Properties of Graphene-Thermoplastic Polyurethane Flexible Conductive Film
Abstract
1. Introduction
2. Experimental Approach
2.1. Materials
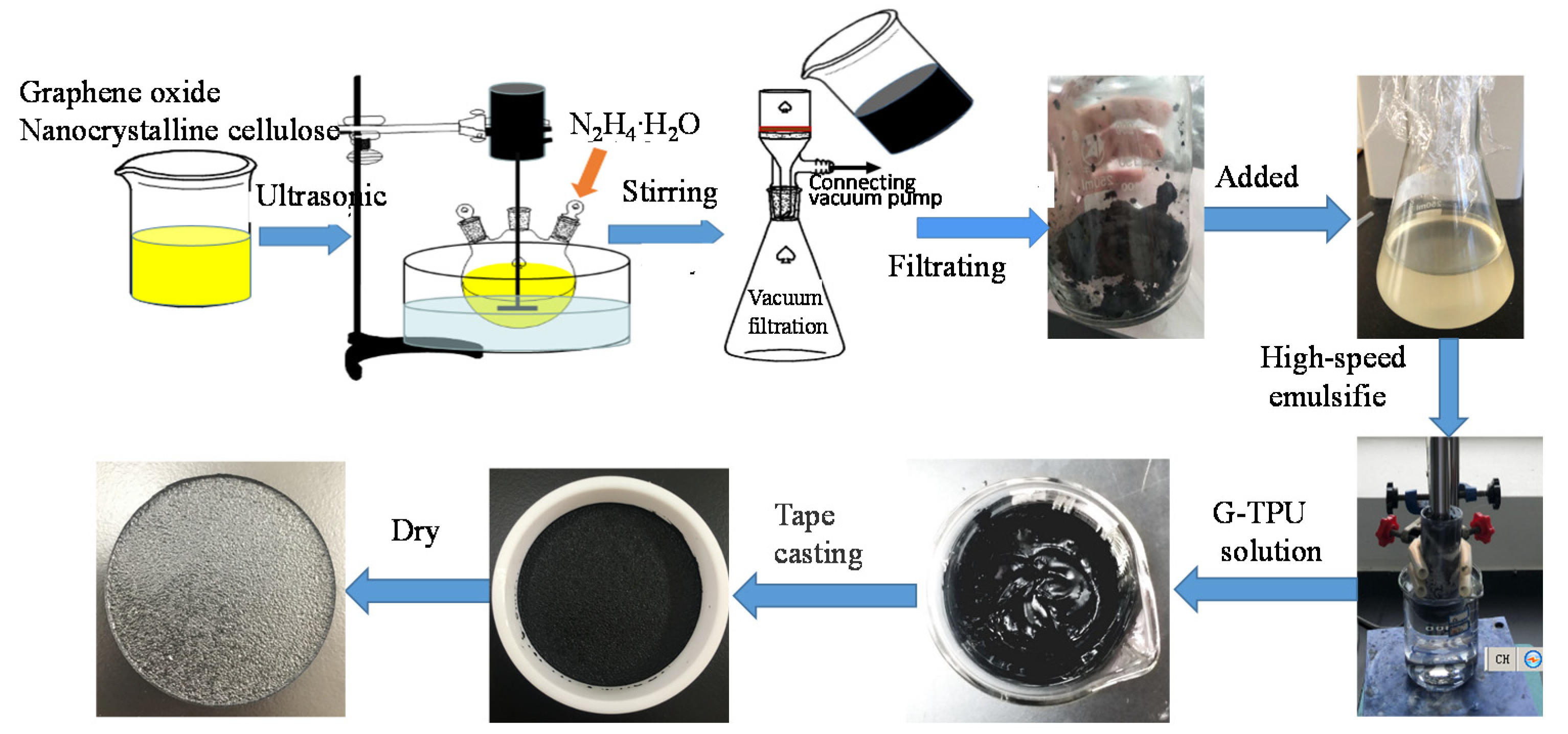
2.2. Preparation of G-TPU Composite Film
2.2.1. Preparation of RGO/NCC Slurry
2.2.2. Preparation of RGO/NCC-TPU Composite Film
2.2.3. Preparation of Superhydrophobic Conductive Composite film of RGO/NCC-TPU
2.3. Characterization
3. Results and Discussion
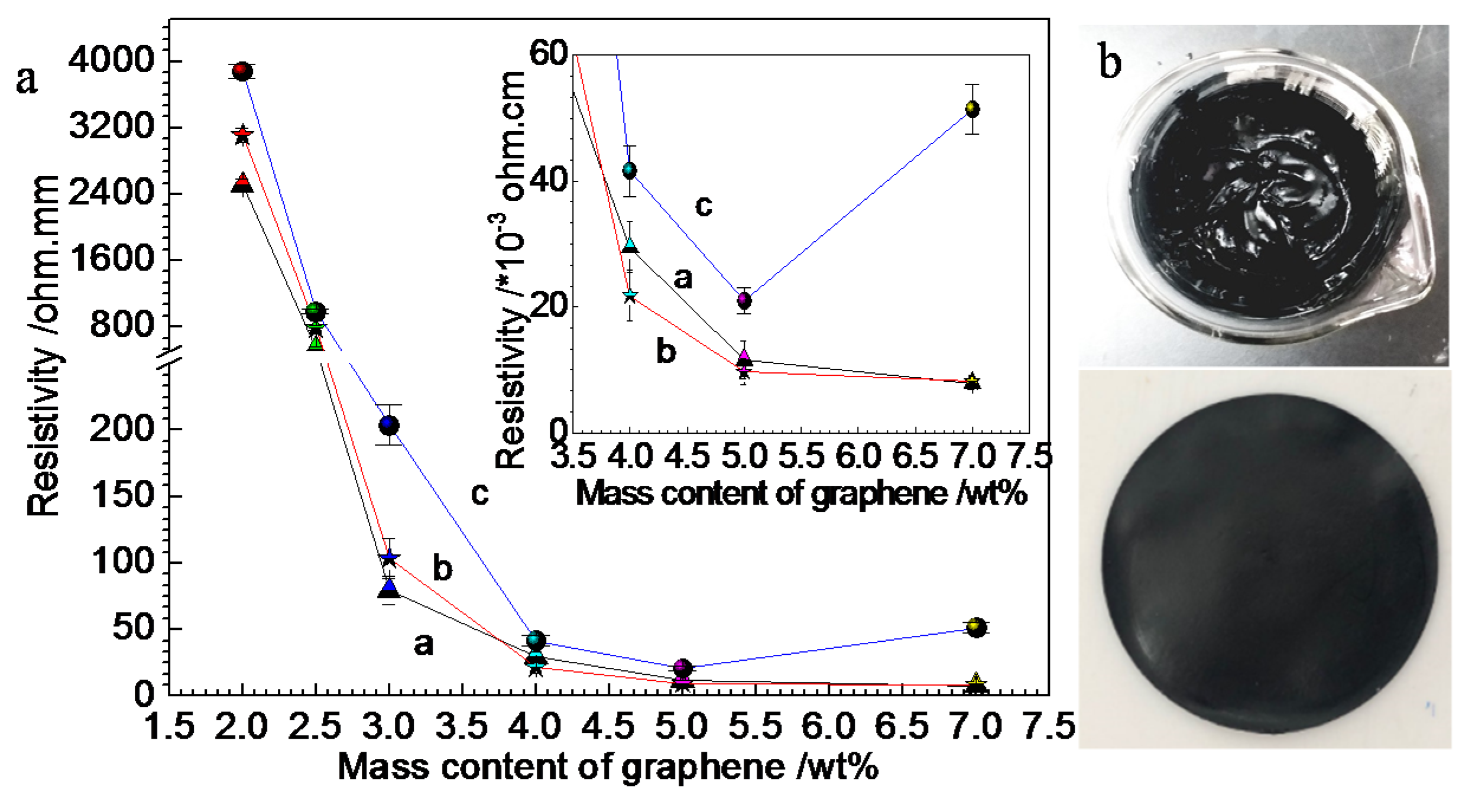
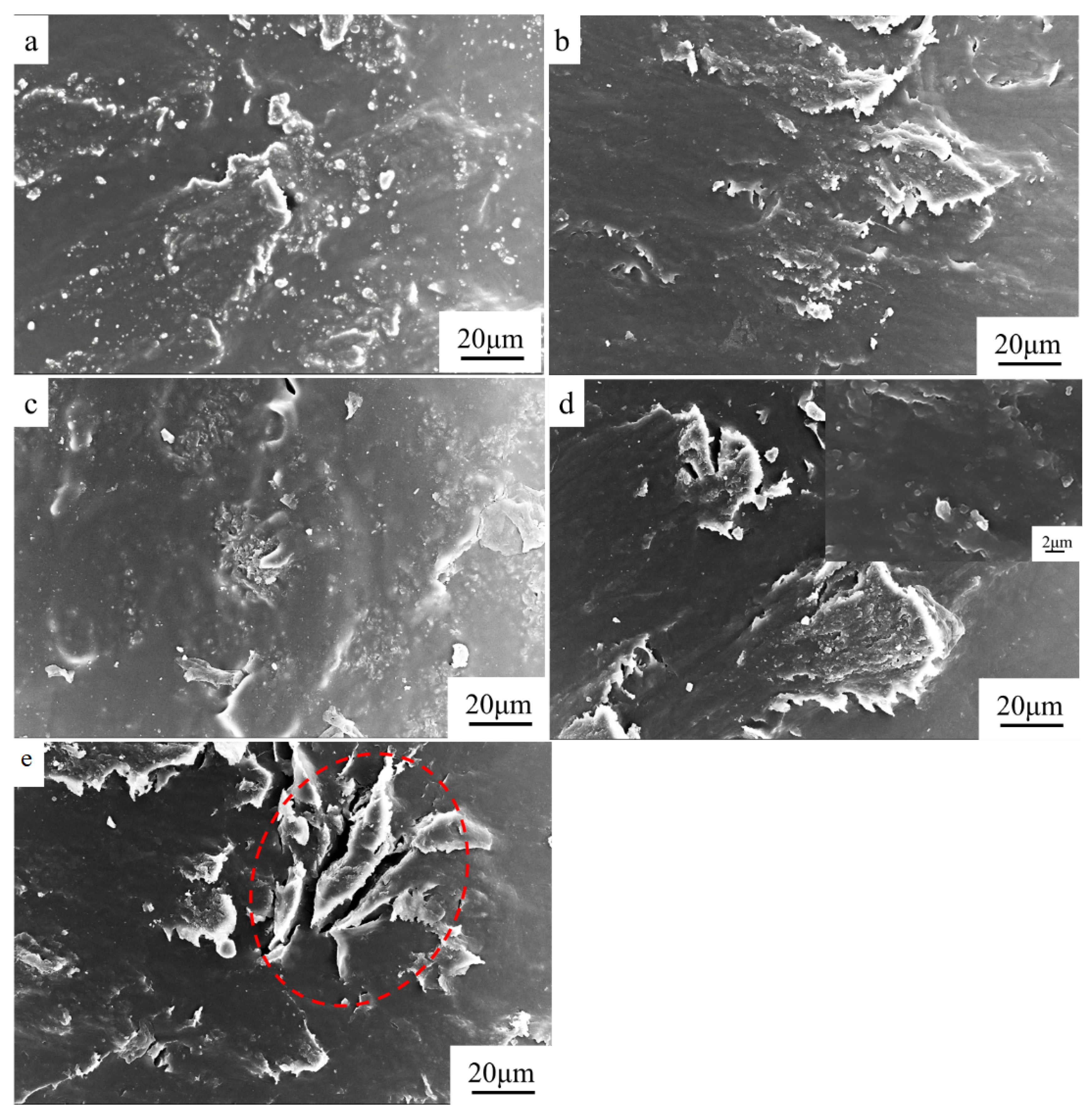
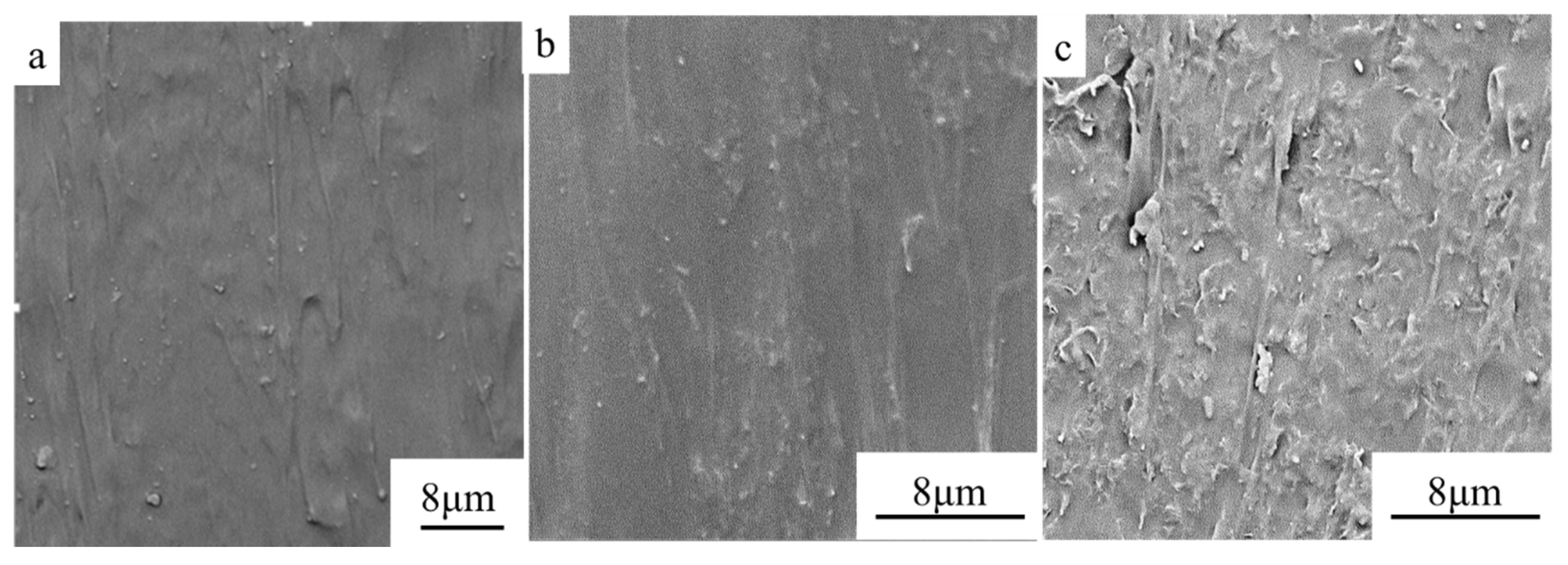
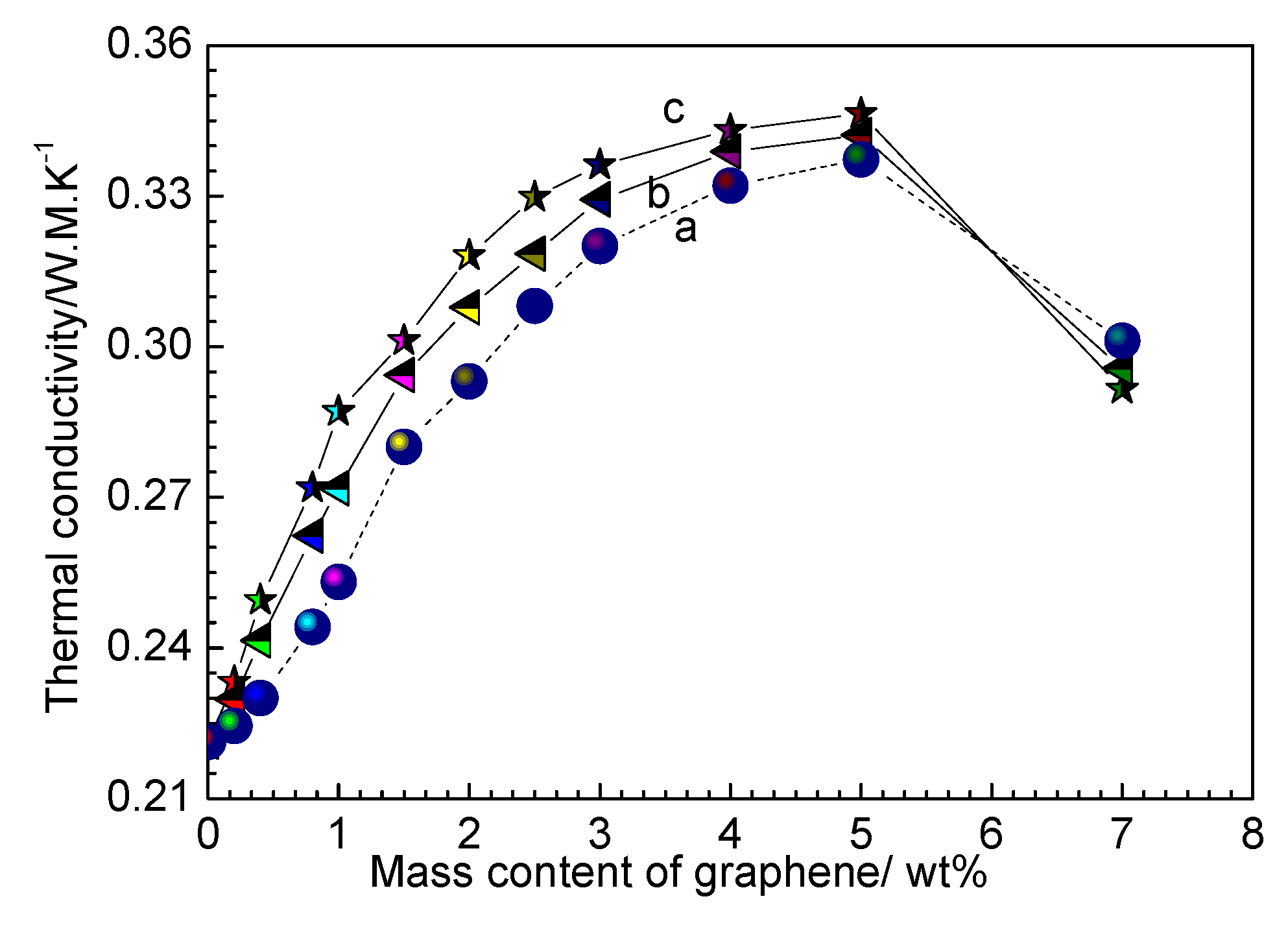
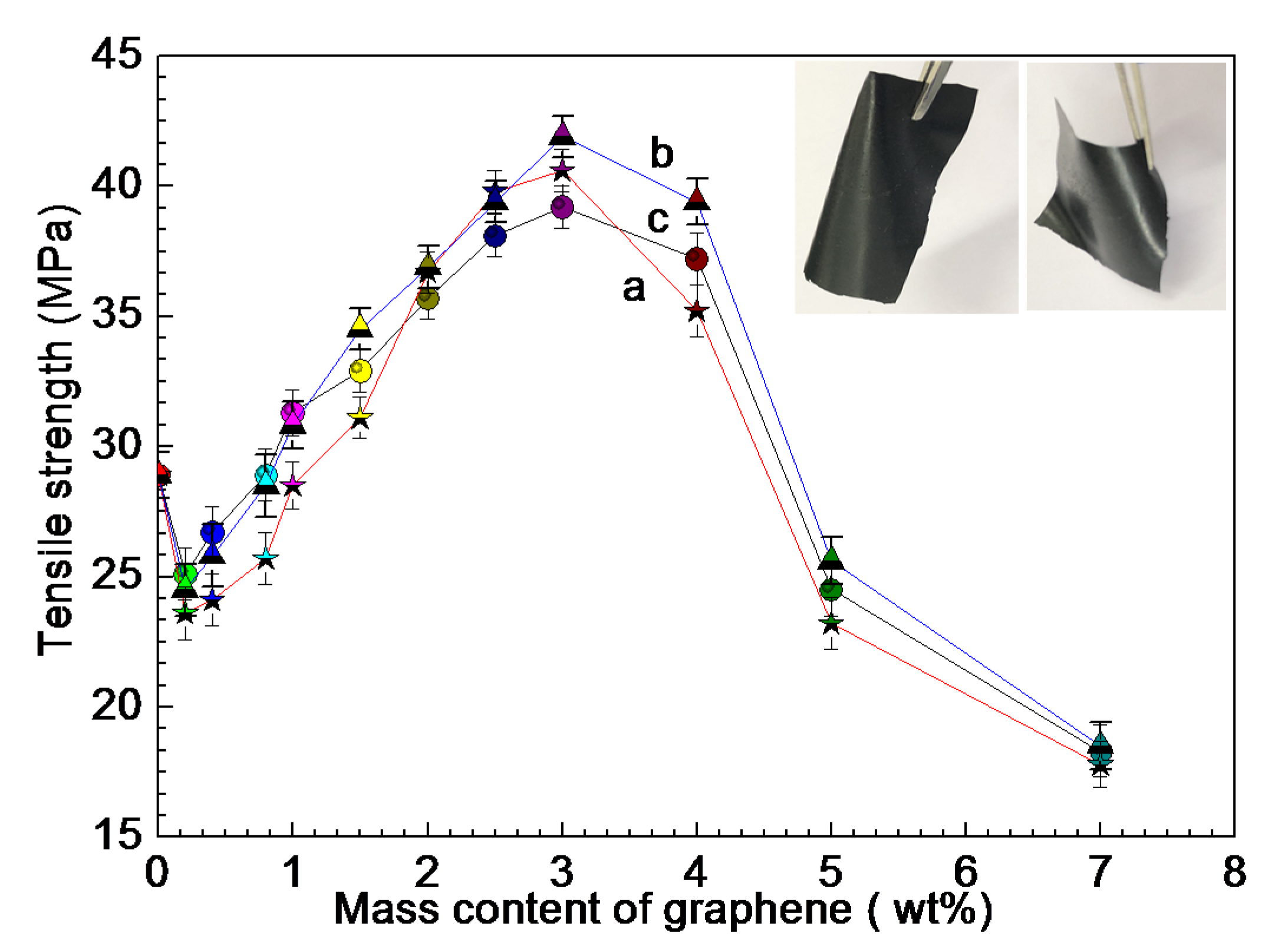
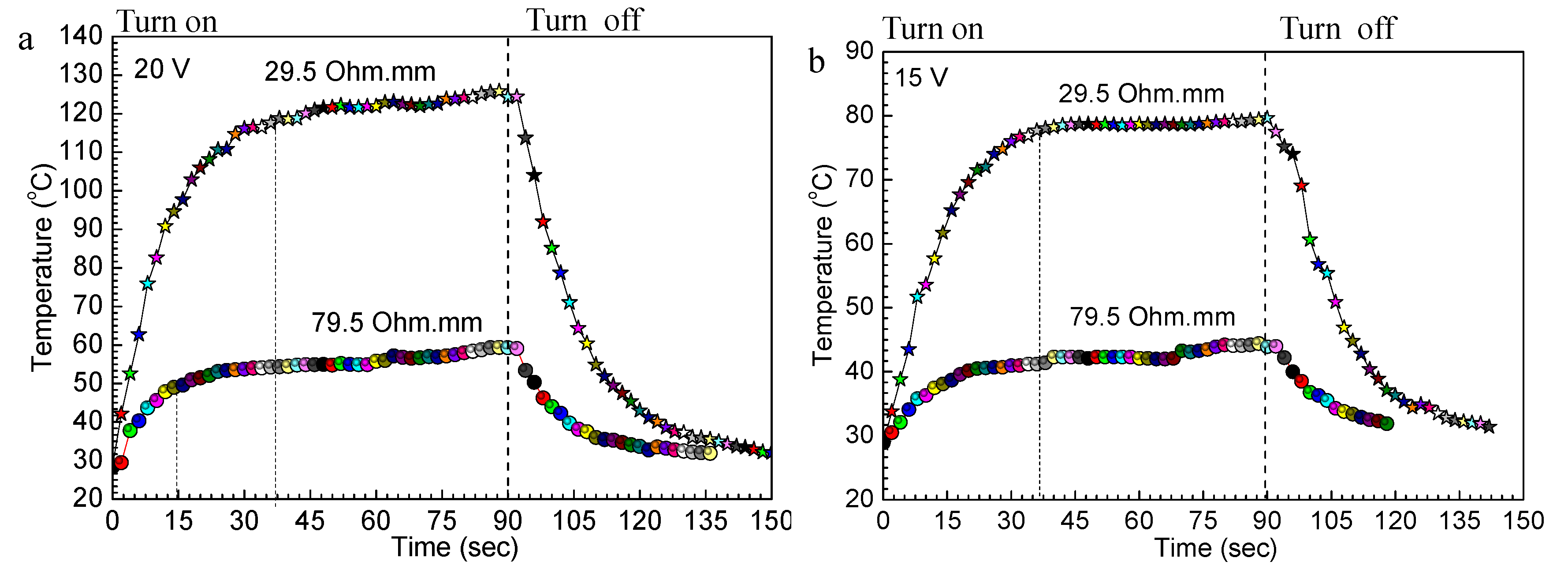
3.1. Electrical and Thermal Properties of RGO/NCC-TPU Film
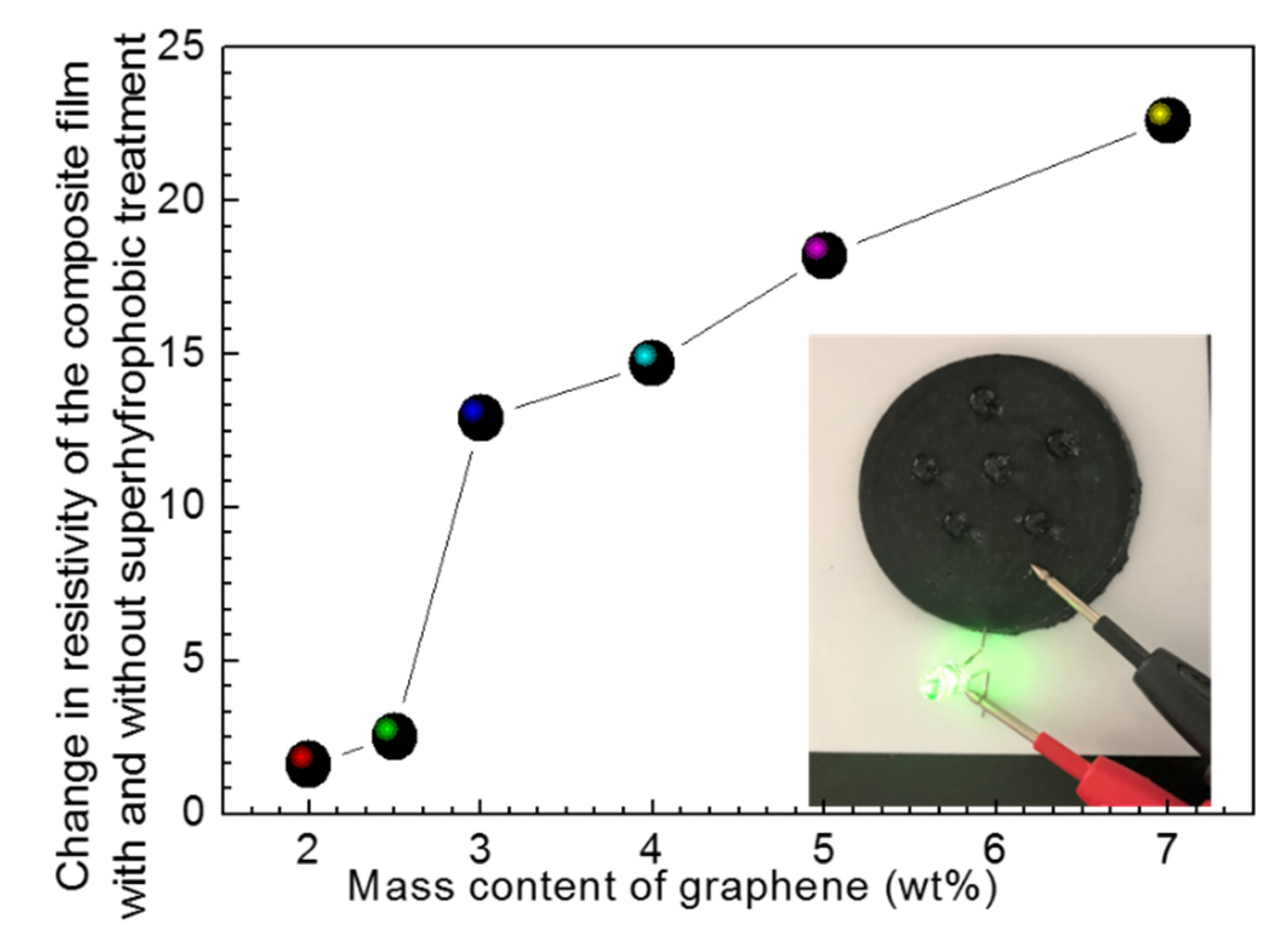
3.2. Superhydrophobic Conductive Composite Film of RGO/NCC-TPU
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, C.; Hu, S.; Zhang, R.; Hu, H.; Ying, C.; Zhang, F.; Liu, Q.; Fu, X. Preparation and properties of flexible conductive polydimethylsiloxane composites containing hybrid fillers. Polym. Bulletin. 2019, 76, 6487–6501. [Google Scholar] [CrossRef]
- Cuttaz, E.; Goding, J.; Vallejo-Giraldo, C.; Aregueta-Robles, U.; Lovell, N.; Ghezzi, D.; Green, R.A. Conductive elastomer composites for fully polymeric, flexible bioelectronics. Biomater. Sci. 2019, 7, 1372–1385. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, Y.; Yan, D.; Duan, H.; Zhao, G.; Liu, Y. Flexible and conductive polyurethane composites for electromagnetic shielding and printable circuit. Chem. Eng. Jl. 2019, 360, 1427–1436. [Google Scholar] [CrossRef]
- Maya, M.G.; George, S.C.; Jose, T.; Kailas, L.; Thomas, S. Development of a flexible and conductive elastomeric composite based on chloroprene rubber. Polym. Test. 2018, 65, 256–263. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.; Wang, H.; Zeng, B.; Wang, J.; Chi, F. Flexible organic light-emitting devices with copper nanowire composite transparent conductive electrode. J. Mater. Sci. 2019, 54, 2343–2350. [Google Scholar] [CrossRef]
- Gu, X.; Li, B.; Li, F.; Zhang, K.; Guo, M. Transparent and flexible vermiculite–cellulose nanofiber composite membranes with high-temperature proton conduction. J. Mater. Sci. 2019, 54, 5528–5535. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, X.; Du, D.X.; Zhang, X.F. A Comprehensive Study of High-Performance of Flexible Transparent Conductive Silver Nanowire Films. J. Mater. Sci. Mater. El. 2019, 30, 13238–13246. [Google Scholar] [CrossRef]
- Yang, X.; Du, D.; Wang, Y.; Zhao, Y. Silver Nanowires Inks for Flexible Circuit on Photographic Paper Substrate. Micromachines 2019, 10, 22. [Google Scholar] [CrossRef]
- Husameldin, A.E.; Sharul, A.K.R.; Xavier, C.; Mohamed, H. Flexible conductive fabric/E-glass fibre composite ultra-wideband antenna for future wireless networks. IET Microw. Antennas Propag. 2019, 13, 455–459. [Google Scholar]
- Ji, T.; Feng, Y.; Qin, M.; Li, S.; Zhang, F.; Lv, F.; Feng, W. Thermal conductive and flexible silastic composite based on a hierarchical framework of aligned carbon fibers-carbon nanotube. Carbon 2018, 131, 149–159. [Google Scholar] [CrossRef]
- Singh, B.P.; Nayak, S.; Nanda, K.K.; Singh, A.; Takai, C.; Takashi, S.; Fuji, M. Transparent, flexible, and conducting films based on graphene–polymer composites. Polym. Compos. 2018, 39, 297–304. [Google Scholar] [CrossRef]
- Xu, W.; Zhong, L.; Xu, F.; Shen, W.; Song, W.; Chou, S. Silver Nanowire-Based Flexible Transparent Composite Film for Curvature Measurements. ACS Appl. Nano Mater. 2018, 1, 3859–3866. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Liu, H.; Wang, J.; Zhao, X.; Xie, Z. Efficient flexible polymer solar cells based on solution-processed reduced graphene oxide–Assisted silver nanowire transparent electrode. Org. Electron. 2017, 50, 255–263. [Google Scholar] [CrossRef]
- Wang, P.; Peng, Z.; Li, M. Stretchable Transparent Conductive Films from Long Carbon Nanotube Metals. Small 2018, 14, 1802625. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Le, T.-S.D.; Huang, Y.; Zhan, Z.; Li, Y.; Zheng, L.; Huang, W.; Sun, G.; Kim, Y.-J. All Graphene-Based Highly Flexible Non-Contact Electronic Skin. ACS Appl. Mater. Interfaces 2017, 9, 44593–44601. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Han, T.; Ruan, H. Solution-Assembled Ordered Grids Constructed with Silver Nanowires as Transparent Conductive Electrodes. ACS Omega 2018, 7, 7191–7195. [Google Scholar] [CrossRef]
- Das, T.; Sharma, B.K.; Katiyar, A.K. Engineering in-plane silicon nanowire springs for highly stretchable electronics. J. Semicond. 2018, 39, 011007. [Google Scholar] [CrossRef]
- Sohn, H.; Woo, Y.S.; Shin, W.; Yun, D.J.; Lee, T.; Kim, F.S.; Hwang, J. Novel transparent conductor with enhanced conductivity: Hybrid of silver nanowires and dual-doped graphene. Appl. Surf. Sci. 2017, 419, 63–69. [Google Scholar] [CrossRef]
- Raji, A.-R.O.; Varadhachary, T.; Nan, K.; Wang, T.; Lin, J.; Ji, Y.; Genorio, B.; Zhu, Y.; Kittrell, C.; Tour, J.M. Composites of graphene nanoribbon stacks and epoxy for Joule heating and deicing of surfaces. ACS Appl. Mater. Interfaces 2016, 8, 3551–3556. [Google Scholar] [CrossRef]
- Kea, K.; McMasterb, M.; Christophersonb, W.; Singerb, K.D. Effects of branched carbon nanotubes and graphene nanoplatelets on dielectric properties of thermoplastic polyurethane at different temperatures. Compos. Part B 2019, 166, 673–680. [Google Scholar] [CrossRef]
- Liang, A.; Jiang, X.; Hong, X.; Jiang, Y.; Shao, Z.; Zhu, D. Recent Developments Concerning the Dispersion Methods and Mechanisms of Graphene. Coatings 2018, 8, 33. [Google Scholar] [CrossRef]
- Ghivela, G.G.; Sengupta, J. The Promise of Graphene: A Survey of Microwave Devices Based on Graphene. IEEE Microw. Mag. 2020, 21, 48–65. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, R. Nanomechanics of graphene. Natl. Sci. Rev. 2019, 6, 324–334. [Google Scholar] [CrossRef]
- Pang, Y.; Yang, Z.; Xie, X.; Tao, L. Graphene Oxide Modified Porous Graphene for Aqueous Alcohol Detection. IEEE Sens. Lett. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Tung, T.T.; Nine, M.J.; Krebsz, M.; Pasinszki, T.; Coghlan, C.J.; Tran, D.N.H.; Losic, D. Recent Advances in Sensing Applications of Graphene Assemblies and Their Composites. Adv. Funct. Mater. 2017, 27, 1702891. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kariper, İ.A. Graphene and graphene oxide based aerogels: Synthesis, characteristics and supercapacitor applications. J. Energy Storage 2020, 7, 101038. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Pham, V.H.; Cuong, T.V.; Dang, T.D.; Hur, S.H.; Kong, B.-S.; Kim, E.J.; Shin, E.W.; Chung, J.S. Superior conductive polystyrene-chemically converted graphene nanocomposite. J. Mater. Chem. 2011, 2, 11312–11316. [Google Scholar] [CrossRef]
- Dhakal, D.R.; Lamichhane, P.; Mishra, K.; Nelson, T.L.; Vaidyanathan, R.K. Influence of graphene reinforcement in conductive polymer: Synthesis and characterization. Polym. Adv. Technol. 2019, 30, 2172–2182. [Google Scholar] [CrossRef]
- Barjasteh, E.; Sutanto, C.; Nepal, D. Conductive polyamide-graphene composite fabric via interface engineering. Langmuir 2019, 35, 2261–2269. [Google Scholar] [CrossRef]
- Kim, J.T.; Kim, B.K.; Kim, E.Y.; Kwon, S.H.; Jeong, H.M. Synthesis and properties of near Ir induced self-healable polyurethane/graphene nanocomposites. Eur. Polym. J. 2013, 49, 3889–3896. [Google Scholar] [CrossRef]
- Huang, L.; Yi, N.; Wu, Y.; Zhang, Y.; Zhang, Q.; Huang, Y.; Ma, Y.; Chen, Y. Multichannel and repeatable self-healing of mechanical enhanced graphene-thermoplastic polyurethane composites. Adv. Mater. 2013, 25, 2224–2228. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Gao, F.; Li, Y.; Yang, J.; Hu, Y.; Guo, Z.; Wang, Z.; Zhou, A. Healing mechanisms induced by synergy of Graphene-CNTs and microwave focusing effect for the thermoplastic polyurethane composites. Compos. Part A Appl. Sci. Manuf. 2018, 106A, 34–41. [Google Scholar] [CrossRef]
- Li, A.; Zhang, C.; Zhang, Y. Thermal Conductivity of Graphene-Polymer Composites: Mechanisms, Properties, and Applications. Polymers 2017, 9, 437. [Google Scholar]
- Sakairi, N.; Suzuki, S.; Ueno, K.; Han, S.-M.; Nishi, N.; Tokura, S. Biosynthesis of hetero polysaccharides by acetobacter xylinum-synthesis and characterization of metalion adsorptive properties of partially carboxyl methylated cellulose. Carbohydr. Polym. 1998, 37, 409–414. [Google Scholar] [CrossRef]
- Kamisan, A.I.; Kamisan, A.S.; Ali, R.M.; Kudin, T.I.T.; Hassan, O.H.; Halim, N.A.; Yahya, M.F. Synthesis of Graphene via Green Reduction of Graphene Oxide with Simple Sugars. Adv. Mater. Res. 2015, 1107, 542–546. [Google Scholar] [CrossRef]
- Xu, P.; Loomis, J.; Bradshaw, R.D.; Panchapakesan, B. Load transfer and mechanical properties of chemically reduced graphene reinforcements in polymer composites. Nanotechnology 2012, 23, 3847–3856. [Google Scholar] [CrossRef]
- Garzón, C.; Palza, H. Electrical behavior of polypropylene composites melt mixed with carbon-based particles: Effect of the kind of particle and annealing process. Compos. Sci. Technol. 2014, 99, 117–123. [Google Scholar] [CrossRef]
- Hu, Z.; Li, N.; Li, J.; Zhang, C.; Song, Y.; Li, X.; Wu, G.; Xie, F.; Huang, Y. Facile preparation of poly(p-phenylene benzobisoxazole)/graphene composite films via one-pot in situ polymerization. Polymer 2015, 71, 8–14. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of thermal conductivity in composites: Mechanisms, parameters and theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Meng, X.; Chen, T.; Li, Y.; Liu, S.; Pan, H.; Ma, Y.; Chen, Z.; Zhang, Y.; Zhu, S. Assembly of carbon nanodots in graphene-based composite for flexible electro-thermal heater with ultrahigh efficiency. Nano Res. 2019, 12, 2498–2508. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, H.; Liu, G.; Su, Z.; Wu, J.; Liu, J.; Zhang, X.; Chen, Y.; Zhou, W. Light-weight, flexible, low-voltage electro-thermal film using graphite nanoplatelets for wearable/smart electronics and deicing devices. J. Alloy. Comp. 2017, 699, 1049–1056. [Google Scholar] [CrossRef]
- Chang, H.; Jia, Y.; Xiao, L.; Chen, H.; Zhao, K.; Chen, Y.; Ma, Y. Three dimensional cross-linked and flexible graphene composite paper with ultrafast electrothermal response at ultra-low voltage. Carbon 2019, 154, 150–155. [Google Scholar] [CrossRef]
- Wang, Y.; Du, D.; Yang, X.; Zhang, X.; Zhao, Y. Optoelectronic and Electrothermal Properties of Transparent Conductive Silver Nanowires Films. Nanomaterials 2019, 9, 904. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhou, Z.; Zhang, J.; Tang, J.; Wu, P.; Wang, K.; Zhao, Y. Properties of Graphene-Thermoplastic Polyurethane Flexible Conductive Film. Coatings 2020, 10, 400. https://doi.org/10.3390/coatings10040400
Wang Y, Zhou Z, Zhang J, Tang J, Wu P, Wang K, Zhao Y. Properties of Graphene-Thermoplastic Polyurethane Flexible Conductive Film. Coatings. 2020; 10(4):400. https://doi.org/10.3390/coatings10040400
Chicago/Turabian StyleWang, Yuehui, Zhimin Zhou, Jiahao Zhang, Jinyuan Tang, Peiyu Wu, Ke Wang, and Yuzhen Zhao. 2020. "Properties of Graphene-Thermoplastic Polyurethane Flexible Conductive Film" Coatings 10, no. 4: 400. https://doi.org/10.3390/coatings10040400
APA StyleWang, Y., Zhou, Z., Zhang, J., Tang, J., Wu, P., Wang, K., & Zhao, Y. (2020). Properties of Graphene-Thermoplastic Polyurethane Flexible Conductive Film. Coatings, 10(4), 400. https://doi.org/10.3390/coatings10040400




