The Trends of TiZr Alloy Research as a Viable Alternative for Ti and Ti16 Zr Roxolid Dental Implants
Abstract
:1. Introduction
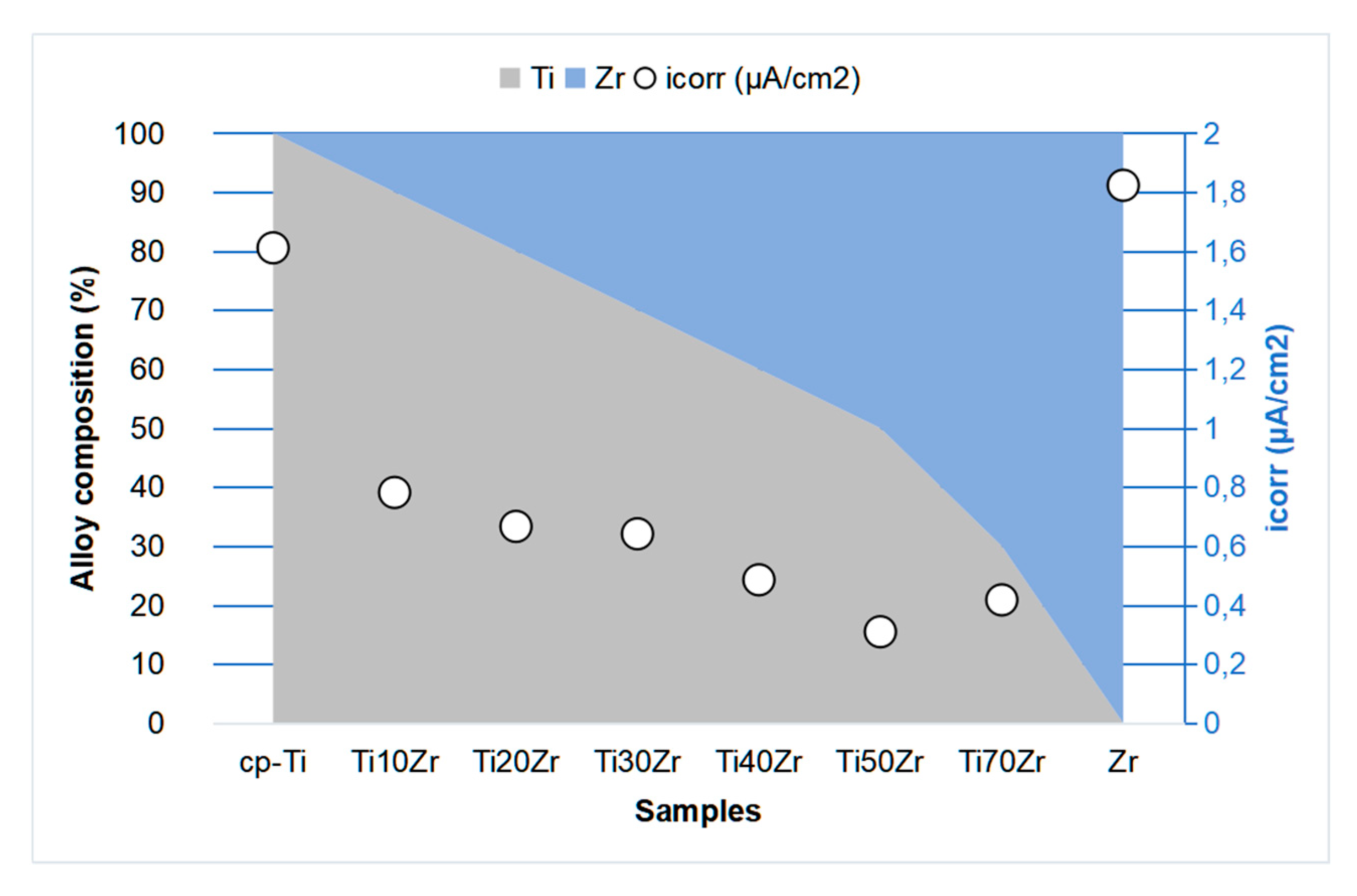
2. TixZr Alloys
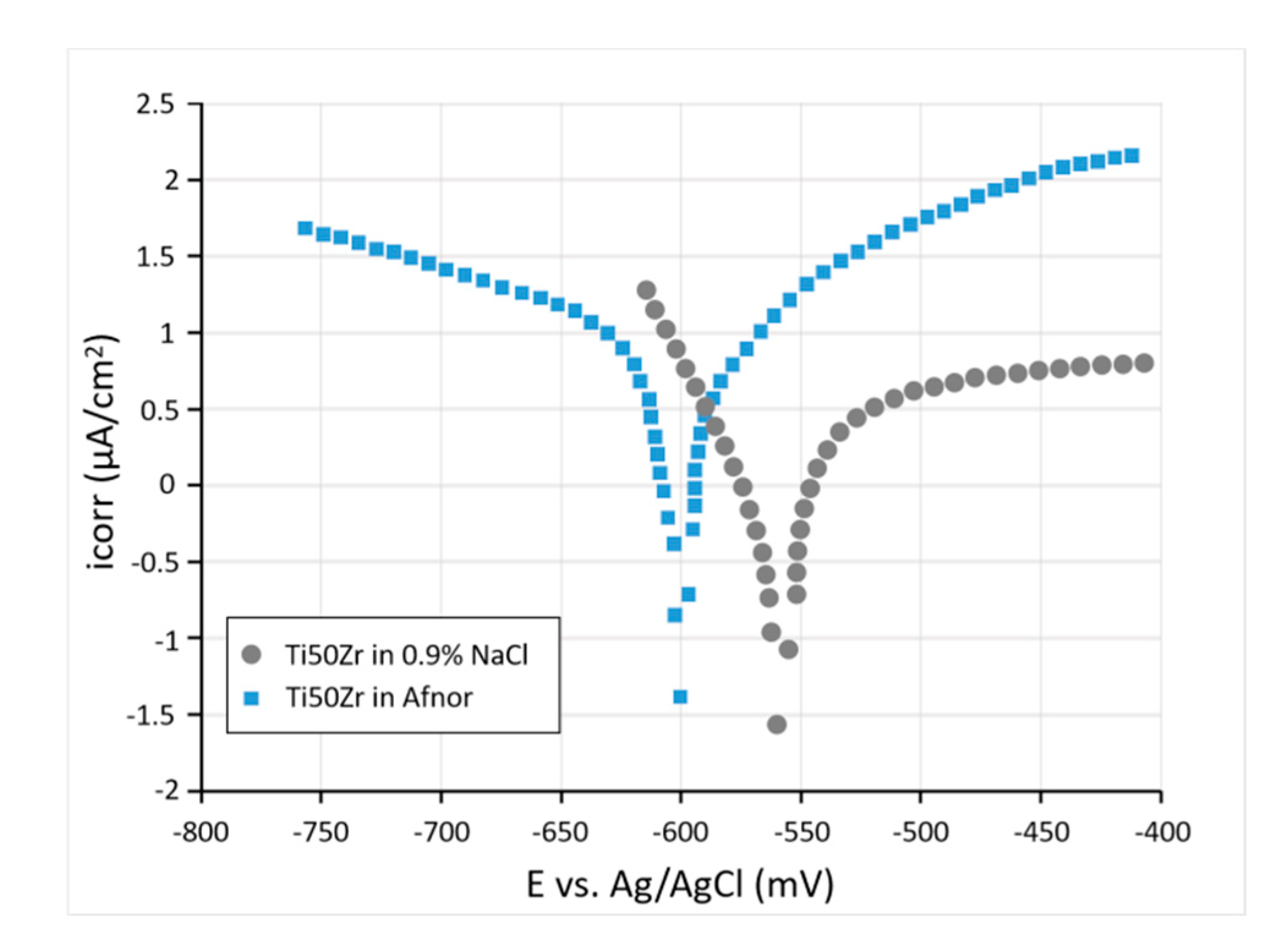
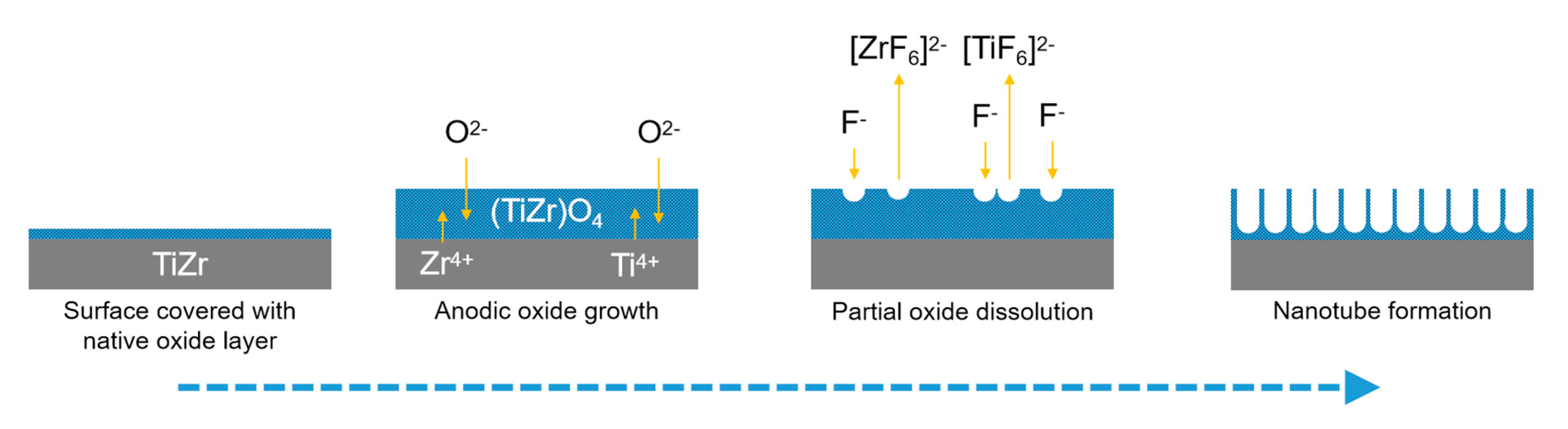
3. Ti50Zr Alloy Surface Modification
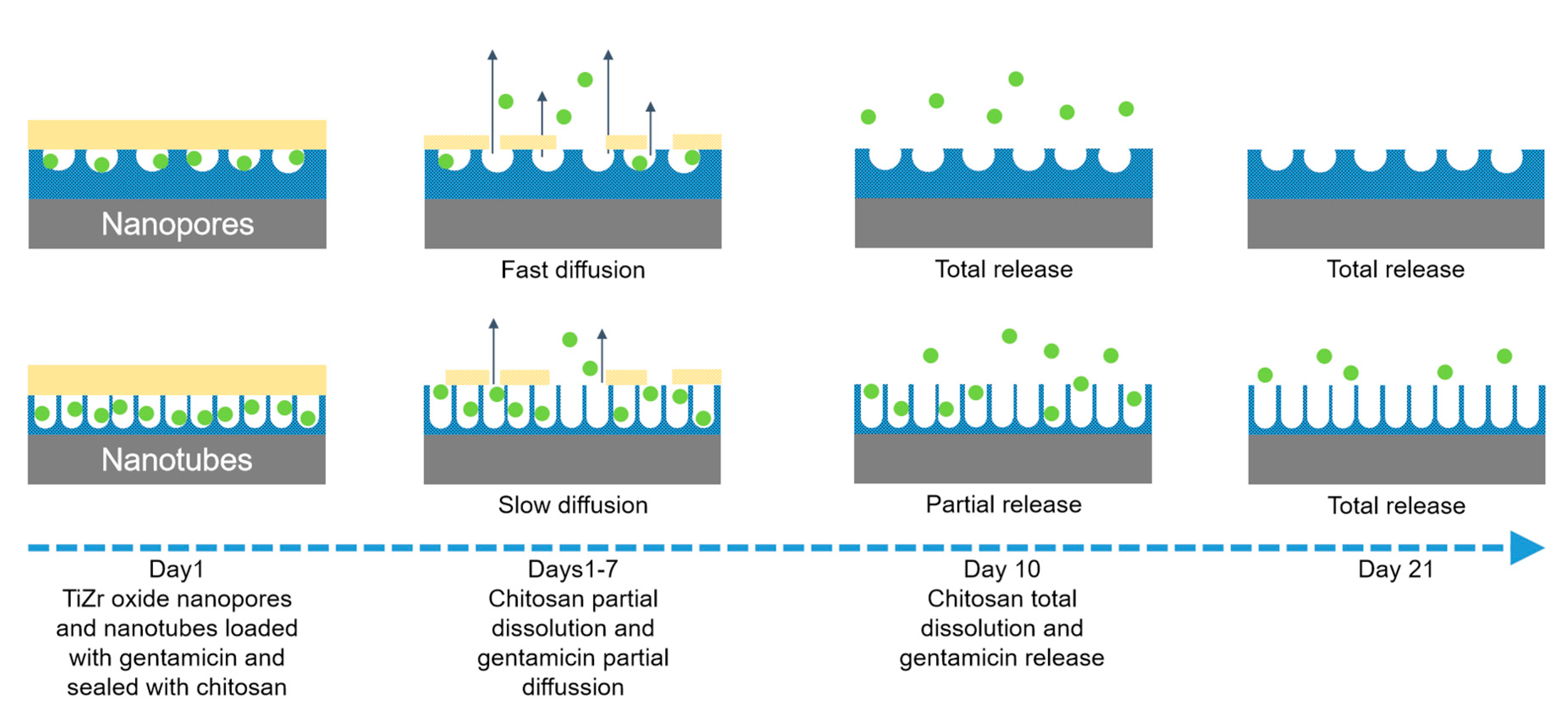
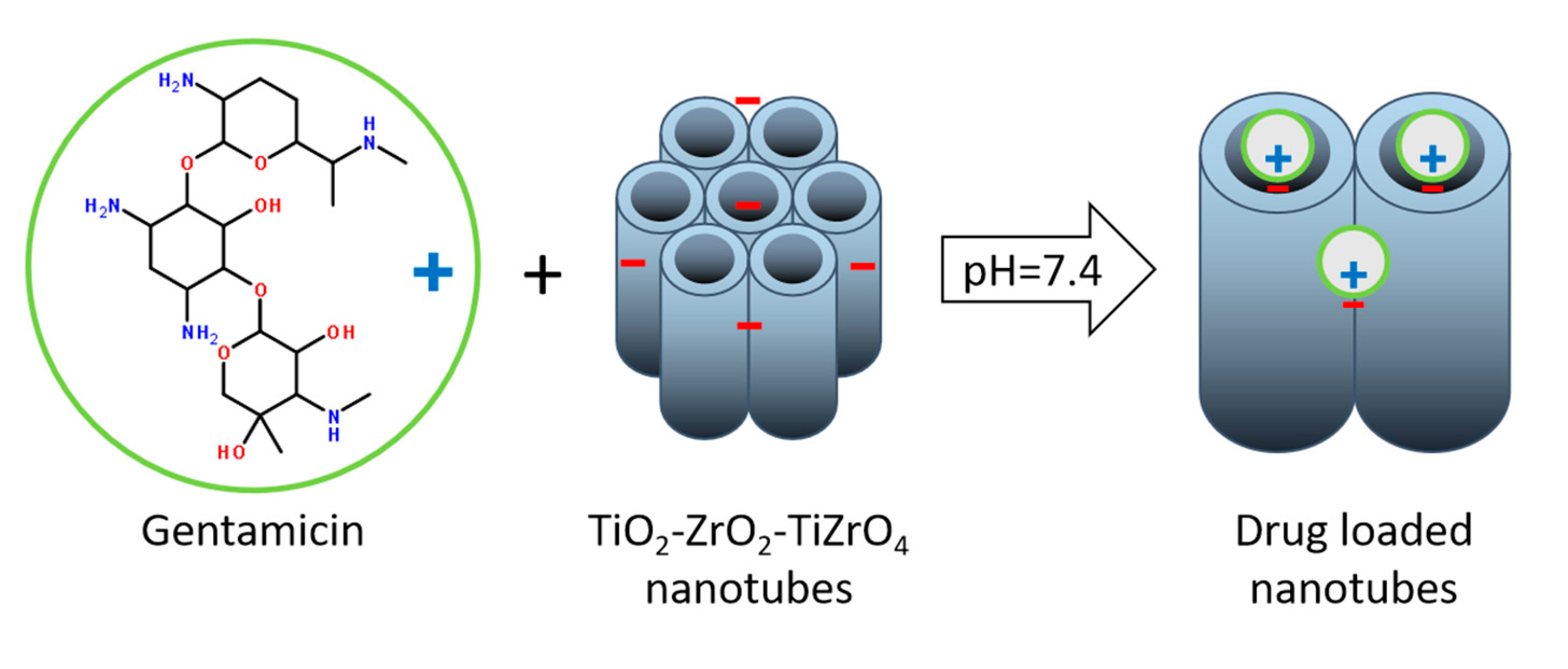
4. Drug Loading and Release from Anodized Ti50Zr
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, L.C.; Chen, L.Y. A review on biomedical titanium alloys: Recent progress and prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef] [Green Version]
- Daniela, I.; Man, I.; Demetrescu, I. The behaviour of electrochemical deposition of phosphate coating on CoCr bio alloys. Key. Eng. Mater. 2007, 330–332, 545–548. [Google Scholar] [CrossRef]
- Hanawa, T. Zirconia versus titanium in dentistry: A review. Dent. Mater. J 2019, 39, 24–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis-a review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linetskiy, I.; Demenko, V.; Linetska, L.; Yefremov, O. Impact of annual bone loss and different bone quality on dental implant success—A finite element study. Comput. Biol. Med. 2017, 91, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Bunoiu, I.; Andrei, M.; Scheau, C.; Manole, C.C.; Stoian, A.B.; Cioranu, V.; Didilescu, A. Electrochemical behavior of rejected dental implants in peri-implantitis. Coatings 2020, 10, 209. [Google Scholar] [CrossRef] [Green Version]
- Popa, M.; Vasilescu, E.; Drob, P.; Demetrescu, I.; Popescu, B.; Ionescu, D.; Vasilescu, C. In vitro assessment and monitoring of the implant titanium materials—Physiological environment interactions. Mater. Corros. 2003, 54, 215–221. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, S.; Tsoi, J.K.H.; Matinlinna, J.P. Binary titanium alloys as dental implant materials—a review. Regen. Biomater. 2017, 4, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Fisher, D.J. Additive Manufacturing of Metals. Mater. Res. Found. 2020, 64, 97–120. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Standard Specification for Wrought Titanium 6Al 4V ELI Alloy for Surgical Implants; ASTM Designation F136-82; ASTM: Philadelphia, PA, USA, 1994; pp. 19–20.
- Okazaki, Y.; Gotoh, E.; Manabe, T.; Kobayashi, K. Comparison of metal concentrations in rat tibia tissues with various metallic implants. Biomaterials 2004, 25, 5913–5920. [Google Scholar] [CrossRef] [PubMed]
- Standard Specification for Wrought Titanium 6Al 7Nb Alloy for Surgical Implants; ASTM Designation F1295-92; ASTM: Philadelphia, PA, USA, 1994; pp. 687–689.
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Bernhard, N.; Berner, S.; de Wild, M.; Wieland, M. The binary TiZr Alloy—A newly developed Ti alloy for the use in dental implants. Forum. Implantol. 2009, 5, 30–39. [Google Scholar]
- Jimbo, R.; Naito, Y.; Galli, S.; Berner, S.; Dard, M.; Wennerberg, A. Biomechanical and histomorphometrical evaluation of TiZr alloy implants: An in vivo study in the rabbit. Clin. Implant Dent. Relat. Res. 2015, 17, 670. [Google Scholar] [CrossRef] [PubMed]
- Grandin, H.M.; Berner, S.; Dard, M. A review of titanium zirconium (TiZr) alloys for use in endosseous dental implants. Materials 2012, 5, 1348–1360. [Google Scholar] [CrossRef] [Green Version]
- Gottlow, J.; Dard, M.; Kjellson, F.; Obrecht, M.; Sennerby, L. Evaluation of a new titanium-zirconium dental implant: A biomechanical and histological comparative study in the mini pig. Clin. Implant Dent. Relat. Res. 2012, 14, 538–545. [Google Scholar] [CrossRef]
- Wang, B.; Ruan, W.; Liu, J.; Zhang, T.; Hailin, Y.; Ruan, J. Microstructure mechanical properties, and preliminary biocompatibility evaluation of binary Ti–Zr alloys for dental application. J. Biomater. Appl. 2019, 33, 766–775. [Google Scholar] [CrossRef]
- Gottlow, J. (Ed.) Preclinical Data Presented at the 23rd Annual Meeting of the Academy of Osseointegration (AO); Straumann Holding AG: Boston, MA, USA; Basel, Switzerland, 2008; Available online: www.straumann.com (accessed on 20 April 2020).
- Araki, H.; Nakano, T.; Ono, S.; Yatani, H. Three dimensional finite element analysis of extra short implant focusing on implant designs and materials. Int. J. Implant Dent 2020, 29, 5. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.B.; Vardaki, M.; Ionita, D.; Enachescu, M.; Brancoveanu, O.; Demetrescu, I. Nanopores and nanotubes ceramic oxides elaborated on titanium alloy with zirconium by changing anodization by changing anodization potentials. Ceram. Int. 2018, 44, 7026–7033. [Google Scholar] [CrossRef]
- Iegami, C.M.; Uehara, P.N.; Sesma, N.; Pannuti, C.M.; Tortamano Neto, P.; Mukai, M.K. Survival rate of titanium-zirconium narrow diameter dental implants versus commercially pure diameter dental implants versus commercially pure titanium diameter dental implants. Clin. Implant Dent. Relat. Res. 2017, 19, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Habazaki, H.; Uozumi, M.; Konno, H.; Shimizu, K.; Nagata, S.; Asami, K.; Matsumoto, K.; Takayama, K.; Oda, Y.; Skeldon, P.; et al. Influences of structure and composition on growth of anodic oxide films on TiZr alloys. Electrochim. Acta 2003, 48, 3257–3266. [Google Scholar] [CrossRef]
- Akimoto, T.; Ueno, T.; Tsutsumi, Y.; Doi, H.; Hanawa, T.; Wakabayashi, N. Evaluation of corrosion resistance of implant-use Ti-Zr binary alloys with a range of compositions. J. Biomed. Mater. Res. B 2018, 106B, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Liu, Y. Fatigue behavior of Ti50Zr alloy for dental implant application. J Alloys Compd. 2019, 793, 212–219. [Google Scholar] [CrossRef]
- Mareci, D.; Bolat, G.; Chelariu, R.; Sutiman, D.; Munteanu, C. The estimation of corrosion behaviour of ZrTi binary alloys for dental applications using electrochemical techniques. Mater. Chem. Phys. 2013, 141, 362–369. [Google Scholar] [CrossRef]
- Ion, R.; Stoian, A.B.; Dumitriu, C.; Grigorescu, S.; Mazare, A.; Cimpean, A.; Demetrescu, I.; Schmuki, P. Nanochannels formed on TiZr alloy improve biological response. Acta Biomater. 2015, 24, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.; Mazare, A.; Dumitriu, C.; Pirvu, C.; Schmuki, P.; Cimpean, A. Nanochannelar topography positively modulates osteoblast differentiation and inhibits osteoclastogenesis. Coatings 2018, 8, 294. [Google Scholar] [CrossRef] [Green Version]
- Stoian, A.B.; Surdu-Bob, C.; Anghel, A.; Ionita, D.; Demetrescu, I. Investigation of High Voltage Anodic Plasma (HVAP) Ag-DLC coatings on Ti50Zr with different Ag amounts. Coatings 2019, 9, 792. [Google Scholar] [CrossRef] [Green Version]
- Gulec, F.; Sher, F.; Karaduman, A. Catalytic performance of Cu- and Zr-modified beta zeolite catalysts in the methylation of 2-methylnaphthalene. Pet. Sci. 2019, 16, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Zarren, G.; Nisar, B.; Sher, F. Synthesis of anthraquinone-based electroactive polymers: A critical review. Mater. Today Sust. 2019, 5, 100019. [Google Scholar] [CrossRef]
- Navarro Amador, R.; Carboni, M.; Meyer, D. Photosensitive titanium and zirconium Metal Organic Frameworks: Current research and future possibilities. Mater. Lett. 2016, 166, 327–338. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J. Dye-sensitized solar cells based on surficial TiO2 modification. Sol. Energy 2019, 184, 454–465. [Google Scholar] [CrossRef]
- Vardaki, M.; Mohajernia, S.; Pantazi, A.; Nica, I.C.; Enachescu, M.; Mazare, A.; Demetrescu, I.; Schmuki, P. Post treatments effect on TiZr nanostructures fabricated via anodizing. J. Mater. Res. Technol. 2019, 8, 5802–5812. [Google Scholar] [CrossRef]
- Ho, W.F.; Chen, W.K.; Wu, S.C.; Hsu, H.C. Structure, mechanical properties, and grindability of dental Ti-Zr alloys. J. Mater. Sci. Mater. Med. 2008, 19, 3179. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, A.; Vardaki, M.; Mihai, G.; Ionita, D.; Stoian, A.B.; Enachescu, M.; Demetrescu, I. Understanding surface and interface properties of modified Ti50Zr with nanotubes. Appl. Surf. Sci. 2020, 506, 144661. [Google Scholar] [CrossRef]
- Manole, C.C.; Dinischiotu, A.; Nica, I.C.; Demetrescu, I.; Pirvu, C. Influence of electrospun TiO2 nanowires on corrosion resistance and cell response of Ti50Zr alloy. Mater. Corros. 2018, 69, 1609–1619. [Google Scholar] [CrossRef]
- Stoian, A.B.; Demetrescu, I.; Ionita, D. Nanotubes and nano pores with chitosan construct on TiZr serving as drug reservoir. Colloids Surf. B Biointerfaces 2020, 185, 110535. [Google Scholar] [CrossRef]
- Demetrescu, I.; Dumitriu, C.; Totea, G.; Nica, I.C.; Dinischiotu, A.; Ionita, D. Zwitterionic cysteine drug coating influence in functionalization of implantable Ti50Zr Alloy for antibacterial, biocompatibility and stability properties. Pharmaceutics 2018, 10, 220. [Google Scholar] [CrossRef] [Green Version]
- Vardaki, M.; Ionita, D.; Stoian, A.B.; Demetrescu, I. Increasing corrosion resistance of a ZrTi alloy with a bioinspired coating with low porosity. Mater. Corros. 2017, 68, 988–994. [Google Scholar] [CrossRef]
- Bolat, G.; Izquierdo, J.; Santana, J.; Mareci, D.; Souto, R.M. Electrochemical characterization of ZrTi alloys for biomedical applications. Electrochim. Acta 2013, 88, 447–456. [Google Scholar] [CrossRef]
- Kim, W.G.; Choe, H.C.; Ko, Y.M.; Brantley, W.A. Nanotube morphology changes for Ti-Zr alloys as Zr content increases. Thin Solid Films 2009, 517, 5033–5037. [Google Scholar] [CrossRef]
- Calderon Moreno, J.M.; Popa, M.; Ivanescu, S.; Vasilescu, C.; Drob, S.I.; Neacsu, E.I.; Popa, M.V. Microstructure, mechanical properties, and corrosion resistance of Ti-20Zr alloy in undoped and NaF doped artificial saliva. Met. Mater. Int. 2014, 20, 177–187. [Google Scholar] [CrossRef]
- Zatkalíkov, V.; Palcek, P.; Markovicova, L.; Chalupov, M. Analysis of fractured screw shaped Ti6Al4V dental implant. Mater. Today: Proc. 2016, 3, 1216–1219. [Google Scholar] [CrossRef]
- Croitoru, S.M.; Popovici, I.A. R&D on dental implants breakage. Appl. Surf. Sci. 2017, 417, 262–268. [Google Scholar] [CrossRef]
- Barbosa, C.; Nascimento, J.L.; Centeno, R.O.; Caminha, I.M.V.; Abud, I.C. Failure analysis of titanium-based dental implant. J. Fail. Anal. Prev. 2010, 10, 138–142. [Google Scholar] [CrossRef]
- Hernandez-Rodriguez, M.A.L.; Contreras-Hernandez, G.R.; Juarez-Hernandez, A.; Beltran-Ramirez, B.; Garcia-Sanchez, E. Failure analysis in a dental implant. Eng. Fail. Anal. 2015, 57, 236–242. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Dentistry-Implants-Dynamic Loading Test for Endosseous Dental Implants; ISO 14801:2016; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Sista, S.; Wen, C.; Hodgson, P.D.; Pande, G. The influence of surface energy of titanium-zirconium alloy on osteoblast cell functions in vitro. J. Biomed. Mater. Res. A 2011, 97a, 27. [Google Scholar] [CrossRef]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, P. Corrosion of titanium: Part 1: Aggressive environments and main forms of degradation. J. Appl. Biomater. Func. 2017, 15, E291–E302. [Google Scholar] [CrossRef]
- Zielinski, A.; Sobieszczyk, S. Corrosion of Titanium biomaterials, mechanisms, effects and modelisation. Corros. Rev. 2008, 26, 1–22. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wang, Q.T.; Zhao, Y.M.; Guo, T.W.; Li, Z.C.; Hornez, J.C.; Hildebrand, H.F.; Tralsnel, M. Electrochemical Behavior of Titanium Alloys under Biological Conditions. Rare Metal Mat. Eng. 2004, 33, 19–22. [Google Scholar]
- Siddiqui, D.A.; Guida, L.; Sridhar, S.; Valderrama, P.; Wilson, T.G.; Rodrigues, D.C. Evaluation of oral microbial corrosion on the surface degradation of dental implant materials. J. Periodontol. 2019, 90, 72–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumitriu, C.; Popescu, S.; Ungureanu, C.; Pirvu, C. Antibacterial efficiencies of TiO2 nanostructured layers prepared in organic viscous electrolytes. Appl. Surf. Sci. 2015, 341, 157–165. [Google Scholar] [CrossRef]
- Mindroiu, M.; Pirvu, C.; Galateanu, B.; Demetrescu, I. Corrosion behaviour and cell viability of untreated and laser treated Ti6Al7Nb alloys. Rev. Chim. –Buchar. 2014, 65, 328–334. [Google Scholar]
- Pirvu, C.; Demetrescu, I.; Drob, P.; Vasilescu, E.; Ivanescu, S.; Mindroiu, M.; Vasilescu, C.; Drob, S.I. Corrosion behaviour of a new Ti-6Al-2Nb-1Ta alloy in various solutions. Mater. Corros. 2011, 62, 948–955. [Google Scholar] [CrossRef]
- Demetrescu, I.; Pirvu, C.; Mitran, V. Effect of nano-topographical features of Ti/TiO2 electrode surface on cell response and electrochemical stability in artificial saliva. Bioelectrochemistry 2010, 79, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Demetrescu, I.; Ionita, D.; Pirvu, C.; Portan, D. Present and future trends in TiO2 nanotubes elaboration, characterization and potential applications. Mol. Cryst. Liq. Cryst. 2010, 521, 195–203. [Google Scholar] [CrossRef]
- Manole, C.C.; Pirvu, C.; Demetrescu, I. Evaluation of TiO2 nanotubes changes after ultrasonication treatment. Mol. Cryst. Liq. Cryst. 2010, 521, 84–92. [Google Scholar] [CrossRef]
- Manole, C.C.; Pirvu, C.; Demetrescu, I. TiO2: From nanotubes to nanopores by changing the anodizing voltage in floride-glycerol electrolyte. Key. Eng. Mater. 2009, 415, 5–8. [Google Scholar] [CrossRef]
- Man, I.; Pirvu, C.; Demetrescu, I. Enhancing titanium stability in Fusayama saliva using electrochemical elaboration of TiO2 nanotubes. Rev. Chim. -Buchar. 2008, 59, 615–617. [Google Scholar] [CrossRef]
- Halley, I.; Ciosmak, D.; Lallemant, M.; Claude, J.M. Oxydation des alliages TiZr sous air et sous oxygéne. III: Particularités morphologiques de l’interface substrat/oxyde de l’alliage Ti52Zr48. J. Alloy Compd. 1994, 215, 35–44. [Google Scholar] [CrossRef]
- Halleydemoulin, I.; Ciosmak, D.; Lallemant, M. Oxydation des alliages TiZr sous air et sous oxygène II. Rôle de la composition des alliages sur l’évolution cinétique et morphologique du titane au zirconium. J. Alloy Compd. 1994, 204, 133–143. [Google Scholar] [CrossRef]
- Wen, C.E.; Yamada, Y.; Hodgson, P.D. Fabrication of novel TiZr alloy foams for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1439–1444. [Google Scholar] [CrossRef]
- Chen, X.B.; Nouri, A.; Hodgson, P.D.; Wen, C.E. Surface modification of TiZr alloy for biomedical application. Adv. Mater. Res. 2007, 15, 89–94. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Huo, W.; Zhang, W.; Zhao, Y.; Zhang, Y. Electrochemical corrosion characteristics and biocompatibility of nanostructured titanium for implants. Appl. Surf. Sci. 2018, 434, 63–72. [Google Scholar] [CrossRef]
- Chen, X.B.; Nouri, A.; Li, Y.C.; Lin, J.G.; Hodgson, P.D.; Wen, C.E. Effect of surface roughness of Ti, Zr, and TiZr on apatite precipitation from simulated body fluid. Biotechnol. Bioeng. 2008, 101, 378–387. [Google Scholar] [CrossRef]
- Wen, C.E.; Xu, W.; Hu, W.Y.; Hodgson, P.D. Hydroxiapatite /titania solgel coatings on titanium zirconia alloys for biomeddical applications. Acta Biomater. 2007, 3, 403–410. [Google Scholar] [CrossRef]
- Vardaki, M.; Prodana, M.; Stoian, A.B.; Ionita, D. Corrosion and bioactivity of a bioinspired coating on TiZr alloys. Sci. Bull. Univ. Politeh. Buchar. 2018, 80, 209–217. [Google Scholar]
- Ma, J.; Wong, H.; Kong, L.B.; Peng, K.W. Biomimetic processing nanocrystalline apatite coatings on titanium zirconium alloy. Nanotechnology 2003, 14, 619–623. [Google Scholar] [CrossRef]
- Minagar, S.; Berndt, C.; Gengenbach, T.; Wen, C. Fabrication and characterization of TiO2–ZrO2 –ZrTiO4 nanotubes on TiZr alloy manufactured via anodization. J. Mater. Chem. B 2014, 2, 71–83. [Google Scholar] [CrossRef]
- Minagar, S.; Li, Y.; Berndt, C.C.; Wen, C. The influence of Titania-Zirconia-Zirconium titanate nanotube characteristics on osteoblast cell adhesion. Acta Biomater. 2014, 12, 281–289. [Google Scholar] [CrossRef]
- Yun, K.D.; Yang, Y.; Lim, H.P.; Oh, G.J.; Koh, J.T.; Bae, I.H.; Kim, J.; Lee, K.M.; Park, S.W. Effect of nanotubular-micro-roughened titanium surface on cell response in vitro and osseointegration in vivo. Mater. Sci. Eng. C 2010, 30, 27–33. [Google Scholar] [CrossRef]
- Sista, S.; Nouri, A.; Li, Y.; Wen, C.; Hodgson, P.D.; Pande, G. Cell biological responses of osteoblasts on anodized nanotubular surface of a titanium—zirconium alloy. J. Biomed. Mater. Res. A 2013, 101, 3416–3430. [Google Scholar] [CrossRef] [PubMed]
- Jha, H.; Hahn, R.; Schmuki, P. Ultrafast oxide nanotube formation on TiNb, TiZr and TiTa alloys by rapid breakdown anodization. Electrochim. Acta 2010, 55, 8883–8887. [Google Scholar] [CrossRef]
- Grigorescu, S.; Pruna, V.; Titorencu, I.; Jinga, V.; Mazare, A.; Schmuki, P.; Demetrescu, I. The two step nano tube formation on TiZr as scaffolds for cell growth. Bioelectrochem 2014, 98, 39–45. [Google Scholar] [CrossRef]
- Grigorescu, S.; Ungureanu, C.; Kirchgeorg, R.; Schmuki, P.; Demetrescu, I. Various sized nanotubes on TiZr for antibacterial surfaces. Appl. Surf. Sci. 2013, 270, 190–196. [Google Scholar] [CrossRef]
- Murphy, M.; Walczak, M.S.; Thomas, A.G.; Silikas, N.; Berner, S.; Lindsay, R. Toward optimizing dental implant performance: Surface characterization of Ti and TiZr implant materials. Dent. Mater. 2017, 33, 43–53. [Google Scholar] [CrossRef]
- Cordeiro, M.; Pantaroto, H.N.; Paschoaleto, E.M.; Rangel, E.C.; da Cruz, N.C.; Sukotjo, C.; Barao, V.A.R. Synthesis of biofunctional coating for a TiZr alloy: Surface, electrochemical, and biological characterizations. Appl. Surf. Sci. 2018, 452, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Kaluderovic, M.R.; Krajnovic, T.; Maksimovic-Ivanic, D.; Graf, H.L.; Mijatovic, S. Ti-SLActive and TiZr-SLActive dental implant surfaces promote fast osteoblast differentiation. Coatings 2017, 7, 102. [Google Scholar] [CrossRef]
- Yin, L.H.; Chang, Y.R.; You, Y.H.; Liu, C.; Li, J.; Lai, H.C. Biological responses of human bone mesenchymal stem cells to Ti and TiZr implant materials. Clin. Implant Dent. R 2019, 21, 550–564. [Google Scholar] [CrossRef]
- Roy, S.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Agew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Macak, J.; Hildebrand, H.; Marten-Jahns, U.; Schmuki, P. Mechanistic aspects and growth of large diameter self-organized TiO2 nanotubes. J. Electroanal. Chem. 2008, 621, 254–266. [Google Scholar] [CrossRef]
- Gulati, K.; Kogawa, M.; Prideaux, M.; Findlay, D.M.; Atkins, G.J.; Losic, D. Drug-releasing nanoengineered titanium implants: Therapeutics efficacy in 3D cell culture model, controlled release and stability. Mater. Sci. Eng. C 2016, 69, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Maher, S.; Chandrasekaran, S.; Findlay, D.M.; Losic, D. Conversion of titania (TiO2) in conductive titanium (Ti) nanotube arrays for combined drug-delivery and electrical simulation therapy. J. Mater. Chem. B 2015, 14, 371–375. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained—action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Lindner, W.D.; Lippold, B.C. Drug release from hydrocolloid embeddings with high or low susceptibility to hydrodynamic stress. Pharm. Res. 1995, 12, 1781–1785. [Google Scholar] [CrossRef]
- Popat, K.; Eltgroth, M.; LaTempa, T.; Grimes, C.; Desai, T. Titania nanotubes: A novel platform for drug-eluting coatings for medical implants? Small 2007, 3, 1878–1881. [Google Scholar] [CrossRef]
- Peng, L.; Mendelsohn, A.; LaTempa, T.; Yoriya, S.; Grimes, C.; Desai, T. Long-term small molecule and protein elution from TiO2 nanotubes. Nano Lett. 2009, 9, 1932–1936. [Google Scholar] [CrossRef]
- Nemati, S.H.; Hadjizadeh, A. Gentamicin-eluting titanium dioxide nanotubes grown on the ultrafine-grained titanium. AAPS Pharm. Sci. Tech. 2017, 18, 2180–2187. [Google Scholar] [CrossRef]
- Aw, M.; Gulati, K.; Losic, D. Controlling drug release from titania nanotube arrays using polymer nanocarriers and biopolymer coating. J. Biomater. Nanobiotechnol. 2011, 2, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Kumeria, T.; Mon, H.; Aw, M.S.; Gulati, K.; Santos, A.; Griesser, H.J.; Losic, D. Advanced biopolymer-coated drug-releasing titania nanotubes (TNTs) implants with simultaneously enhanced osteoblast adhesion and antibacterial properties. Colloid. Surf. B: Biointerfaces 2015, 1, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Vasilev, K.; Cook, J.; Griesser, H.J. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Devices 2009, 6, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Schmidt-Stein, F.; Bauer, S.; Schmuki, P. Amphiphilic TiO2 nanotube arrays: An actively controllable drug delivery system. J. Am. Chem. Soc. 2009, 131, 4230–4232. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Okada, S.; Wakiguchi, H.; Kudo, K.; Kittaka, S.; Hara, M.; Furukawa, S. Cysteine, Histidine and Glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin. Exp. Immunol. 2012, 167, 269–274. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionita, D.; Pirvu, C.; Stoian, A.B.; Demetrescu, I. The Trends of TiZr Alloy Research as a Viable Alternative for Ti and Ti16 Zr Roxolid Dental Implants. Coatings 2020, 10, 422. https://doi.org/10.3390/coatings10040422
Ionita D, Pirvu C, Stoian AB, Demetrescu I. The Trends of TiZr Alloy Research as a Viable Alternative for Ti and Ti16 Zr Roxolid Dental Implants. Coatings. 2020; 10(4):422. https://doi.org/10.3390/coatings10040422
Chicago/Turabian StyleIonita, Daniela, Cristian Pirvu, Andrei Bogdan Stoian, and Ioana Demetrescu. 2020. "The Trends of TiZr Alloy Research as a Viable Alternative for Ti and Ti16 Zr Roxolid Dental Implants" Coatings 10, no. 4: 422. https://doi.org/10.3390/coatings10040422
APA StyleIonita, D., Pirvu, C., Stoian, A. B., & Demetrescu, I. (2020). The Trends of TiZr Alloy Research as a Viable Alternative for Ti and Ti16 Zr Roxolid Dental Implants. Coatings, 10(4), 422. https://doi.org/10.3390/coatings10040422








