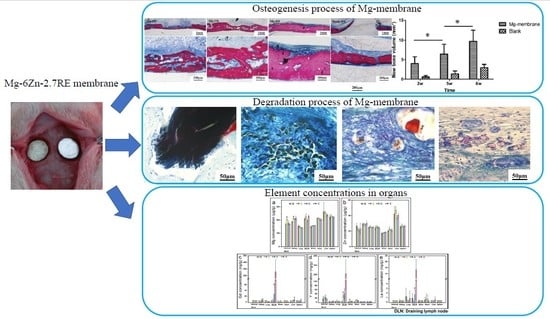
Degradation Behavior, Transport Mechanism and Osteogenic Activity of Mg–Zn–RE Alloy Membranes in Critical-Sized Rat Calvarial Defects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
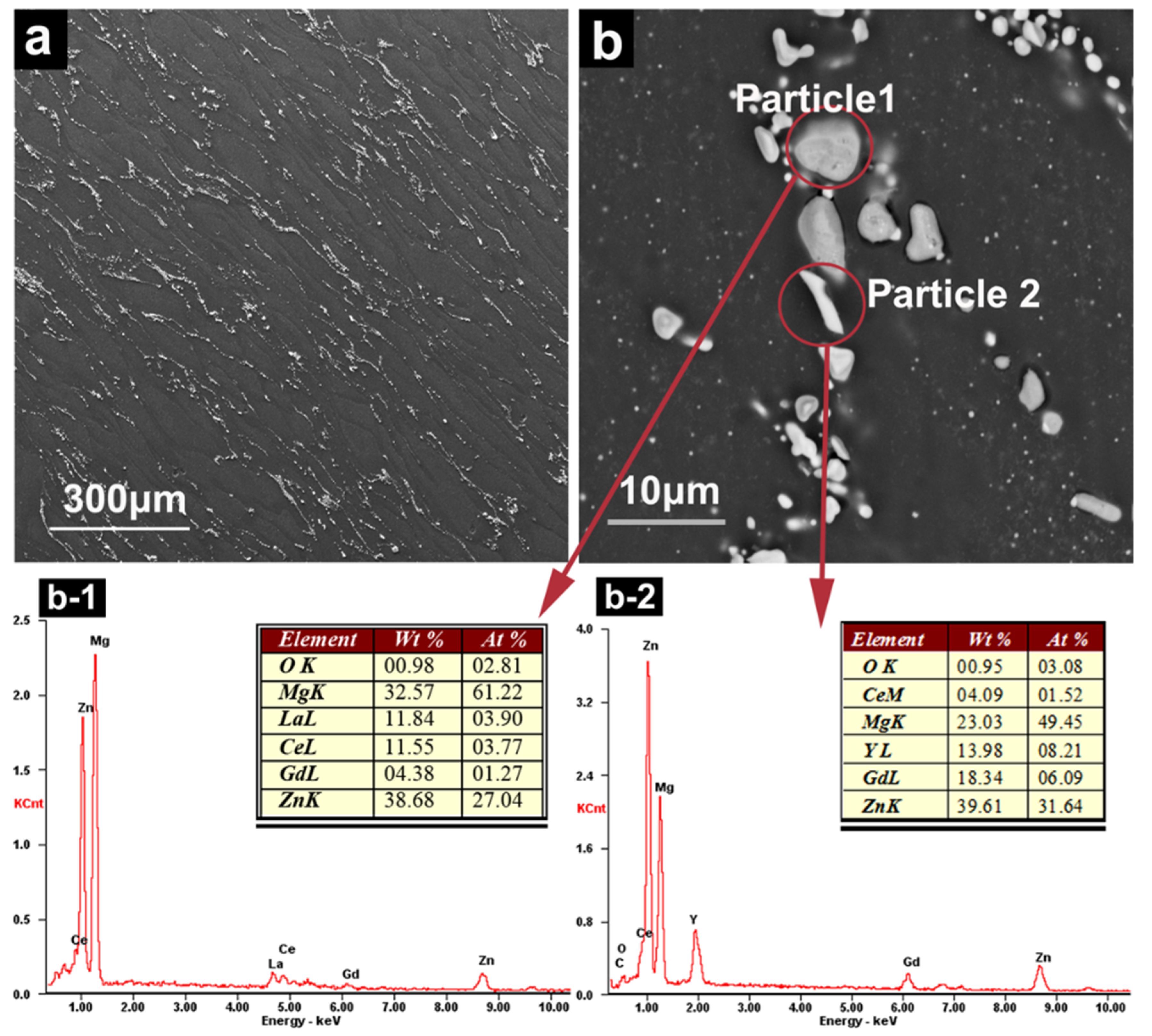
2.2. Characteristics of Precipitates
2.3. Degradation and Histological Evaluation In Vivo
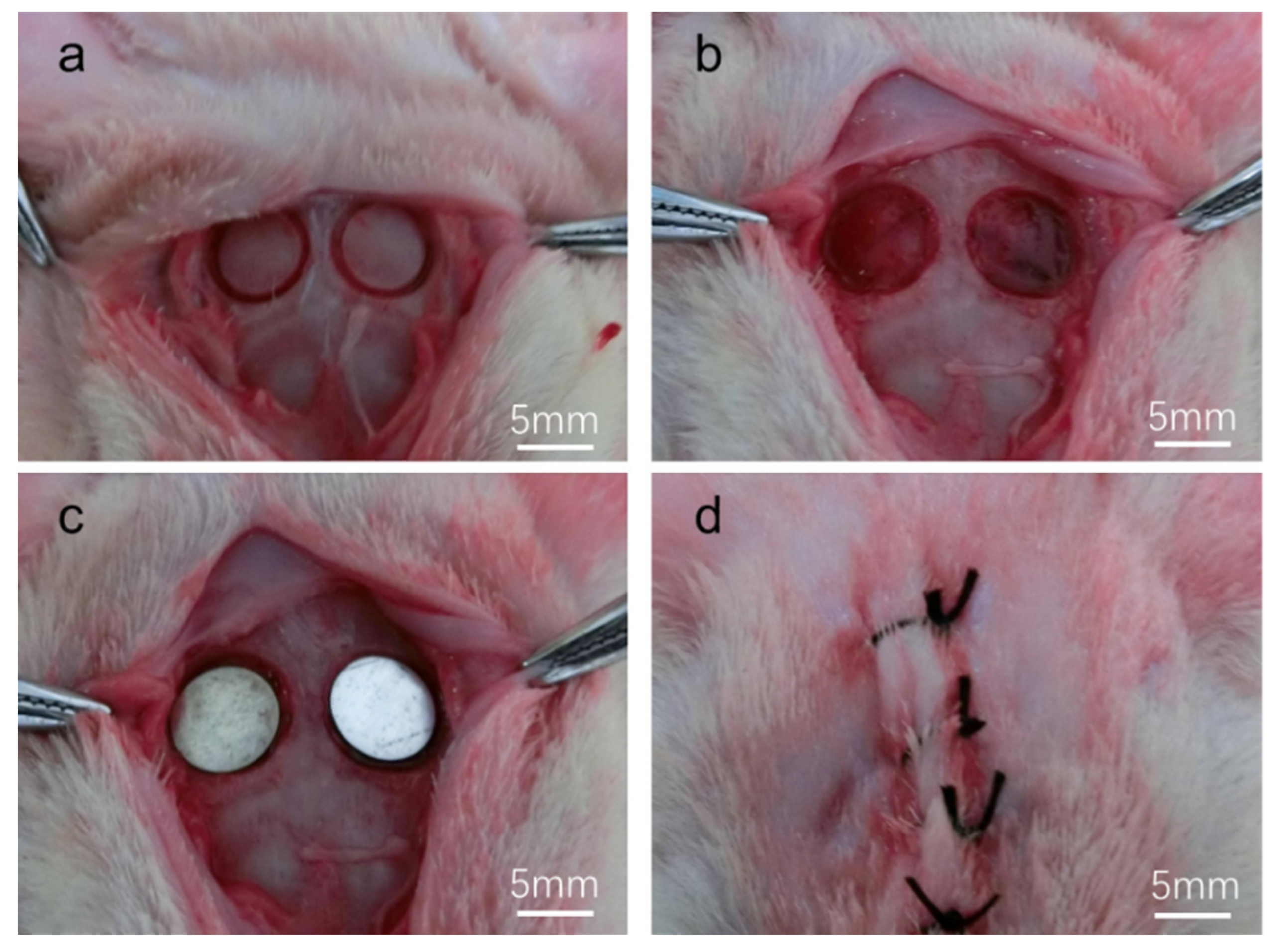
2.3.1. Surgical Procedure
2.3.2. Radiographic Evaluation (Microcomputed Tomography (Micro-CT) Analyses)
2.3.3. Histological Processing
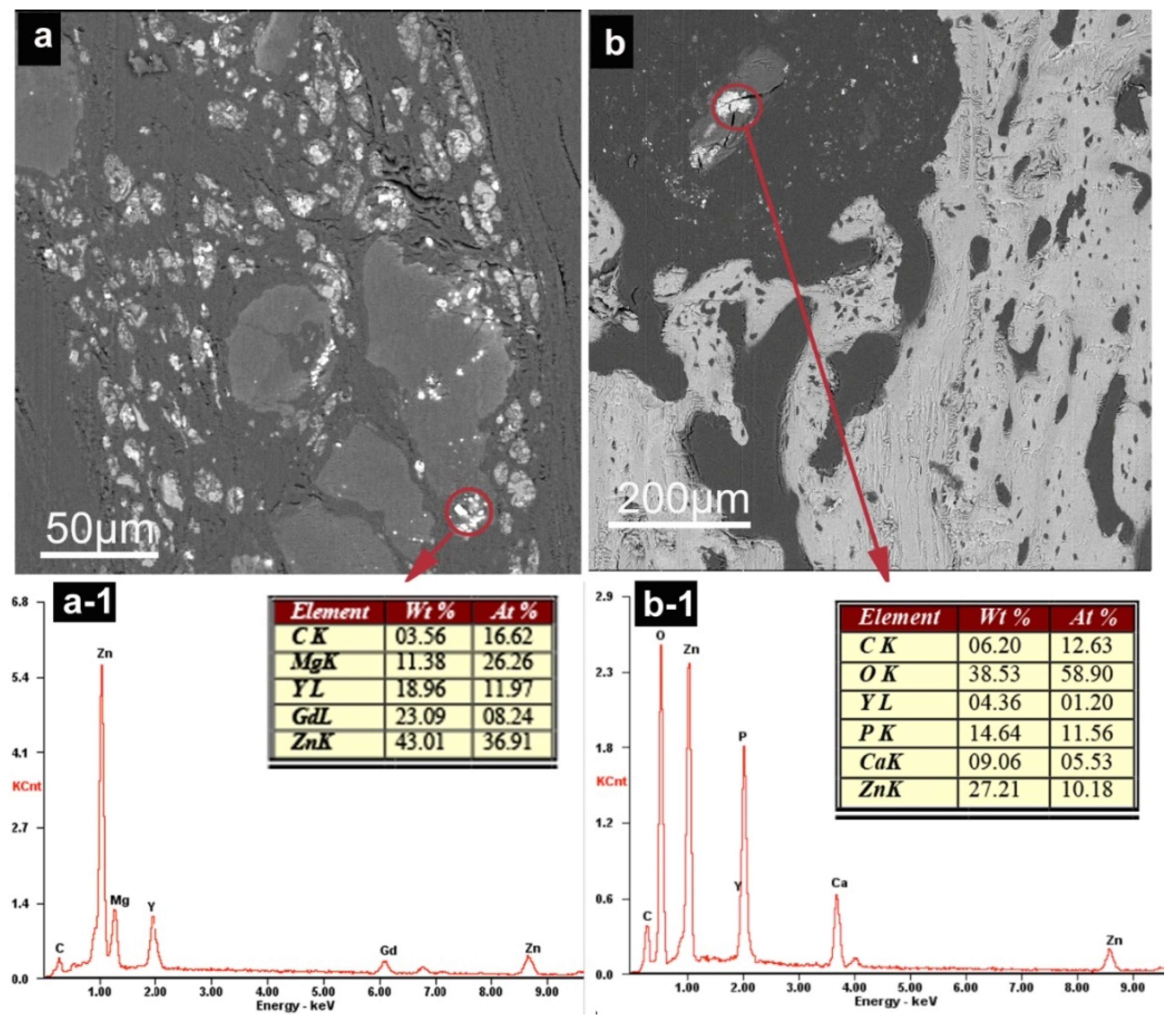
2.3.4. Degradation Characterization In Vivo (SEM Examination)
2.3.5. Determination of REEs, Mg and Zn in Organs
2.3.6. Statistical Analysis
3. Results
3.1. Precipitates of Mg–6Zn–2.7RE Alloy
3.2. Degradation of Mg–6Zn–2.7RE Alloy and Osteogenesis Processes In Vivo
3.2.1. Clinical Examination
3.2.2. Micro-CT Examination
3.2.3. Histological Evaluation
3.2.4. SEM Observation and EDS Analysis
3.2.5. Element Concentrations in Organs
4. Discussion
4.1. Degradation of Mg-6Zn-RE Membrane In Vivo
4.2. Analysis of Mg, Zn and REES in Organs
4.3. Mg Alloy Membrane-Guided Bone Regeneration
5. Conclusions
- (1)
- In the Mg–6Zn–2.7RE membrane degradation process, the Mg matrix degraded first; then, Zn dissolved and REEs were released slowly. The release rate of REEs depended on the type and composition of the precipitates;
- (2)
- REEs accumulated in draining lymph node, and may be transported mainly through the lymph system;
- (3)
- The Mg-membrane used in the present study showed excellent biocompatibility and enhanced bone formation in the vicinity of the implants. This result demonstrates that Mg–membranes are a suitable osteoinduction material for cranio-maxillofacial surgery applications.
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Liu, X.; Wu, S.; Yeung, K.W.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Witte, F. Reprint of: The history of biodegradable magnesium implants: A review. Acta Biomater. 2015, 23, S28–S40. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Miao, H.; Zhang, D.; Chen, C.; Zhang, L.; Pei, J.; Su, Y.; Huang, H.; Wang, Z.; Kang, B.; Ding, W.; et al. Research on biodegradable Mg-Zn-Gd alloys for potential orthopedic implants: In vitro and in vivo evaluations. ACS Biomater. Sci. Eng. 2019, 5, 1623–1634. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, T.; Nahan, K.; Guo, X.; Zhang, Z.; Dong, Z.; Chen, S.; Chou, D.T.; Hong, D.; Kumta, P.N.; et al. In vivo characterization of magnesium alloy biodegradation using electrochemical H2 monitoring, ICP-MS, and XPS. Acta Biomater. 2017, 50, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Zhou, W.; Deng, J.; Liu, Y.; Li, W.; Chu, X.; Xiu, P.; Cai, H.; Kou, Y.; Jiang, B.; et al. Development of magnesium-based biodegradable metals with dietary trace element germanium as orthopaedic implant applications. Acta Biomater. 2017, 64, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Huang, Y. Assessment of magnesium-based biomaterials: From bench to clinic. Biomater. Sci. 2019, 7, 2241–2263. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef]
- Revell, P.A.; Damien, E.; Zhang, X.S.; Evans, P.J.; Howlett, C.R. The Effect of Magnesium Ions on Bone Bonding to Hydroxyapatite Coating on Titanium Alloy Implants. Key Eng. Mater. 2003, 254, 447–450. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, L.; Zhang, X.; Guan, S. Enhancing biocompatibility and corrosion resistance of biodegradable Mg-Zn-Y-Nd alloy by preparing PDA/HA coating for potential application of cardiovascular biomaterials. Mater. Sci. Eng. 2020, 109, 110607. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Sharon, N.M.L. Magnesium, Magnesium Alloys, and Magnesium Composites; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 41–42. [Google Scholar]
- Trivedi, P.; Nune, K.C.; Misra, R.D.K. Degradation behaviour of magnesium rare earth biomedical alloys. Mater. Technol. 2016, 31, 726–731. [Google Scholar] [CrossRef]
- Gusieva, K.; Davies, C.H.J.; Scully, J.R.; Birbilis, N. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2015, 60, 169–194. [Google Scholar] [CrossRef]
- Leng, Z.; Zhang, J.; Zhang, M.; Liu, X.; Zhan, H.; Wu, R. Microstructure and high mechanical properties of Mg–9RY–4Zn (RY: Y-rich misch metal) alloy with long period stacking ordered phase. Mater. Sci. Eng. A 2012, 540, 38–45. [Google Scholar] [CrossRef]
- Eggebrecht, H.; Rodermann, J.; Hunold, P.; Schmermund, A.; Bose, D.; Haude, M.; Erbel, R. Images in cardiovascular medicine. Novel magnetic resonance-compatible coronary stent: The absorbable magnesium-alloy stent. Circulation 2005, 112, 303–304. [Google Scholar] [CrossRef]
- Reifenrath, J.; Angrisani, N.; Erdmann, N.; Lucas, A.; Waizy, H.; Seitz, J.M.; Bondarenko, A.; Meyer-Lindenberg, A. Degrading magnesium screws ZEK100: Biomechanical testing, degradation analysis and soft-tissue biocompatibility in a rabbit model. Biomed. Mater. 2013, 8, 045012. [Google Scholar] [CrossRef]
- Wolters, L.; Besdo, S.; Angrisani, N.; Wriggers, P.; Hering, B.; Seitz, J.M.; Reifenrath, J. Degradation behaviour of LAE442-based plate-screw-systems in an in vitro bone model. Mater. Sci. Eng. C 2015, 49, 305–315. [Google Scholar] [CrossRef]
- Rettig, R.; Virtanen, S. Composition of corrosion layers on a magnesium rare-earth alloy in simulated body fluids. J. Biomed. Mater. Res. A 2009, 88A, 359–369. [Google Scholar] [CrossRef]
- He, W.; Zhang, E.; Yang, K. Effect of Y on the bio-corrosion behavior of extruded Mg–Zn–Mn alloy in Hank’s solution. Mater. Sci. Eng. C 2010, 30, 167–174. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.F.; Hanzi, A.C.; Uggowitzer, P.J.; Loffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Dziuba, D.; Meyer-Lindenberg, A.; Seitz, J.M.; Waizy, H.; Angrisani, N.; Reifenrath, J. Long-term In vivo degradation behaviour and biocompatibility of the magnesium alloy ZEK100 for use as a biodegradable bone implant. Acta Biomater. 2013, 9, 8548–8560. [Google Scholar] [CrossRef] [PubMed]
- Amerstorfer, F.; Fischerauer, S.F.; Fischer, L.; Eichler, J.; Draxler, J.; Zitek, A.; Meischel, M.; Martinelli, E.; Kraus, T.; Hann, S.; et al. Long-term In vivo degradation behavior and near-implant distribution of resorbed elements for magnesium alloys WZ21 and ZX50. Acta Biomater. 2016, 42, 440–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, H.; Wang, W.; Huang, H.; Pei, J.; Qu, H.; Yuan, G.; Li, Y. The degradation and transport mechanism of a Mg-Nd-Zn-Zr stent in rabbit common carotid artery: A 20-month study. Acta Biomater. 2018, 69, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Myrissa, A.; Braeuer, S.; Martinelli, E.; Willumeit-Romer, R.; Goessler, W.; Weinberg, A.M. Gadolinium accumulation in organs of Sprague-Dawley(R) rats after implantation of a biodegradable magnesium-gadolinium alloy. Acta Biomater. 2017, 48, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg-Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application A Review. J. Mater. Sci. Tech. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Watanabe, H.; Moriwaki, K.; Mukai, T.; Ohsuna, T.; Hiraga, K.; Higashi, K. Materials processing for structural stability in a ZK60 magnesium alloy. Mater. Trans. 2003, 44, 7. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Song, D.; Zhang, S.; Tang, Y.; Zhao, D. Effect of heat-treatment on the stability of the quasicrystal in a Mg-Zn-Zr-Y alloy. Mater. Lett. 1994, 21, 85–88. [Google Scholar] [CrossRef]
- Ghosh, P.; Mezbahul-Islam, M.; Medraj, M. Critical assessment and thermodynamic modeling of Mg–Zn, Mg–Sn, Sn–Zn and Mg–Sn–Zn systems. Calphad 2012, 36, 28–43. [Google Scholar] [CrossRef]
- Rossig, C.; Angrisani, N.; Helmecke, P.; Besdo, S.; Seitz, J.M.; Welke, B.; Fedchenko, N.; Kock, H.; Reifenrath, J. In vivo evaluation of a magnesium-based degradable intramedullary nailing system in a sheep model. Acta Biomater. 2015, 25, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, H.; Seitz, J.M.; Acil, Y.; Damm, T.; Moller, I.; Gulses, A.; Wiltfang, J. Osteosynthesis of a cranio-osteoplasty with a biodegradable magnesium plate system in miniature pigs. Acta Biomater. 2017, 62, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and In vivo bone formation by activating Wnt/catenin signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Atrens, A.; Dargusch, M. Influence of microstructure on the corrosion of diecast AZ91D. Corros. Sci. 1998, 41, 249–273. [Google Scholar] [CrossRef]
- Deshpande, K.B. Numerical modeling of micro-galvanic corrosion. Electrochim. Acta 2011, 56, 1737–1745. [Google Scholar] [CrossRef]
- Walter, R.; Kannan, M.B. A mechanistic in vitro study of the microgalvanic degradation of secondary phase particles in magnesium alloys. J. Biomed. Mater. Res. 2015, 103, 990–1000. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Seitz, J.M.; Eifler, R.; Bach, F.W.; Maier, H.J. Magnesium degradation products: Effects on tissue and human metabolism. J. Biomed. Mater. Res. 2014, 102, 3744–3753. [Google Scholar] [CrossRef]
- Velard, F.; Braux, J.; Amedee, J.; Laquerriere, P. Inflammatory cell response to calcium phosphate biomaterial particles: An overview. Acta Biomater. 2013, 9, 4956–4963. [Google Scholar] [CrossRef]
- Quamme, G.; Rouffignac, C. Epithelial magnesium transport and regulation by the kidney. Front. Biosci. 2000, 5, 694–711. [Google Scholar] [CrossRef] [Green Version]
- Kemp, P.A.; Gardiner, S.M.; March, J.E.; Rubin, P.C.; Bennett, T. Assessment of the effect of endothelin-1 and magnesium sulphate on regional blood flows in conscious rats, by the coloured microsphere reference technique. Br. J. Pharmacol. 1999, 126, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, S.; Suzuki, K.T. Exposure, Metabolism, and Toxicity of Rare Earths and Related Compounds. Environ. Health Perspect. 1996, 104, 85–95. [Google Scholar] [PubMed]
- Schaller, B.; Saulacic, N.; Imwinkelried, T.; Beck, S.; Liu, E.W.; Gralla, J.; Nakahara, K.; Hofstetter, W.; Iizuka, T. In vivo degradation of magnesium plate/screw osteosynthesis implant systems: Soft and hard tissue response in a calvarial model in miniature pigs. J. Craniomaxillofac. Surg. 2016, 44, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, D.; Li, X.; Hu, S. Mechanical properties, corrosion resistance and biocompatibilities of degradable Mg-RE alloys: A review. J. Mater. Res. Technol. 2019, 8, 1538–1549. [Google Scholar] [CrossRef]
- Kang, M.H.; Lee, H.M.; Jang, T.; Seong, Y.J.; Kim, H.; Koh, Y.; Song, J.; Jung, H. Biomimetic porous Mg with tunable mechanical properties and biodegradation rates for bone regeneration. Acta Biomater. 2019, 84, 453–467. [Google Scholar] [CrossRef]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Myers, N.; Costello, B.J.; Chou, D.T.; Pal, S.; Maiti, S.; Kumta, P.N.; Sfeir, C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 2015, 18, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Luthringer, B.J.C.; Feyerabend, F.; Schilling, A.F.; Willumeit, R. Effects of extracellular magnesium on the differentiation and function of human osteoclasts. Acta Biomater. 2014, 10, 2843–2854. [Google Scholar] [CrossRef] [Green Version]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Liu, G.; Li, Y.; Yu, X.; Yuan, S.; Nie, Z.; Wang, J.; Han, J.; Tan, C.; Guo, C. Degradation Behavior, Transport Mechanism and Osteogenic Activity of Mg–Zn–RE Alloy Membranes in Critical-Sized Rat Calvarial Defects. Coatings 2020, 10, 496. https://doi.org/10.3390/coatings10050496
Zhao M, Liu G, Li Y, Yu X, Yuan S, Nie Z, Wang J, Han J, Tan C, Guo C. Degradation Behavior, Transport Mechanism and Osteogenic Activity of Mg–Zn–RE Alloy Membranes in Critical-Sized Rat Calvarial Defects. Coatings. 2020; 10(5):496. https://doi.org/10.3390/coatings10050496
Chicago/Turabian StyleZhao, Mingyu, Guanqi Liu, Ying Li, Xiaodong Yu, Shenpo Yuan, Zhihua Nie, Jiewen Wang, Jianmin Han, Chengwen Tan, and Chuanbin Guo. 2020. "Degradation Behavior, Transport Mechanism and Osteogenic Activity of Mg–Zn–RE Alloy Membranes in Critical-Sized Rat Calvarial Defects" Coatings 10, no. 5: 496. https://doi.org/10.3390/coatings10050496