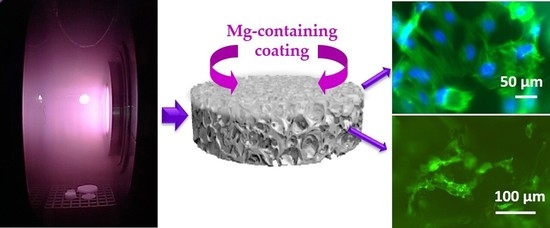
Plasma-Assisted Deposition of Magnesium-Containing Coatings on Porous Scaffolds for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. PCL Sample Preparation
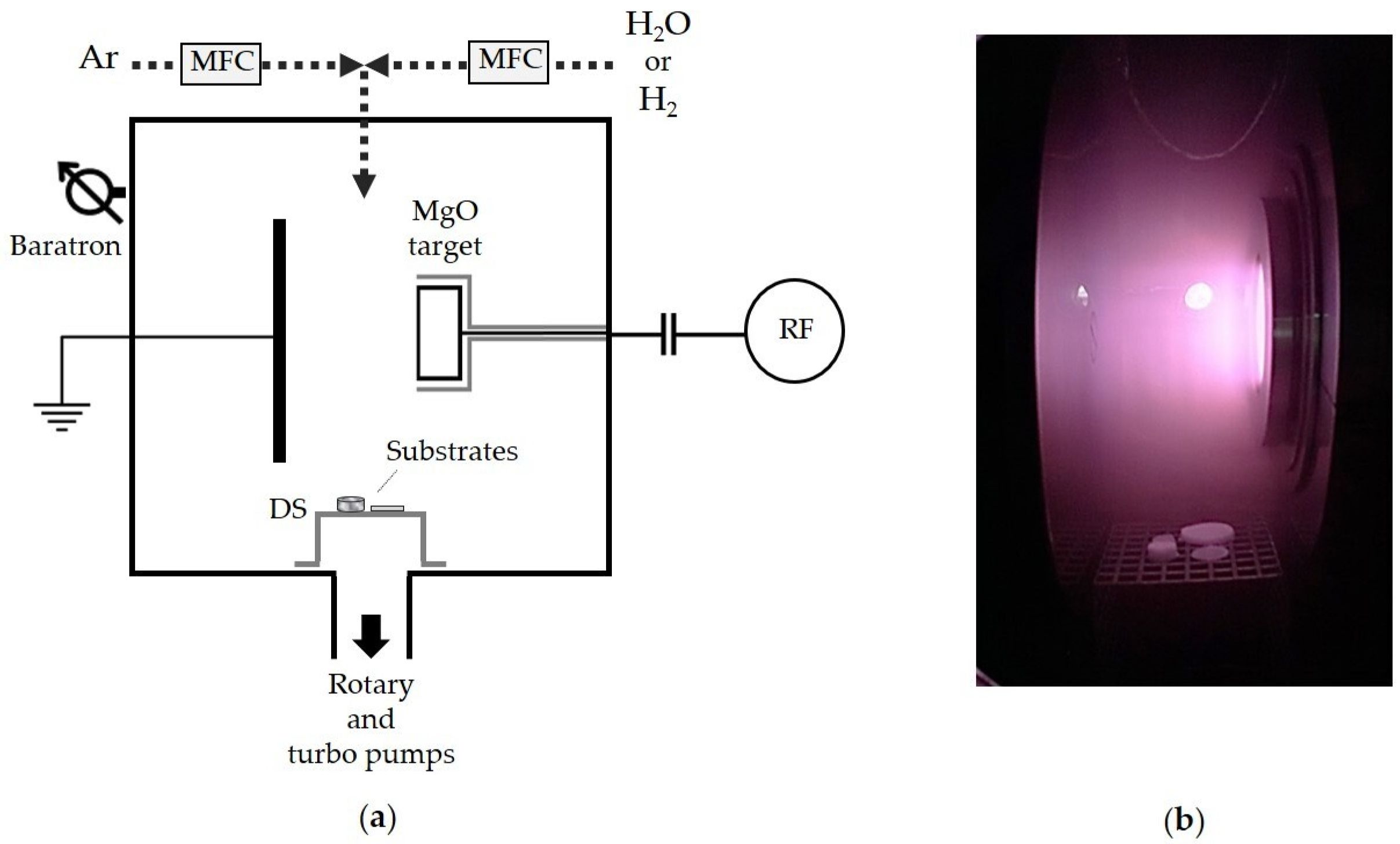
2.2. Plasma-Assisted Deposition of Magnesium-Containing Coatings
2.3. XPS Analyses
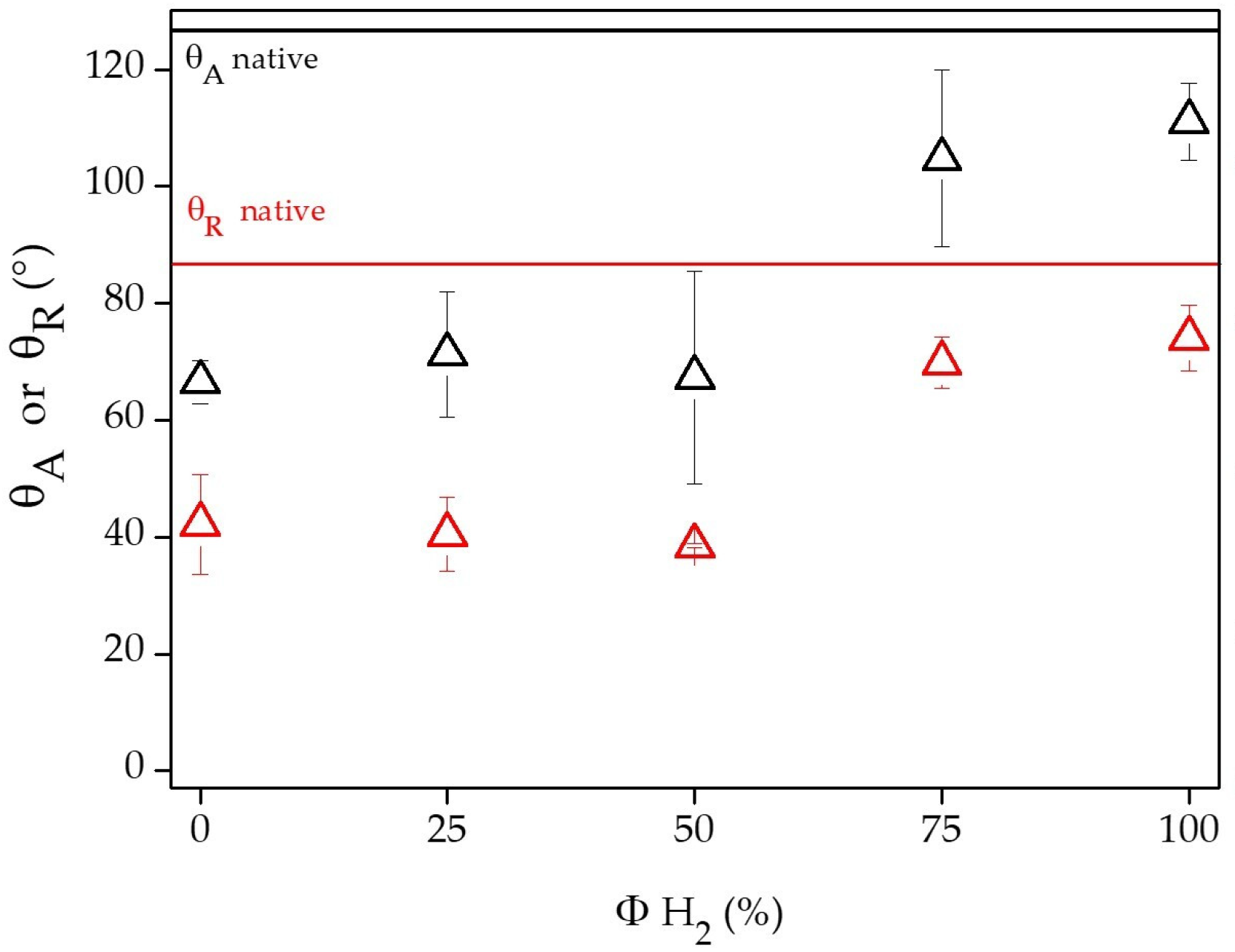
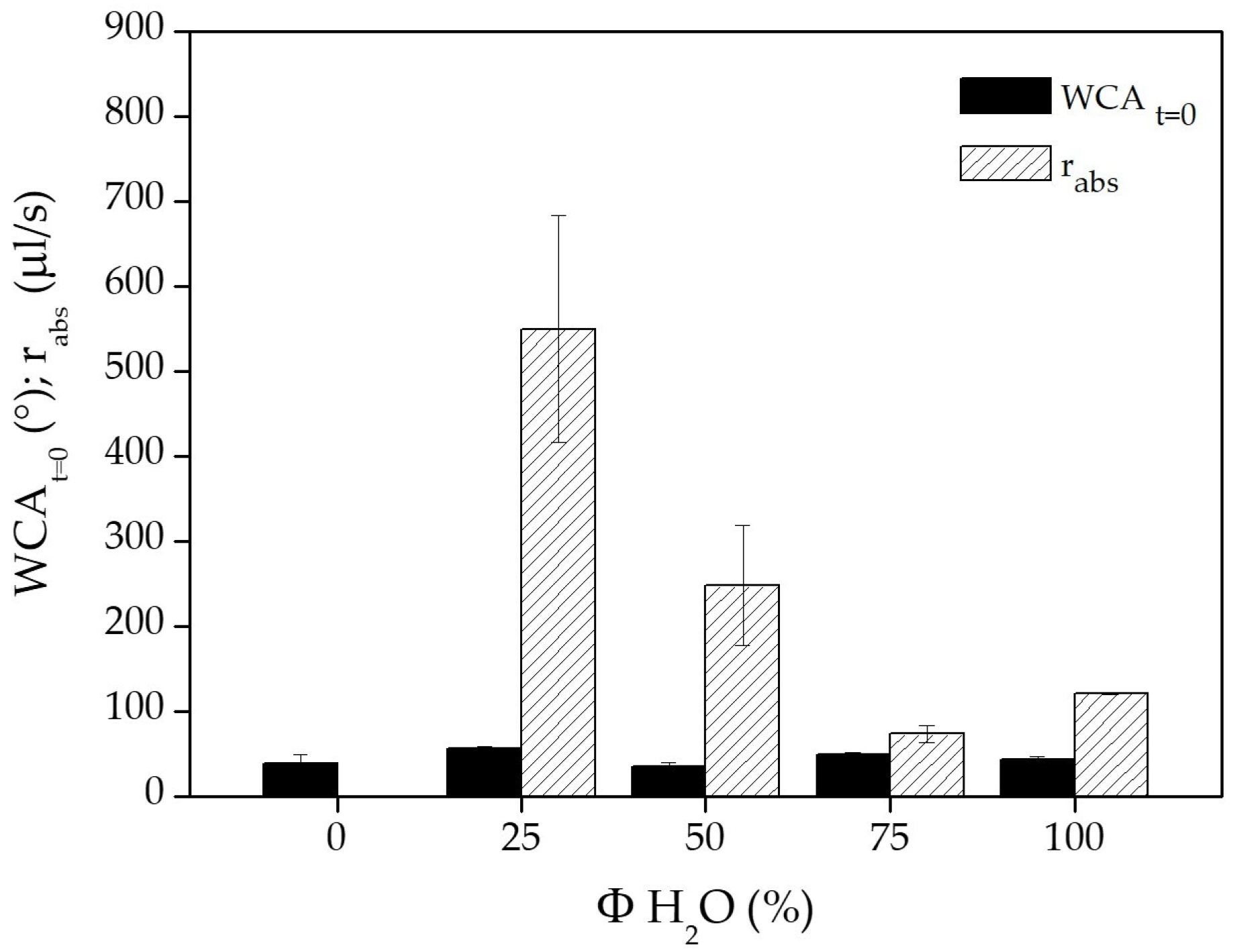
2.4. WCA Measurements
2.5. Cytocompatibility Assays
3. Results and Discussion
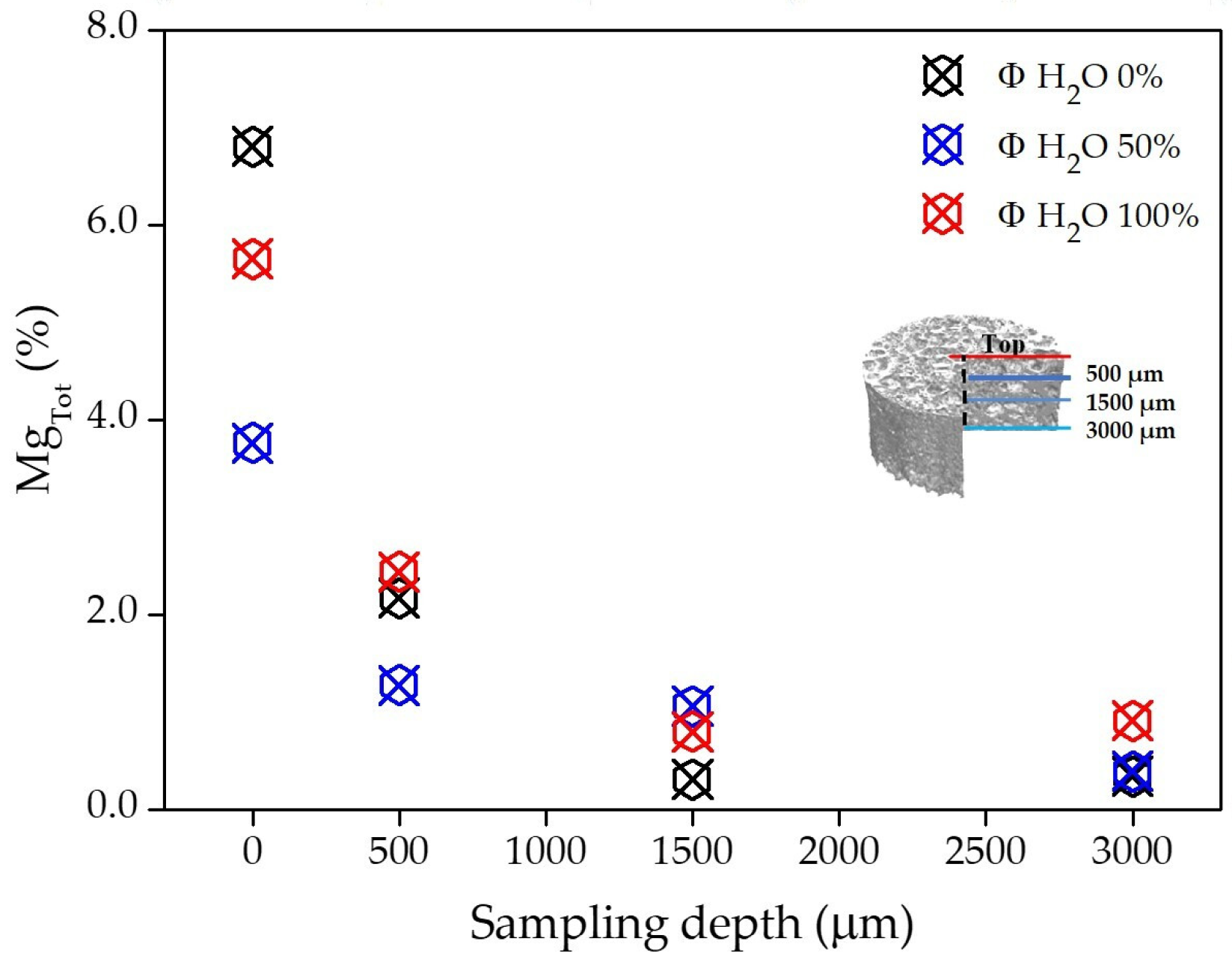
3.1. Chemical and Physical Characterization of PCL Flat Samples and Scaffolds
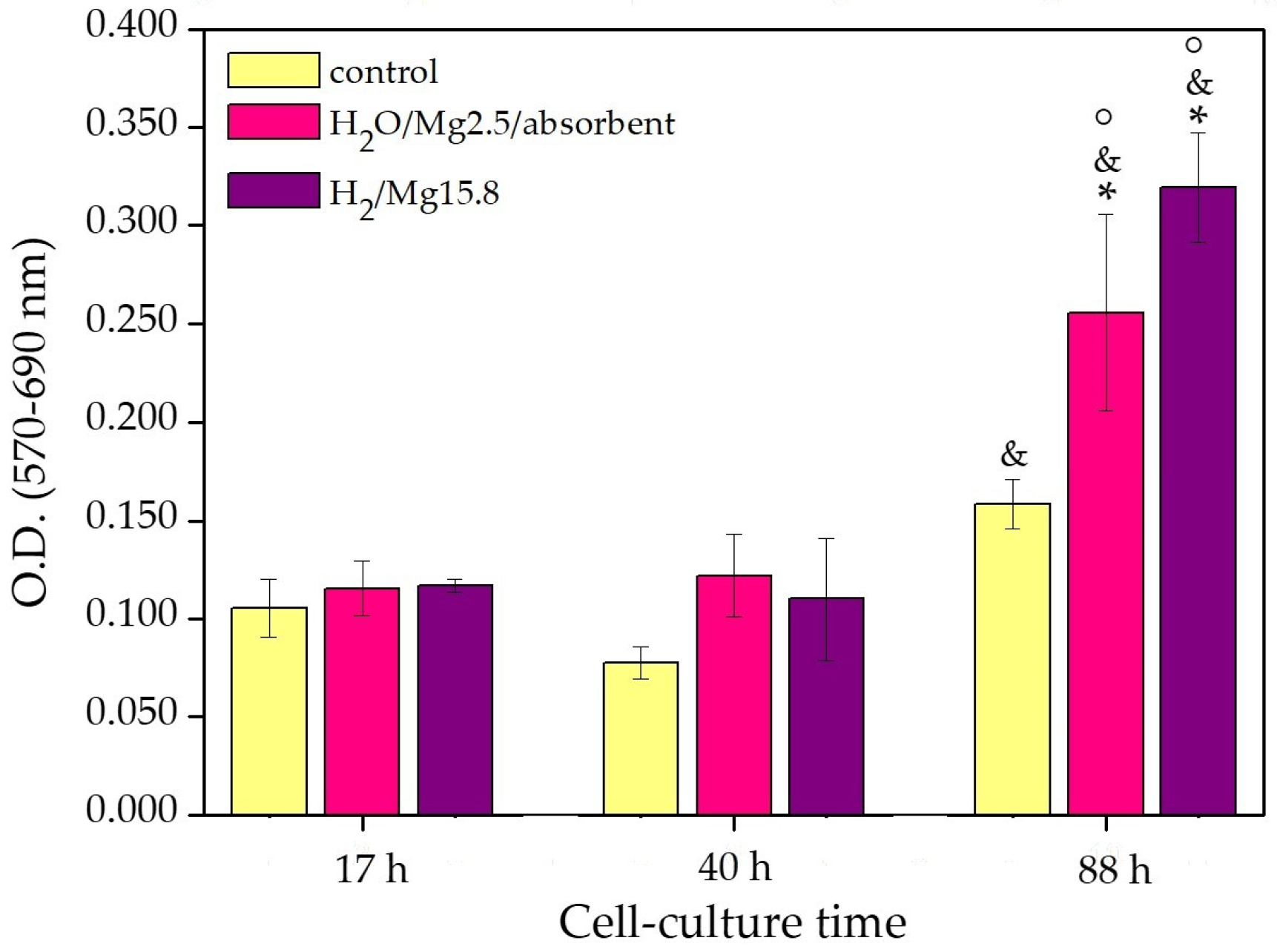
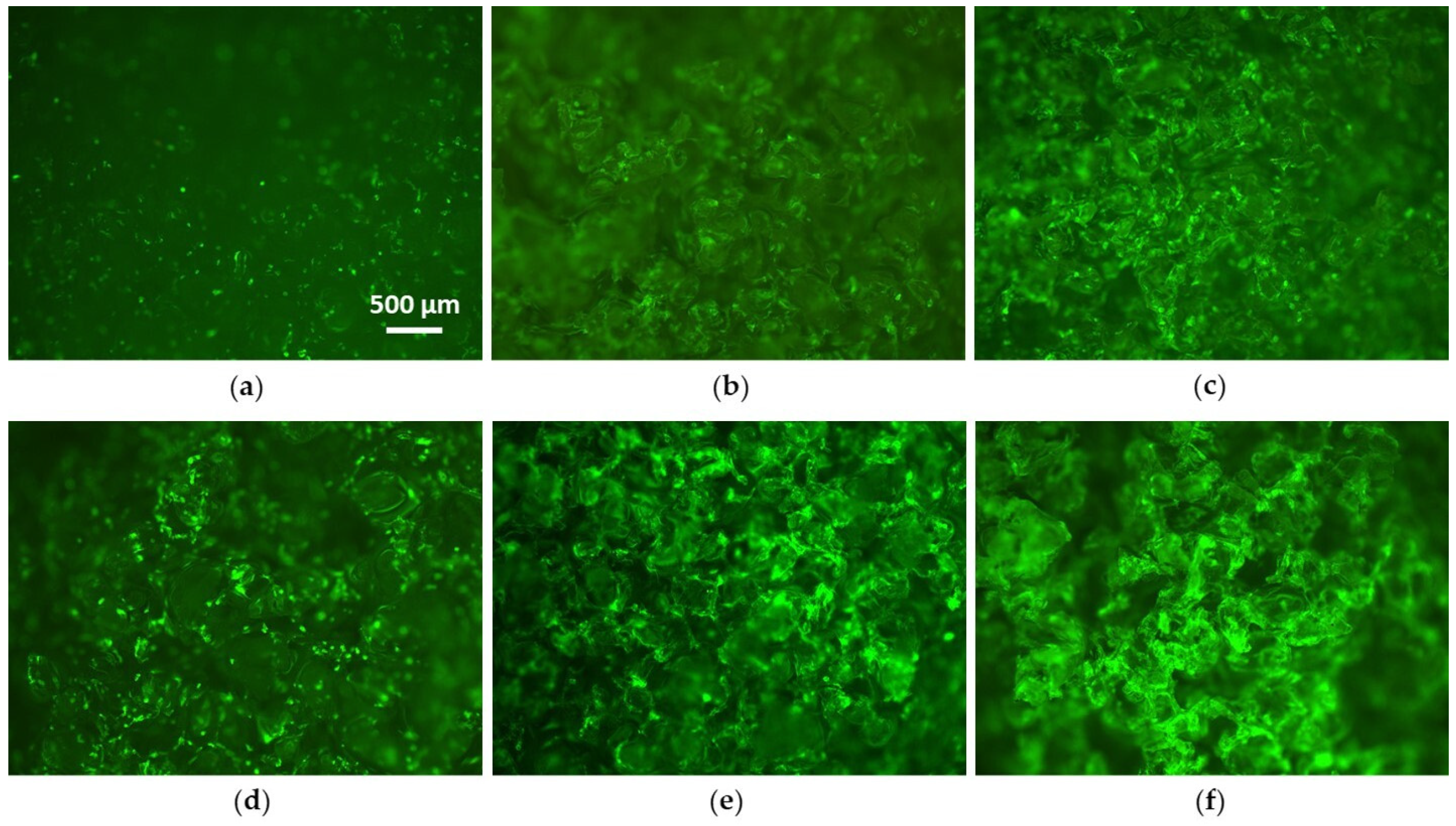
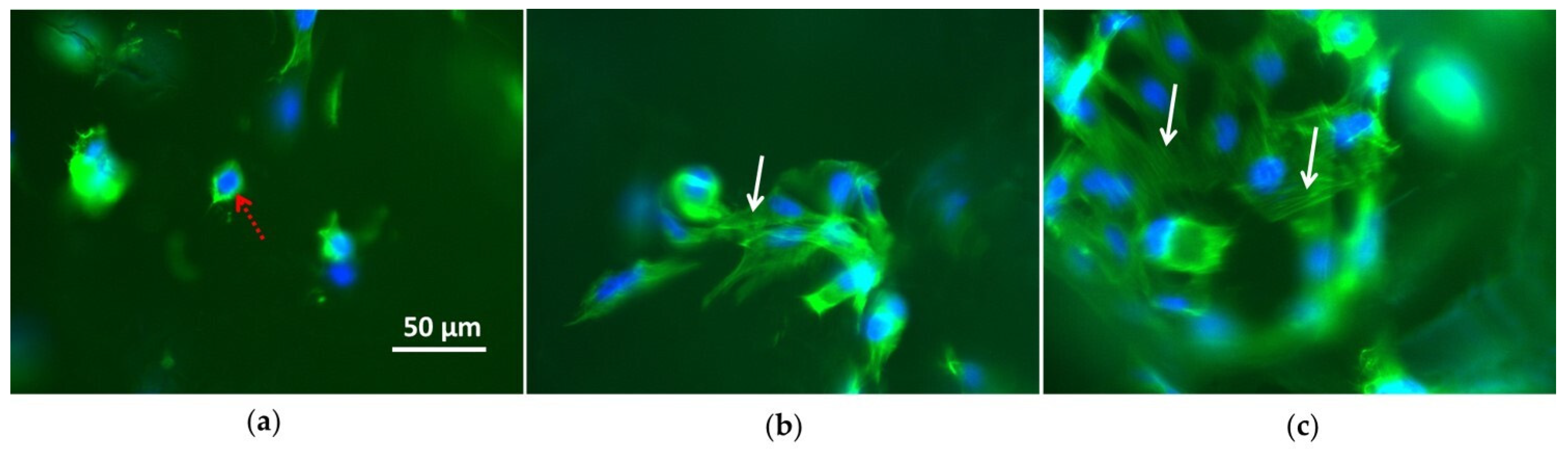
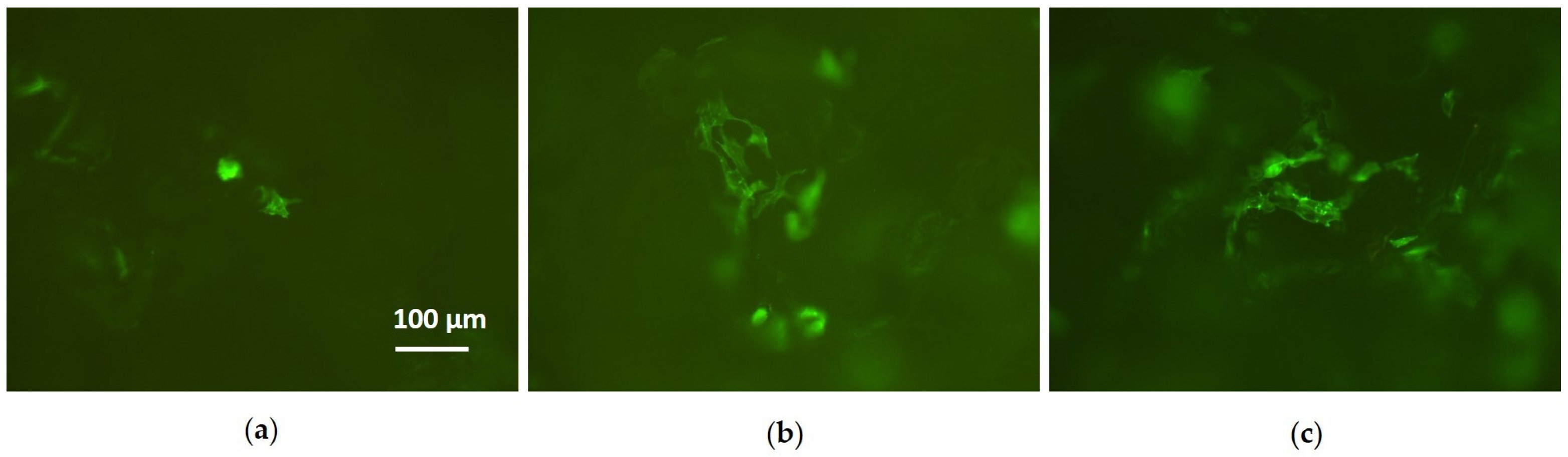
3.2. Cell Culture Tests
- 1)
- Control—native 3D PCL porous scaffolds;
- 2)
- H2O/Mg2.5/absorbent—3D PCL porous scaffolds processed with Φ H2O 100%;
- 3)
- H2/Mg15.8—3D PCL porous scaffolds processed with Φ H2 100%.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application: A Review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Craciunescu, O.; Tardei, C.; Moldovan, L.; Zarnescu, O. Magnesium substitution effect on porous scaffolds for bone repair. Cent. Eur. J. Biol. 2011, 6, 301–311. [Google Scholar] [CrossRef]
- Salma-Ancane, K.; Stipniece, L.; Putnins, A.; Berzina-Cimdina, L. Development of Mg-containing porous β-tricalcium phosphate scaffolds for bone repair. Ceram. Int. 2015, 41, 4996–5004. [Google Scholar] [CrossRef]
- Dittler, M.L.; Unalan, I.; Grünewald, A.; Beltrán, A.M.; Grillo, C.A.; Destch, R.; Gonzalez, M.C.; Boccaccini, A.R. Bioactive glass (45S5)-based 3D scaffolds coated with magnesium and zinc loadedhydroxyapatite nanoparticles for tissue engineering applications. Colloids Surf. B Biointerfaces 2019, 182, 110346–110355. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Brundavanam, S.; Fawcett, D. Biomedical Magnesium Alloys: A Review of Material Properties, Surface Modifications and Potential as a Biodegradable Orthopaedic Implant. Am. J. Biomed. Eng. 2012, 2, 218–240. [Google Scholar] [CrossRef] [Green Version]
- Lambotte, A. L’utilisation du magnesium comme material perdu dans l’osteosynthese. Bull. Mem. Soc. Nat. Chir. 1932, 28, 1325–1334. [Google Scholar]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef]
- Yang, J.; Cui, F.; Lee, I.S. Surface Modifications of Magnesium Alloys for Biomedical Applications. Ann. Biomed. Eng. 2011, 39, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Golzar, H.; Mohammadrezaei, D.; Yadegari, A.; Rasoulianboroujeni, M.; Hashemi, M.; Omidi, M.; Yazdian, F.; Shalbaf, M.; Tayebi, L. Incorporation of functionalized reduced graphene oxide/magnesium nanohybrid to enhance the osteoinductivity capability of 3D printed calcium phosphate-based scaffolds. Compos. Part B 2020, 185, 107749–107759. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, J.; Zhang, X.; Liang, G.; Xu, T.; Niu, W. Three-dimensional Printed Mg-Doped b-TCP Bone Tissue Engineering Scaffolds: Effects of Magnesium Ion Concentration on Osteogenesis and Angiogenesis In Vitro. Tissue Eng. Regen. Med. 2019, 16, 415–429. [Google Scholar] [CrossRef]
- Zamani, D.; Moztarzadeh, F.; Bizari, D. Alginate-bioactive glass containing Zn and Mg composite scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2019, 137, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.K. Development and characterization of gelatin/chitosan/montmorillonite composite scaffold enriched with magnesium. Rev. Roum. Chim. 2019, 64, 327–334. [Google Scholar] [CrossRef]
- Baradaran, T.; Shafiei, S.S.; Mohammadi, S.; Moztarzadeh, F. Poly (ε-caprolactone)/layered double hydroxide microspheres-aggregated nanocomposite scaffold for osteogenic differentiation of mesenchymal stem cell. Mater. Today Commun. 2020, 23, 100913–100924. [Google Scholar] [CrossRef]
- Kim, K.J.; Choi, S.; Cho, Y.S.; Yang, S.J.; Cho, Y.S.; Kim, K.K. Magnesium ions enhance infiltration of osteoblasts in scaffolds via increasing cell motility. Mater. Sci: Mater. Med. 2017, 28, 96. [Google Scholar] [CrossRef]
- Perumal, G.; Sivakumar, P.M.; Nandkumar, A.M.; Doble, M. Synthesis of magnesium phosphate nanoflakes and its PCL composite electrospun nanofiber scaffolds for bone tissue regeneration. Mater. Sci. Eng. C 2020, 109, 110527–110537. [Google Scholar] [CrossRef]
- Roh, H.S.; Lee, C.M.; Hwang, Y.H.; Kook, M.S.; Yang, S.W.; Lee, D.; Kim, B.H. Addition of MgO nanoparticles and plasma surface treatment of three-dimensional printed polycaprolactone/hydroxyapatite scaffolds for improving bone regeneration. Mater. Sci. Eng. C 2017, 74, 525–535. [Google Scholar] [CrossRef]
- Vandrovcova, M.; Douglas, T.E.L.; Mróz, W.; Musial, O.; Schaubroeck, D.; Budner, B.; Syroka, R.; Dubruel, P.; Bacakova, L. Pulsed laser deposition of magnesium-doped calcium phosphate coatings on porous polycaprolactone scaffolds produced by rapid prototyping. Mater. Lett. 2015, 148, 178–183. [Google Scholar] [CrossRef]
- Shen, J.; Wang, W.; Zhai, X.; Chen, B.; Qiao, W.; Li, W.; Li, P.; Zhao, Y.; Meng, Y.; Qian, S.; et al. 3D-printed nanocomposite scaffolds with tunable magnesium ionic microenvironment induce in situ bone tissue regeneration. Appl. Mater. Today 2019, 16, 493–507. [Google Scholar] [CrossRef]
- Go, E.Z.; Kang, E.Y.; Lee, S.K.; Park, S.; Kim, J.H.; Park, W.; Kim, I.W.; Choi, B.; Han, D.K. An osteoconductive PLGA scaffold with bioactive β-TCP and anti-inflammatory Mg(OH)2 to improve in vivo bone regeneration. Biomater. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sardella, E.; Fisher, E.R.; Shearer, J.C.; Garzia Trulli, M.; Gristina, R.; Favia, P. N2/H2O Plasma Assisted Functionalization of Poly(ε-caprolactone) Porous Scaffolds: Acidic/Basic Character versus Cell Behavior. Plasma Process. Polym. 2015, 12, 786–798. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Intranuovo, F.; Gristina, R.; Fracassi, L.; Lacitignola, L.; Crovace, A.; Favia, P. Plasma Processing of Scaffolds for Tissue Engineering and Regenerative Medicine. Plasma Chem. Plasma Process. 2016, 36, 269–280. [Google Scholar] [CrossRef]
- Sardella, E.; Salama, R.A.; Waly, G.H.; Habib, A.N.; Favia, P.; Gristina, R. Improving Internal Cell Colonization of Porous Scaffolds with Chemical Gradients Produced by Plasma Assisted Approaches. ACS Appl. Mater. Interfaces 2017, 9, 4966–4975. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Domingos, M.; Intranuovo, F.; Gloria, A.; Gristina, R.; Ambrosio, L.; Bártolo, P.J.; Favia, P. Improved osteoblast cell affinity on plasma-modified 3-D extruded PCL scaffolds. Acta Biomater. 2013, 9, 5997–6005. [Google Scholar] [CrossRef]
- Trizio, I.; Garzia Trulli, M.; Lo Porto, C.; Pignatelli, D.; Camporeale, G.; Palumbo, F.; Sardella, E.; Gristina, R.; Favia, P. Plasma Processes for Life Sciences. In Elsevier Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Reedijk, J., Ed.; Elsevier: Waltham, MA, USA, 2018. [Google Scholar]
- Intranuovo, F.; Sardella, E.; Gristina, R.; Nardulli, M.; White, L.; Howard, D.; Shakesheff, K.M.; Alexander, M.R.; Favia, P. PE-CVD processes improve cell affinity of polymer scaffolds for tissue engineering. Surf. Coat Technol. 2011, 205, S548–S551. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Chapman, B. Glow Discharge Processes: Sputtering and Plasma Etching; John Wiley and Sons: New York, NY, USA, 1980. [Google Scholar]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; Wiley: Chichester, UK, 1992. [Google Scholar]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics, Inc.: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Cui, N.; Wang, F. Ti-doped MgO thin film by magnetron sputtering for cesium frequency standard. Int. J. Mod. Phys. B 2019, 33, 1950202–1950211. [Google Scholar] [CrossRef]
- Sirghi, L.; Hatanaka, Y.; Sakaguchi, K. Photocatalytic property of titanium dioxide thin films deposited by radio frequency magnetron sputtering in argon and water vapour plasma. Appl. Surf. Sci. 2015, 352, 38–41. [Google Scholar] [CrossRef]
- Platzer-Björkman, C.; Mongstad, T.; Mæhlen, J.P.; Baldi, A.; Karazhanov, S.; Holt, A. Deposition of magnesium hydride thin films using radio frequency reactive sputtering. Thin Solid Film 2011, 519, 5949–5954. [Google Scholar] [CrossRef]
- Laidani, N.; Bartali, R.; Tosi, P.; Anderle, M. Argon–hydrogen rf plasma study for carbon film deposition. J. Phys. D Appl. Phys. 2004, 37, 2593–2606. [Google Scholar] [CrossRef]
- Terauchi, M.; Hashimoto, J.; Nishitani, H.; Fukui, Y.; Okafuji, M.; Yamashita, H.; Hayata, H.; Okuma, T.; Yamanishi, H.; Nishitani, M.; et al. High-performance MgO thin films for PDPs with a high-rate sputtering-deposition Process. J. Soc. Inf. Disp. 2008, 16, 1195–1201. [Google Scholar] [CrossRef]
- Hine, K.; Yoshimura, S.; Ikuse, K.; Kiuchi, M.; Hashimoto, J.; Terauchi, M.; Nishitani, M.; Hamaguchi, S. Experimental evaluation of MgO sputtering yields by monochromatic Ne, Kr, or Xe ion beams. Thin Solid Film 2008, 517, 835–840. [Google Scholar] [CrossRef]
- Janning, C.; Willbold, E.; Vogt, C.; Nellesen, J.; Meyer-Lindenberg, A.; Windhagen, H.; Thorey, F.; Witte, F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater. 2010, 6, 1861–1868. [Google Scholar] [CrossRef]
- Fisher, E.R. Challenges in the Characterization of Plasma-Processed Three-Dimensional Polymeric Scaffolds for Biomedical Applications. ACS Appl. Mater. Interfaces 2013, 5, 9312–9321. [Google Scholar] [CrossRef]
- Wang, C.; Qiao, C.; Song, W.; Sun, H. Ultrafast Spreading Effect Induced Rapid Cell Trapping into Porous Scaffold with Superhydrophilic Surface. ACS Appl. Mater. Interfaces 2015, 7, 17545–17551. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma Methods for the Generation of Chemically Reactive Surfaces for Biomolecule Immobilization and Cell Colonization—A Review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Mirzadeh, H.; Irani, S. Plasma surface modification of poly (L-lactic acid) and poly(lactic-co-glycolic acid) films for improvement of nerve cells adhesion. Radiat. Phys. Chem. 2008, 77, 280–287. [Google Scholar] [CrossRef]
| Φ H2O or Φ H2 (%) | Φ Ar (sccm) | Φ H2O or Φ H2 (sccm) |
|---|---|---|
| 0 | 20 | 0 |
| 25 | 15 | 5 |
| 50 | 10 | 10 |
| 75 | 5 | 15 |
| 100 | 0 | 20 |
| Sample/Conditions of Deposition | PCL Flat | PCL Scaffold | |||||
|---|---|---|---|---|---|---|---|
| C (%) | O (%) | Mg (%) | C (%) | O (%) | Mg (%) | ||
| Native | 73.6 ± 3.7 | 26.4 ± 1.3 | – | 75.2 ± 3.8 | 24.8 ± 1.2 | – | |
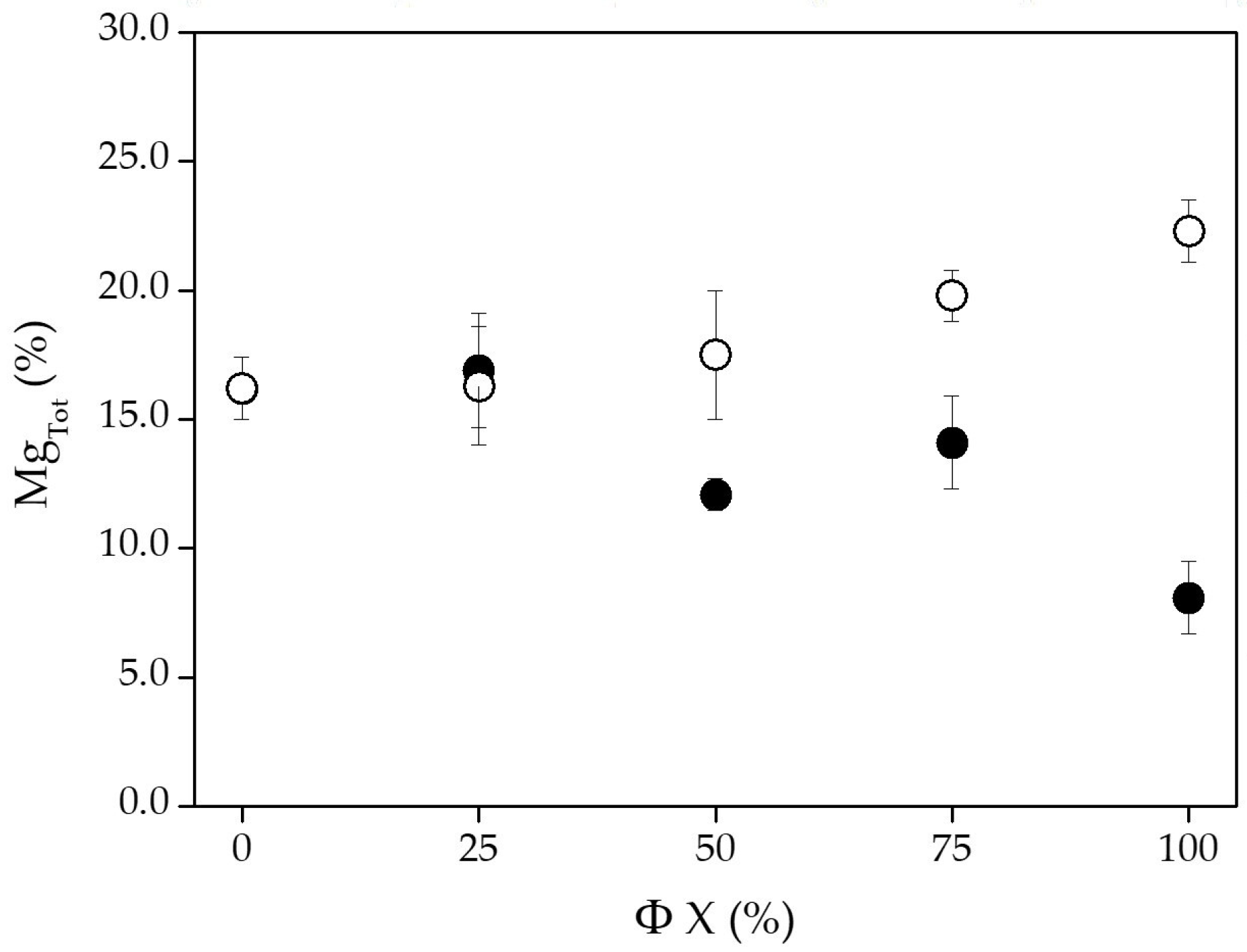
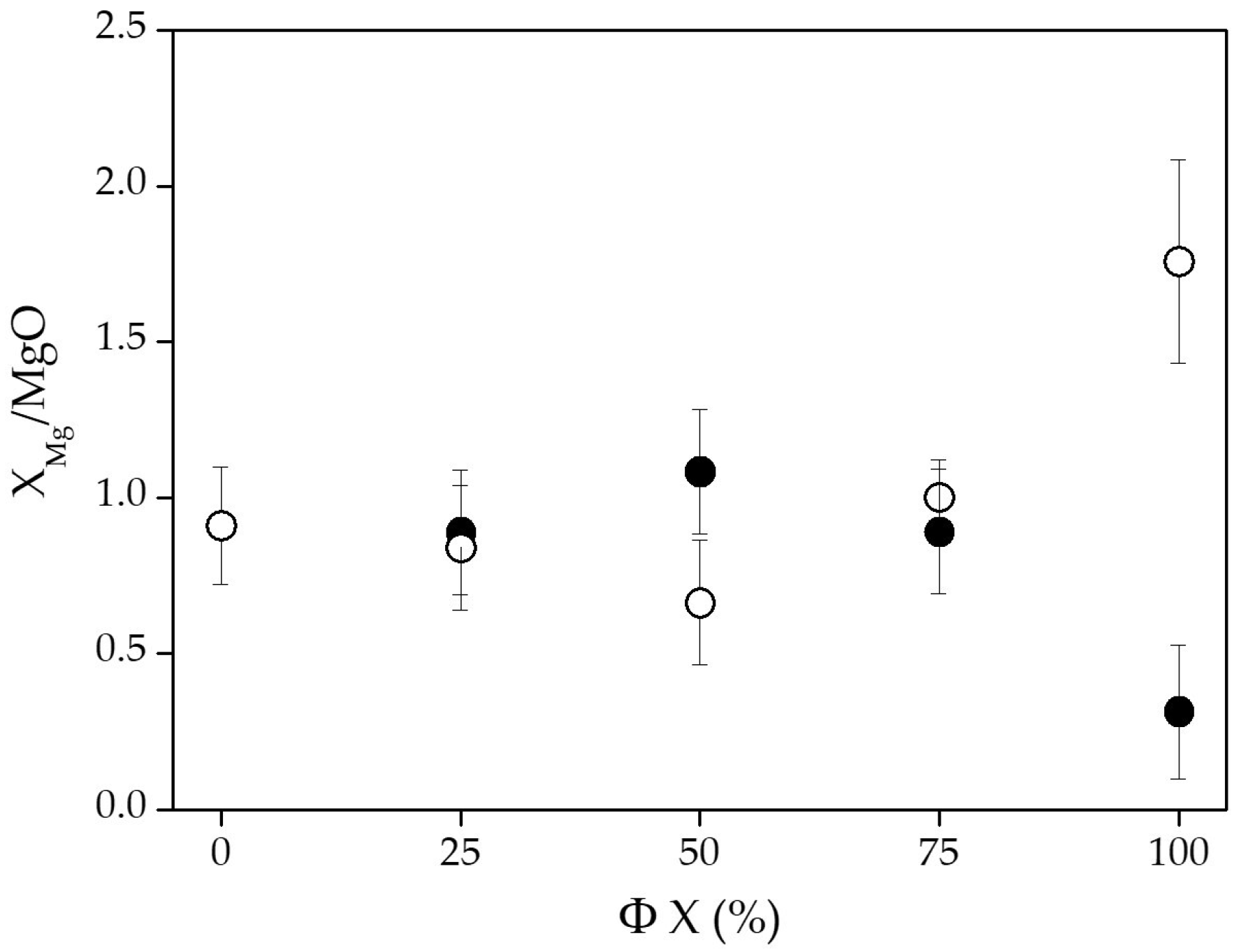
| Φ H2O (%) | 0 | 51.3 ± 2.5 | 32.5 ± 1.6 | 16.2 ± 1.2 | 64.9 ± 4.1 | 28.3 ± 4.6 | 6.8 ± 3.5 |
| 25 | 54.5 ± 2.7 | 28.6 ± 1.4 | 16.9 ± 2.2 | 70.1 ± 2.9 | 26.1 ± 3.1 | 3.8 ± 0.6 | |
| 50 | 57.1 ± 2.9 | 30.8 ± 1.5 | 12.1 ± 0.6 | 66.6 ± 3.7 | 28.1 ± 3.4 | 5.3 ± 0.8 | |
| 75 | 56.9 ± 2.8 | 29.0 ± 1.5 | 14.1 ± 1.8 | 65.3 ± 4.6 | 29.0 ± 3.5 | 5.7 ± 0.8 | |
| 100 | 64.6 ± 1.5 | 27.3 ± 0.1 | 8.1 ± 1.4 | 68.3 ± 0.4 | 29.2 ± 0.4 | 2.5 ± 0.1 | |
| Φ H2 (%) | 0 | 51.3 ± 2.5 | 32.6 ± 1.6 | 16.2 ± 1.2 | 64.9 ± 4.1 | 28.4 ± 4.6 | 6.8 ± 3.5 |
| 25 | 50.5 ± 2.5 | 33.2 ± 3.3 | 16.3 ± 2.3 | 48.6 ± 3.6 | 37.4 ± 4.5 | 14.0 ± 2.0 | |
| 50 | 49.4 ± 2.5 | 33.1 ± 3.3 | 17.5 ± 2.5 | 48.0 ± 3.3 | 37.7 ± 4.5 | 14.3 ± 0.5 | |
| 75 | 50.7 ± 0.2 | 29.5 ± 1.9 | 19.8 ± 1.0 | 51.7 ± 2.6 | 34.0 ± 1.5 | 14.3 ± 0.6 | |
| 100 | 46.2 ± 0.4 | 31.5 ± 2.6 | 22.3 ± 1.2 | 52.6 ± 4.3 | 31.6 ± 3.8 | 15.8 ± 0.7 | |
| Sample/Conditions of Deposition | C0 (%) | C1 (%) | C2 (%) | C3 (%) | C4 (%) | νCO/CC | |
|---|---|---|---|---|---|---|---|
| C–C/C–H | C–COOR/H | COR/H | C=O/O–C–O | COOR/H | |||
| Native | 50.5 ± 2.5 | 8.7 ± 0.4 | 4.8 ± 0.2 | – | 9.6 ± 0.5 | 0.46 ± 0.20 | |
| Φ H2O (%) | 0 | 30.2 ± 1.9 | 9.5 ± 1.2 | 2.9 ± 0.4 | 2.1 ± 0.5 | 6.6 ± 1.0 | 0.70 ± 0.10 |
| 25 | 31.1 ± 1.5 | 9.2 ± 0.5 | 3.9 ± 0.2 | 4.4 ± 0.2 | 5.9 ± 0.3 | 0.75 ± 0.02 | |
| 50 | 34.4 ± 1.7 | 8.5 ± 0.4 | 3.9 ± 0.2 | 3.4 ± 0.2 | 6.9 ± 0.3 | 0.66 ± 0.02 | |
| 75 | 37.7 ± 1.9 | 6.2 ± 0.3 | 2.9 ± 0.5 | 3.6 ± 0.2 | 6.5 ± 0.3 | 0.51 ± 0.04 | |
| 100 | 40.7 ± 2.0 | 8.5 ± 0.4 | 4.0 ± 0.2 | 3.8 ± 0.2 | 7.6 ± 0.4 | 0.60 ± 0.02 | |
| Φ H2 (%) | 0 | 30.2 ± 1.9 | 9.5 ± 1.2 | 2.9 ± 0.4 | 2.1 ± 0.5 | 6.6 ± 1.0 | 0.70 ± 0.10 |
| 25 | 31.2 ± 2.5 | 8.4 ± 0.7 | 3.0 ± 0.2 | 2.2 ± 0.2 | 5.7 ± 0.5 | 0.62 ± 0.03 | |
| 50 | 31.5 ± 2.5 | 8.1 ± 0.6 | 1.6 ± 0.1 | 2.6 ± 0.2 | 5.6 ± 0.4 | 0.57 ± 0.02 | |
| 75 | 36.4 ± 2.9 | 6.4 ± 0.5 | 1.9 ± 0.1 | 1.5 ± 0.1 | 4.5 ± 0.4 | 0.39 ± 0.01 | |
| 100 | 31.5 ± 2.5 | 6.7 ± 0.5 | 2.1 ± 0.2 | 1.2 ± 0.1 | 4.7 ± 0.4 | 0.47 ± 0.02 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armenise, V.; Gristina, R.; Favia, P.; Cosmai, S.; Fracassi, F.; Sardella, E. Plasma-Assisted Deposition of Magnesium-Containing Coatings on Porous Scaffolds for Bone Tissue Engineering. Coatings 2020, 10, 356. https://doi.org/10.3390/coatings10040356
Armenise V, Gristina R, Favia P, Cosmai S, Fracassi F, Sardella E. Plasma-Assisted Deposition of Magnesium-Containing Coatings on Porous Scaffolds for Bone Tissue Engineering. Coatings. 2020; 10(4):356. https://doi.org/10.3390/coatings10040356
Chicago/Turabian StyleArmenise, Vincenza, Roberto Gristina, Pietro Favia, Savino Cosmai, Francesco Fracassi, and Eloisa Sardella. 2020. "Plasma-Assisted Deposition of Magnesium-Containing Coatings on Porous Scaffolds for Bone Tissue Engineering" Coatings 10, no. 4: 356. https://doi.org/10.3390/coatings10040356
APA StyleArmenise, V., Gristina, R., Favia, P., Cosmai, S., Fracassi, F., & Sardella, E. (2020). Plasma-Assisted Deposition of Magnesium-Containing Coatings on Porous Scaffolds for Bone Tissue Engineering. Coatings, 10(4), 356. https://doi.org/10.3390/coatings10040356