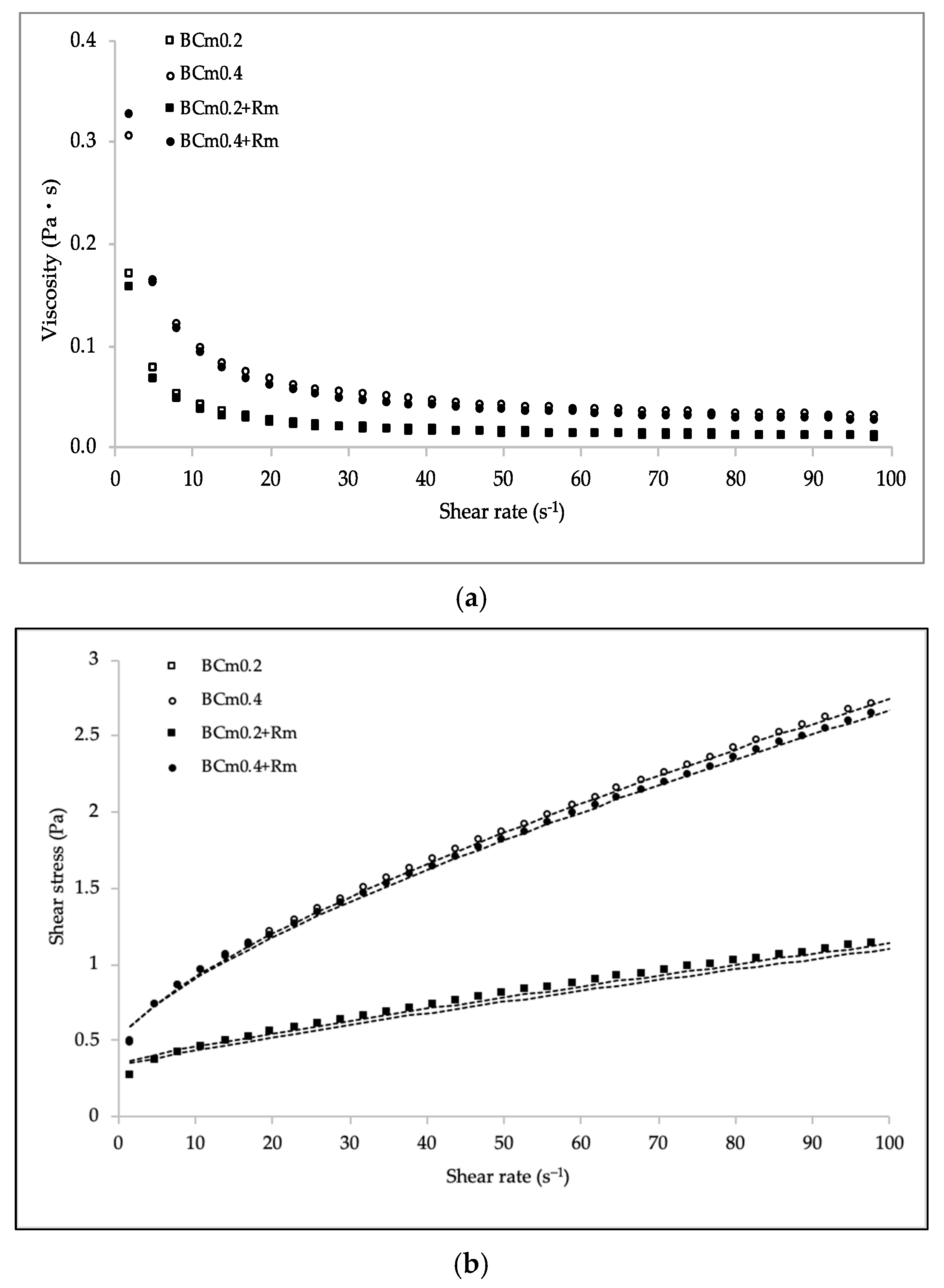
3.2.6. Rheological Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flores-López, M.L.; Cerqueira, M.A.; Jasso de Rodríguez, D.; Vicente, A.A. Perspectives on utilization of edible coatings and nano-laminate coatings for extension of postharvest storage of fruits and vegetables. Food Eng. Rev. 2016, 8, 292–305. [Google Scholar] [CrossRef] [Green Version]
- Jasso de Rodríguez, D.; Salas-Méndez, E.d.J.; Rodríguez-García, R.; Hernández-Castillo, F.D.; Díaz-Jiménez, M.L.V.; Sáenz-Galindo, A.; González-Morales, S.; Flores-López, M.L.; Villarreal-Quintanilla, J.A.; Peña-Ramos, F.M.; et al. Antifungal activity in vitro of ethanol and aqueous extracts of leaves and branches of Flourensia spp. against postharvest fungi. Ind. Crops Prod. 2017, 107, 499–508. [Google Scholar] [CrossRef]
- Kotan, R.; Cakir, A.; Ozer, H.; Kordali, S.; Cakmakci, R.; Dadasoglu, F.; Dikbas, N.; Aydin, T.; Kazaz, C. Antibacterial effects of Origanum onites against phytopathogenic bacteria: Possible use of the extracts from protection of disease caused by some phytopathogenic bacteria. Sci. Hortic. 2014, 172, 210–220. [Google Scholar] [CrossRef]
- De, A.; Bose, R.; Kumar, A.; Mozumdar, S. Worldwide pesticide use. In Targeted Delivery of Pesticides Using Biodegradable Polymeric Nanoparticles; Springer: New Delhi, India, 2014; pp. 5–6. [Google Scholar]
- Adwan, G.; Abu-Shanab, B.; Adwan, K. Antibacterial activities of some plant extracts alone and in combination with different antimicrobials against multidrug-resistant Pseudomonas aeruginosa strains. Asian Pac. J. Trop. Med. 2010, 3, 266–269. [Google Scholar] [CrossRef] [Green Version]
- Jasso de Rodríguez, D.; Trejo-González, F.A.; Rodríguez-García, R.; Díaz-Jimenez, M.L.V.; Sáenz-Galindo, A.; Hernández-Castillo, F.D.; Villarreal-Quintanilla, J.A.; Peña-Ramos, F.M. Antifungal activity in vitro of Rhus muelleri against Fusarium oxysporum f. sp. lycopersici. Ind. Crops Prod. 2015, 75, 150–158. [Google Scholar] [CrossRef]
- Dolores, L.; Latorre, F.A. Plants used by the Mexican Kickapoo Indians. Econ. Bot. 1977, 31, 340–357. [Google Scholar] [CrossRef]
- Fabra, M.J.; Flores-López, M.L.; Cerqueira, M.A.; Jasso de Rodríguez, D.; Lagaron, J.M.; Vicente, A.A. Layer-by-Layer technique to developing functional nanolaminate films with antifungal activity. Food Bioprocess Technol. 2016, 9, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Vieira, J.M.; Flores-López, M.L.; Jasso de Rodríguez, D.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan–Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Torres-León, C.; Vicente, A.A.; Flores-López, M.L.; Rojas, R.; Serna-Cock, L.; Alvarez-Pérez, O.B.; Aguilar, C.N. Edible films and coatings based on mango (var. Ataulfo) by-products to improve gas transfer rate of peach. LWT Food Sci. Technol. 2018, 97, 624–631. [Google Scholar]
- Cerqueira, M.A.; Souza, B.W.S.; Martins, J.T.; Teixeira, J.A.; Vicente, A.A. Seed extracts of Gleditsia triacanthos: Functional properties evaluation and incorporation into galactomannan films. Food Res. Int. 2010, 43, 2031–2038. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.J.; Cerqueira, M.A.; Ruiz, H.A.; Fougnies, C.; Richel, A.; Vicente, A.A.; Teixeira, J.A.; Aguedo, M. Use of wheat bran arabinoxylans in chitosan-based films: Effect on physicochemical properties. Ind. Crops Prod. 2015, 66, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Capitani, M.I.; Matus-Basto, A.; Ruiz-Ruiz, J.C.; Santiago-García, J.L.; Betancur-Ancona, D.A.; Nolasco, S.M.; Tomás, M.C.; Segura-Campos, M.R. Characterization of biodegradable films based on Salvia hispanica L. protein and mucilage. Food Bioprocess Technol. 2016, 9, 1276–1286. [Google Scholar] [CrossRef]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; Rios, A.D.O.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, L.A.; Aguilera, J.M.; Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Characterization and microstructure of films made from mucilage of Salvia hispanica and whey protein concentrate. J. Food Eng. 2012, 111, 511–518. [Google Scholar] [CrossRef]
- Valdivia-López, M.A.; Tecante, A. Chia (Salvia hispanica): A review of native Mexican seed and its nutritional and functional properties. In Advances in Food and Nutrition Research, 1st ed.; Elsevier Inc.: Oxford, UK, 2015; Volume 15, pp. 53–75. [Google Scholar]
- de Falco, B.; Fiore, A.; Rossi, R.; Amato, M.; Lanzotti, V. Metabolomics driven analysis by UAEGC-MS and antioxidant activity of chia (Salvia hispanica L.) commercial and mutant seeds. Food Chem. 2018, 254, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Jasso De Rodríguez, D.; García-Hernández, L.C.; Rocha-Guzmán, N.E.; Moreno-Jiménez, M.R.; Rodríguez-García, R.; Díaz-Jiménez, M.L.V.; Flores-López, M.L.; Villarreal-Quintanilla, J.A.; Peña-Ramos, F.M.; Carrillo-Lomelí, D.A. Hypoglycemic and anti-inflammatory effects of Psacalium paucicapitatum corms infusions. Ind. Crops Prod. 2017, 107, 482–488. [Google Scholar] [CrossRef]
- Jesus, M.S.; Genisheva, Z.; Romaní, A.; Pereira, R.N.; Teixeira, J.A.; Domingues, L. Bioactive compounds recovery optimization from vine pruning residues using conventional heating and microwave-assisted extraction methods. Ind. Crops Prod. 2019, 132, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Arquelau, P.B.d.F.; Silva, V.D.M.; Garcia, M.A.V.T.; de Araújo, R.L.B.; Fante, C.A. Characterization of edible coatings based on ripe ‘Prata’ banana peel flour. Food Hydrocoll. 2018, 89, 570–578. [Google Scholar] [CrossRef]
- do Prado, A.C.P.; da Silva, H.S.; da Silveira, S.M.; Barreto, P.L.M.; Vieira, C.R.W.; Maraschin, M.; Ferreira, S.R.S.; Block, J.M. Effect of the extraction process on the phenolic compounds profile and the antioxidant and antimicrobial activity of extracts of pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell. Ind. Crops Prod. 2013, 52, 552–561. [Google Scholar] [CrossRef]
- Wu, T.; McCallum, J.L.; Wang, S.; Liu, R.; Zhu, H.; Tsao, R. Evaluation of antioxidant activities and chemical characterisation of staghorn sumac fruit (Rhus hirta L.). Food Chem. 2013, 138, 1333–1340. [Google Scholar] [CrossRef]
- Bursal, E.; Köksal, E. Evaluation of reducing power and radical scavenging activities of water and ethanol extracts from sumac (Rhus coriaria L.). Food Res. Int. 2011, 44, 2217–2221. [Google Scholar] [CrossRef]
- Nguyen, D.M.C.; Seo, D.J.; Lee, H.B.; Kim, I.S.; Kim, K.Y.; Park, R.D.; Jung, W.J. Antifungal activity of gallic acid purified from Terminalia nigrovenulosa bark against Fusarium solani. Microb. Pathog. 2013, 56, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Osorio, E.; Flores, M.; Hernández, D.; Ventura, J.; Rodríguez, R.; Aguilar, C.N. Biological efficiency of polyphenolic extracts from pecan nuts shell (Carya Illinoensis), pomegranate husk (Punica granatum) and creosote bush leaves (Larrea tridentata Cov.) against plant pathogenic fungi. Ind. Crops Prod. 2010, 31, 153–157. [Google Scholar] [CrossRef]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Zhu, J.; Jiang, J.; Zhao, Y.; Li, B.; Mu, W.; Liu, F. Evaluation of bioactivity and control efficacy of tetramycin against Corynespora cassiicola. Pestic. Biochem. Physiol. 2018, 152, 106–113. [Google Scholar] [CrossRef]
- McGovern, R.J. Management of tomato diseases caused by Fusarium oxysporum. Crop Prot. 2015, 73, 78–92. [Google Scholar] [CrossRef]
- Ribeiro, C.; Vicente, A.A.; Teixeira, J.A.; Miranda, C. Optimization of edible coating composition to retard strawberry fruit senescence. Postharvest Biol. Technol. 2007, 44, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Hauser, C.; Peñaloza, A.; Guarda, A.; Galotto, M.J.; Bruna, J.E.; Rodríguez, F.J. Development of an active packaging film based on a methylcellulose coating containing murta (Ugni molinae Turcz) leaf extract. Food Bioprocess Technol. 2016, 9, 298–307. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H.D. Tolerance of ‘Baladi’ mandarin fruits to cold storage by postharvest pectin/PVA blend with ascorbic acid treatment. Sci. Hortic. 2019, 256, 108637. [Google Scholar] [CrossRef]
- Capitani, M.I.; Corzo-Rios, L.J.; Chel-Guerrero, L.A.; Betancur-Ancona, D.A.; Nolasco, S.M.; Tomás, M.C. Rheological properties of aqueous dispersions of chia (Salvia hispanica L.) mucilage. J. Food Eng. 2015, 149, 70–77. [Google Scholar] [CrossRef]
- Steffolani, E.; Martinez, M.M.; León, A.E.; Gómez, M. Effect of pre-hydration of chia (Salvia hispanica L.), seeds and flour on the quality of wheat flour breads. LWT Food Sci. Technol. 2015, 61, 401–406. [Google Scholar] [CrossRef]
- Chiumarelli, M.; Hubinger, M.D. Evaluation of edible films and coatings formulated with cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocoll. 2014, 38, 20–27. [Google Scholar] [CrossRef]
- Fabra, M.J.; Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A. Antiviral and antioxidant properties of active alginate edible films containing phenolic extracts. Food Hydrocoll. 2018, 81, 96–103. [Google Scholar] [CrossRef]
- Kurek, M.; Galus, S.; Debeaufort, F. Surface, mechanical and barrier properties of bio-based composite films based on chitosan and whey protein. Food Packag. Shelf Life 2014, 1, 56–67. [Google Scholar] [CrossRef]
- Vieira, J.M.; Mantovani, R.A.; Raposo, M.F.J.; Coimbra, M.A.; Vicente, A.A. Effect of extraction temperature on rheological behavior and antioxidant capacity of flaxseed gum. Carbohydr. Polym. 2019, 213, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Szabo, K.; Teleky, B.-E.; Mitrea, L.; Călinoiu, L.-F.; Martău, G.-A.; Simon, E.; Varvara, R.-A.; Vodnar, D.C. Active packaging—Poly (Vinyl alcohol) films enriched with tomato by-products extract. Coatings 2020, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Orozco, J.P.; Sánchez-Herrera, L.M.; Ortiz-Basurto, R.I. Effect of concentration, temperature, pH, co-solutes on the rheological properties of Hyptis suaveolens L. mucilage dispersions. Food Hydrocoll. 2019, 87, 297–306. [Google Scholar] [CrossRef]
| AE | HAE | |
|---|---|---|
| Total phenolic content, TPC (g GA kg−1 extract) | 146.8 ± 0.1a | 151.0 ± 3.9a |
| Antiradical activity, IC50 (mg L−1) | 130.0 ± 10.0b | 170.0 ± 10.0a |
| Phenolic compound (mg L−1) | ||
| Catechin | n.d. | 10.4 ± 0.4 |
| Ferulic acid | 5.7 ± 0.1a | 6.1 ± 1.3a |
| Gallic acid | 203.2 ± 0.7a | 98.6 ± 4.4b |
| p-coumaric acid + epicatechin | 7.4 ± 0.2b | 78.2 ± 1.5a |
| Ellagic acid | n.d. | 2.9 ± 0.1 |
| Apigenin | n.d. | 0.3 ± 0.0 |
| Quercetin | n.d. | 2.1 ± 0.0 |
| Resveratrol | n.d. | 2.9 ± 0.0 |
| Extracts | 95% Fiducial Limits | 95% Fiducial Limits | ||||
|---|---|---|---|---|---|---|
| MIC50 (mg L−1) | Lower | Upper | MIC90 (mg L−1) | Lower | Upper | |
| F. oxysporum | ||||||
| AE | 5780.8 | 5387.8 | 6173.8 | 9314.5 | 8909.0 | 9719.9 |
| HAE | 2522.5 | 2093.2 | 3181.8 | 4251.1 | 3493.4 | 5874.3 |
| C. cassiicola | ||||||
| AE | 3116.7 | 2901.7 | 3331.7 | 5741.2 | 5524.9 | 5957.5 |
| HAE | 1802.9 | 978.4 | 2935.5 | 3086.3 | 2256.3 | 6268.0 |
| Film Formulation | Surface tension (mN m−1) | Zp (mV) | WVP × 10−10 (g m−1 s−1 Pa−1) | Moisture (%) | Solubility (%) |
|---|---|---|---|---|---|
| BCm0.2 | 51.6 ± 0.4a | −33.3 ± 2.8a | 3.7 ± 0.2a | 81.0 ± 1.7a | 15.0 ± 3.3a |
| BCm0.4 | 50.6 ± 0.2a | −34.4 ± 3.0a | 3.7 ± 0.1a | 68.4 ± 1.3c | 25.0 ± 2.4a |
| BCm0.2+Rm | 43.0 ± 0.8b | −32.8 ± 3.8a | 3.8 ± 0.2a | 79.0 ± 0.7b | 23.3 ± 1.5a |
| BCm0.4+Rm | 43.9 ± 1.6b | −26.9 ± 3.4a | 4.0 ± 0.2a | 66.0 ± 0.9d | 27.3 ± 4.3a |
| Film Formulation | L* | a* | b* | Opacity (%) | |
|---|---|---|---|---|---|
| BCm0.2 | 92.3 ± 0.3a | 0.4 ± 0.1d | 10.2 ± 0.6d | 5.2 ± 0.3c | 12.3 ± 0.4d |
| BCm0.4 | 85.4 ± 0.5b | 1.8 ± 0.1c | 17.8 ± 0.4c | 6.3 ± 0.4b | 17.3 ± 0.4c |
| BCm0.2+Rm | 83.6 ± 0.6c | 4.5 ± 0.3b | 29.8 ± 1.0b | 4.5 ± 0.2d | 29.6 ± 1.0b |
| BCm0.4+Rm | 79.8 ± 0.8d | 5.6 ± 0.4a | 32.1 ± 0.8a | 6.8 ± 0.3a | 32.4 ± 1.0a |
| Film Formulation | Thickness (µm) | TS (MPa) | EB (%) |
|---|---|---|---|
| BCm0.2 | 40.0 ± 0.0c | 0.5 ± 0.2c | 14.0 ± 3.0a |
| BCm0.4 | 50.0 ± 0.0b | 1.1 ± 0.1b | 9.5 ± 1.0b |
| BCm0.2+Rm | 50.0 ± 0.0b | 0.6 ± 0.0c | 5.5 ± 0.5c |
| BCm0.4+Rm | 60.0 ± 0.0a | 1.6 ± 0.4a | 7.2 ± 1.4bc |
| Film Formulation | n | k (Pa sn) | R2 | RSME |
|---|---|---|---|---|
| BCm0.2 | 0.86 ± 0.00a | 0.02 ± 0.00b | 0.999 | 0.6 × 10−4 |
| BCm0.4 | 0.70 ± 0.00b | 0.09 ± 0.01a | 0.999 | 1.3 × 10−4 |
| BCm0.2+Rm | 0.87 ± 0.04a | 0.02 ± 0.01b | 0.998 | 1.1 × 10−4 |
| BCm0.4+Rm | 0.70 ± 0.00b | 0.08 ± 0.01a | 0.999 | 1.4 × 10−4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charles-Rodríguez, A.V.; Rivera-Solís, L.L.; Martins, J.T.; Genisheva, Z.; Robledo-Olivo, A.; González-Morales, S.; López-Guarin, G.; Martínez-Vázquez, D.G.; Vicente, A.A.; Flores-López, M.L. Edible Films Based on Black Chia (Salvia hispanica L.) Seed Mucilage Containing Rhus microphylla Fruit Phenolic Extract. Coatings 2020, 10, 326. https://doi.org/10.3390/coatings10040326
Charles-Rodríguez AV, Rivera-Solís LL, Martins JT, Genisheva Z, Robledo-Olivo A, González-Morales S, López-Guarin G, Martínez-Vázquez DG, Vicente AA, Flores-López ML. Edible Films Based on Black Chia (Salvia hispanica L.) Seed Mucilage Containing Rhus microphylla Fruit Phenolic Extract. Coatings. 2020; 10(4):326. https://doi.org/10.3390/coatings10040326
Chicago/Turabian StyleCharles-Rodríguez, Ana V., Luz L. Rivera-Solís, Joana T. Martins, Zlatina Genisheva, Armando Robledo-Olivo, Susana González-Morales, Gustavo López-Guarin, Dolores G. Martínez-Vázquez, António A. Vicente, and María L. Flores-López. 2020. "Edible Films Based on Black Chia (Salvia hispanica L.) Seed Mucilage Containing Rhus microphylla Fruit Phenolic Extract" Coatings 10, no. 4: 326. https://doi.org/10.3390/coatings10040326









