Icariin/Aspirin Composite Coating on TiO2 Nanotubes Surface Induce Immunomodulatory Effect of Macrophage and Improve Osteoblast Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Specimen Preparation and Surface Characterization
2.2.1. TiO2 Nanotubes Fabrication
2.2.2. Construction of Icariin/Aspirin Composite Coating on the TiO2 Nanotubes Surface
2.2.3. Surface Characterization
2.3. Icariin and Aspirin Drug Release Amount Measurement
2.4. Behaviors of RAW 264.7 Cells Cultured on Different Surfaces
2.4.1. Cell Culture of Macrophage Cells
2.4.2. Proinflammatory (M1) and Proregenerative (M2) Marker Gene Expression
2.4.3. Enzyme-Linked Immunosorbent (ELISA) Assay
2.5. Behaviors of MC3T3-E1 Cells on Various Surfaces in Conditioned Medium (CM)
2.5.1. Collection and Preparation of CM
2.5.2. Cell Culture of Osteoblast Cells
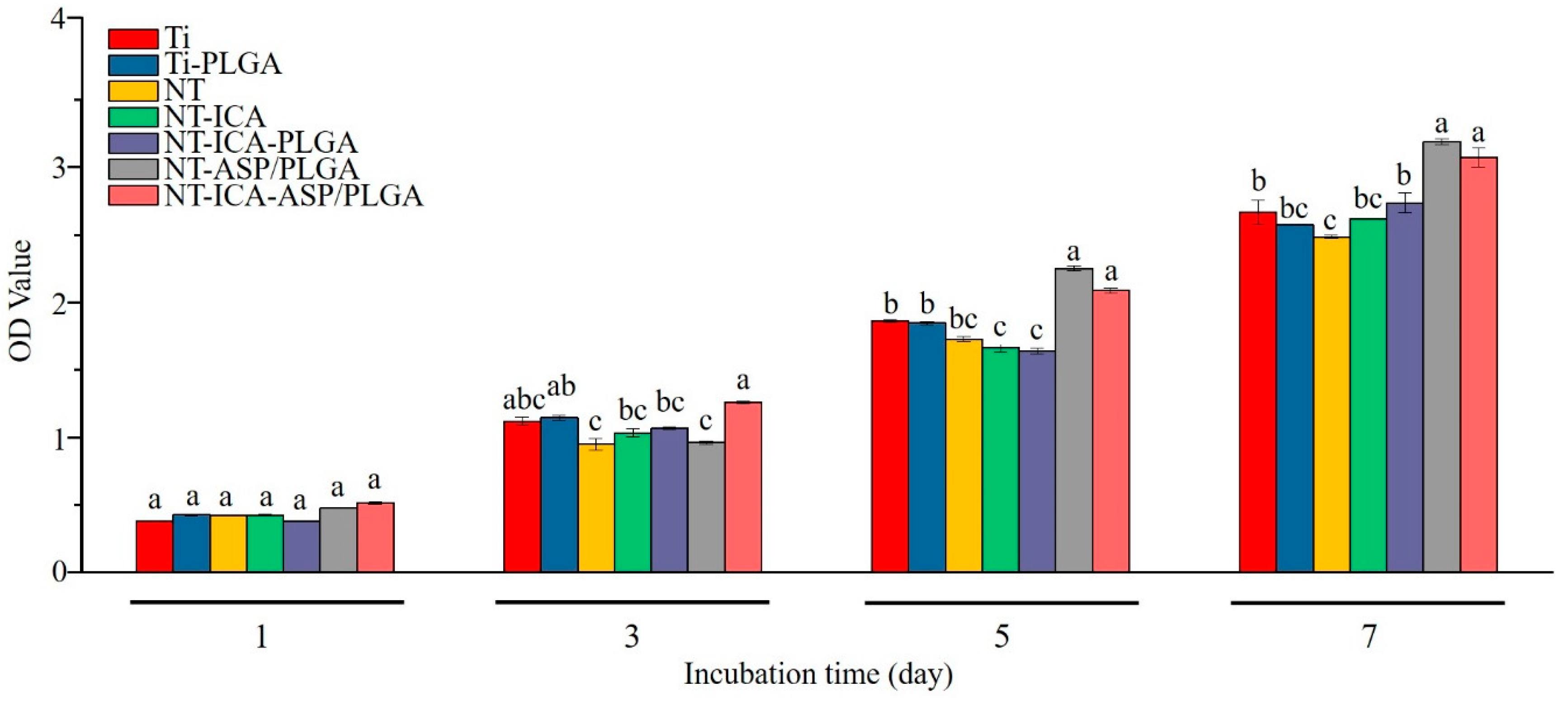
2.5.3. Cell Proliferation
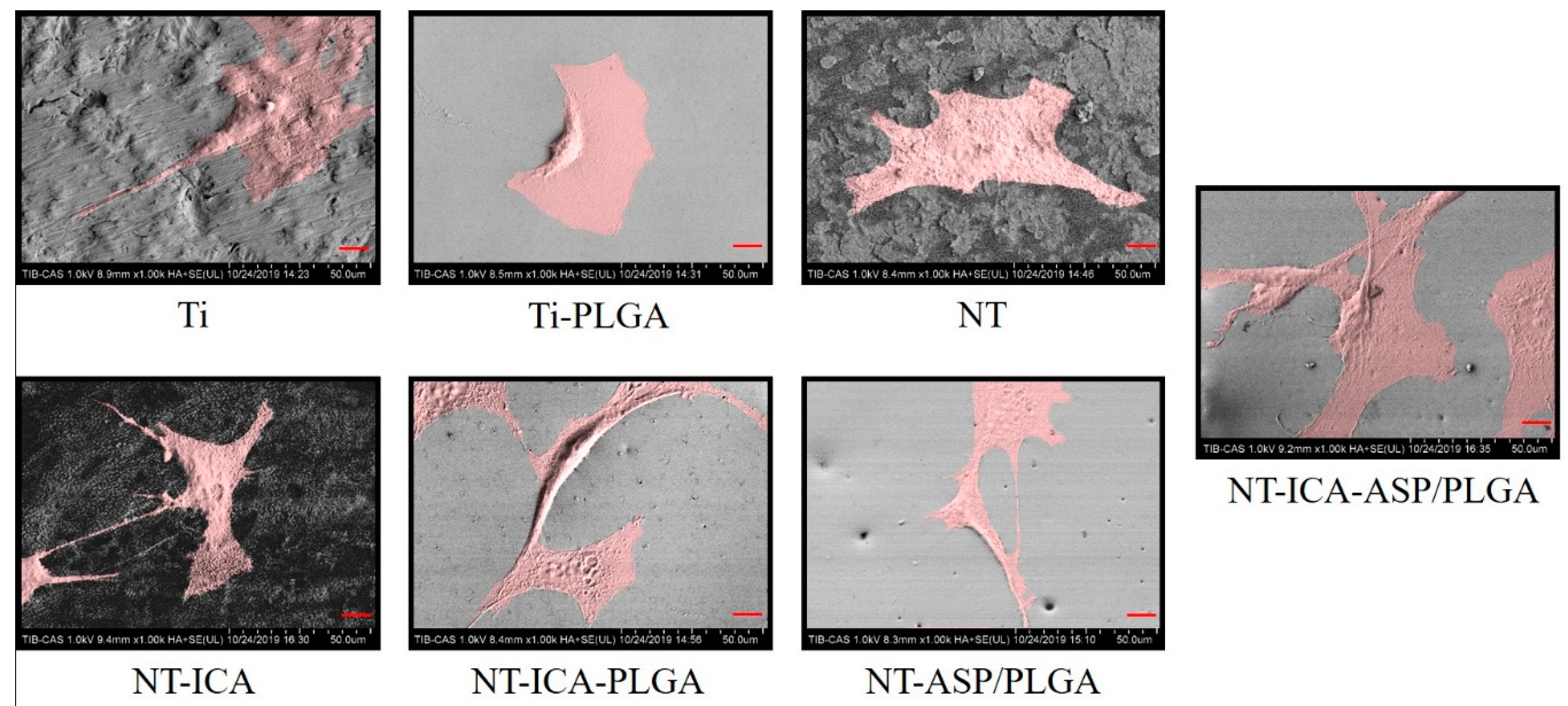
2.5.4. Cell Morphology
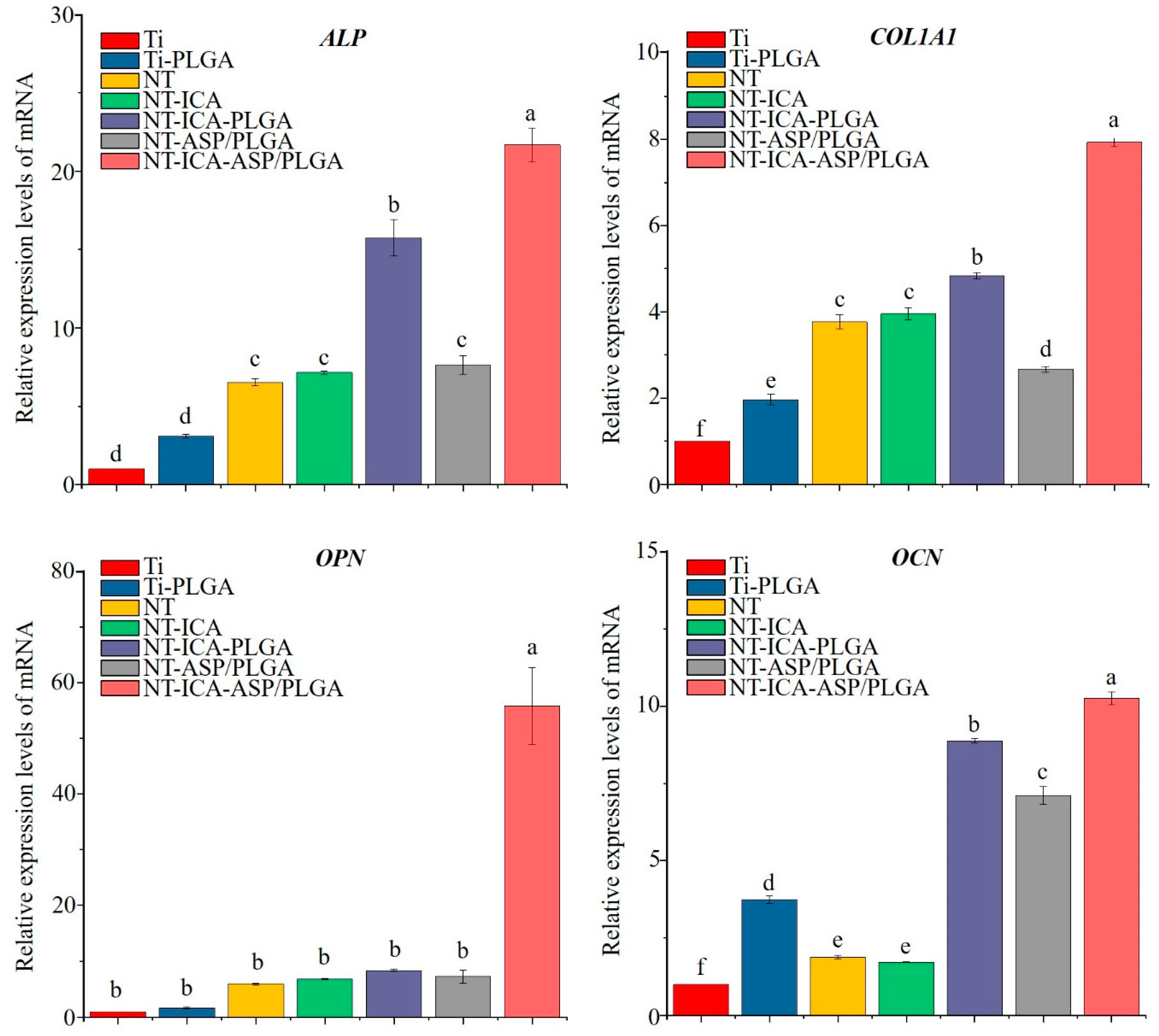
2.5.5. Osteogenic-Related Gene Expression
2.5.6. Western Blot Test
2.6. Statistical Analysis
3. Results
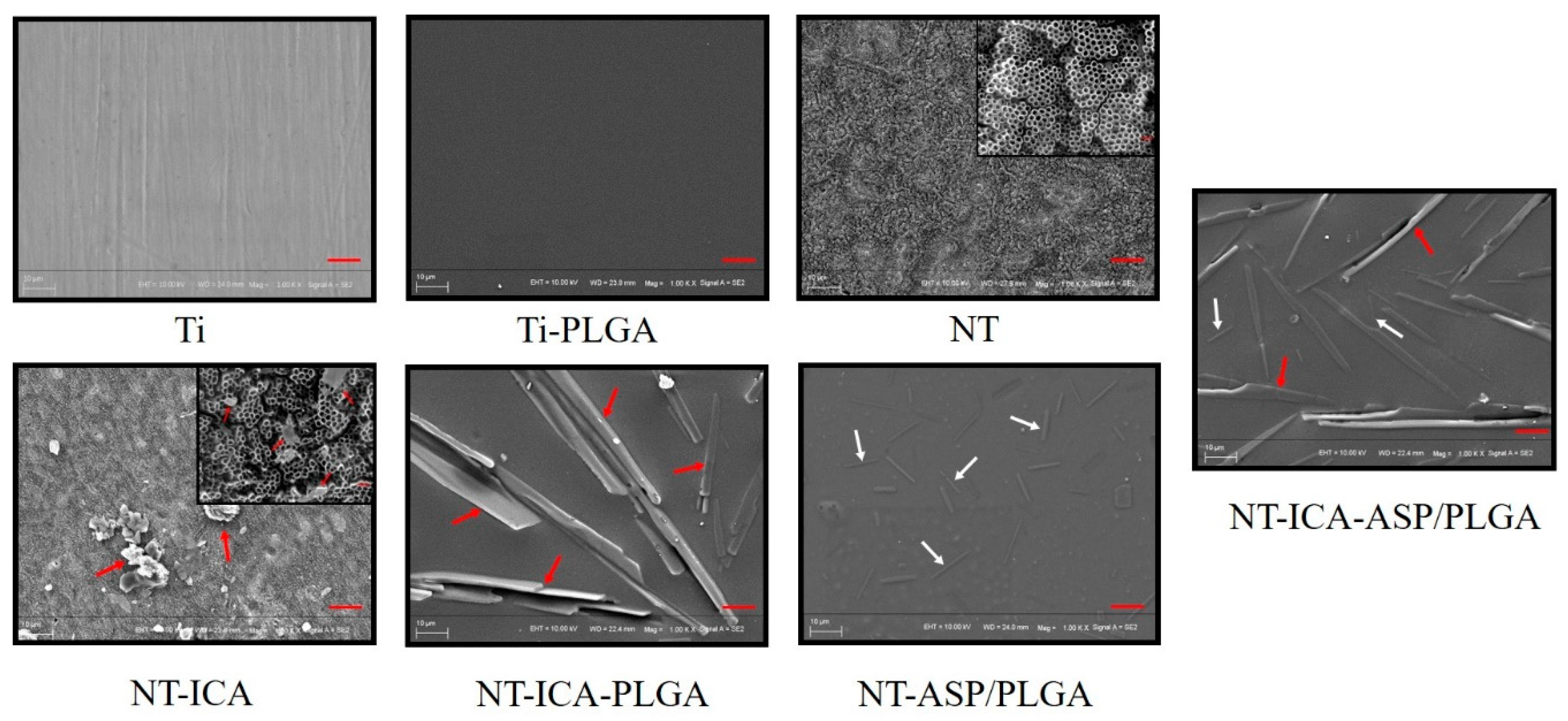
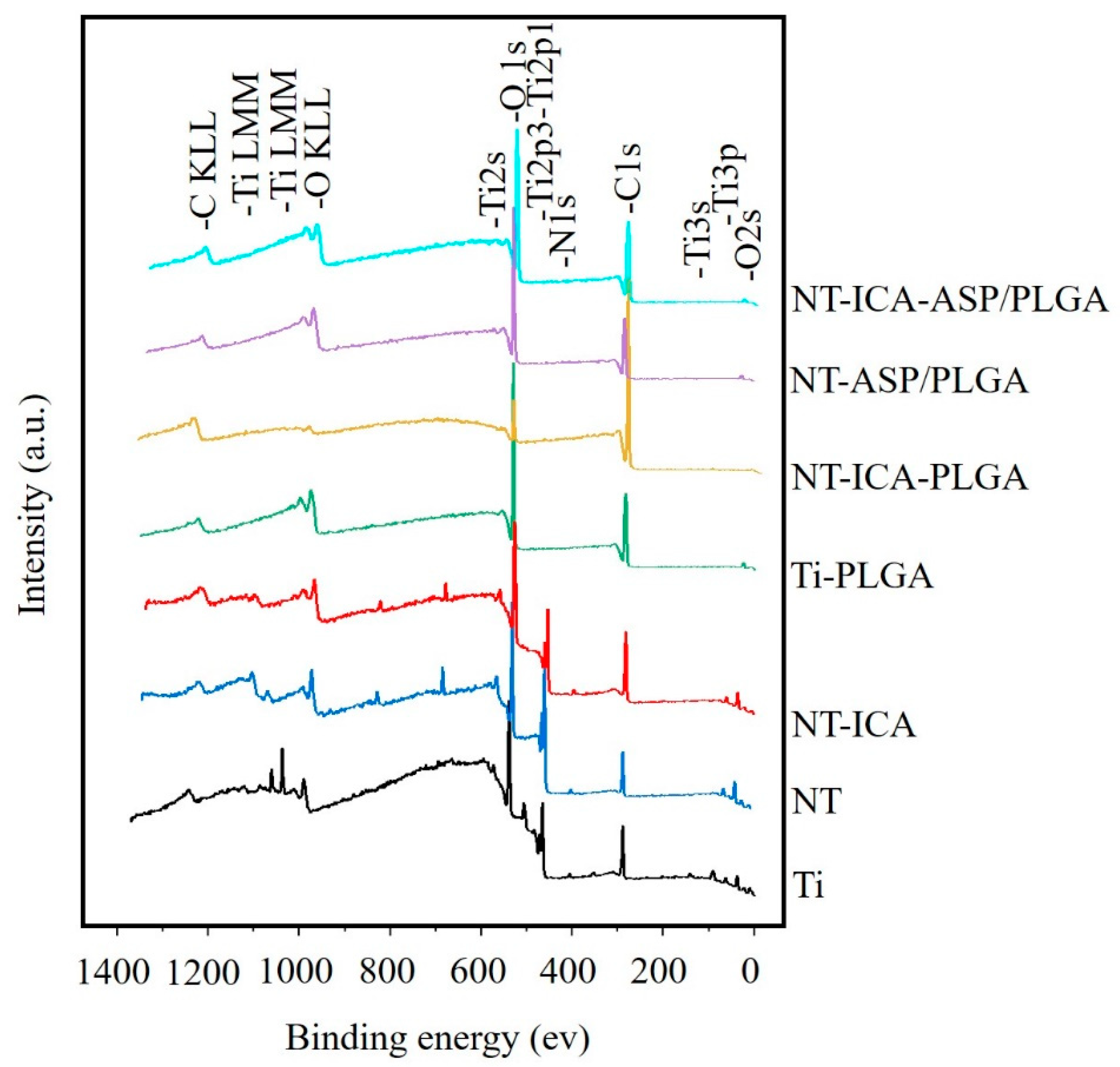
3.1. Surface Characterization
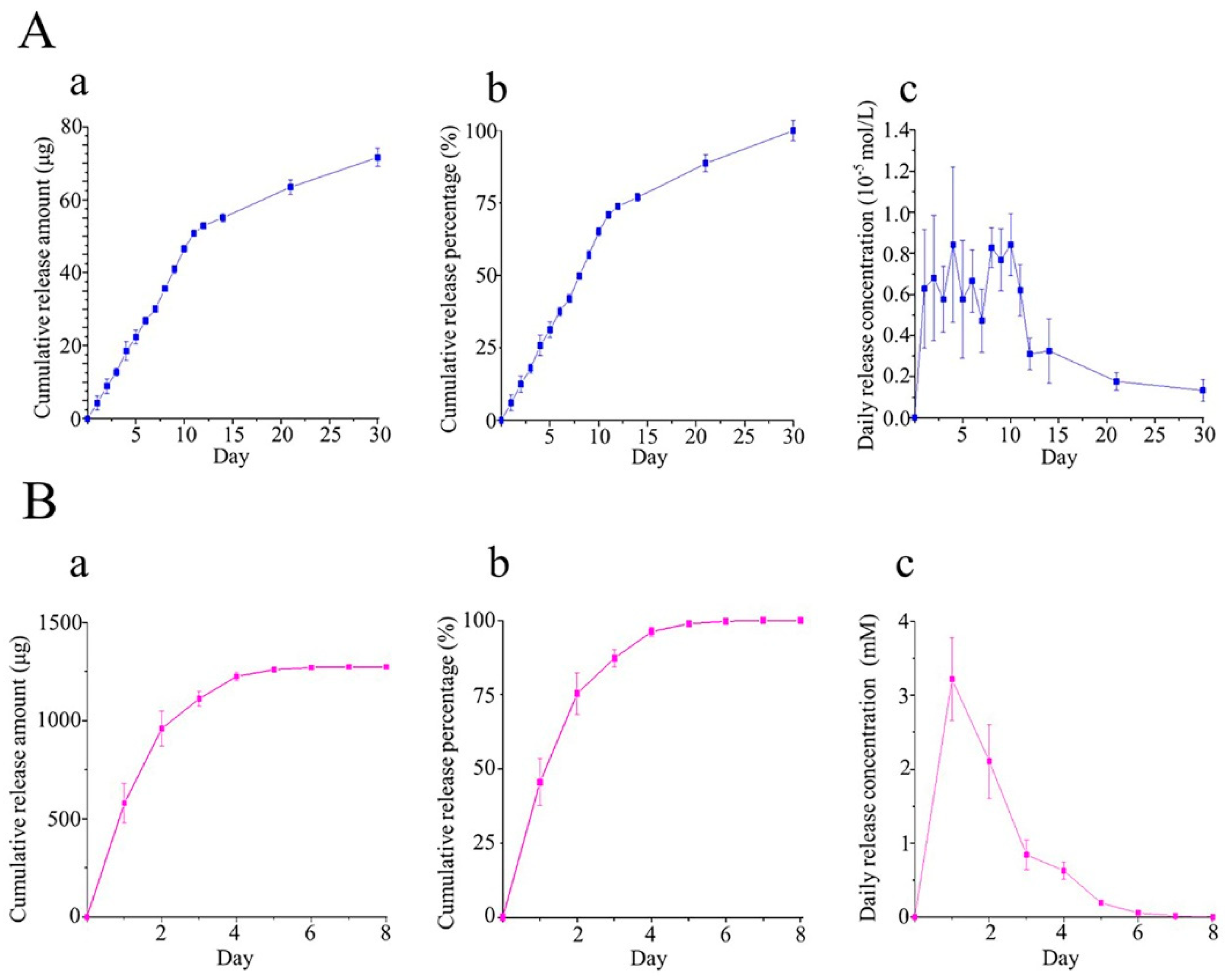
3.2. In Vitro Drug Release Profile
3.3. Polarization Status of RAW 264.7 Cells Cultured on Different Surfaces
3.3.1. Proinflammatory (M1) and Proregenerative (M2) Marker Genes Expression
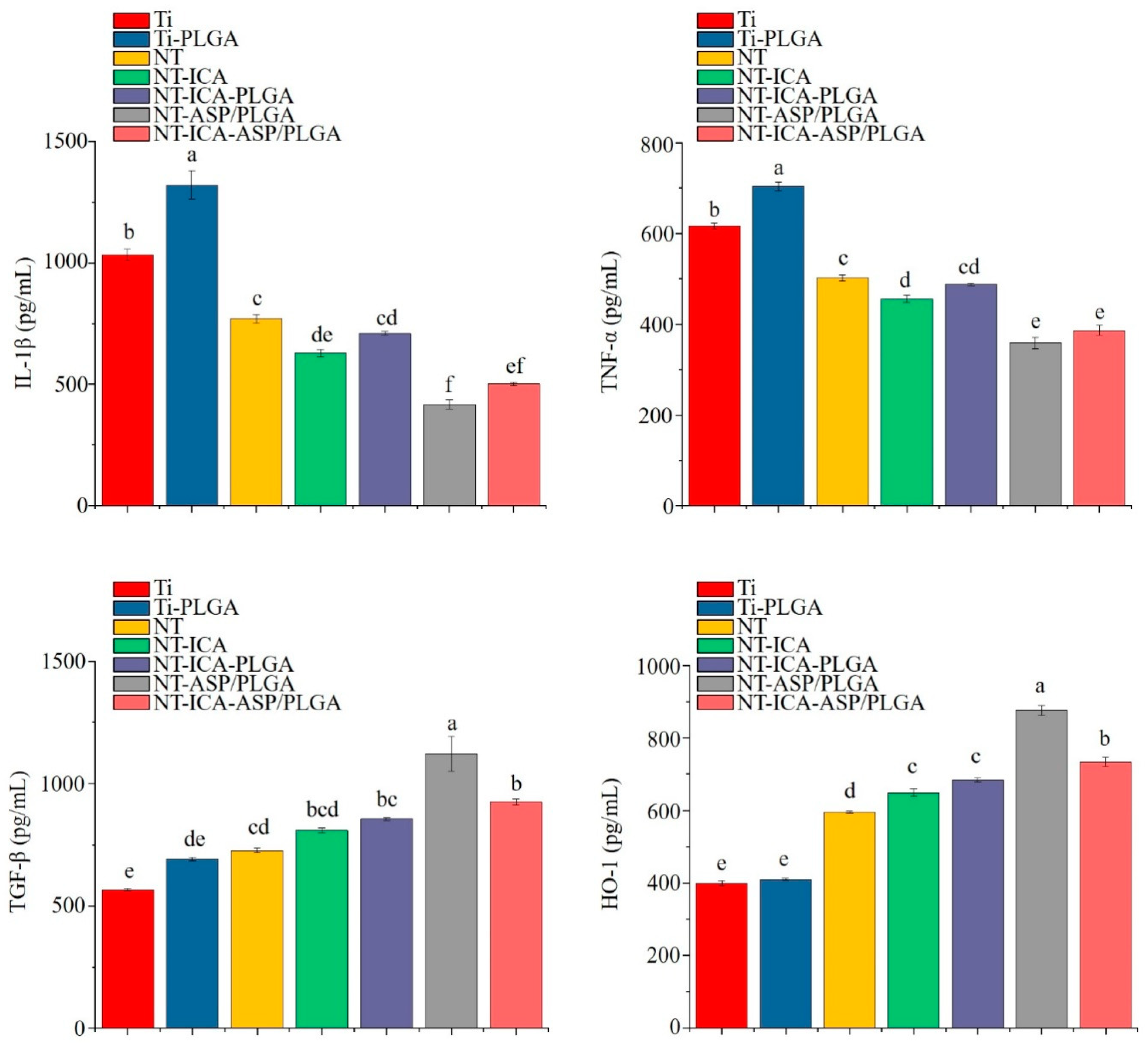
3.3.2. Enzyme-Linked Immunosorbent (ELISA) Assay
3.4. Behaviors of MC3T3-E1 Cells on Various Surfaces in Conditioned Medium (CM)
3.4.1. Cell Proliferation
3.4.2. Cell Morphology
3.4.3. Osteogenesis-Related Gene Expression
3.4.4. Western Blot Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.V.; Bose, S.; Bandyopadhyay, A. Low stiffness porous Ti structures for load-bearing implants. Acta Biomater. 2007, 3, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.V.; Vinoth-Kumar, L.; Anitha, V.C.; Manzoor, K.; Deepthy, M.; Shantikumar, V.N. Osteointegration of titanium implant is sensitive to specific nanostructure morphology. Acta Biomater. 2012, 8, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.C.; Zhou, X.D.; Yu, H.Y.; Wu, Y.; Bao, C.Y.; Man, Y.; Cheng, L.; Sun, Y. Advances in titanium dental implant surface modification. Hua Xi Kou Qiang Yi Xue Za Zhi 2019, 37, 124–129. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.; Ma, A.; Li, C. Surface Immobilization of TiO2 Nanotubes with Bone Morphogenetic Protein-2 Synergistically Enhances Initial Preosteoblast Adhesion and Osseointegration. BioMed Res. Int. 2019, 2019, 5697250. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; Garcia, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.; Cai, K.; Zhao, L.; Chen, X.; Hou, Y.; Yang, Z. Surface functionalization of TiO2 nanotubes with bone morphogenetic protein 2 and its synergistic effect on the differentiation of mesenchymal stem cells. Biomacromolecules 2011, 12, 1097–1105. [Google Scholar] [CrossRef]
- Ren, L.; Pan, S.; Li, H.; Li, Y.; He, L.; Zhang, S.; Che, J.; Niu, Y. Effects of aspirin-loaded graphene oxide coating of a titanium surface on proliferation and osteogenic differentiation of MC3T3-E1 cells. Sci. Rep. 2018, 8, 15143. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.V.; Puleo, D.A. Infection, inflammation, and bone regeneration: A paradoxical relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Huang, Y.; Wismeijer, D.; Liu, Y. Icariin: Does it have an osteoinductive potential for bone tissue engineering? Phytother. Res. 2014, 28, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ma, A.; Ning, J.; Zhong, X.; Zhang, Q.; Zhang, X.; Hong, G.; Li, Y.; Sasaki, K.; Li, C. Loading icariin on titanium surfaces by phase-transited lysozyme priming and layer-by-layer self-assembly of hyaluronic acid/chitosan to improve surface osteogenesis ability. Int. J. Nanomed. 2018, 13, 6751–6767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Shu, T.; Kang, L.; Wu, J.; Xing, J.; Lu, Z.; Chen, S.; Lv, J. Icaritin, a novel plant-derived osteoinductive agent, enhances the osteogenic differentiation of human bone marrow- and human adipose tissue-derived mesenchymal stem cells. Int. J. Mol. Med. 2017, 39, 984–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.N.; Zhou, J.; Ge, B.F.; Zhen, P.; Ma, H.P.; Shi, W.G.; Cheng, K.; Xian, C.J.; Chen, K.M. Icariin induces osteoblast differentiation and mineralization without dexamethasone in vitro. Planta Med. 2013, 79, 1501–1508. [Google Scholar] [CrossRef]
- Song, L.; Zhao, J.; Zhang, X.; Li, H.; Zhou, Y. Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur. J. Pharmacol. 2013, 714, 15–22. [Google Scholar] [CrossRef]
- Zhao, J.; Ohba, S.; Komiyama, Y.; Shinkai, M.; Chung, U.I.; Nagamune, T. Icariin: A potential osteoinductive compound for bone tissue engineering. Tissue Eng. Part A 2010, 16, 233–243. [Google Scholar] [CrossRef]
- Ma, A.; Shang, H.; Song, Y.; Chen, B.; You, Y.; Han, W.; Zhang, X.; Zhang, W.; Li, Y.; Li, C. Icariin-Functionalized Coating on TiO2 Nanotubes Surface to Improve Osteoblast Activity In Vitro and Osteogenesis Ability In Vivo. Coatings 2019, 9, 327. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wu, J.; Chen, X.; Fortenbery, N.; Eksioglu, E.; Kodumudi, K.N.; Pk, E.B.; Dong, J.; Djeu, J.Y.; Wei, S. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int. Immunopharmacol. 2011, 11, 890–898. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.; Shen, J.; Wang, M.; Cui, J.; Wang, Y.; Zhu, S.; Zhang, W.; Yang, H.; Xu, Y.; Geng, D. Icariin protects against titanium particle-induced osteolysis and inflammatory response in a mouse calvarial model. Biomaterials 2015, 60, 92–99. [Google Scholar] [CrossRef]
- Chi, L.; Gao, W.; Shu, X.; Lu, X. A natural flavonoid glucoside, icariin, regulates Th17 and alleviates rheumatoid arthritis in a murine model. Mediat. Inflamm. 2014, 2014, 392062. [Google Scholar] [CrossRef]
- Derry, S.; Moore, R.A. Single dose oral aspirin for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2012, 18, CD002067. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xiong, J.; Wu, T.; Tang, Z.; Ding, G.; Zhang, C.; Wang, S.; Liu, Y. Aspirin treatment improved mesenchymal stem cell immunomodulatory properties via the 15d-PGJ2/PPARgamma/TGF-beta1 pathway. Stem Cells Dev. 2014, 23, 2093–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Xiong, J.; Mei, S.; Wang, F.; Zhao, Z.; Wang, S.; Liu, Y. Aspirin promotes bone marrow mesenchymal stem cell-based calvarial bone regeneration in mini swine. Stem Cell Res. 2015, 6, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Pan, M.; Gao, Y.; Zhang, L.; Ge, W.; Tang, P. Dose-dependent roles of aspirin and other non-steroidal anti-inflammatory drugs in abnormal bone remodeling and skeletal regeneration. Cell Biosci. 2019, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Yamaza, T.; Akiyama, K. Is aspirin treatment an appropriate intervention to osteoporosis? Future Rheumatol. 2008, 3, 499–502. [Google Scholar] [CrossRef] [Green Version]
- Yamaza, T.; Miura, Y.; Bi, Y.; Liu, Y.; Akiyama, K.; Sonoyama, W.; Patel, V.; Gutkind, S.; Young, M.; Gronthos, S.; et al. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS ONE 2008, 3, e2615. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Li, Y.; Bai, Y.; Pan, J.; Wang, H.; Li, H.; Xu, X.; Fu, X.; Shi, R.; Luo, Z.; et al. A hybrid 3D-printed aspirin-laden liposome composite scaffold for bone tissue engineering. J. Mater. Chem. B 2018, 7. [Google Scholar] [CrossRef]
- Maher, S.; Mazinani, A.; Barati, M.R.; Losic, D. Engineered titanium implants for localized drug delivery: Recent advances and perspectives of Titania nanotubes arrays. Expert Opin. Drug Deliv. 2018, 15, 1021–1037. [Google Scholar] [CrossRef]
- Ahn, T.K.; Lee, D.H.; Kim, T.S.; Jang, G.C.; Choi, S.; Oh, J.B.; Ye, G.; Lee, S. Modification of Titanium Implant and Titanium Dioxide for Bone Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1077, 355–368. [Google Scholar] [CrossRef]
- Gulati, K.; Ivanovski, S. Dental implants modified with drug releasing titania nanotubes: Therapeutic potential and developmental challenges. Expert Opin. Drug Deliv. 2017, 14, 1009–1024. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Uludag, H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning and functionalization for improved performance. Biomater. Sci. 2018, 6, 1312–1338. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, J.Y.; Li, H.Q.; Chen, Z.; Zhao, A.Z.; Wang, Y.; Zhang, K.Q.; Sun, H.T.; Al-Deyab, S.S.; Lai, Y.K. TiO2 nanotube platforms for smart drug delivery: A review. Int. J. Nanomed. 2016, 11, 4819–4834. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Pagels, R.F.; Prud’homme, R.K. Polymeric nanoparticles and microparticles for the delivery of peptides, biologics, and soluble therapeutics. J. Control. Release 2015, 219, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Meng, F.; Song, W.; Jin, J.; Ma, Q.; Fei, D.; Fang, L.; Chen, L.; Wang, Q.; Zhang, Y. Nanostructured titanium regulates osseointegration via influencing macrophage polarization in the osteogenic environment. Int. J. Nanomed. 2018, 13, 4029–4043. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Liu, X.; Xiong, Z.; Huang, Q.; Yang, X.; Yan, H.; Ma, J.; Feng, Q.; Shen, Z. The immunomodulatory effects of Zn-incorporated micro/nanostructured coating in inducing osteogenesis. Artif. CellsNanomed. Biotechnol. 2018, 46, 1123–1130. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Peiseler, M.; Kubes, P. Macrophages play an essential role in trauma-induced sterile inflammation and tissue repair. Eur. J. Trauma Emerg. Surg. Off. Publ. Eur. Trauma Soc. 2018, 44, 335–349. [Google Scholar] [CrossRef]
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M. Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol. 2017, 61, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Sun, X.; Wei, J.; Lyu, J.; Bian, T.; Liu, Z.; Huang, J.; Pi, F.; Li, C.; Zhong, Z. Bone-targeting drug delivery system of biomineral-binding liposomes loaded with icariin enhances the treatment for osteoporosis. J. Nanobiotechnol. 2019, 17, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Fakhrullin, R.; Lazzara, G. Core/Shell Gel Beads with Embedded Halloysite Nanotubes for Controlled Drug Release. Coatings 2019, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhu, G.; He, J.; Wang, G.; Li, D.; Zhang, F. Icariin targets Nrf2 signaling to inhibit microglia-mediated neuroinflammation. Int. Immunopharmacol. 2019, 73, 304–311. [Google Scholar] [CrossRef] [PubMed]
- El-Shitany, N.A.; Eid, B.G. Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signaling pathways. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 120, 109567. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mei, S.; Chu, P.K.; Zhang, Y.; Wu, Z. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials 2010, 31, 5072–5082. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, J.; Jin, K.; Liu, W.; Qiu, X.; Li, C. Fabrication of functional PLGA-based electrospun scaffolds and their applications in biomedical engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 1181–1194. [Google Scholar] [CrossRef]
- Chen, Z.; Klein, T.; Murray, R.Z.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today 2016, 19, 304–321. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Yang, H.; Yang, Y.; Huang, J.; Wu, K.; Chen, Z.; Wang, X.; Lin, C.; Lai, Y. Progress in TiO2 nanotube coatings for biomedical applications: A review. J. Mater. Chem. B 2018, 6, 1862–1886. [Google Scholar] [CrossRef]
- von Wilmowsky, C.; Bauer, S.; Roedl, S.; Neukam, F.W.; Schmuki, P.; Schlegel, K.A. The diameter of anodic TiO2 nanotubes affects bone formation and correlates with the bone morphogenetic protein-2 expression in vivo. Clin. Oral Implant. Res. 2012, 23, 359–366. [Google Scholar] [CrossRef]
- Yu, W.-Q.; Jiang, X.-Q.; Zhang, F.-Q.; Xu, L. The effect of anatase TiO2 nanotube layers on MC3T3-E1 preosteoblast adhesion, proliferation, and differentiation. J. Biomed. Mater. Res. A 2010, 94A, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.C.; Campos, C.H.; Diáz, C.; Jiménez, V.A.; Vidal, F.; Guzmán, L.; Alderete, J.B. PAMAM-grafted TiO2 nanotubes as novel versatile materials for drug delivery applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xi, X.; Ran, Q.; Liu, P.; Hu, Y.; Cai, K. Influence of the titania nanotubes dimensions on adsorption of collagen: An experimental and computational study. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Mazare, A.; Park, J.; Gongadze, E.; Killian, M.S.; Kralj, S.; von der Mark, K.; Iglic, A.; Schmuki, P. Protein interactions with layers of TiO2 nanotube and nanopore arrays: Morphology and surface charge influence. Acta Biomater. 2016, 45, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Ya, J.; An, L.; Liu, Z.; E, L.; Zhao, W.; Zhao, D.; Liu, C. Investigation of surface morphologies of TiO2 nanotube arrays by anodization in ethylene glycol electrolytes. J. Optoelectron. Adv. Mater. 2011, 13, 684–688. [Google Scholar]
- Bao, H.; Lv, F.; Liu, T. A pro-angiogenic degradable Mg-poly(lactic-co-glycolic acid) implant combined with rhbFGF in a rat limb ischemia model. Acta Biomater. 2017, 64, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Mehrasa, M.; Asadollahi, M.A.; Ghaedi, K.; Salehi, H.; Arpanaei, A. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int. J. Biol. Macromol 2015, 79, 687–695. [Google Scholar] [CrossRef]
- Daristotle, J.L.; Lau, L.W.; Erdi, M.; Hunter, J.; Djoum, A., Jr.; Srinivasan, P.; Wu, X.; Basu, M.; Ayyub, O.B.; Sandler, A.D.; et al. Sprayable and biodegradable, intrinsically adhesive wound dressing with antimicrobial properties. Bioeng. Transl. Med. 2020, 5, e10149. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ren, S.; Zhang, X.; Yu, Y.; Liu, C.; Yang, J.; Miao, L. Safety and efficacy of PLGA(Ag-Fe3O4)-coated dental implants in inhibiting bacteria adherence and osteogenic inducement under a magnetic field. Int. J. Nanomed. 2018, 13, 3751–3762. [Google Scholar] [CrossRef] [Green Version]
- Kazek-Kesik, A.; Nosol, A.; Plonka, J.; Smiga-Matuszowicz, M.; Golda-Cepa, M.; Krok-Borkowicz, M.; Brzychczy-Wloch, M.; Pamula, E.; Simka, W. PLGA-amoxicillin-loaded layer formed on anodized Ti alloy as a hybrid material for dental implant applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 998–1008. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B-Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Ramakrishnan, S.; Aw, M.S.; Atkins, G.J.; Findlay, D.M.; Losic, D. Biocompatible polymer coating of titania nanotube arrays for improved drug elution and osteoblast adhesion. Acta Biomater. 2012, 8, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Nan, K.; Chen, J.; Jin, D.; Jiang, S.; Zhao, P.; Xu, J.; Du, H.; Zhang, X.; Li, J.; et al. A new bone repair scaffold combined with chitosan/hydroxyapatite and sustained releasing icariin. Chin. Sci. Bull. 2009, 54, 2953–2961. [Google Scholar] [CrossRef]
- Banerjee, P.K.; Amidon, G.L. Physicochemical property modification strategies based on enzyme substrate specificities I: Rationale, synthesis, and pharmaceutical properties of aspirin derivatives. J. Pharm. Sci. 1981, 70, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Botting, R.M. The mechanism of action of aspirin. Thromb Res. 2003, 110, 255–258. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, S.; Li, X.; Feng, J.; Du, J.; Guo, L.; Su, Y.; Zhou, J.; Ding, G.; Bai, Y.; et al. Aspirin inhibits LPS-induced macrophage activation via the NF-kappaB pathway. Sci. Rep. 2017, 7, 11549. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Wang, Z.; Guo, X.; Shen, J.; Sun, H.; Bai, J.; Yu, B.; Wang, L.; Zhou, W.; Liu, Y.; et al. Aspirin inhibits osteoclast formation and wear-debris-induced bone destruction by suppressing mitogen-activated protein kinases. J. Cell. Physiol. 2020, 235, 2599–2608. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, X.; Yuan, Z.; Shen, M.; Song, Y.; Liu, H.; Deng, J.; Zhong, X.; Zhang, X. Establishing an osteoimmunomodulatory coating loaded with aspirin on the surface of titanium primed with phase-transited lysozyme. Int. J. Nanomed. 2019, 14, 977–991. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef] [Green Version]
- Miron, R.J.; Bosshardt, D.D. OsteoMacs: Key players around bone biomaterials. Biomaterials 2016, 82, 1–19. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Wagener, F.; Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharm. 2018, 153, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Nakamura, K.; Kageyama, S.; Lawal, A.O.; Gong, K.W.; Bhetraratana, M.; Fujii, T.; Sulaiman, D.; Hirao, H.; Bolisetty, S.; et al. Myeloid HO-1 modulates macrophage polarization and protects against ischemia-reperfusion injury. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, I.; Vanella, A.; Peterson, S.J.; Kim, D.H.; Tibullo, D.; Giallongo, C.; Vanella, L.; Parrinello, N.; Palumbo, G.A.; Di Raimondo, F.; et al. Overexpression of heme oxygenase-1 increases human osteoblast stem cell differentiation. J. Bone Min. Metab. 2010, 28, 276–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Aghaloo, T.; Pi-Anfruns, J.; Moshaverinia, A.; Sim, D.; Grogan, T.; Hadaya, D. The Effects of Systemic Diseases and Medications on Implant Osseointegration: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2019, 34, s35–s49. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammacher, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis-a review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Eleniste, P.P.; Patel, V.; Posritong, S.; Zero, O.; Largura, H.; Cheng, Y.H.; Himes, E.R.; Hamilton, M.; Baughman, J.; Kacena, M.A.; et al. Pyk2 and Megakaryocytes Regulate Osteoblast Differentiation and Migration Via Distinct and Overlapping Mechanisms. J. Cell Biochem. 2016, 117, 1396–1406. [Google Scholar] [CrossRef] [Green Version]





| Gene | Gene Bank ID | DNA Primer | Sequence | Size (bp) |
|---|---|---|---|---|
| TNF–α | NM_001278601.1 | Forward | 5’-TGCCTATGTCTCAGCCTCTTC-3’ | 117 |
| Reverse | 5’-GAGGCCATTTGGGAACTTCT-3’ | |||
| IL–1β | NM_008361.4 | Forward | 5’-TGTGCAAGTGTCTGAAGCAGC-3’ | 129 |
| Reverse | 5’-TGGAAGCAGCCCTTCATCTT-3’ | |||
| TGF–β | NM_011577.2 | Forward | 5’-TTGCTTCAGCTCCACAGAGA-3’ | 183 |
| Reverse | 5’-TGGTTGTAGAGGGCAAGGAC-3’ | |||
| HO–1 | NM_010442.2 | Forward | 5’-GCCGAGAATGCTGAGTTCATG-3’ | 86 |
| Reverse | 5’-TGGTACAAGGAAGCCATCACC-3’ | |||
| GAPDH | NM_008084.3 | Forward | 5’-GGTGAAGGTCGGTGTGAACG-3’ | 233 |
| Reverse | 5’-CTCGCTCCTGGAAGATGGTG-3’ |
| Gene | Gene Bank ID | DNA Primer | Sequence | Size (bp) |
|---|---|---|---|---|
| ALP | NM_007431.3 | Forward | 5’-ATCTTTGGTCTGGCTCCCATG-3’ | 106 |
| Reverse | 5’-TTTCCCGTTCACCGTCCAC-3’ | |||
| COL1A1 | NM_007742.4 | Forward | 5’-TAAGGGTCCCCAATGGTGAGA-3’ | 203 |
| Reverse | 5’-GGGTCCCTCGACTCCTACAT-3’ | |||
| OPN | NM_001204203.1 | Forward | 5’-CTCACATGAAGAGCGGTGAG-3’ | 174 |
| Reverse | 5’-TCTCCTGGCTCTCTTTGGAA-3’ | |||
| OCN | NM_007541.3 | Forward | 5’-GGACCATCTTTCTGCTCACTCTG-3’ | 131 |
| Reverse | 5’-GTTCACTACCTTATTGCCCTCCTG-3’ | |||
| GAPDH | NM_008084.3 | Forward | 5’-GGTGAAGGTCGGTGTGAACG-3’ | 233 |
| Reverse | 5’-CTCGCTCCTGGAAGATGGTG-3’ |
| Substrates | C% | N% | O% | Ti% |
|---|---|---|---|---|
| Ti | 47.19 ± 0.05 | 3.57 ± 0.01 | 39.27 ± 0.05 | 9.97 ± 0.02 |
| Ti–PLGA | 61.17 ± 0.05 | 0.34 ± 0.01 | 38.49 ± 0.03 | 0 |
| NT | 38.2 ± 0.03 | 2.97 ± 0.01 | 43.19 ± 0.03 | 15.63 ± 0.02 |
| NT–ICA | 50.49 ± 0.03 | 2.16 ± 0.01 | 39.47 ± 0.02 | 7.87 ± 0.02 |
| NT–ICA–PLGA | 90.66 ± 0.05 | 0 | 9.34 ± 0.02 | 0 |
| NT–ASP/PLGA | 72.3 ± 0.05 | 0 | 27.71 ± 0.02 | 0 |
| NT–ICA–ASP/PLGA | 64.53 ± 0.01 | 0 | 35.47 ± 0.05 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, A.; You, Y.; Chen, B.; Wang, W.; Liu, J.; Qi, H.; Liang, Y.; Li, Y.; Li, C. Icariin/Aspirin Composite Coating on TiO2 Nanotubes Surface Induce Immunomodulatory Effect of Macrophage and Improve Osteoblast Activity. Coatings 2020, 10, 427. https://doi.org/10.3390/coatings10040427
Ma A, You Y, Chen B, Wang W, Liu J, Qi H, Liang Y, Li Y, Li C. Icariin/Aspirin Composite Coating on TiO2 Nanotubes Surface Induce Immunomodulatory Effect of Macrophage and Improve Osteoblast Activity. Coatings. 2020; 10(4):427. https://doi.org/10.3390/coatings10040427
Chicago/Turabian StyleMa, Aobo, Yapeng You, Bo Chen, Wanmeng Wang, Jialin Liu, Hui Qi, Yunkai Liang, Ying Li, and Changyi Li. 2020. "Icariin/Aspirin Composite Coating on TiO2 Nanotubes Surface Induce Immunomodulatory Effect of Macrophage and Improve Osteoblast Activity" Coatings 10, no. 4: 427. https://doi.org/10.3390/coatings10040427
APA StyleMa, A., You, Y., Chen, B., Wang, W., Liu, J., Qi, H., Liang, Y., Li, Y., & Li, C. (2020). Icariin/Aspirin Composite Coating on TiO2 Nanotubes Surface Induce Immunomodulatory Effect of Macrophage and Improve Osteoblast Activity. Coatings, 10(4), 427. https://doi.org/10.3390/coatings10040427





