Fabrication of Robust Water-Repellent Technology on Cotton Fabric via Reaction of Thiol-ene Click Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
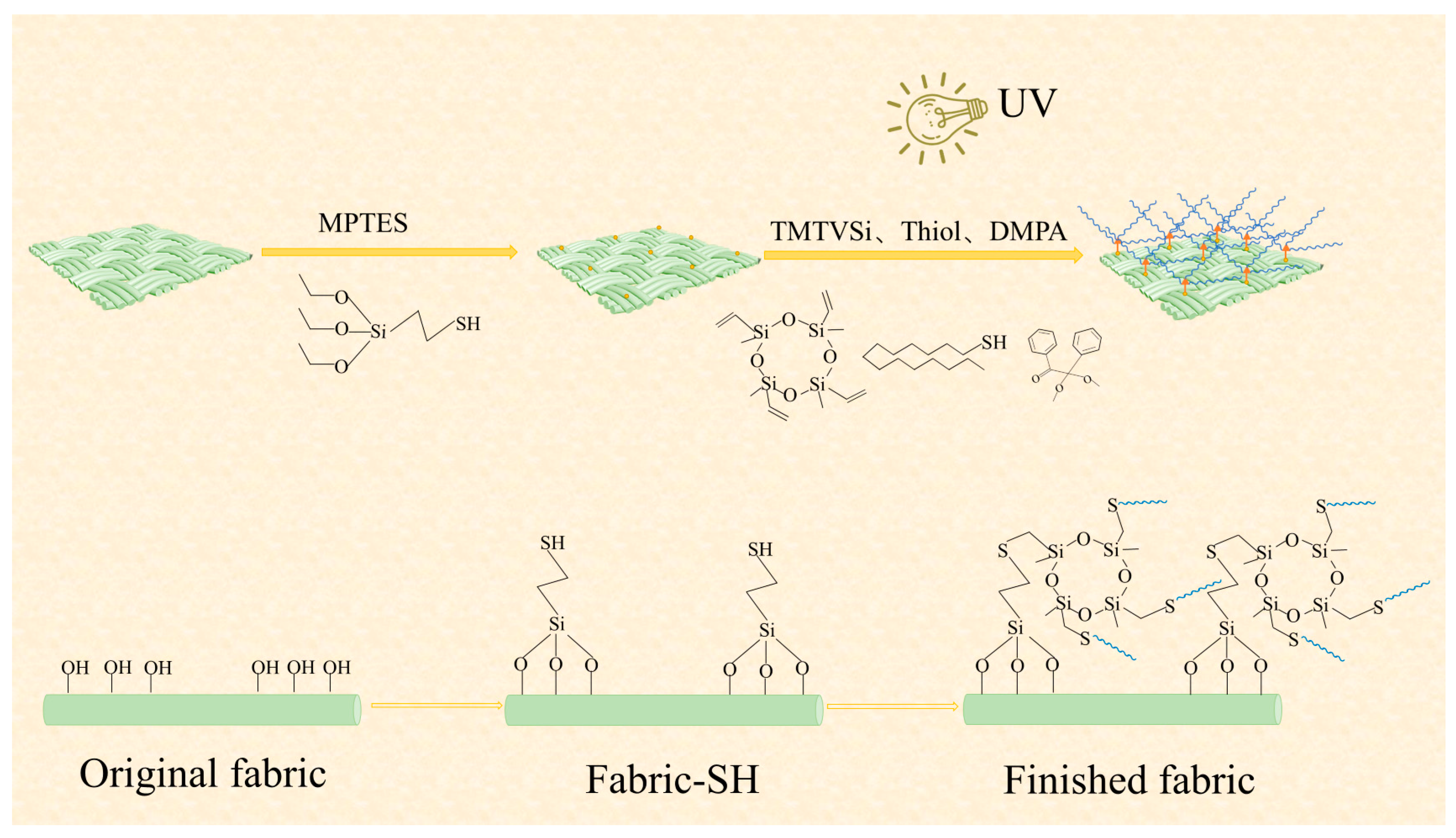
2.2. Preparation of Mercapto-Modified Cotton Fabric
2.3. Superhydrophobic Fabric Finishing Process
2.4. Characterization
3. Results and Discussion
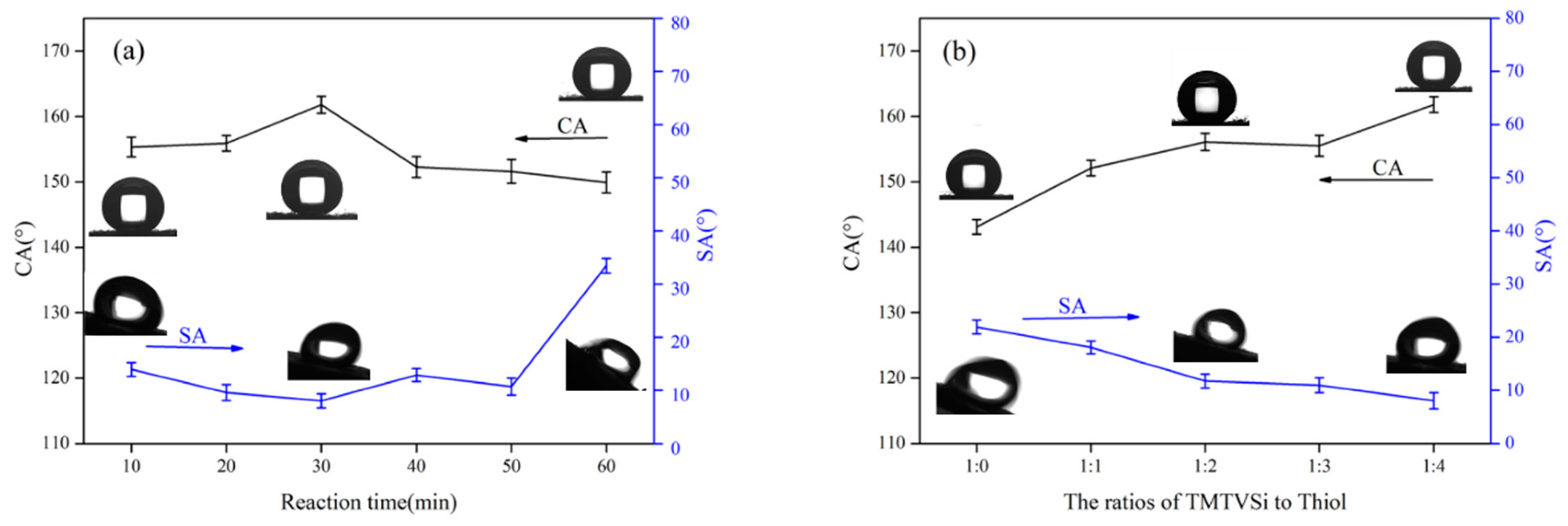
3.1. Effects of UV Irradiation Time and the Ratios of TMTVSi to Octadecyl Mercaptan on the Superhydrophobicity of Cotton Fabric
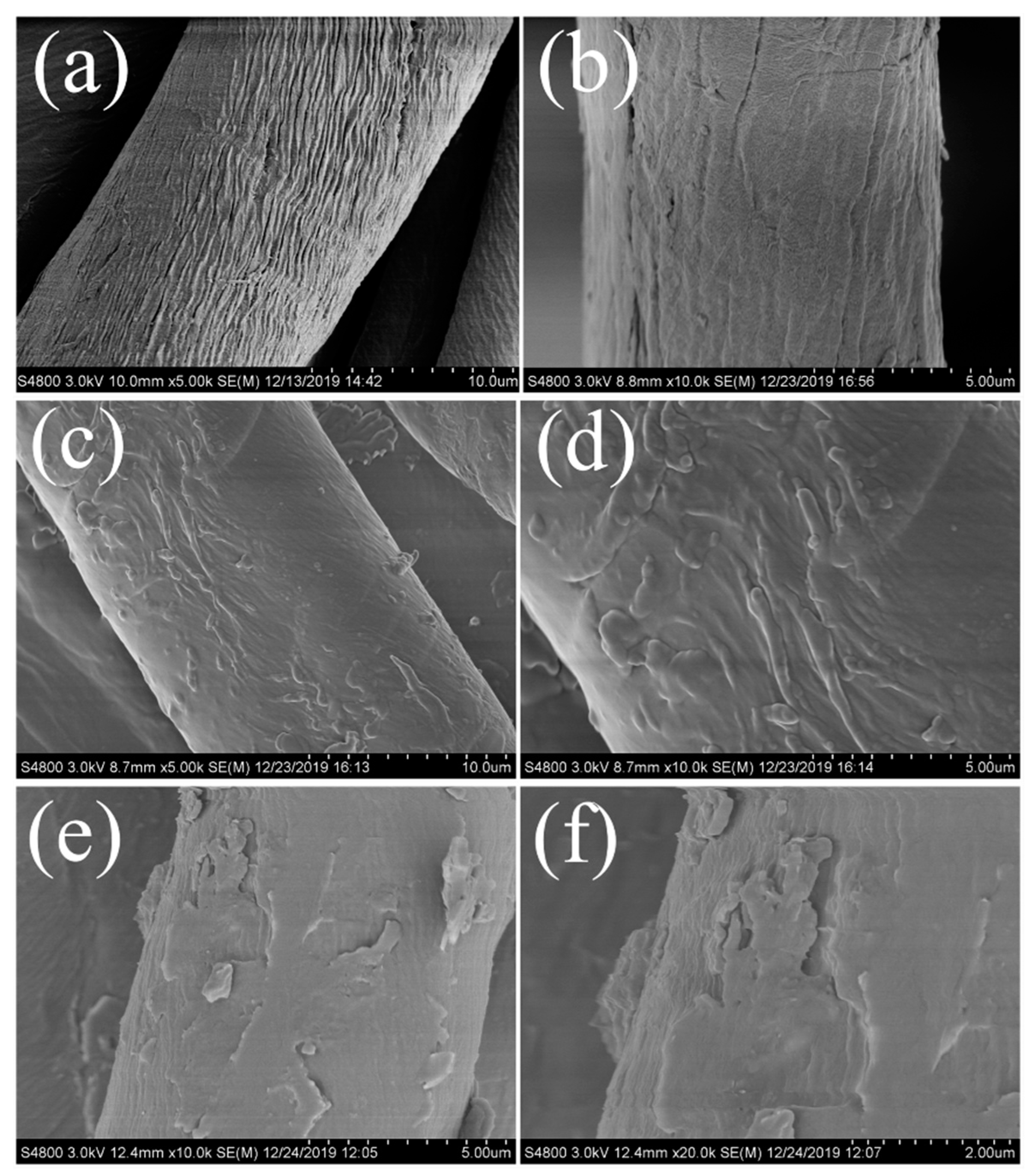
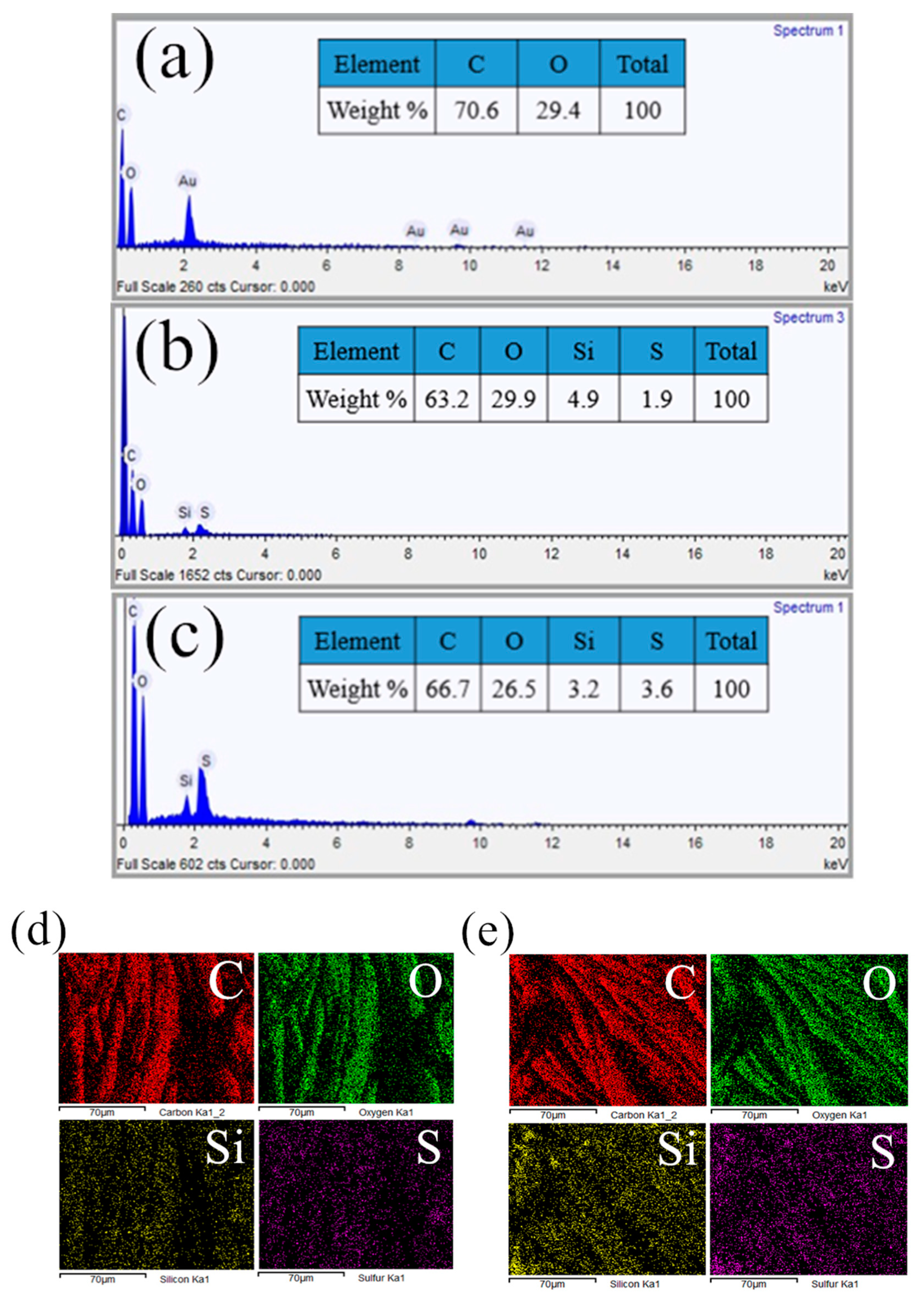
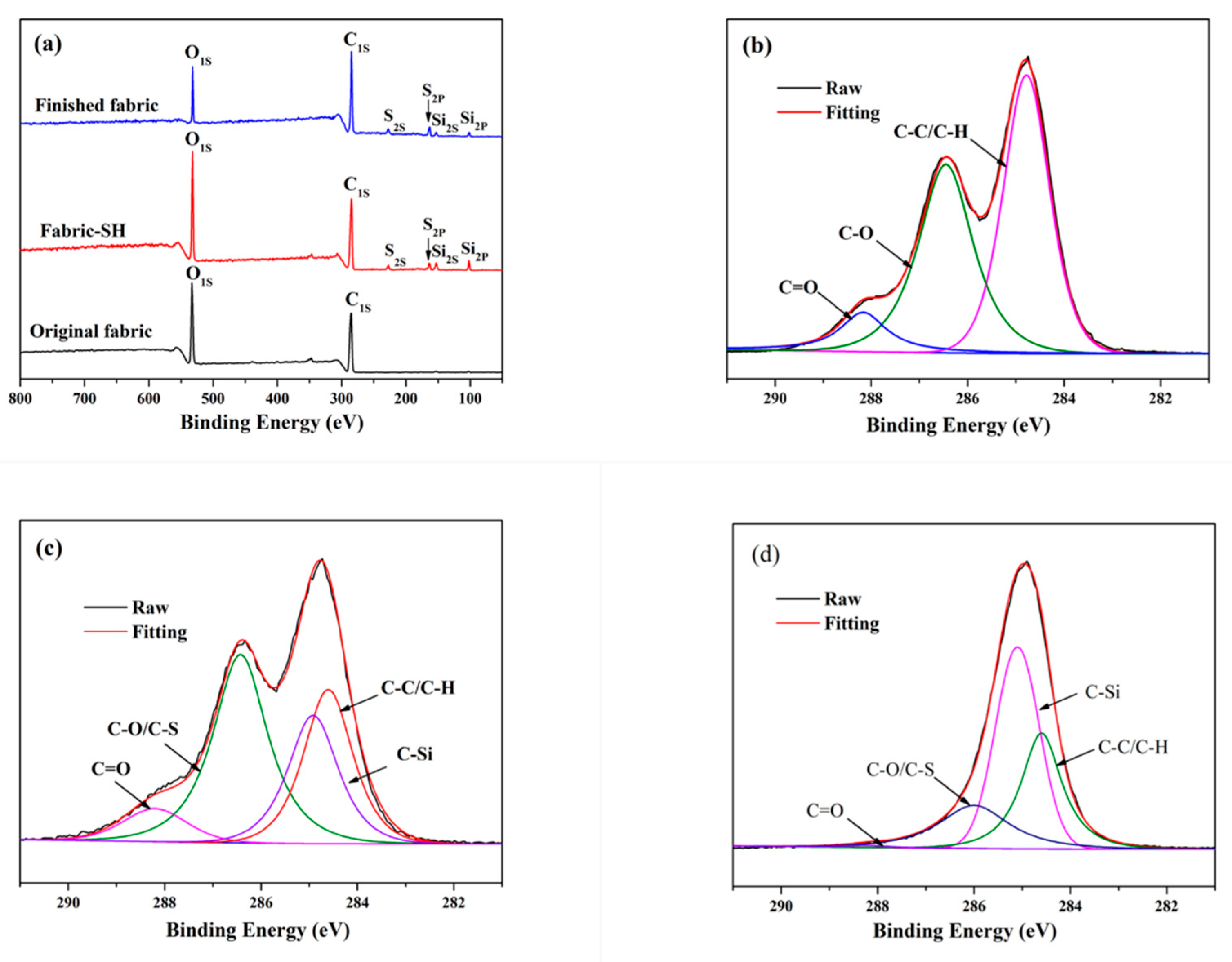
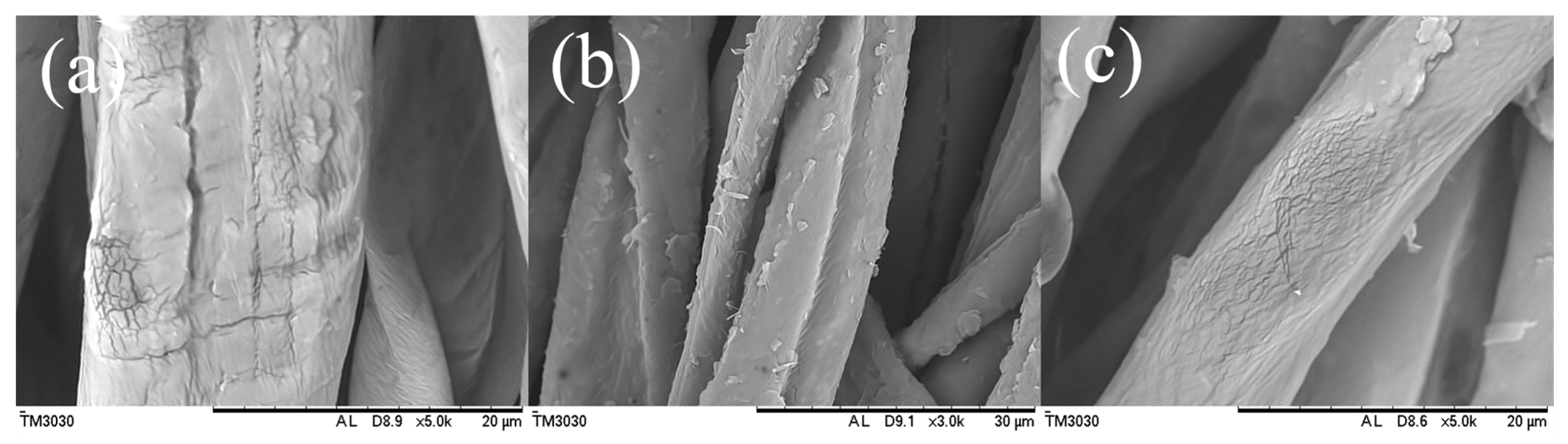
3.2. Morphology and Composition of the Fabric Samples
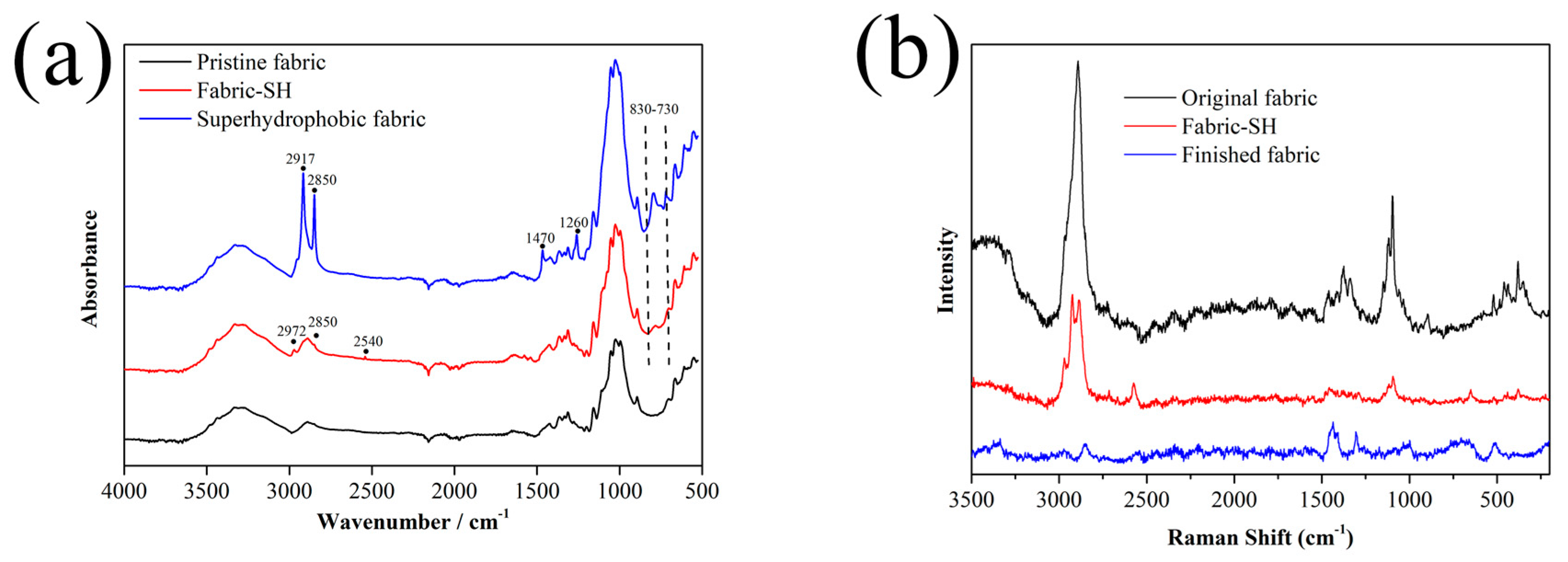
3.3. FTIR-ATR and Raman Spectroscopy
3.4. Durability and Stability
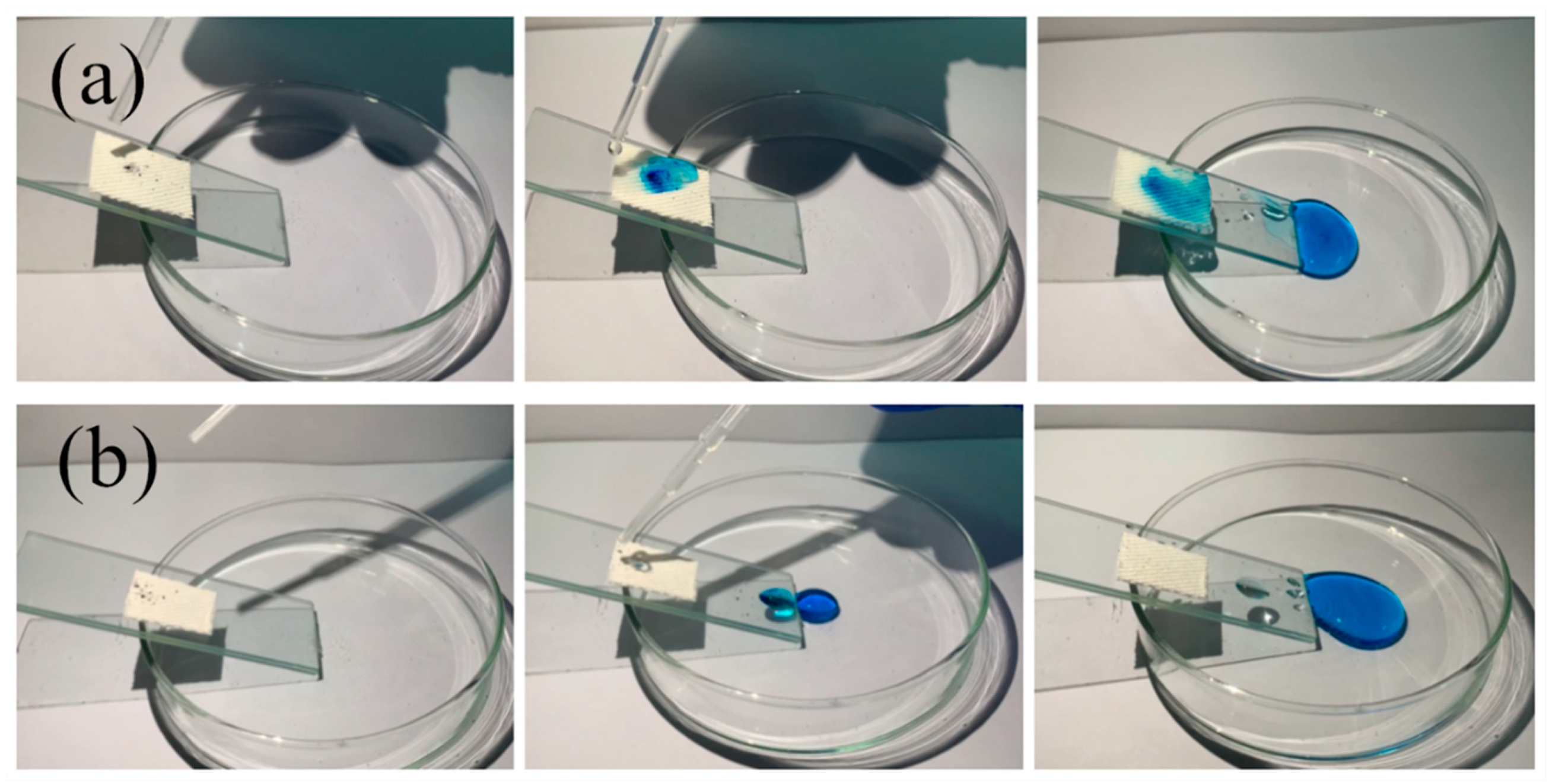
3.5. Self-cleaning Performance of the Modified Fabric
3.6. Oil/water Separation Property of the Modified Fabric
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Razaq, A.; Asif, M.H.; Kalsoom, R.; Khan, A.F.; Awan, M.S.; Ishrat, S.; Ramay, S.M. Conductive and electroactive composite paper reinforced by coating of polyaniline on lignocelluloses fibers. J. Appl. Polym. Sci. 2015, 132, 42293. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Li, S.; Huang, J.; Mihailiasa, M.; Lai, Y. Rational design of multi-layered superhydrophobic coating on cotton fabrics for UV shielding, self-cleaning and oil-water separation. Mater. Des. 2017, 134, 342–351. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Ge, M.; Cao, C.; Deng, S.; Zhang, S.; Lai, Y. Robust flower-like TiO2@ cotton fabrics with special wettability for effective self-cleaning and versatile oil/water separation. Adv. Mater. Interfaces 2015, 2, 1500220. [Google Scholar] [CrossRef]
- Wang, J.; Han, F.; Liang, B.; Geng, G. Hydrothermal fabrication of robustly superhydrophobic cotton fibers for efficient separation of oil/water mixtures and oil-in-water emulsions. J. Ind. Eng. Chem. 2017, 54, 174–183. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Li, X.M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef]
- Yang, Y.; He, H.; Li, Y.; Qiu, J. Using Nanoimprint Lithography to Create Robust, Buoyant, Superhydrophobic PVB/SiO2 Coatings on wood Surfaces Inspired by Red roses petal. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Yao, Y.; Gellerich, A.; Zauner, M.; Wang, X.; Zhang, K. Differential anti-fungal effects from hydrophobic and superhydrophobic wood based on cellulose and glycerol stearoyl esters. Cellulose 2018, 25, 1329–1338. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Lai, X.; Su, X.; Liang, T.; Zeng, X. Thiolated graphene-based superhydrophobic sponges for oil-water separation. Chem. Eng. J. 2017, 316, 736–743. [Google Scholar] [CrossRef]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Superhydrophobic fabrics with mechanical durability prepared by a two-step plasma processing method. Coatings 2018, 8, 351. [Google Scholar] [CrossRef] [Green Version]
- Makowski, T.; Svyntkivska, M.; Piorkowska, E.; Mizerska, U.; Fortuniak, W.; Kowalczyk, D.; Brzezinski, S. Conductive and superhydrophobic cotton fabric through pentaerythritol tetrakis (3-(3, 5-di-tert-butyl-4-hydroxyphenyl) propionate) assisted thermal reduction of graphene oxide and modification with methyltrichlorosilane. Cellulose 2018, 25, 5377–5388. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Gao, B.; Bian, Y.; Liu, X.; He, Z.; Zeng, Y.; Du, X.; Gu, Z. Fast Strategy to Functional Paper Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 14445–14456. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yao, T.; Wu, J.; Ma, C.; Fan, Z.; Wang, Z.; Yang, B. Facile approach in fabricating superhydrophobic and superoleophilic surface for water and oil mixture separation. ACS Appl. Mater. Interfaces 2009, 1, 2613–2617. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.Y.; Guan, C.S.; Li, Y.D.; Zhu, J.J.; Zeng, B. Robust and durable superhydrophobic cotton fabrics via a one-step solvothermal method for efficient oil/water separation. Cellulose 2019, 26, 2861–2872. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, Z.; Yang, H.; Hou, K.; Zeng, X.; Zheng, Y.; Cheng, J. Nature-inspired strategy toward superhydrophobic fabrics for versatile oil/water separation. ACS Appl. Mater. Interfaces 2017, 9, 9184–9194. [Google Scholar] [CrossRef]
- Xu, C.L.; Song, F.; Wang, X.L.; Wang, Y.Z. Surface modification with hierarchical CuO arrays toward a flexible, durable superhydrophobic and self-cleaning material. Chem. Eng. J. 2017, 313, 1328–1334. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Feng, X.; Yue, G.; Yang, W. Fabrication of superhydrophobicity on cotton fabric by sol–gel. Appl. Surf. Sci. 2012, 258, 8134–8138. [Google Scholar] [CrossRef]
- Lau, K.K.; Bico, J.; Teo, K.B.; Chhowalla, M.G.; Amaratunga, A.; Milne, W.I.; Gleason, K.K. Superhydrophobic carbon nanotube forests. Nano Lett. 2003, 3, 1701–1705. [Google Scholar] [CrossRef] [Green Version]
- Xiong, M.; Ren, Z.; Liu, W. Fabrication of UV-resistant and superhydrophobic surface on cotton fabric by functionalized polyethyleneimine/SiO2 via layer-by-layer assembly and dip-coating. Cellulose 2019, 26, 8951–8962. [Google Scholar] [CrossRef]
- Barthwal, S.; Lim, S.H. Rapid fabrication of a dual-scale micro-nanostructured superhydrophobic aluminum surface with delayed condensation and ice formation properties. Soft Matter 2019, 15, 7945–7955. [Google Scholar] [CrossRef] [PubMed]
- Tarrade, J.; Darmanin, T.E.; Guittard, F.; Debarnot, D.F. Poncin-Epaillard, Texturation and superhydrophobicity of polyethylene terephthalate thanks to plasma technology. Appl. Surf. Sci. 2014, 292, 782–789. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Hong, T.; Liu, W.; Li, M.; Chen, C. Click chemistry at the microscale. Analyst 2019, 144, 1492–1512. [Google Scholar] [CrossRef] [PubMed]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol–ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Tucker-Schwartz, A.K.; Farrell, R.A.; Garrell, R.L. Thiol–ene click reaction as a general route to functional trialkoxysilanes for surface coating applications. J. Am. Chem. Soc. 2011, 133, 11026–11029. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol–ene “click” reactions and recent applications in polymer and materials synthesis: A first update. Polym. Chem. 2014, 5, 4820–4870. [Google Scholar] [CrossRef]
- Dondoni, A.; Marra, A. Recent applications of thiol–ene coupling as a click process for glycoconjugation. Chem. Soc. Rev. 2012, 41, 573–586. [Google Scholar] [CrossRef]
- Rissing, C.; Son, D.Y. Thiol-ene chemistry of vinylsilanes. Main Group Chem. 2009, 8, 251–262. [Google Scholar] [CrossRef]
- Drozdov, F.V.; Cherkaev, G.V.; Muzafarov, A.M. Synthesis of new functional siloxane derivatives of limonene. Part I: Combination of hydrosilylation and hydrothiolation reactions. J. Organomet. Chem. 2019, 880, 293–299. [Google Scholar] [CrossRef]
- Resetco, C.; Hendriks, B.; Badi, N.; Du Prez, F. Thiol–ene chemistry for polymer coatings and surface modification–building in sustainability and performance. Mater. Horiz. 2017, 4, 1041–1053. [Google Scholar] [CrossRef]
- Liu, G.; Wang, W.; Yu, D. Robust and self-healing superhydrophobic cotton fabric via UV induced click chemistry for oil/water separation. Cellulose 2019, 26, 3529–3541. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Li, Y.; Sun, J. Intumescent flame-retardant and self-healing superhydrophobic coatings on cotton fabric. ACS Nano 2015, 9, 4070–4076. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.H.; Guo, X.J.; Zhang, M.M.; Ma, J.Z.; Jia, S.T. Fabrication of robust superhydrophobic surfaces by modification of chemically roughened fibers via thiol–ene click chemistry. J. Mater. Chem. A 2015, 3, 21797–21804. [Google Scholar] [CrossRef]
- Taghavikish, M.; Subianto, S.; Dutta, N.K.; Roy Choudhury, N. Novel thiol-ene hybrid coating for metal protection. Coatings 2016, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- American Association of Textile Chemists and Colorists. AATCC Technical Manual; American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 2007. [Google Scholar]
- Xue, C.H.; Fan, Q.Q.; Guo, X.J.; Jia, S.T. Fabrication of superhydrophobic cotton fabrics by grafting of POSS-based polymers on fibers. Appl. Surf. Sci. 2019, 465, 241–248. [Google Scholar] [CrossRef]
- Hou, K.; Zeng, Y.; Zhou, C.; Chen, J.; Wen, X.; Xu, S.; Pi, P. Facile generation of robust POSS-based superhydrophobic fabrics via thiol-ene click chemistry. Chem. Eng. J. 2018, 332, 150–159. [Google Scholar] [CrossRef]
- Zaharescu, M.; Jitianu, A.; Braileanu, A.; Madarász, J.; Pokol, G. Ageing effect on the SiO2-based inorganic-organic hybrid materials. J. Therm. Anal. Calorim. 2001, 64, 689–696. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, W.; Yang, M.; Zhang, F.; Shi, H.; Xie, Y.; Wang, Z. Robust fabrication of superhydrophobic and photocatalytic self-cleaning cotton textiles for oil–water separation via thiol-ene click reaction. J. Mater. Sci. 2019, 54, 7369–7382. [Google Scholar] [CrossRef]
- Bao, B.; Bai, S.; Fan, J.; Su, J.; Wang, W.; Yu, D. A novel and durable photochromic cotton-based fabric prepared via thiol-ene click chemistry. Dyes Pigments 2019, 171, 107778. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, B.; Chu, R.; Xing, T.; Chen, G. Fabrication of Robust Water-Repellent Technology on Cotton Fabric via Reaction of Thiol-ene Click Chemistry. Coatings 2020, 10, 508. https://doi.org/10.3390/coatings10060508
Chen X, Wang B, Chu R, Xing T, Chen G. Fabrication of Robust Water-Repellent Technology on Cotton Fabric via Reaction of Thiol-ene Click Chemistry. Coatings. 2020; 10(6):508. https://doi.org/10.3390/coatings10060508
Chicago/Turabian StyleChen, Xinpeng, Baoliang Wang, Runshan Chu, Tieling Xing, and Guoqiang Chen. 2020. "Fabrication of Robust Water-Repellent Technology on Cotton Fabric via Reaction of Thiol-ene Click Chemistry" Coatings 10, no. 6: 508. https://doi.org/10.3390/coatings10060508





