Oxidation Behavior and Structural Transformation of (CrTaTiVZr)N Coatings
Abstract
:1. Introduction
2. Experimental
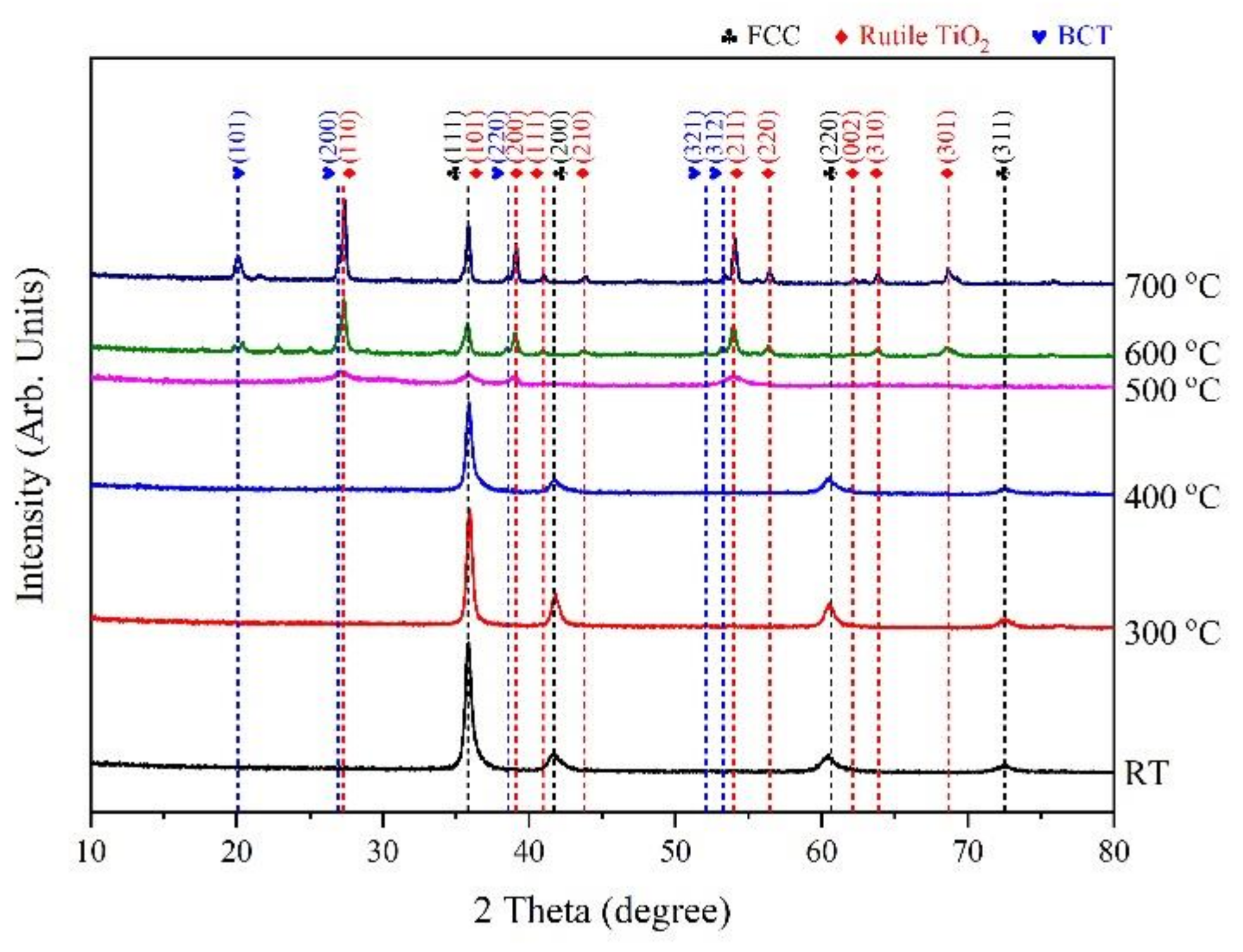
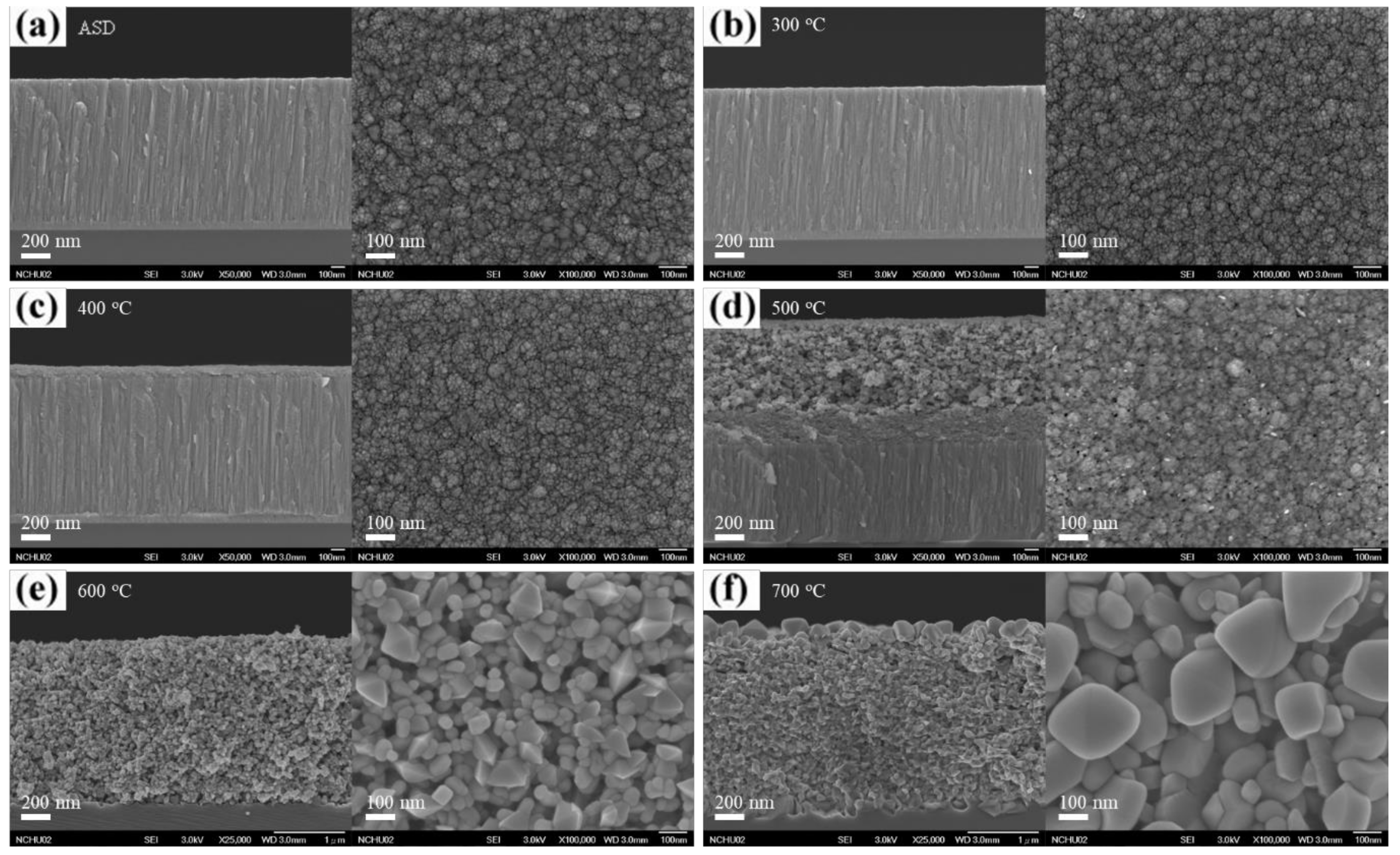
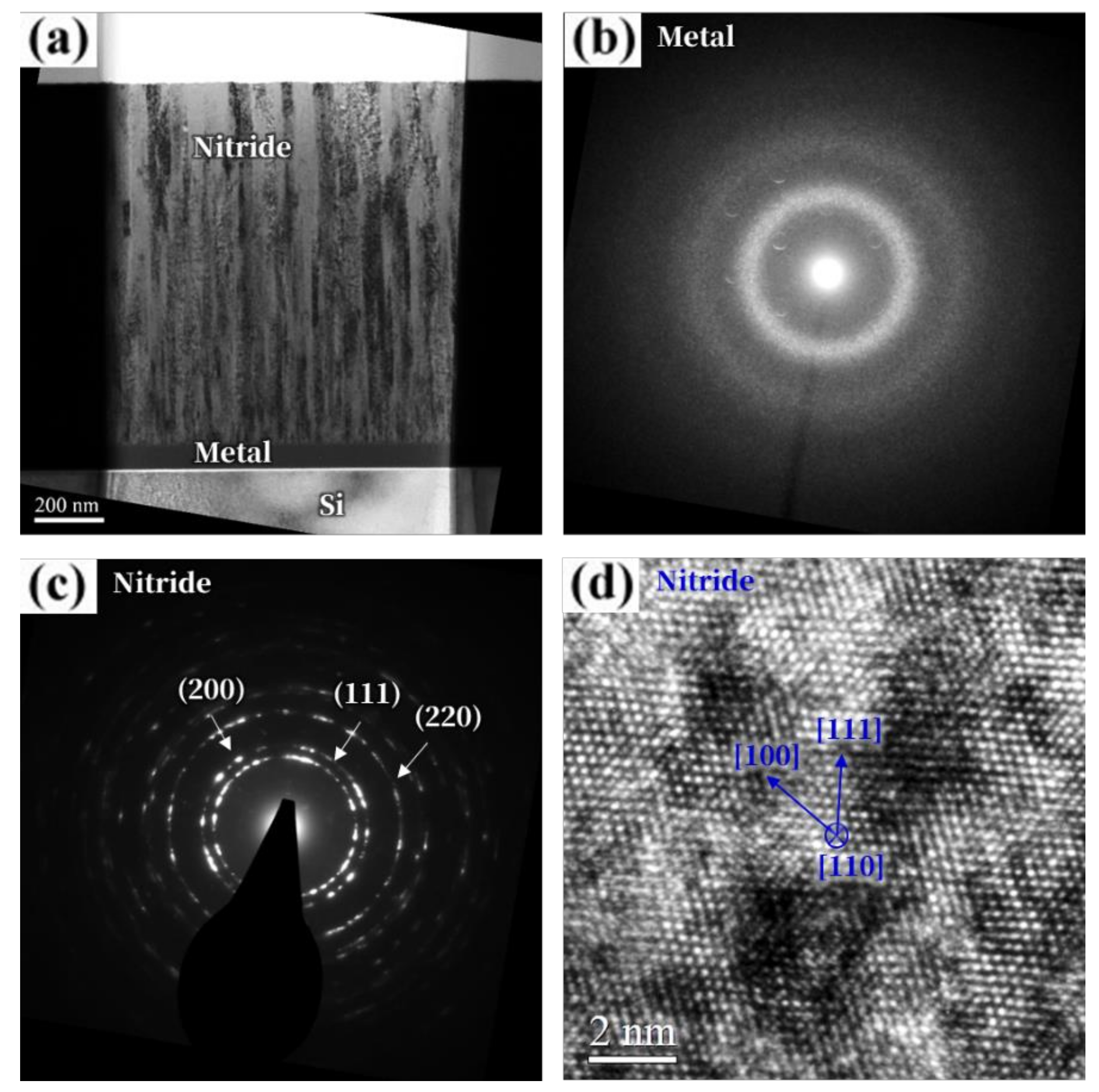
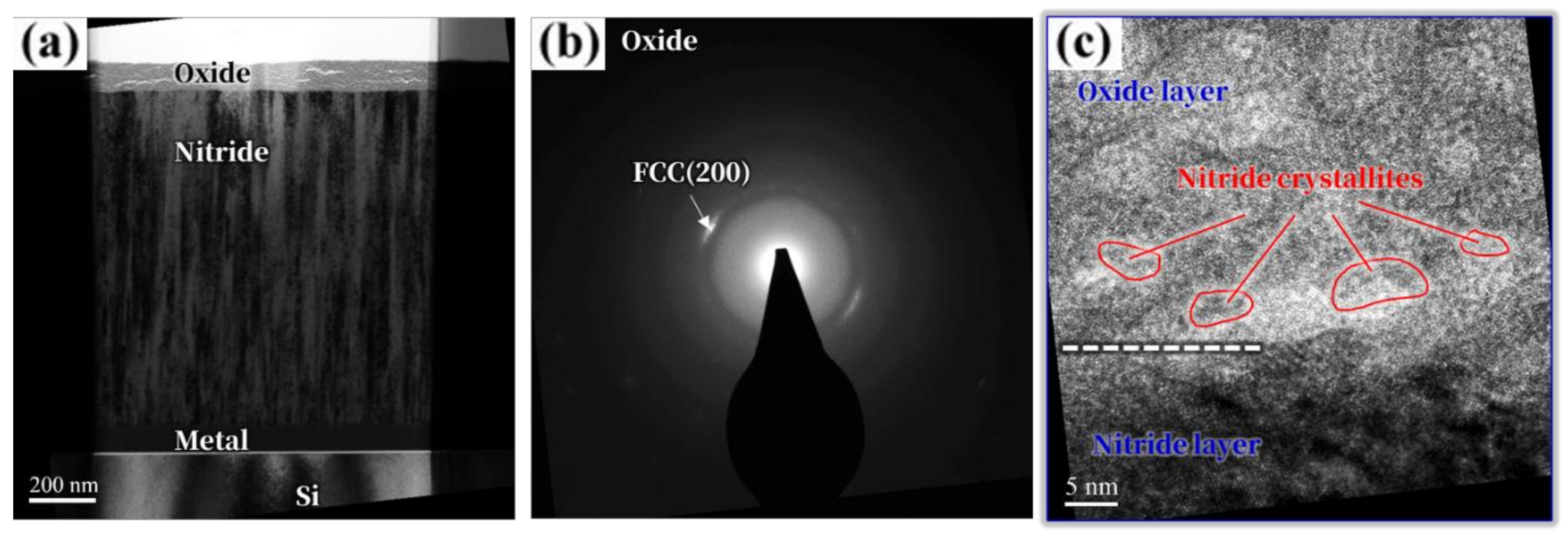
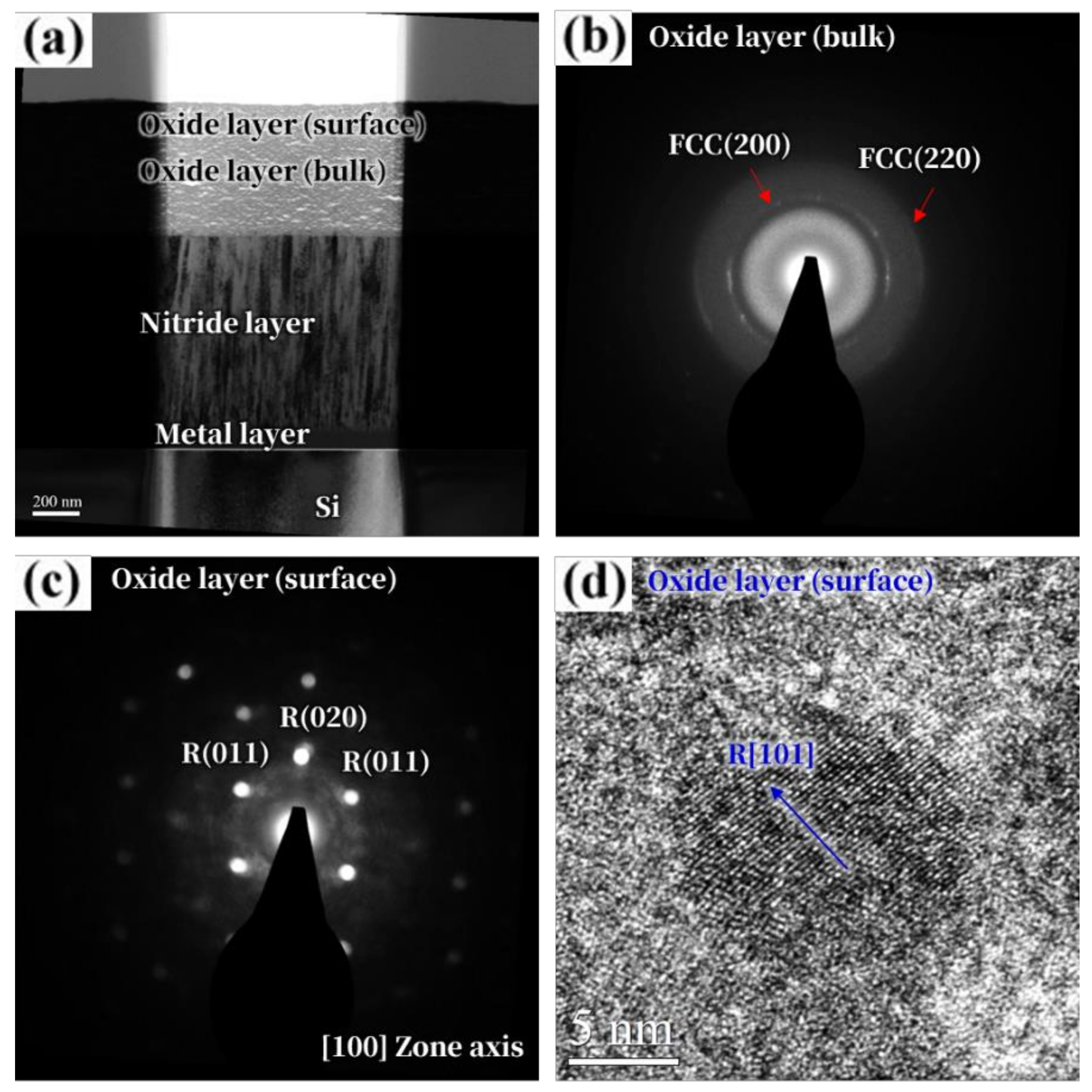
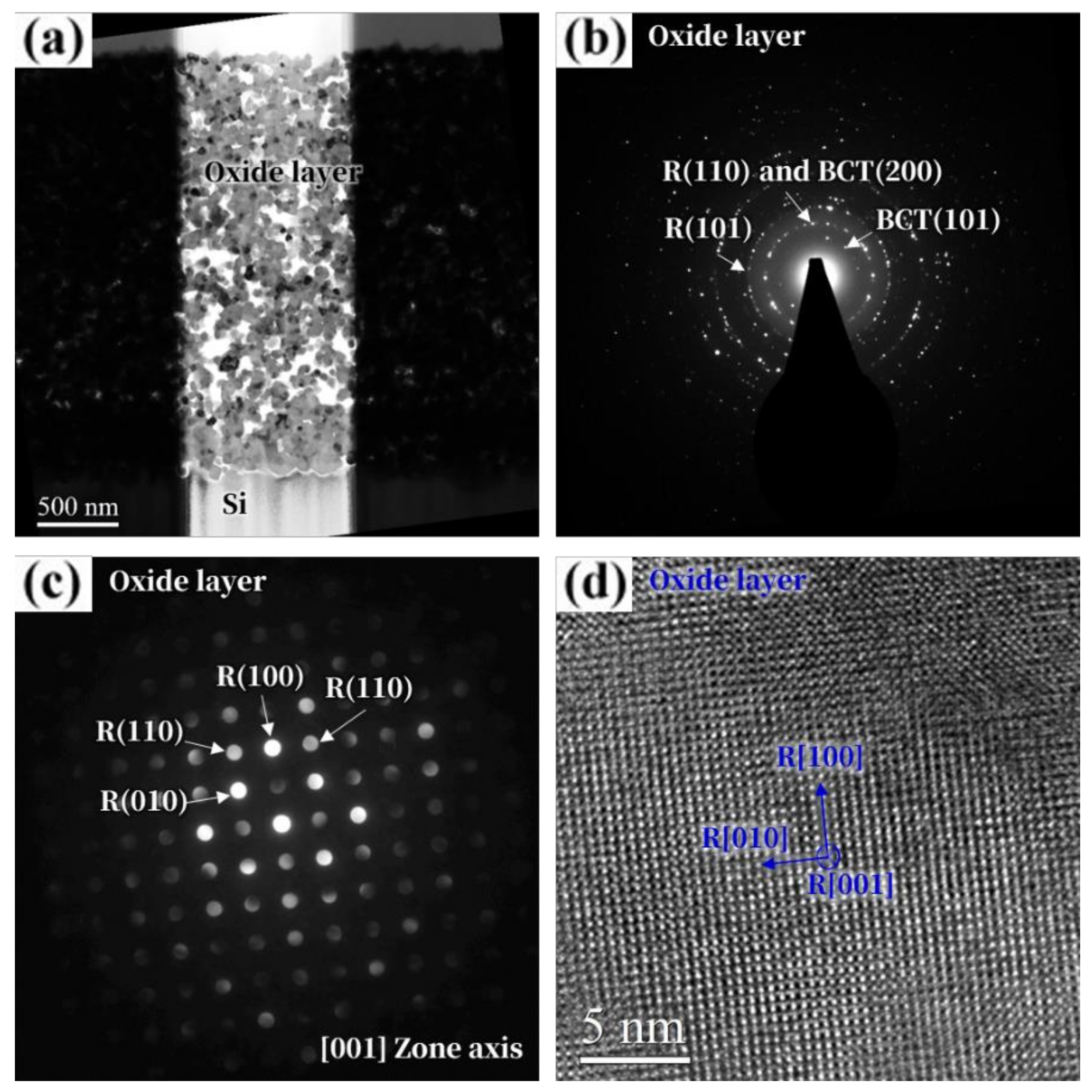
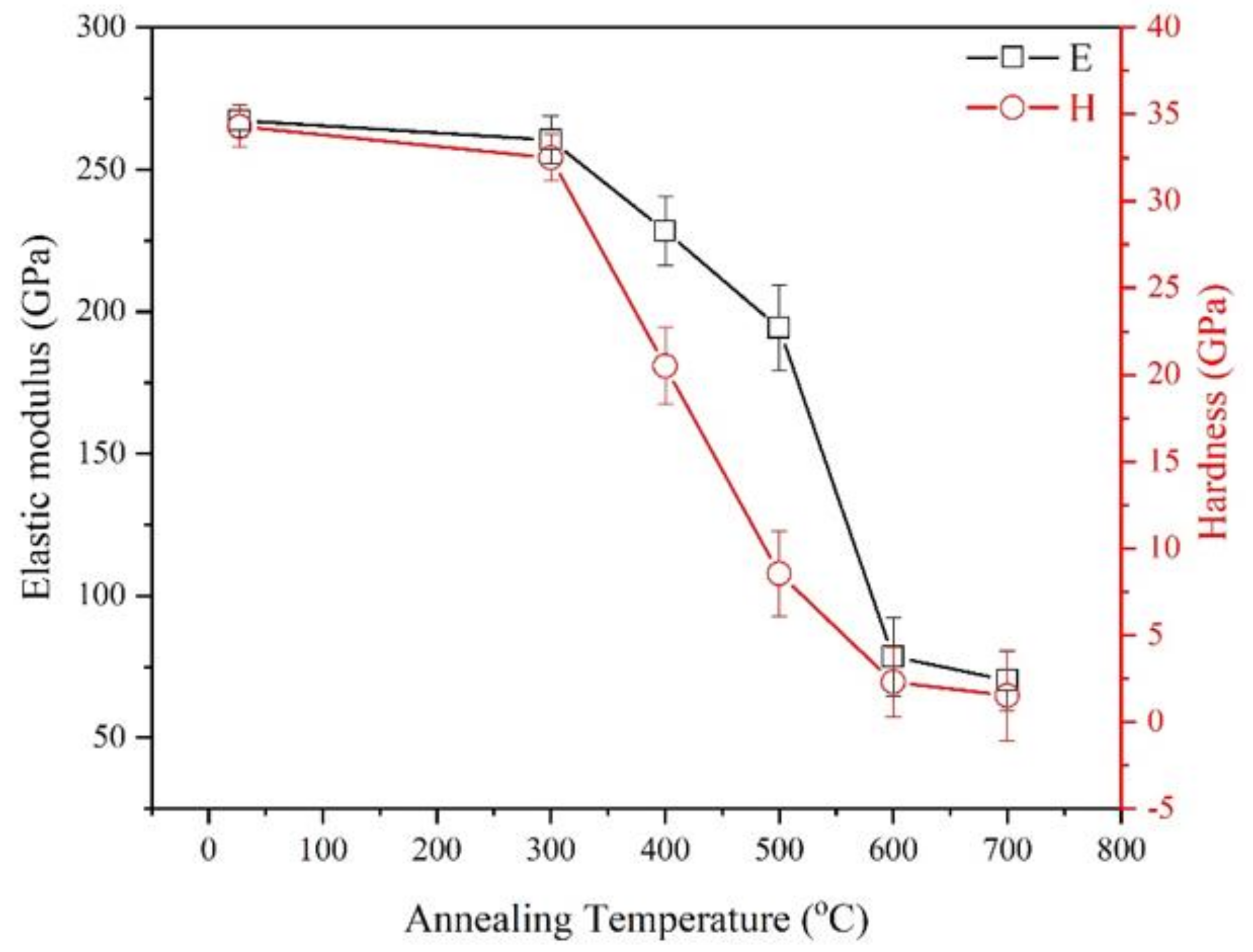
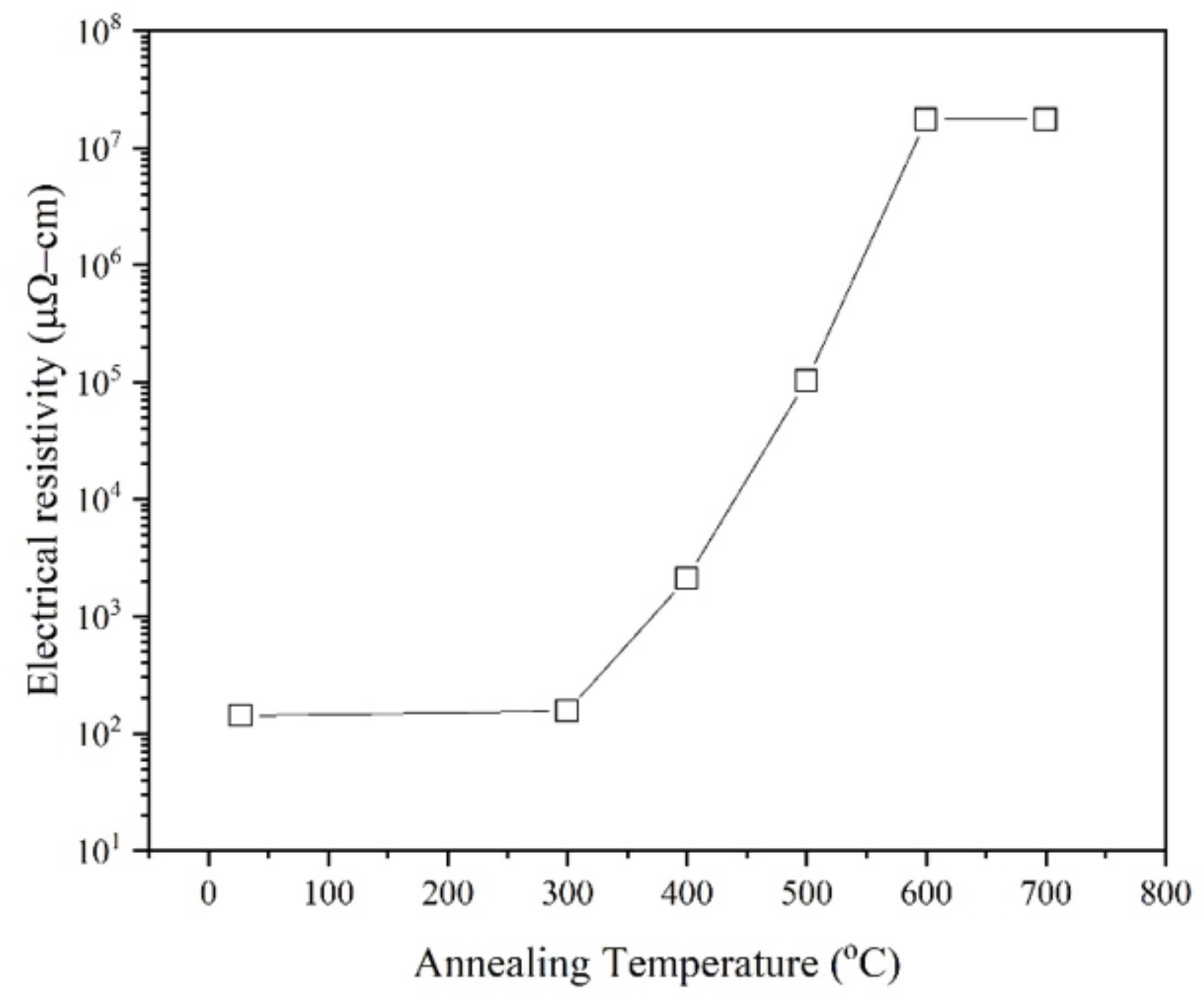
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murty, B.S.; Yeh, J.W.; Ranganathan, S. High-Entropy Alloys; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Gao, M.C.; Yeh, J.W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys Fundamentals and Applications; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Park, H.J.; Lim, K.S.; Hong, S.H.; Kim, K.B. Structural and Mechanical Properties of AlCoCrNi High Entropy Nitride Films: Influence of Process Pressure. Coatings 2020, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Park, H.J.; Mun, S.C.; Jumaev, E.; Hong, S.H.; Song, G.; Kim, J.T.; Park, Y.K.; Kim, K.S.; Jeong, S.I.; et al. Investigation of structure and mechanical properties of TiZrHfNiCuCo high entropy alloy thin films synthesized by magnetron sputtering. J. Alloys Compd. 2019, 797, 834–841. [Google Scholar] [CrossRef]
- Chang, Z.-C.; Tsai, D.-C.; Chen, E.-C. Effect of N2 flow on the structure and mechanical properties of (CrTaTiVZr)Nx coatings processed by reactive magnetron sputtering. J. Mater. Res. 2015, 30, 924–934. [Google Scholar] [CrossRef]
- Chang, Z.-C.; Tsai, D.-C.; Chen, E.-C. Structure and characteristics of reactive magnetron sputtered (CrTaTiVZr)N coatings. Mater. Sci. Semicond. Process. 2015, 39, 30–39. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Beresnev, V.M.; Smyrnova, K.V.; Kravchenko, Y.; Zukowski, P.V.; Bondarenko, G.G. The influence of nitrogen pressure on the fabrication of the two-phase superhard nanocomposite (TiZrNbAlYCr)N coatings. Mater. Lett. 2018, 211, 316–318. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.H.; Li, P.W.; Wu, Q.Q.; Wang, M.H.; Sung, C.C.; Hsu, C.-Y. Nanostructured and mechanical properties of high-entropy alloy nitride films prepared by magnetron sputtering at different substrate temperatures. Mater. Technol. 2018, 34, 343–349. [Google Scholar] [CrossRef]
- Chen, T.K.; Shun, T.T.; Yeh, J.W.; Wong, M.S. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2004, 188–189, 193–200. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, J.B.; Hou, C.; Li, J.C.; Jiang, Q. Dense and smooth amorphous films of multicomponent FeCoNiCuVZrAl high-entropy alloy deposited by direct current magnetron sputtering. Mater. Des. 2013, 46, 675–679. [Google Scholar] [CrossRef]
- Chang, Z.-C. Structure and properties of duodenary (TiVCrZrNbMoHfTaWAlSi)N coatings by reactive magnetron sputtering. Mater. Chem. Phys. 2018, 220, 98–110. [Google Scholar] [CrossRef]
- Shu, F.Y.; Liu, S.; Zhao, H.Y.; He, W.X.; Sui, S.H.; Zhang, J.; He, P.; Xu, B.S. Structure and high-temperature property of amorphous composite coating synthesized by laser cladding FeCrCoNiSiB high-entropy alloy powder. J. Alloys Compd. 2018, 731, 662–666. [Google Scholar] [CrossRef]
- Jin, G.; Cai, Z.; Guan, Y.; Cui, X.; Liu, Z.; Li, Y.; Dong, M.; Zhang, D. High temperature wear performance of laser-cladded FeNiCoAlCu high-entropy alloy coating. Appl. Surf. Sci. 2018, 445, 113–122. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Wang, L.; Liang, J.; Liu, C. Laser cladding FeCrCoNiTiAl high entropy alloy coatings reinforced with self-generated TiC particles. J. Laser Appl. 2016, 29, 012004. [Google Scholar] [CrossRef]
- Yao, C.; Wei, B.; Zhang, P.; Lu, X.; Liu, P.; Tong, Y. Facile preparation and magnetic study of amorphous Tm-Fe-Co-Ni-Mn multicomponent alloy nanofilm. J. Rare Earths 2011, 29, 133–137. [Google Scholar] [CrossRef]
- Li, Q.; Yue, T.; Guo, Z. Electro-spark Deposition of Multi-Element High Entropy Alloy Coating; ASM International: Almere, The Netherlands, 2010. [Google Scholar]
- Yin, S.; Li, W.; Song, B.; Yan, X.; Kuang, M.; Xu, Y.; Wen, K.; Lupoi, R. Deposition of FeCoNiCrMn high entropy alloy (HEA) coating via cold spraying. J. Mater. Sci. Technol. 2019, 35, 1003–1007. [Google Scholar] [CrossRef]
- Popov, V.V.; Katz-Demyanetz, A.; Koptyug, A.; Bamberger, M. Selective electron beam melting of Al0.5CrMoNbTa0.5 high entropy alloys using elemental powder blend. Heliyon 2019, 5, e01188. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Wang, Y.; Cui, X.; Jin, G.; Li, Y.; Liu, Z.; Dong, M. Design and microstructure characterization of FeCoNiAlCu high-entropy alloy coating by plasma cladding: In comparison with thermodynamic calculation. Surf. Coat. Technol. 2017, 330, 163–169. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Huang, Y.-L.; Lin, S.-R.; Jung, D.-R.; Chang, S.-Y.; Shieu, F.-S. Structure and mechanical properties of (TiVCr)N coatings prepared by energetic bombardment sputtering with different nitrogen flow ratios. J. Alloys Compd. 2011, 509, 3141–3147. [Google Scholar] [CrossRef]
- Abadias, G.; Koutsokeras, L.E.; Siozios, A.; Patsalas, P. Stress, phase stability and oxidation resistance of ternary Ti–Me–N (Me=Zr, Ta) hard coatings. Thin Solid Films 2013, 538, 56–70. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Refractory Carbides and Nitrides; Noyes Publications: Westwood, NJ, USA, 1996. [Google Scholar]
- Dean, J.A. Lange’s Handbook of Chemistry; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Rath, C.; Mohanty, P.; Pandey, A.C.; Mishra, N.C. Oxygen vacancy induced structural phase transformation in TiO2 nanoparticles. J. Phys. D Appl. Phys. 2009, 42, 205101. [Google Scholar] [CrossRef]
- Ghosh, T.B.; Dhabal, S.; Datta, A.K. Erratum: On crystalline size dependence of phase stability of nanocrystalline TiO2. Appl. Phys. 94, 4577 (2003). J. Appl. Phys. 2003, 95, 408. [Google Scholar] [CrossRef] [Green Version]
- Chou, P.-W.; Wang, Y.-S.; Lin, C.-C.; Chen, Y.-J.; Cheng, C.-L.; Wong, M.-S. Effect of carbon and oxygen on phase transformation of titania films during annealing. Surf. Coat. Technol. 2009, 204, 834–839. [Google Scholar] [CrossRef]
- Reidy, D.J.; Holmes, J.D.; Morris, M.A. The critical size mechanism for the anatase to rutile transformation in TiO2 and doped-TiO2. J. Eur. Ceram. Soc. 2006, 26, 1527–1534. [Google Scholar] [CrossRef]
- Batzill, M.; Morales, E.H.; Diebold, U. Influence of Nitrogen Doping on the Defect Formation and Surface Properties of TiO2 Rutile and Anatase. Phys. Rev. Lett. 2006, 96, 026103. [Google Scholar] [CrossRef]
- Ihara, T.; Miyoshi, M.; Iriyama, Y.; Matsumoto, O.; Sugihara, S. Visible-light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Appl. Catal. B Environ. 2003, 42, 403–409. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lu, F.-H. Oxidation behavior of titanium nitride films. J. Vac. Sci. Technol. A 2005, 23, 1006–1009. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-S.; Chen, L.; Lui, H.-W.; Cai, M.-H.; Yeh, J.-W. Microstructure, hardness, resistivity and thermal stability of sputtered oxide films of AlCoCrCu0.5NiFe high-entropy alloy. Mater. Sci. Eng. A 2007, 457, 77–83. [Google Scholar] [CrossRef]
- Unutulmazsoy, Y.; Merkle, R.; Fischer, D.; Mannhart, J.; Maier, J. The oxidation kinetics of thin nickel films between 250 and 500 °C. Phys. Chem. Chem. Phys. 2017, 19, 9045–9052. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, K.; Adachi, H.; Wasa, K. 6—Functional Thin Films. In Handbook of Sputtering Technology, 2nd ed.; Wasa, K., Kanno, I., Kotera, H., Eds.; William Andrew Publishing: Oxford, UK, 2012; p. 495. ISBN 978-1-4377-3483-6. [Google Scholar]
- Zou, C.W.; Yan, X.D.; Patterson, D.; Emanuelsson, E.A.C.; Bian, J.M.; Gao, W. Temperature sensitive crystallization of V2O5: From amorphous film to β-V2O5nanorods. CrystEngComm 2010, 12, 691–693. [Google Scholar] [CrossRef]
- Müller, F.; Gorr, B.; Christ, H.-J.; Müller, J.; Butz, B.; Chen, H.; Kauffmann, A.; Heilmaier, M. On the oxidation mechanism of refractory high entropy alloys. Corros. Sci. 2019, 159, 108161. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Chang, Z.-C.; Kuo, L.-Y.; Lin, T.-J.; Lin, T.-N.; Shiao, M.-H.; Shieu, F.-S. Oxidation resistance and structural evolution of (TiVCrZrHf)N coatings. Thin Solid Films 2013, 544, 580–587. [Google Scholar] [CrossRef]
- Ren, W.; Ouyang, F.; Ding, B.; Zhong, Y.; Yu, J.; Ren, Z.; Zhou, L. The influence of CrTaO4 layer on the oxidation behavior of a directionally-solidified nickel-based superalloy at 850–900 °C. J. Alloys Compd. 2017, 724, 565–574. [Google Scholar] [CrossRef]
- Ondik, H.; McMurdie, H.F. Phase Diagrams for Zirconium and Zirconia Systems; American Ceramic Society: Columbus, DC, USA, 2017. [Google Scholar]
- Xing, X.; Zhu, Z.; Qiu, X.; Liu, G. Zero-Thermal Expansion and Heat Capacity of Zirconium Pyrovanadate Doped with Zirconia and Vanadium (V) Oxide. Rare Met. 2001, 20, 1–4. [Google Scholar]
- Jantschner, O. Investigation on Vanadium Containing Zirconia Coatings for High Temperature Sliding Interfaces; University of Leoben: Leoben, Austria, 2011. [Google Scholar]
- Habel, D.; Stelzer, J.B.; Feike, E.; Schröder, C.; Hösch, A.; Hess, C.; Knop-Gericke, A.; Caro, J.; Schubert, H. Phase development in the catalytic system V2O5/TiO2 under oxidising conditions. J. Eur. Ceram. Soc. 2006, 26, 3287–3294. [Google Scholar] [CrossRef]
- Malan, W.D.; Akdogan, G.; Taskinen, P.; Hamuyuni, J.; Zietsman, J. Phase equilibria and thermodynamic evaluation of the Ti-V-O system in air. Calphad 2018, 63, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Tsai, D.-C.; Chang, Z.-C.; Kuo, B.-H.; Tsao, C.-T.; Chen, E.-C.; Shieu, F.-S. Influence of discharge power on the structural, electro-optical, and mechanical properties of (TiZrHf)N coatings. J. Alloys Compd. 2015, 622, 446–457. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Chang, Z.-C.; Kuo, B.-H.; Chen, B.-C.; Chen, E.-C.; Shieu, F.-S. Wide variation in the structure and physical properties of reactively sputtered (TiZrHf)N coatings under different working pressures. J. Alloys Compd. 2018, 750, 350–359. [Google Scholar] [CrossRef]

| Oxide | Cr2O3 | Ta2O5 | TiO2 | V2O5 | ZrO2 |
|---|---|---|---|---|---|
| ΔG (kJ·mol−1 O2) | 352.7 | 382.2 | 444.4 | 283.86 | 521.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Z.-C.; Liang, J.-Y. Oxidation Behavior and Structural Transformation of (CrTaTiVZr)N Coatings. Coatings 2020, 10, 415. https://doi.org/10.3390/coatings10040415
Chang Z-C, Liang J-Y. Oxidation Behavior and Structural Transformation of (CrTaTiVZr)N Coatings. Coatings. 2020; 10(4):415. https://doi.org/10.3390/coatings10040415
Chicago/Turabian StyleChang, Zue-Chin, and Jun-Yang Liang. 2020. "Oxidation Behavior and Structural Transformation of (CrTaTiVZr)N Coatings" Coatings 10, no. 4: 415. https://doi.org/10.3390/coatings10040415




