Recent Advances in Hydrophobic and Icephobic Surface Treatments of Concrete
Abstract
1. Introduction
1.1. Wetting Theories: The Case of Cement-Based Materials
2. Typologies of Hydrophobic Surface Treatments
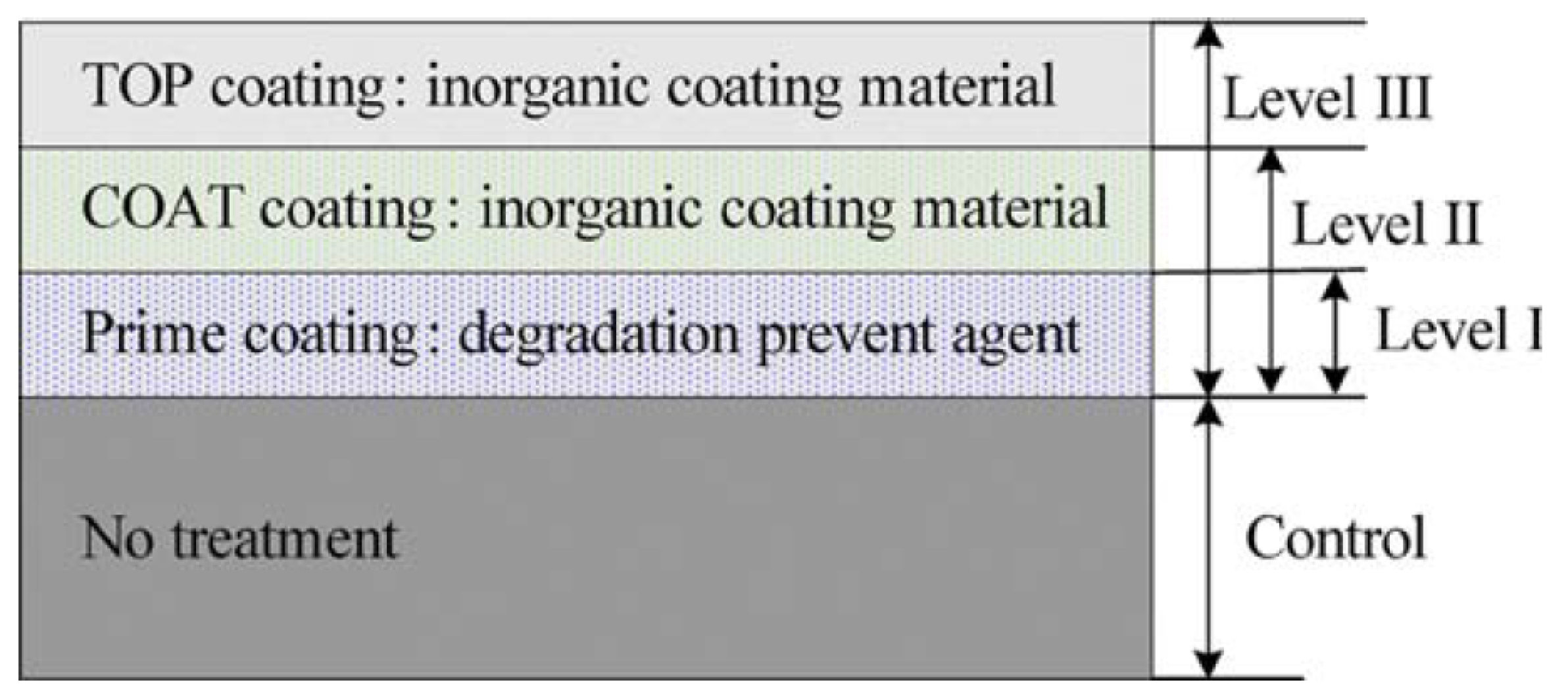
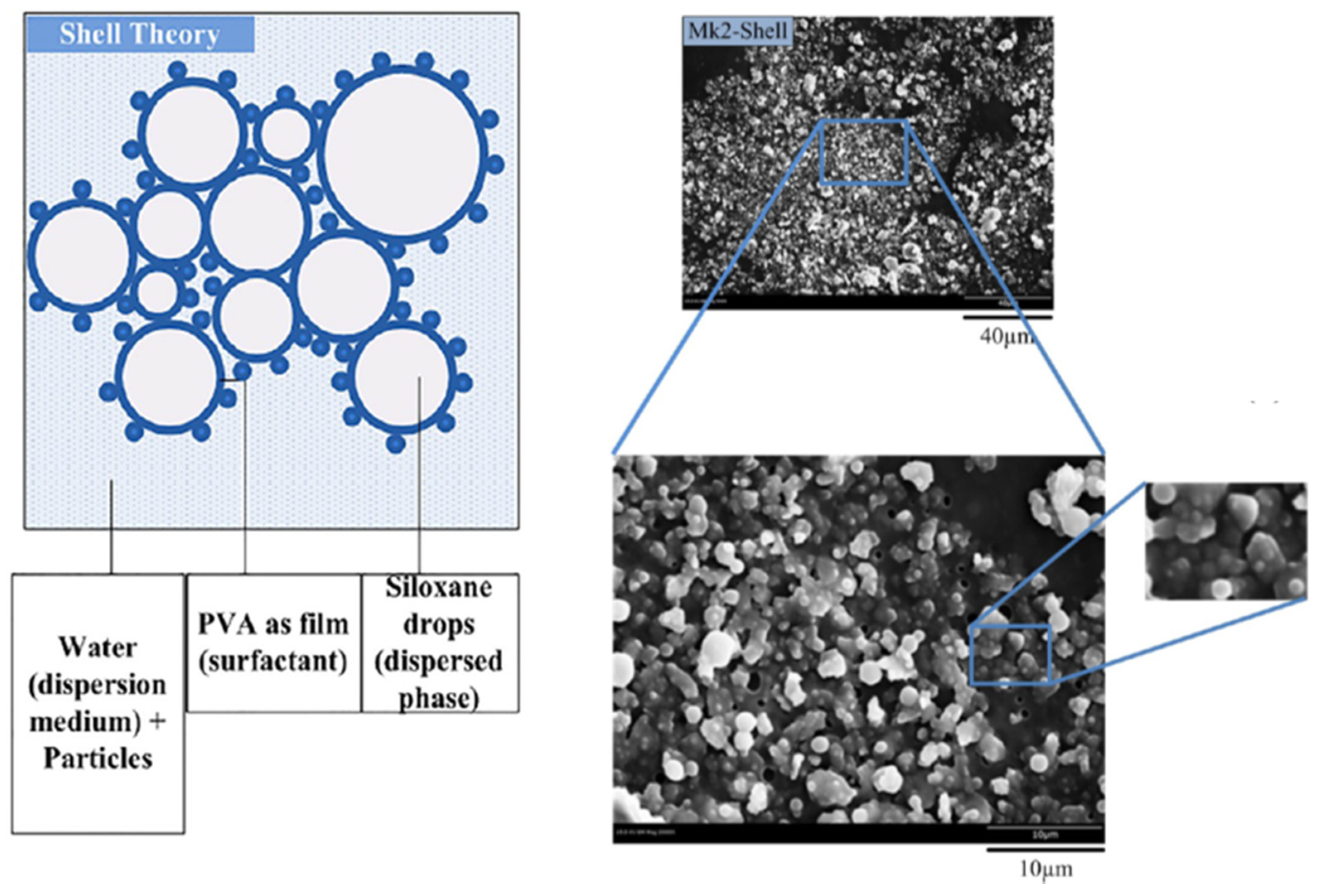
2.1. Coatings
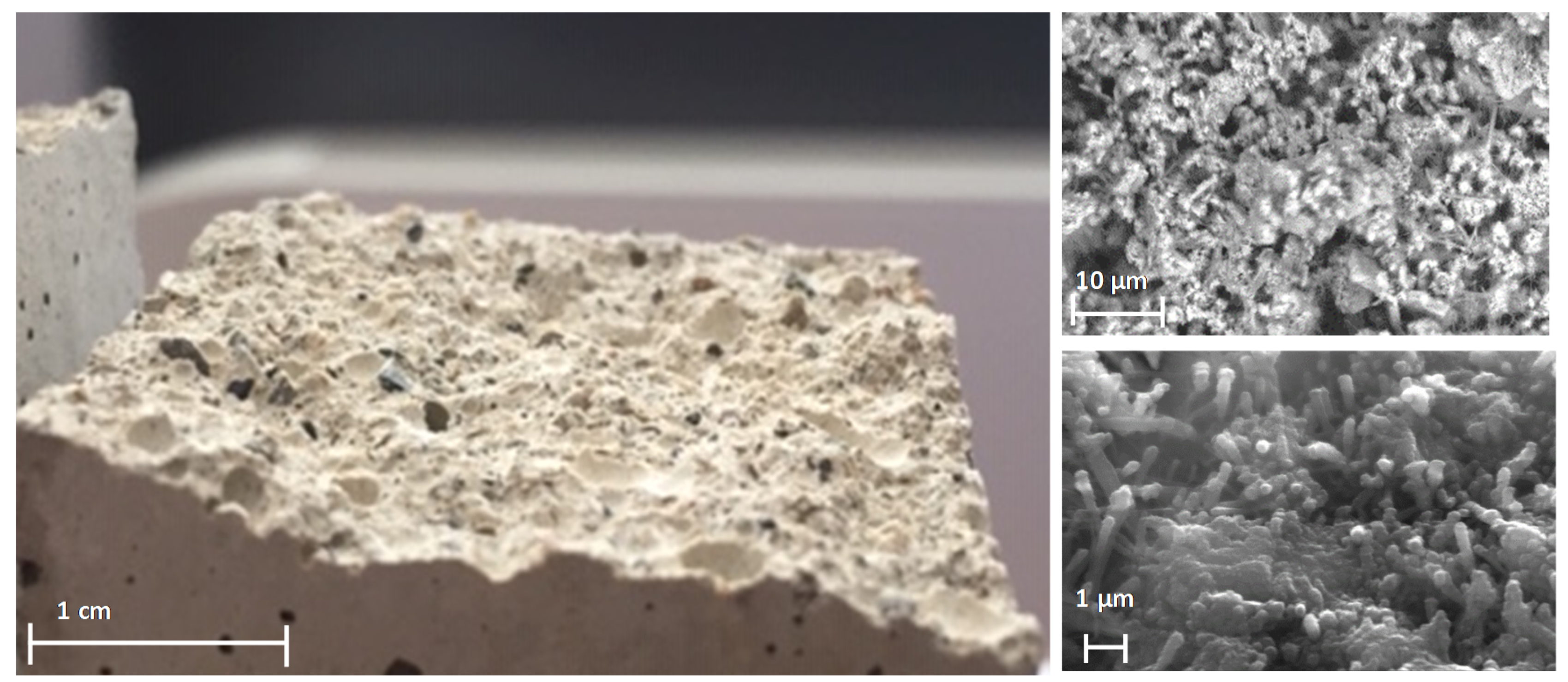
2.2. Pore Blockage
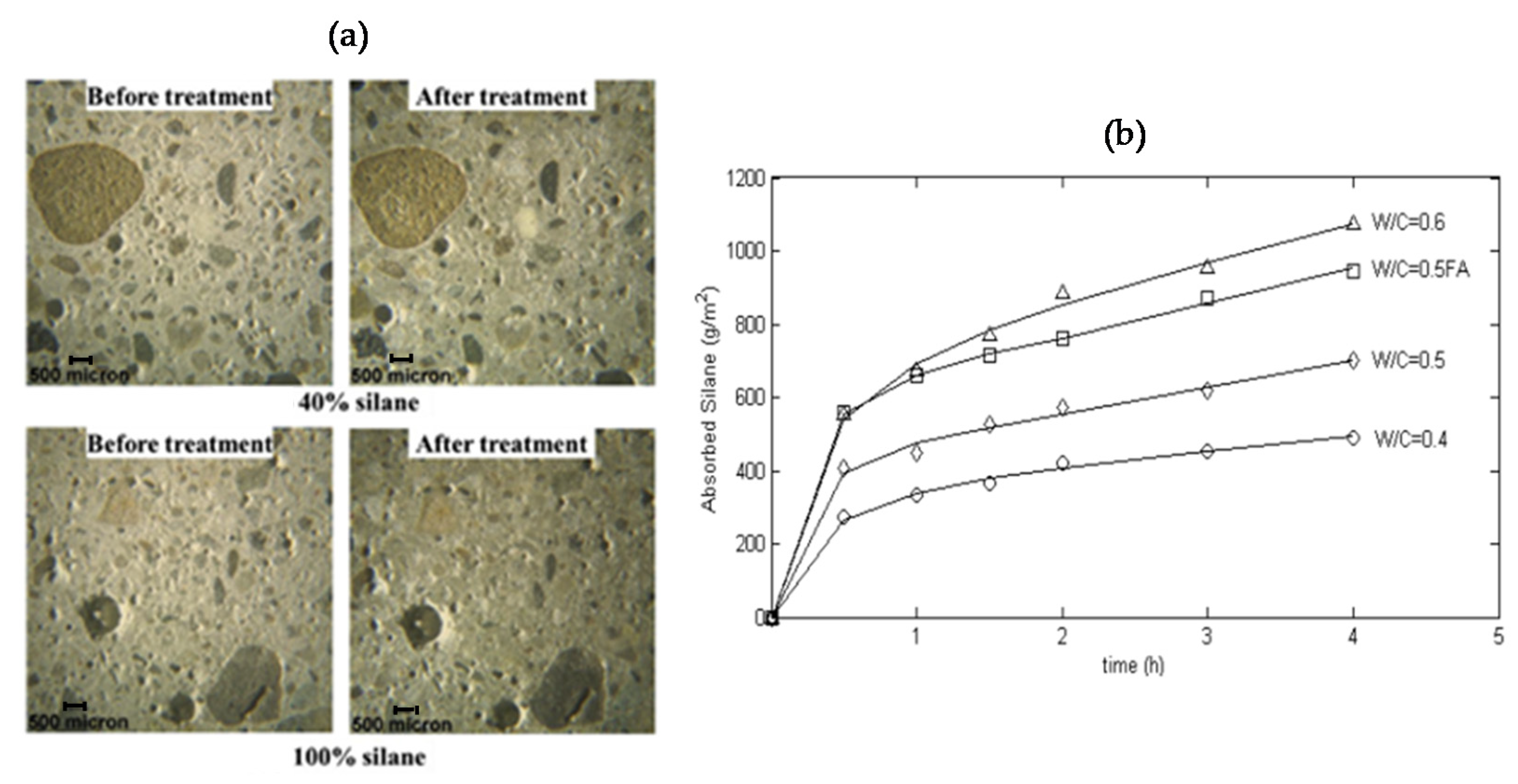
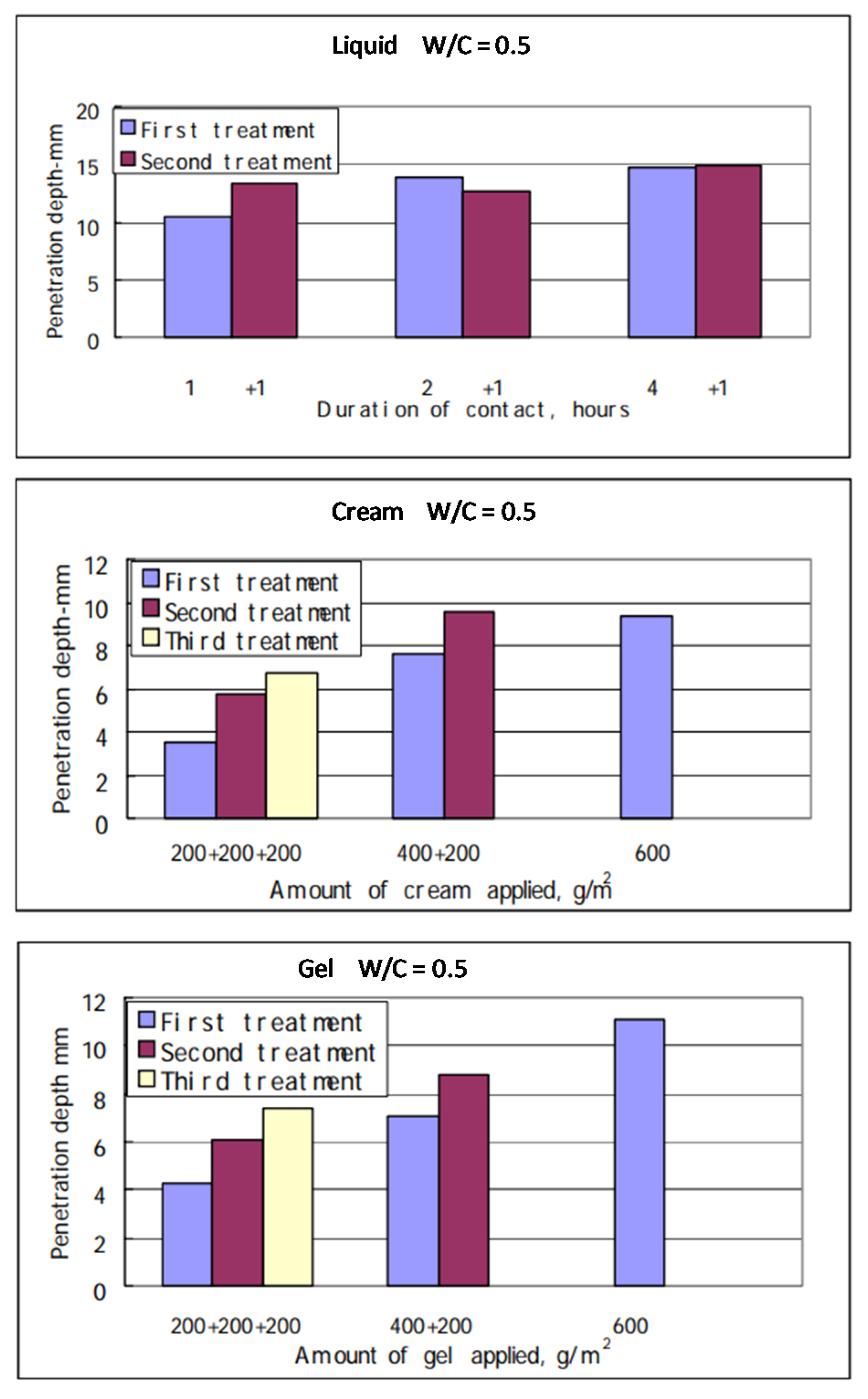
2.3. Hydrophobic Impregnation
3. Comparison with Hydrophobic Bulk Modification Approaches
4. Sustainable Hydrophobic Treatments for Concrete
5. Icephobic Concrete
- Water repellence: an efficient water-repellent surface helps timely removal of the water droplet, so that ice formation can be prevented.
- Ice nucleation delay: under the circumstance that quick removal of water is difficult or impossible, a longer delay in ice formation time or a lower freezing temperature then becomes a useful feature. Often, this phenomenon is referred to as “anti-icing”.
- Ice adhesion reduction: the last defense line is to ensure a low ice-adhesion strength when ice inevitably forms to ensure its easy removal. Often, this phenomenon is specifically referred to as “icephobicity”.
5.1. Water Repellency Line
5.2. Ice Nucleation Delay Line
- The internal frost damage leading to bulk cracking and loss of integrity;
- The surface scaling involving progressive swelling and flaking of the mortar component.
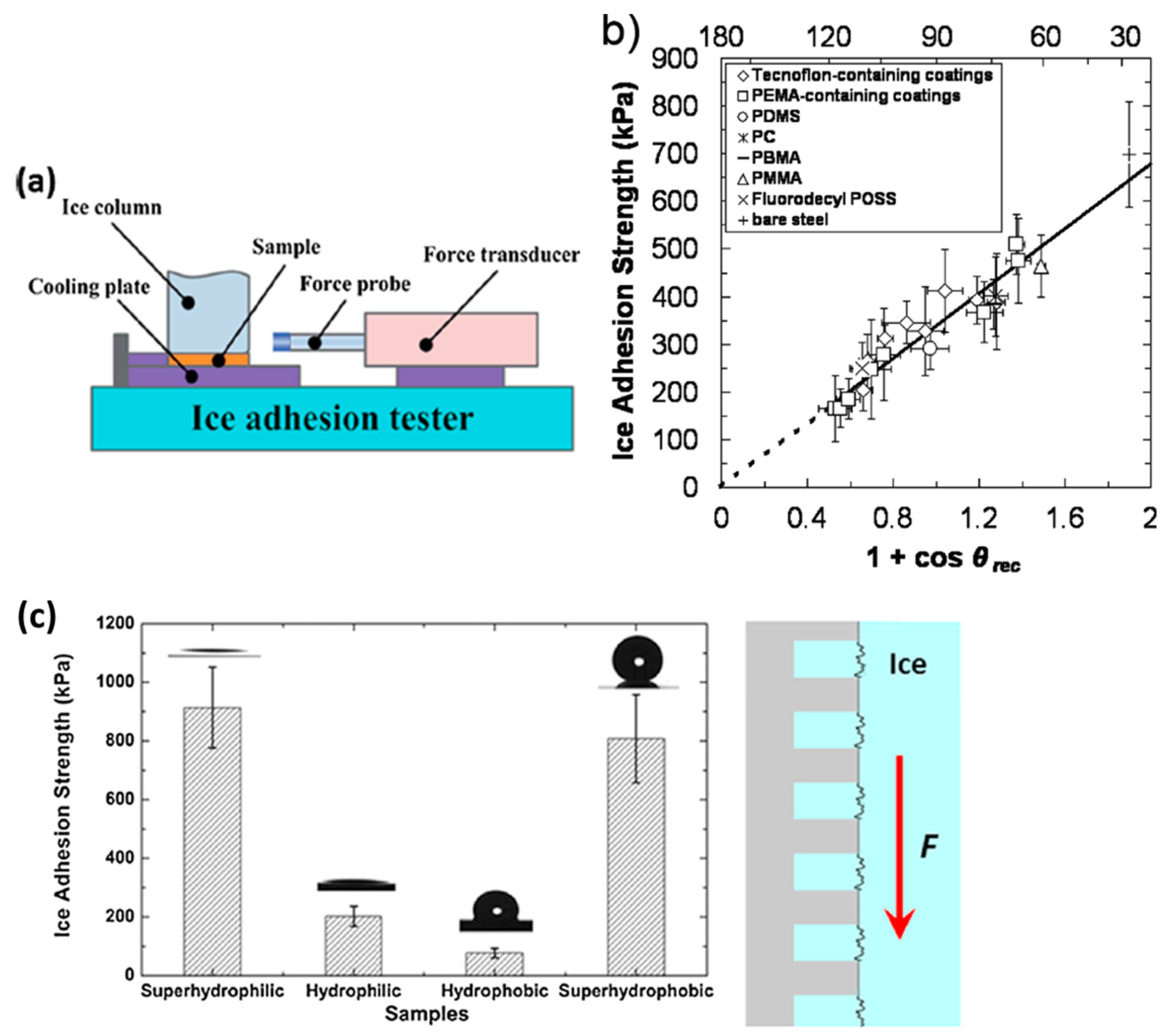
5.3. Adhesion Strength Line
- If ice is formed conforming to the Cassie–Baxter state (liquid water over to protrusions tops), the air voids act as stress concentrators that reduce the ice adhesion strength,
- If it is formed conforming to the Wenzel state (liquid water inside the cavities), the protrusions act as mechanical interlocking for ice, which is even intensified considering that expansion occurs upon solidification.
6. Evaluation of Performances
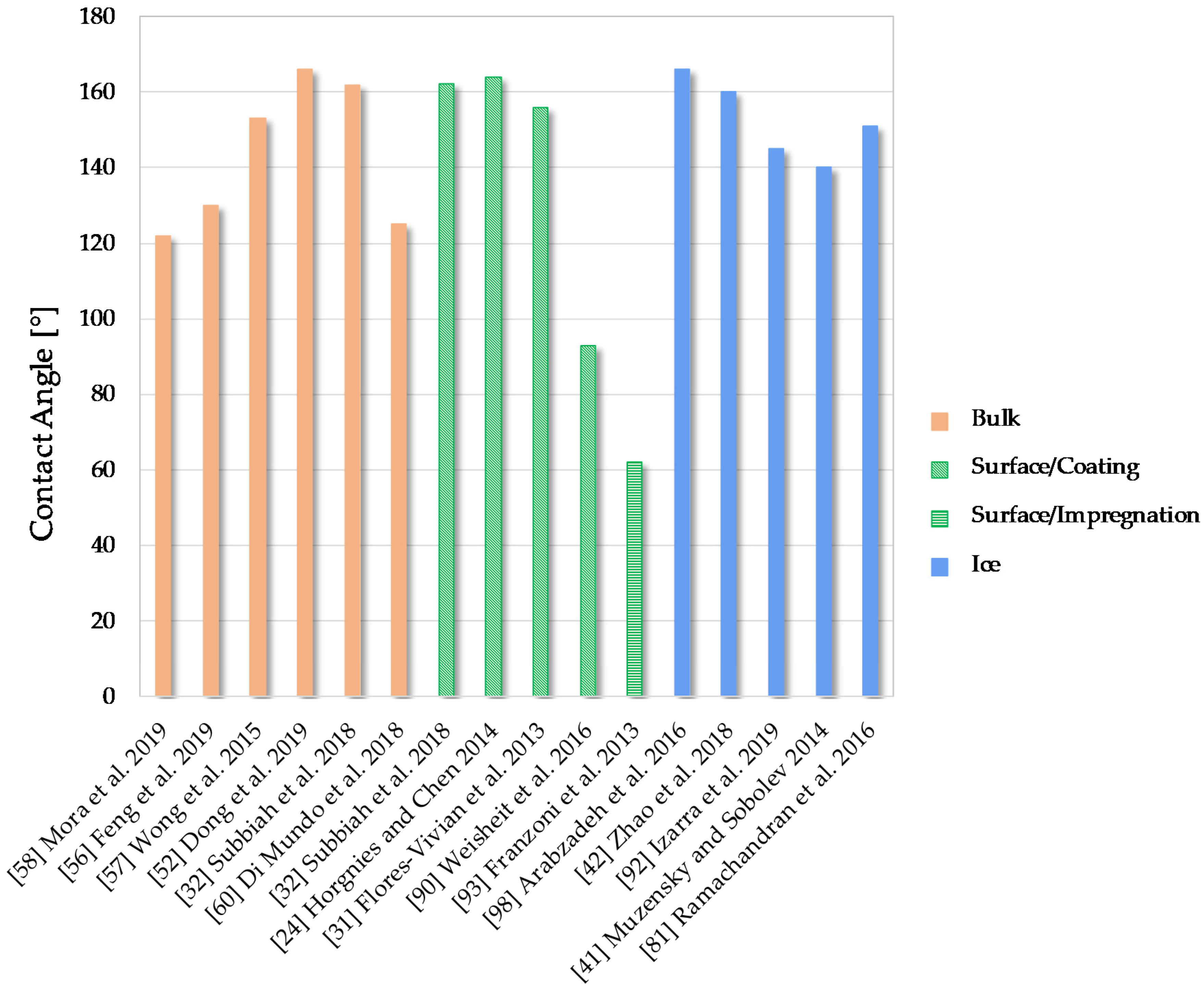
6.1. Water Contact Angle
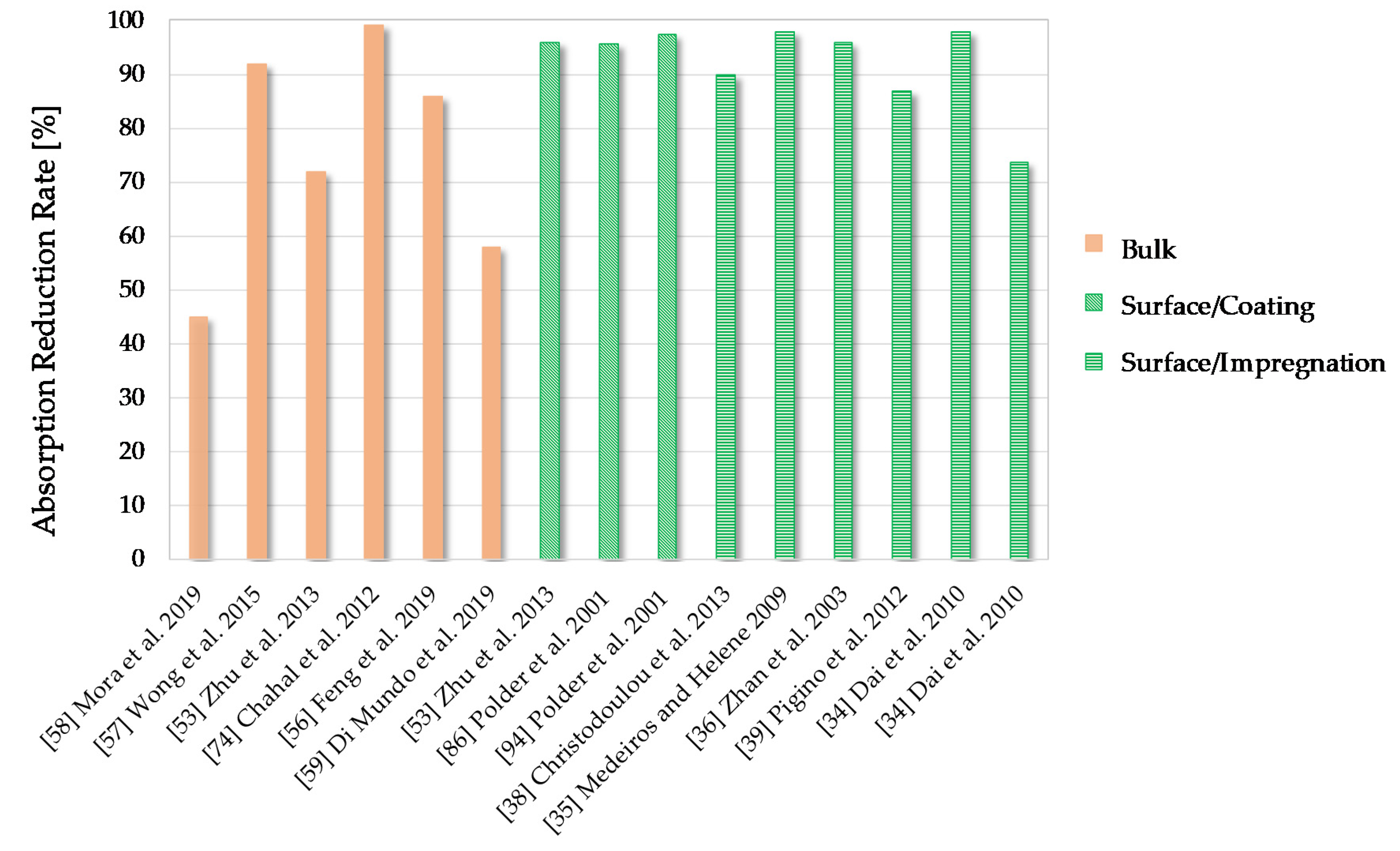
6.2. Water Absorption
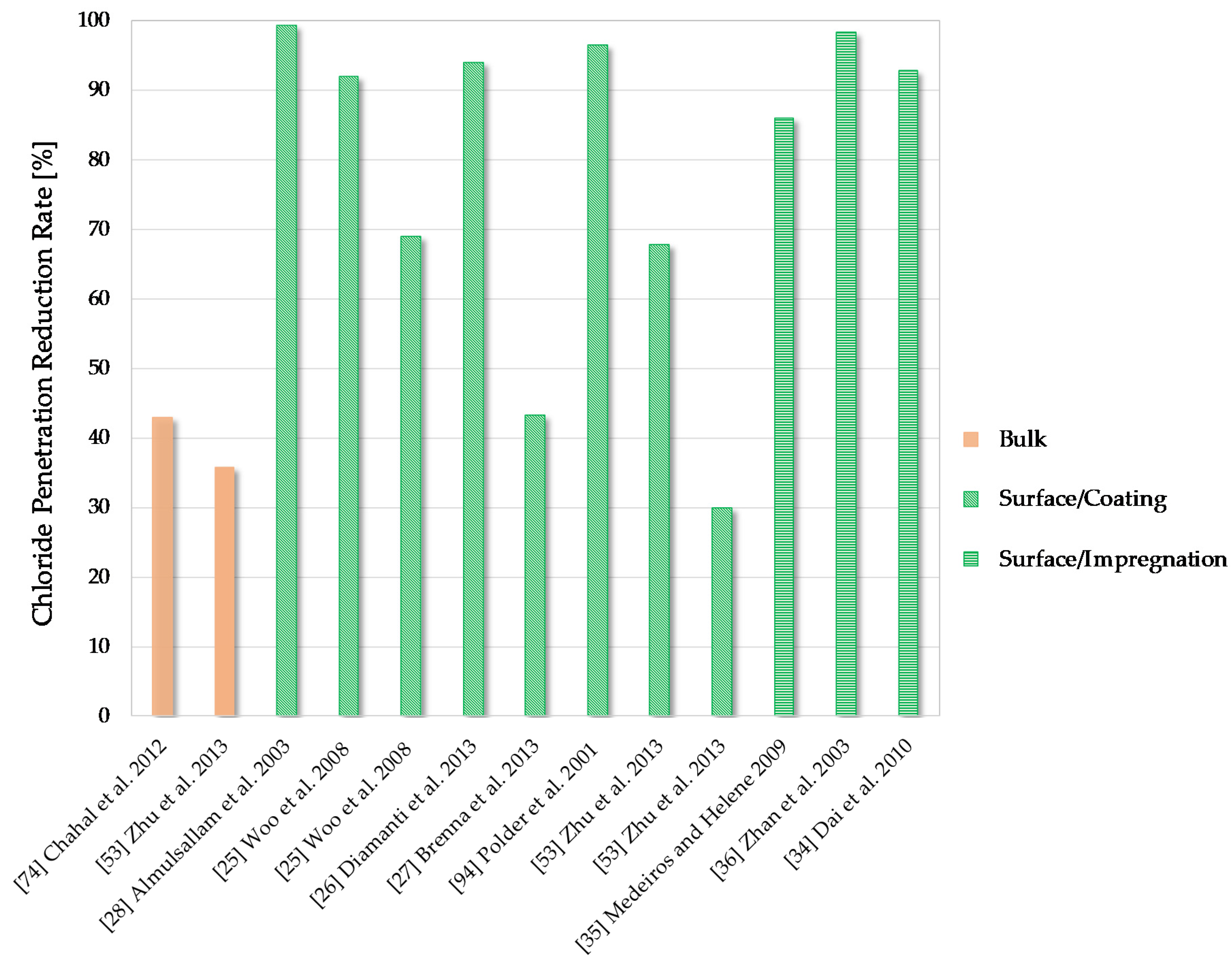
6.3. Chloride Penetration
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Pacheco-Torgal, F.; Labrincha, J. Biotech cementitious materials: Some aspects of an innovative approach for concrete with enhanced durability. Constr. Build. Mater. 2013, 40, 1136–1141. [Google Scholar] [CrossRef]
- Bertolini, L.; Elsener, B.; Pedeferri, P.; Redaelli, E.; Polder, R. Corrosion of Steel in Concrete; Wiley: Weinheim, Germany, 2013; Volume 392. [Google Scholar]
- British Standards Institution. BS EN 1504:2005 Products and Systems for the Protection and Repair of Concrete Structures—Definitions, Requirements, Quality Control and Evaluation of Conformity—Part 3: Structural and Non-structural Repair; BSI: London, UK, 2005. [Google Scholar]
- British Standards Institution. BS EN 1504-2, Products and Systems for the Protection and Repair of Concrete Structures—Definitions, Requirements, Quality Control and Evaluation of Conformity—Part 2: Surface Protection Systems for Concrete; BSI: London, UK, 2004. [Google Scholar]
- Young, T.R. An assay on the cohesion of fluids. Philos. Trans. Soc. R. Lond. 1805, 95, 65–87. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Telecka, A.; Mandsberg, N.K.; Li, T.; Ludvigsen, E.; Ndoni, S.; Di Mundo, R.; Palumbo, F.; Fiutowski, J.; Chiriaev, S.; Taboryski, R.J. Mapping the transition to superwetting state for nanotextured surfaces templated from block-copolymer self-assembly. Nanoscale 2018, 10, 20652–20663. [Google Scholar] [CrossRef] [PubMed]
- Orchard, M.; Kohonen, M.; Humphries, S. The influence of surface energy on the self-cleaning of insect adhesive devices. J. Exp. Biol. 2011, 215, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Quéré, D. Non-sticking drops. Rep. Prog. Phys. 2005, 68, 2495–2532. [Google Scholar] [CrossRef]
- Di Mundo, R.; Palumbo, F. Comments Regarding ‘An Essay on Contact Angle Measurements’. Plasma Process. Polym. 2010, 8, 14–18. [Google Scholar] [CrossRef]
- Furmidge, C. Studies at phase interfaces. I. The sliding of liquid drops on solid surfaces and a theory for spray retention. J. Colloid Sci. 1962, 17, 309–324. [Google Scholar] [CrossRef]
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Relationships between Water Wettability and Ice Adhesion. ACS Appl. Mater. Interfaces 2010, 2, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Di Mundo, R.; Bottiglione, F.; Carbone, G. Cassie state robustness of plasma generated randomly nano-rough surfaces. Appl. Surf. Sci. 2014, 316, 324–332. [Google Scholar] [CrossRef]
- Bormashenko, E.; Pogreb, R.; Whyman, G.; Erlich, M. Cassie−Wenzel Wetting Transition in Vibrating Drops Deposited on Rough Surfaces: Is the Dynamic Cassie−Wenzel Wetting Transition a 2D or 1D Affair? Langmuir 2007, 23, 6501–6503. [Google Scholar] [CrossRef] [PubMed]
- Bottiglione, F.; Di Mundo, R.; Soria, L.; Carbone, G. Wenzel to Cassie Transition in Superhydrophobic Randomly Rough Surfaces. Nanosci. Nanotechnol. Lett. 2015, 7, 74–78. [Google Scholar] [CrossRef]
- Di Mundo, R.; Bottiglione, F.; Palumbo, F.; Favia, P.; Carbone, G. Sphere-on-cone microstructures on Teflon surface: Repulsive behavior against impacting water droplets. Mater. Des. 2016, 92, 1052–1061. [Google Scholar] [CrossRef]
- Di Mundo, R.; Bottiglione, F.; Palumbo, F.; Notarnicola, M.; Carbone, G. Filamentary superhydrophobic Teflon surfaces: Moderate apparent contact angle but superior air-retaining properties. J. Colloid Interface Sci. 2016, 482, 175–182. Available online: https://www.sciencedirect.com/science/article/pii/S0021979716305380 (accessed on 10 January 2020). [CrossRef]
- Kumar, R.; Bhattacharjee, B. Porosity, pore size distribution and in situ strength of concrete. Cem. Concr. Res. 2003, 33, 155–164. [Google Scholar] [CrossRef]
- Favia, P.; De Vietro, N.; Di Mundo, R.; Fracassi, F.; D’Agostino, R. Tuning the Acid/Base Surface Character of Carbonaceous Materials by Means of Cold Plasma Treatments. Plasma Process. Polym. 2006, 3, 66–74. [Google Scholar] [CrossRef]
- Hall, C. Water movement in porous building materials—I. Unsaturated flow theory and its applications. Build. Environ. 1977, 12, 117–125. [Google Scholar] [CrossRef]
- Bittrich, E.; Cometa, S.; de Giglio, E.; Di Mundo, R.; Ditaranto, N.; Eichhorn, K.J.; Keller, B.; Lednický, F.; Mangolini, F.; Palumbo, F.; et al. Polymer Surface Characterization; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2014. [Google Scholar]
- Horgnies, M.; Chen, J. Superhydrophobic concrete surfaces with integrated microtexture. Cem. Concr. Compos. 2014, 52, 81–90. [Google Scholar] [CrossRef]
- Woo, R.S.C.; Zhu, H.; Chow, M.M.; Leung, C.K.; Kim, J.-K. Barrier performance of silane–clay nanocomposite coatings on concrete structure. Compos. Sci. Technol. 2008, 68, 2828–2836. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Brenna, A.; Bolzoni, F.M.; Berra, M.; Pastore, T.; Ormellese, M. Effect of polymer modified cementitious coatings on water and chloride permeability in concrete. Constr. Build. Mater. 2013, 49, 720–728. [Google Scholar] [CrossRef]
- Brenna, A.; Bolzoni, F.M.; Beretta, S.; Ormellese, M. Long-term chloride-induced corrosion monitoring of reinforced concrete coated with commercial polymer-modified mortar and polymeric coatings. Constr. Build. Mater. 2013, 48, 734–744. [Google Scholar] [CrossRef]
- Almusallam, A.; Khan, F.; Dulaijan, S.; Al-Amoudi, O. Effectiveness of surface coatings in improving concrete durability. Cem. Concr. Compos. 2003, 25, 473–481. [Google Scholar] [CrossRef]
- Vipulanandan, C.; Liu, J. Performance of polyurethane-coated concrete in sewer environment. Cem. Concr. Res. 2005, 35, 1754–1763. [Google Scholar] [CrossRef]
- Moon, H.Y.; Shin, D.G.; Choi, D.S. Evaluation of the durability of mortar and concrete applied with inorganic coating material and surface treatment system. Constr. Build. Mater. 2007, 21, 362–369. [Google Scholar] [CrossRef]
- Flores-Vivian, I.; Hejazi, V.; Kozhukhova, M.; Nosonovsky, M.; Sobolev, K. Self-Assembling Particle-Siloxane Coatings for Superhydrophobic Concrete. ACS Appl. Mater. Interfaces 2013, 5, 13284–13294. [Google Scholar] [CrossRef]
- Karthick, S.; Park, D.-J.; Lee, Y.S.; Saraswathy, V.; Lee, H.-S.; Jang, H.-O.; Choi, H.-J. Development of water-repellent cement mortar using silane enriched with nanomaterials. Prog. Org. Coat. 2018, 125, 48–60. [Google Scholar] [CrossRef]
- Medeiros, M.H.F.; Castro-Borges, P.; Aleixo, D.M.; Quarcioni, V.A.; Marcondes, C.G.N.; Helene, P. Reducing water and chloride penetration through silicate treatments for concrete as a mean to control corrosion kinetics. Int. J. Electrochem. Sci. 2012, 7, 9668–9681. [Google Scholar]
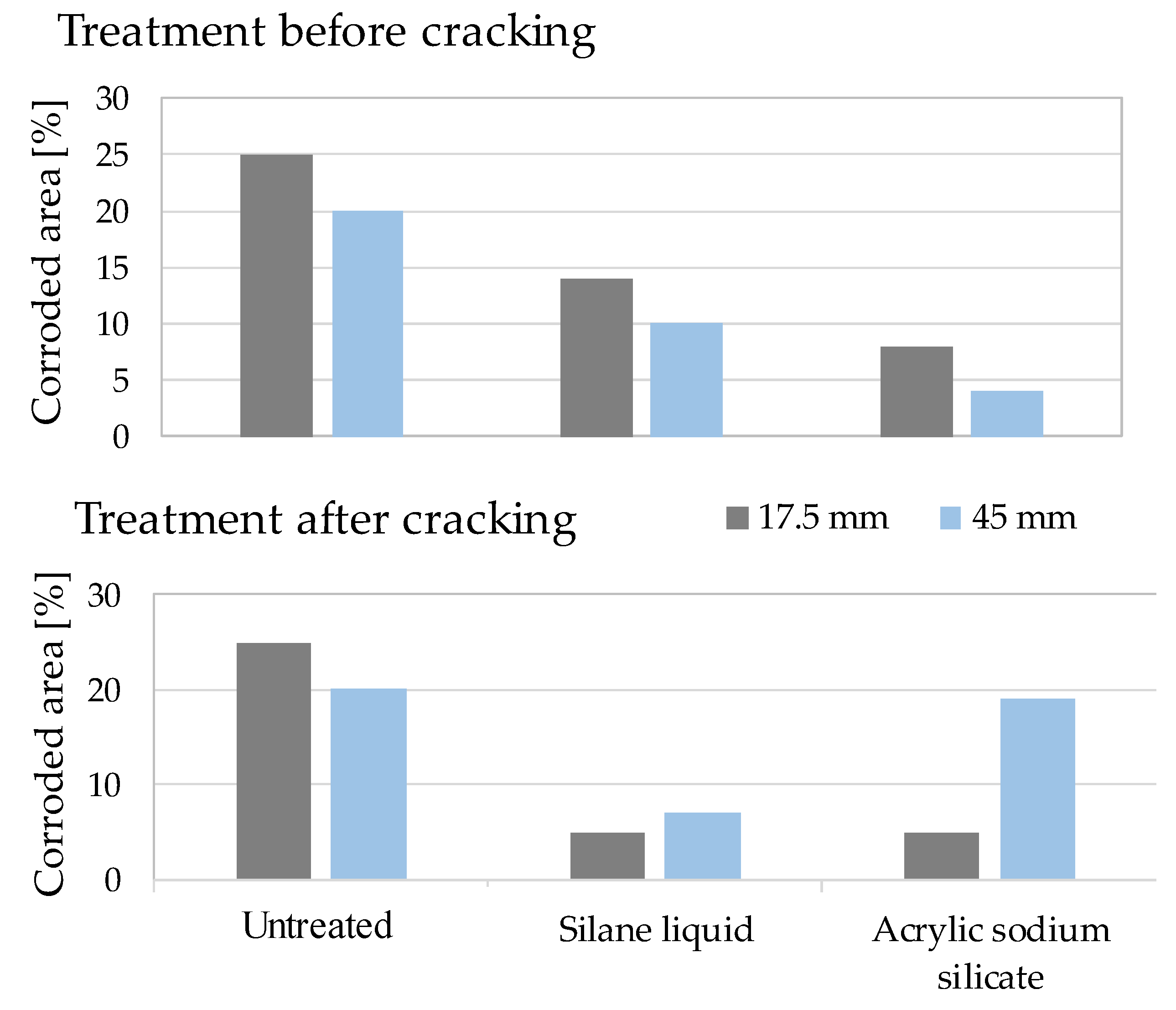
- Dai, J.-G.; Akira, Y.; Wittmann, F.; Yokota, H.; Zhang, P. Water repellent surface impregnation for extension of service life of reinforced concrete structures in marine environments: The role of cracks. Cem. Concr. Compos. 2010, 32, 101–109. [Google Scholar] [CrossRef]
- Medeiros, M.; Helene, P. Efficacy of surface hydrophobic agents in reducing water and chloride ion penetration in concrete. Mater. Struct. 2007, 41, 59–71. [Google Scholar] [CrossRef]
- Zhan, H.; Wittmann, F.H.; Zhao, T. Chloride Barrier for Concrete in Saline Environment Established by Water Repellent Treatment/Durch Hydrophobieren hergestellte Chloridschranke für Beton in Kontakt mit Salzwasser. Restor. Build. Monum. 2003, 9, 535–550. [Google Scholar] [CrossRef]
- Frattolillo, A.; Giovinco, G.; Mascolo, M.; Vitale, A. Effects of hydrophobic treatment on thermophysical properties of lightweight mortars. Exp. Therm. Fluid Sci. 2005, 29, 733–741. [Google Scholar] [CrossRef]
- Christodoulou, C.; Goodier, C.; Austin, S.; Webb, J.; Glass, G. Long-term performance of surface impregnation of reinforced concrete structures with silane. Constr. Build. Mater. 2013, 48, 708–716. [Google Scholar] [CrossRef]
- Pigino, B.; Leemann, A.; Franzoni, E.; Lura, P. Ethyl silicate for surface treatment of concrete—Part II: Characteristics and performance. Cem. Concr. Compos. 2012, 34, 313–321. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Muzenski, S.; Flores-Vivian, I.; Sobolev, K. The Development of Hydrophobic and Superhydrophobic Cementitious Composites. In Proceedings of the 4th International Conference on the Durability of Concrete Structures, Purdue University, West Lafayette, IN, USA, 24–26 July 2014. [Google Scholar]
- Zhao, Y.; Liu, Y.; Liu, Q.; Guo, W.; Yang, D.; Ge, D. Icephobicity studies of superhydrophobic coatings on concrete via spray method. Mater. Lett. 2018, 233, 263–266. [Google Scholar] [CrossRef]
- Kupwade-patil, K.; Cardenas, H.E.; Gordon, K.; Lee, L.S. Corrosion Mitigation in Reinforced Concrete Beams via Nanoparticle Treatment. ACI Mater. J. 2012, 109. [Google Scholar] [CrossRef]
- Jones, J.W.; Maxfield, P. Method of Hardening and Polishing Concrete Floors, Walls, and the Like. U.S. Patent 6,454,632, 24 September 2002. [Google Scholar]
- Thompson, J.; Silsbee, M.; Gill, P.; Scheetz, B. Characterization of silicate sealers on concrete. Cem. Concr. Res. 1997, 27, 1561–1567. [Google Scholar] [CrossRef]
- Meier, S.; Wittmann, F. Recommendations for Water Repellent Surface Impregnation of Concrete. Restor. Build. Monum. 2011, 17, 347–358. [Google Scholar] [CrossRef]
- Bertolini, L.; Elsener, B.; Pedeferri, P.; Polder, R. Corrosive Agents and Their Interaction with Materials; Wiley: Weinheim, Germany, 2004; Volume 392. [Google Scholar]
- Batis, G.; Pantazopoulou, P.; Routoulas, A. Corrosion protection investigation of reinforcement by inorganic coating in the presence of alkanolamine-based inhibitor. Cem. Concr. Compos. 2003, 25, 371–377. [Google Scholar] [CrossRef]
- Liu, Z.; Hansen, W. Effect of hydrophobic surface treatment on freeze-thaw durability of concrete. Cem. Concr. Compos. 2016, 69, 49–60. [Google Scholar] [CrossRef]
- De Medeiros, M.H.F.; Helene, P. Surface treatment of reinforced concrete in marine environment: Influence on chloride diffusion coefficient and capillary water absorption. Constr. Build. Mater. 2009, 23, 1476–1484. [Google Scholar] [CrossRef]
- Al-Kheetan, M.J.; Rahman, M.M.; Chamberlain, D. Development of hydrophobic concrete by adding dual-crystalline admixture at mixing stage. Struct. Concr. 2018, 19, 1504–1511. [Google Scholar] [CrossRef]
- Dong, B.; Wang, F.; Abadikhah, H.; Hao, L.Y.; Xu, X.; Khan, S.A.; Wang, G.; Agathopoulos, S. Simple Fabrication of Concrete with Remarkable Self-Cleaning Ability, Robust Superhydrophobicity, Tailored Porosity, and Highly Thermal and Sound Insulation. ACS Appl. Mater. Interfaces 2019, 11, 42801–42807. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Kou, S.-C.; Poon, C.-S.; Dai, J.-G.; Li, Q.-Y. Influence of silane-based water repellent on the durability properties of recycled aggregate concrete. Cem. Concr. Compos. 2013, 35, 32–38. [Google Scholar] [CrossRef]
- Tittarelli, F.; Moriconi, G. The effect of silane-based hydrophobic admixture on corrosion of reinforcing steel in concrete. Cem. Concr. Res. 2008, 38, 1354–1357. [Google Scholar] [CrossRef]
- Gong, J.; Duan, Z.; Sun, K.; Xiao, M. Waterproof properties of thermal insulation mortar containing vitrified microsphere. Constr. Build. Mater. 2016, 123, 274–280. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, F.; Xie, T.; Ou, J.; Xue, M.; Li, W. Integral hydrophobic concrete without using silane. Constr. Build. Mater. 2019, 227, 116678. [Google Scholar] [CrossRef]
- Wong, H.S.; Barakat, R.; Al Hilali, A.; Saleh, M.; Cheeseman, C.R. Hydrophobic concrete using waste paper sludge ash. Cem. Concr. Res. 2015, 70, 9–20. [Google Scholar] [CrossRef]
- Mora, E.; González, G.; Romero, P.; Castellón, E. Control of water absorption in concrete materials by modification with hybrid hydrophobic silica particles. Constr. Build. Mater. 2019, 221, 210–218. [Google Scholar] [CrossRef]
- Di Mundo, R.; Dilonardo, E.; Nacucchi, M.; Carbone, G.; Notarnicola, M. Water absorption in rubber-cement composites: 3D structure investigation by X-ray computed-tomography. Constr. Build. Mater. 2019, 228, 116602. [Google Scholar] [CrossRef]
- Di Mundo, R.; Petrella, A.; Notarnicola, M. Surface and bulk hydrophobic cement composites by tyre rubber addition. Constr. Build. Mater. 2018, 172, 176–184. [Google Scholar] [CrossRef]
- Creasey, R.; Andrews, J.; Ekolu, S.; Kruger, D. Long-term 20-year performance of surface coating repairs applied to façades of reinforced concrete buildings. Case Stud. Constr. Mater. 2017, 7, 348–360. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Miraldo, S.; Baklouti, S.; Ding, Y. An overview on the potential of geopolymers for concrete infrastructure rehabilitation. Constr. Build. Mater. 2012, 36, 1053–1058. [Google Scholar] [CrossRef]
- Nazier, M.; Arafa, M. Field Implementation of Geopolymer Coatings Disclaimer Statement. F. Implement. Geopolymer Coatings. 2004. Available online: https://cait.rutgers.edu/files/FHWA-NJ-2002-011.pdf (accessed on 4 May 2020).
- Zhang, Z.; Yao, X.; Zhu, H. Potential application of geopolymers as protection coatings for marine concrete I. Basic properties. Appl. Clay Sci. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, X.; Wang, H. Potential application of geopolymers as protection coatings for marine concrete III. Field experiment. Appl. Clay Sci. 2012, 67, 57–60. [Google Scholar] [CrossRef]
- Tilley, S.; Fry, R. Priority Environmental Contaminants: Understanding their sources of exposure, biological mechanisms, and impacts on health. In Systems Biology in Toxicology and Environmental Health; Academic Press: Cambridge, MA, USA, 2015; pp. 117–169. [Google Scholar]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Cyclomethicone, Cyclotetrasiloxane, Cyclopentasiloxane, Cyclohexasiloxane, and Cycloheptasiloxane. Int. J. Toxicol. 2011, 30, 149–227. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Silylates and Surface-Modified Siloxysilicates. Int. J. Toxicol. 2013, 32, 5–24. [Google Scholar] [CrossRef]
- Annual Report. Available online: http://www.ecetoc.org/wp-content/uploads/2012/05/ECETOC_2011_Annual_Report.pdf (accessed on 4 May 2020).
- Franzen, A.C.; Van Landingham, C.; Greene, T.; Plotzke, K.P.; Gentry, P.R. A global human health risk assessment for Decamethylcyclopentasiloxane (D5). Regul. Toxicol. Pharmacol. 2016, 74, S25–S43. [Google Scholar] [CrossRef]
- Mojsiewicz-Pieńkowska, K.; Krenczkowska, D. Evolution of consciousness of exposure to siloxanes—Review of publications. Chemosphere 2018, 191, 204–217. [Google Scholar] [CrossRef] [PubMed]
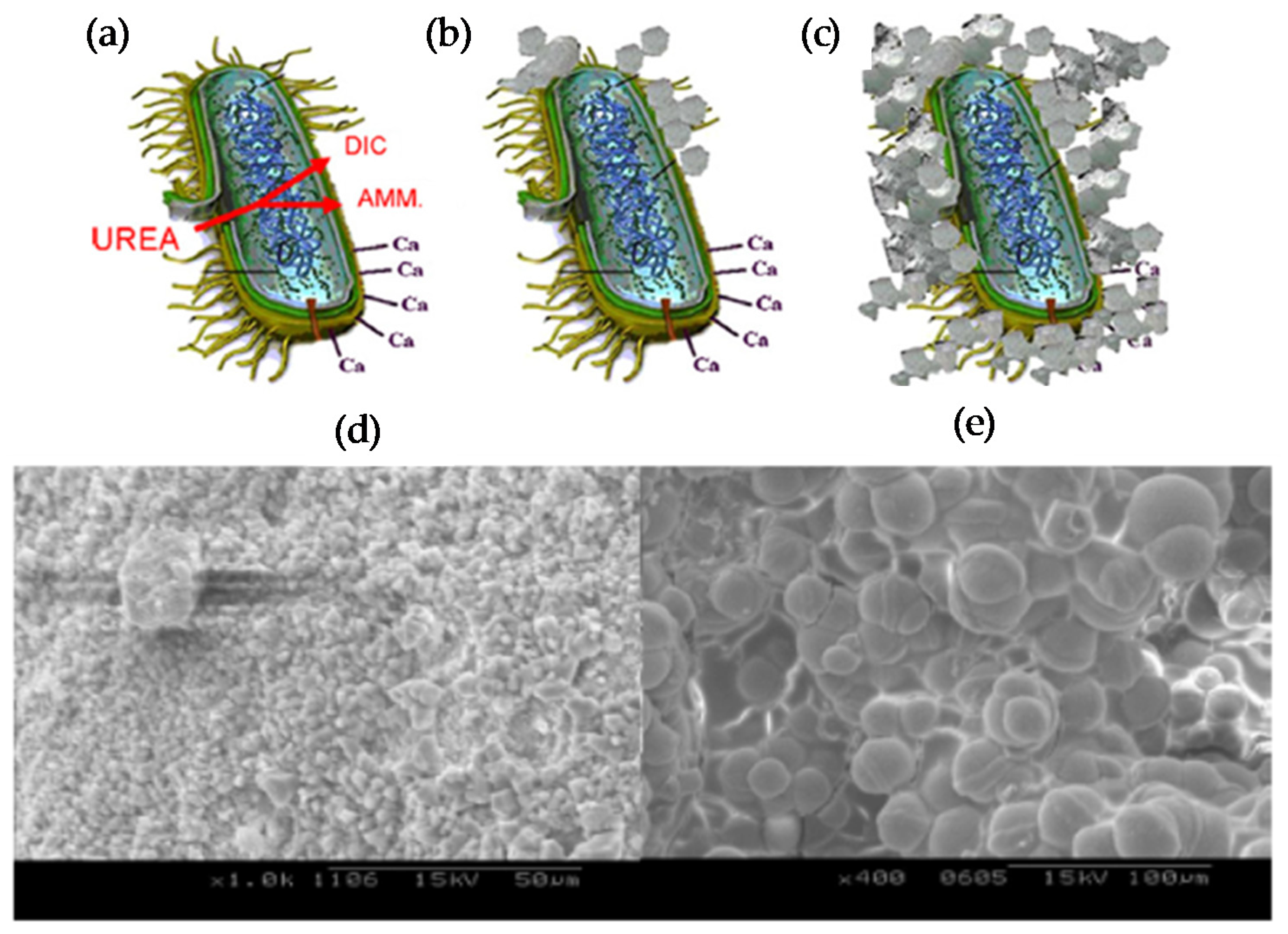
- Li, P.H.; Qu, W.J. Microbial Carbonate Mineralization as an Improvement Method for Durability of Concrete Structures. Adv. Mater. Res. 2011, 365, 280–286. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Chahal, N.; Siddique, R.; Rajor, A. Influence of bacteria on the compressive strength, water absorption and rapid chloride permeability of concrete incorporating silica fume. Constr. Build. Mater. 2012, 37, 645–651. [Google Scholar] [CrossRef]
- De Belie, N.; Wang, J. Bacteria-based repair and self-healing of concrete. J. Sustain. Cem. Mater. 2015, 5, 35–56. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Boil. Biochem. 1999. [Google Scholar] [CrossRef]
- De Muynck, W.; Cox, K.; De Belie, N.; Verstraete, W. Bacterial carbonate precipitation as an alternative surface treatment for concrete. Constr. Build. Mater. 2008, 22, 875–885. [Google Scholar] [CrossRef]
- Bird, J.C.; Dhiman, R.; Kwon, H.-M.; Varanasi, K.K. Reducing the contact time of a bouncing drop. Nature 2013, 503, 385–388. [Google Scholar] [CrossRef]
- Liu, Y.; Moevius, L.; Xu, X.; Qian, T.; Yeomans, J.M.; Wang, Z. Pancake bouncing on superhydrophobic surfaces. Nat. Phys. 2014, 10, 515–519. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic materials: Fundamentals, performance evaluation, and applications. Prog. Mater. Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Ramachandran, R.; Kozhukhova, M.; Sobolev, K.; Nosonovsky, M. Anti-Icing Superhydrophobic Surfaces: Controlling Entropic Molecular Interactions to Design Novel Icephobic Concrete. Entropy 2016. [Google Scholar] [CrossRef]
- Zhang, Y.; Anim-Danso, E.; Bekele, S.; Dhinojwala, A. Effect of Surface Energy on Freezing Temperature of Water. ACS Appl. Mater. Interfaces 2016, 8, 17583–17590. [Google Scholar] [CrossRef] [PubMed]
- Menini, R.; Farzaneh, M. Advanced Icephobic Coatings. J. Adhes. Sci. Technol. 2011, 25, 971–992. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Modin, E.B. Modus Operandi of Protective and Anti-icing Mechanisms Underlying the Design of Longstanding Outdoor Icephobic Coatings. ACS Nano 2019, 13, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, S.; Tao, J.; Pan, L.; Wang, T. Superhydrophobic Ti6Al4V surfaces with regular array patterns for anti-icing applications. RSC Adv. 2015, 5, 32813–32818. [Google Scholar] [CrossRef]
- Basheer, L.; Cleland, D. Freeze–thaw resistance of concretes treated with pore liners. Constr. Build. Mater. 2006, 20, 990–998. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; He, M.; Li, K.; Cui, D.; Zhang, Q.; Zeng, X.; Zhang, Y.; Wang, J.; Song, Y. Superhydrophobic surfaces cannot reduce ice adhesion. Appl. Phys. Lett. 2012, 101, 111603. [Google Scholar] [CrossRef]
- Chen, J.; Dou, R.; Cui, D.; Zhang, Q.; Zhang, Y.; Xu, F.; Zhou, X.; Wang, J.; Song, Y.; Jiang, L. Robust Prototypical Anti-icing Coatings with a Self-lubricating Liquid Water Layer between Ice and Substrate. ACS Appl. Mater. Interfaces 2013, 5, 4026–4030. [Google Scholar] [CrossRef]
- Ge, D.; Yang, L.; Wu, G.; Yang, S. Spray coating of superhydrophobic and angle-independent coloured films. Chem. Commun. 2014, 50, 2469. [Google Scholar] [CrossRef]
- Weisheit, S.; Unterberger, S.H.; Bader, T.; Lackner, R. Assessment of test methods for characterizing the hydrophobic nature of surface-treated High Performance Concrete. Constr. Build. Mater. 2016, 110, 145–153. [Google Scholar] [CrossRef]
- Izarra, I.; Cubillo, J.; Serrano, A.; Rodriguez, J.; Carmona, M. A hydrophobic release agent containing SiO2-CH3 submicron-sized particles for waterproofing mortar structures. Constr. Build. Mater. 2019, 199, 30–39. [Google Scholar] [CrossRef]
- Arabzadeh, A.; Ceylan, H.; Kim, S.; Gopalakrishnan, K.; Sassani, A.; Information, R. Superhydrophobic Coatings on Asphalt Concrete Surfaces: Toward Smart Solutions for Winter Pavement Maintenance. Transp. Res. Rec. J. Transp. Res. Board 2016, 2551, 10–17. [Google Scholar] [CrossRef]
- Franzoni, E.; Pigino, B.; Pistolesi, C. Ethyl silicate for surface protection of concrete: Performance in comparison with other inorganic surface treatments. Cem. Concr. Compos. 2013, 44, 69–76. [Google Scholar] [CrossRef]
- Polder, R.B.; Borsje, H.; Vries, H.D. Prevention of reinforcement corrosion by hydrophobic treatment of concrete. Heron 2001, 46, 227–238. [Google Scholar]
- Page, C.; Short, N.; El Tarras, A. Diffusion of chloride ions in hardened cement pastes. Cem. Concr. Res. 1981, 11, 395–406. [Google Scholar] [CrossRef]
- ASTM, G102, Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. 2010. Available online: https://www.google.com/search?sxsrf=ACYBGNQLJ1RmYS7NjEI48ptTvJiYbc7J9g%3A1578649552913&ei=0EcYXr-mN8G5kwXH-7WgBg&q=ASTM+G102%2C+Standard+practice+for+calculation+of+corrosion+rates+and+related+information+from+electrochemical+measurements%3B+2010.&oq=ASTM (accessed on 10 January 2020).
- Cigna, R.; Andrade, C.; Nünberger, U.; Polder, R.; Weydert, R.; Seitz, E. Cost Action 521. Corrosion of Steel in Reinforced Concrete Structures: Final Report; European communities: Luxembourg, 2003. [Google Scholar]
- Andrade, C.; González, J.A. Quantitative measurements of corrosion rate of reinforcing steels embedded in concrete using polarization resistance measurements. Mater. Corros. 1978, 29, 515–519. [Google Scholar] [CrossRef]
- Amleh, L.; Mirza, S. Corrosion Influence on Bond between Steel and Concrete. ACI Struct. J. 1999, 96, 415–423. [Google Scholar] [CrossRef]
- Yeau, K.Y.; Kim, E.K. An experimental study on corrosion resistance of concrete with ground granulate blast-furnace slag. Cem. Concr. Res. 2005, 35, 1391–1399. [Google Scholar] [CrossRef]
- ASTM International. ASTM, 876-09. Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]











| Hydrophobic Surface Treatments | Compound and Version | Compound Molecular Structure | Reference |
|---|---|---|---|
| Coating | Silane/siloxane polymer ethanol diluted 1:7 w/w (Silres BS290) | Not Specified | [24] |
| Siloxane contamination from microtextured Polydimethylsiloxane (PDMS) mould | Not Specified | ||
| Mixture of octyltriethoxysilanes isomers, with iso-octyltriethoxysilane as the main component (SILRES BS 1701) | 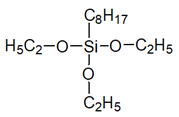 | [25] | |
| Acrylic-based polymer mixed with cement | Not Specified | [26,27] | |
| Hydro-dispersed coating acrylic polymer | Not Specified | [27] | |
| Acrylic-based polymer mixed with sand | Not Specified | ||
| Acrylic resin-based paint in water dispersion | Not Specified | ||
| Polyurethane | Not Specified | [28,29] | |
| Chlorinated rubber | Not Specified | [28] | |
| Epoxy resin | Not Specified | ||
| Acrylic silicone mixture as outer layer and metal silicates as inner layers | Not Specified | [30] | |
| Polymethylhydroxysilane (PMHS) in emulsion with silica fume and polyvinyl alcohol | 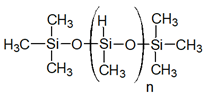 | [31] | |
| 1H,1H,2H,2H-perfluorodecyltriethoxysilane (PFDTS) with nano TiO2 and SiO2 | 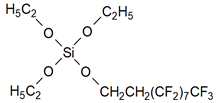 | [32] | |
| Pore Blockage | Sodium silicate | 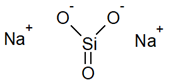 | [33] |
| Acrylic sodium silicate (Silicate + acrylic resin) | Not Specified | [34] | |
| Impregnation | Silane-based (trietoxysilane, alkylalkoxysilane) | 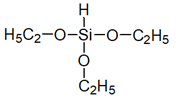 | [34] |
| Silane/siloxane dispersed in water | Not Specified | [35] | |
| Silane/siloxane dispersed in solvent | Not Specified | ||
| Acrylic dispersed in solvent | Not Specified | ||
| Polyurethane | Not Specified | ||
| Isooctyl triethoxy silane/Isobutyl triethoxy silane |  | [36] | |
| Siloxane/oligosiloxane | Not Specified | [37] | |
| Silanes(alkyl alkoxysilanes) | 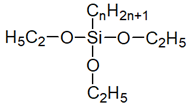 | [38] | |
| Tetraethoxysilane (Estel 1000) |  | [39] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mundo, R.; Labianca, C.; Carbone, G.; Notarnicola, M. Recent Advances in Hydrophobic and Icephobic Surface Treatments of Concrete. Coatings 2020, 10, 449. https://doi.org/10.3390/coatings10050449
Di Mundo R, Labianca C, Carbone G, Notarnicola M. Recent Advances in Hydrophobic and Icephobic Surface Treatments of Concrete. Coatings. 2020; 10(5):449. https://doi.org/10.3390/coatings10050449
Chicago/Turabian StyleDi Mundo, Rosa, Claudia Labianca, Giuseppe Carbone, and Michele Notarnicola. 2020. "Recent Advances in Hydrophobic and Icephobic Surface Treatments of Concrete" Coatings 10, no. 5: 449. https://doi.org/10.3390/coatings10050449
APA StyleDi Mundo, R., Labianca, C., Carbone, G., & Notarnicola, M. (2020). Recent Advances in Hydrophobic and Icephobic Surface Treatments of Concrete. Coatings, 10(5), 449. https://doi.org/10.3390/coatings10050449








