Development of Robust Chitosan–Silica Class II Hybrid Coatings with Antimicrobial Properties for Titanium Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Coating Materials
2.2. Deposition of Hybrid Coatings
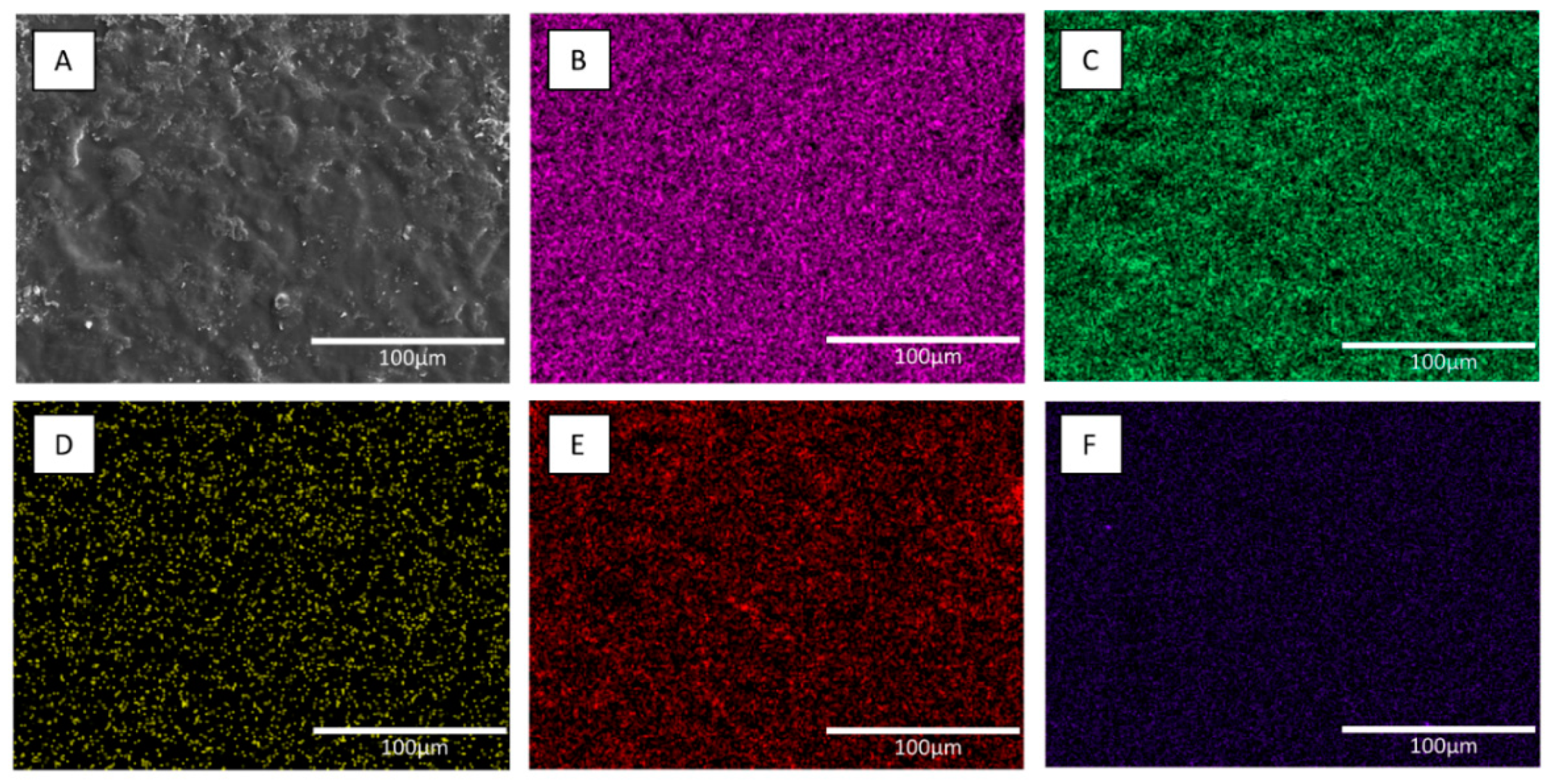
2.3. Characterization of Coating Surfaces
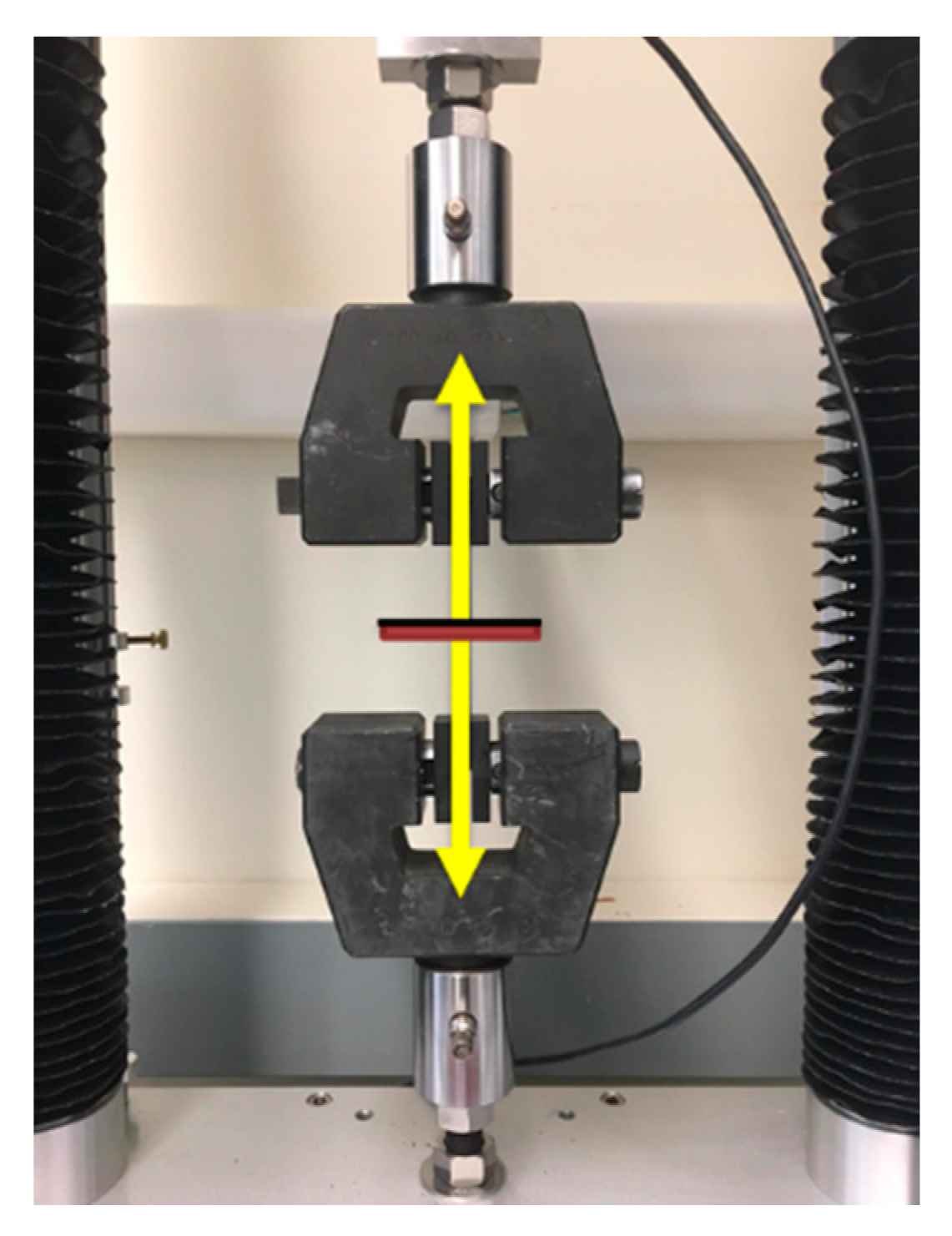
2.4. Evaluation of Mechanical Properties
2.5. Evaluation of Antimicrobial Properties
2.6. Statistical Analysis
3. Results and Discussion
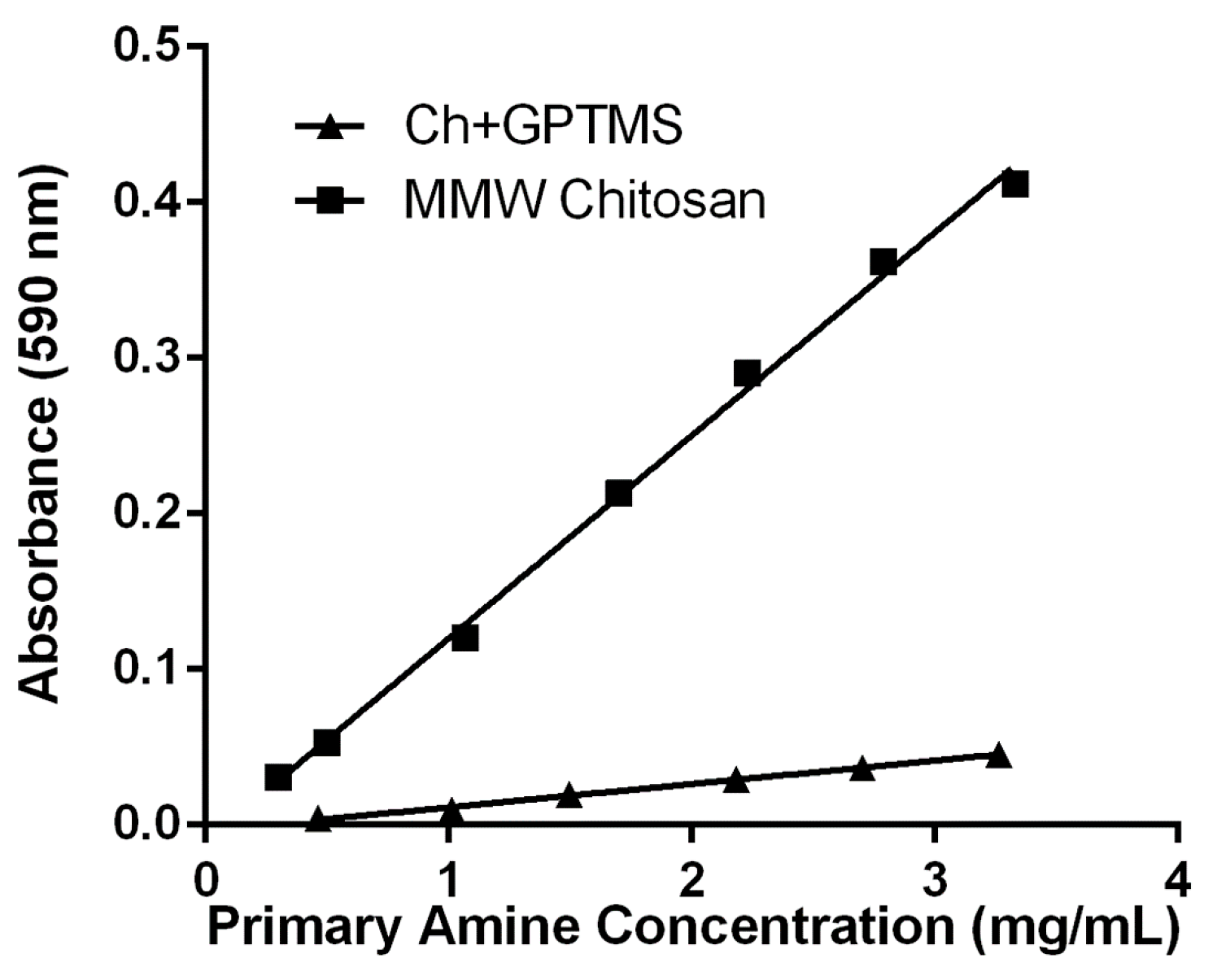
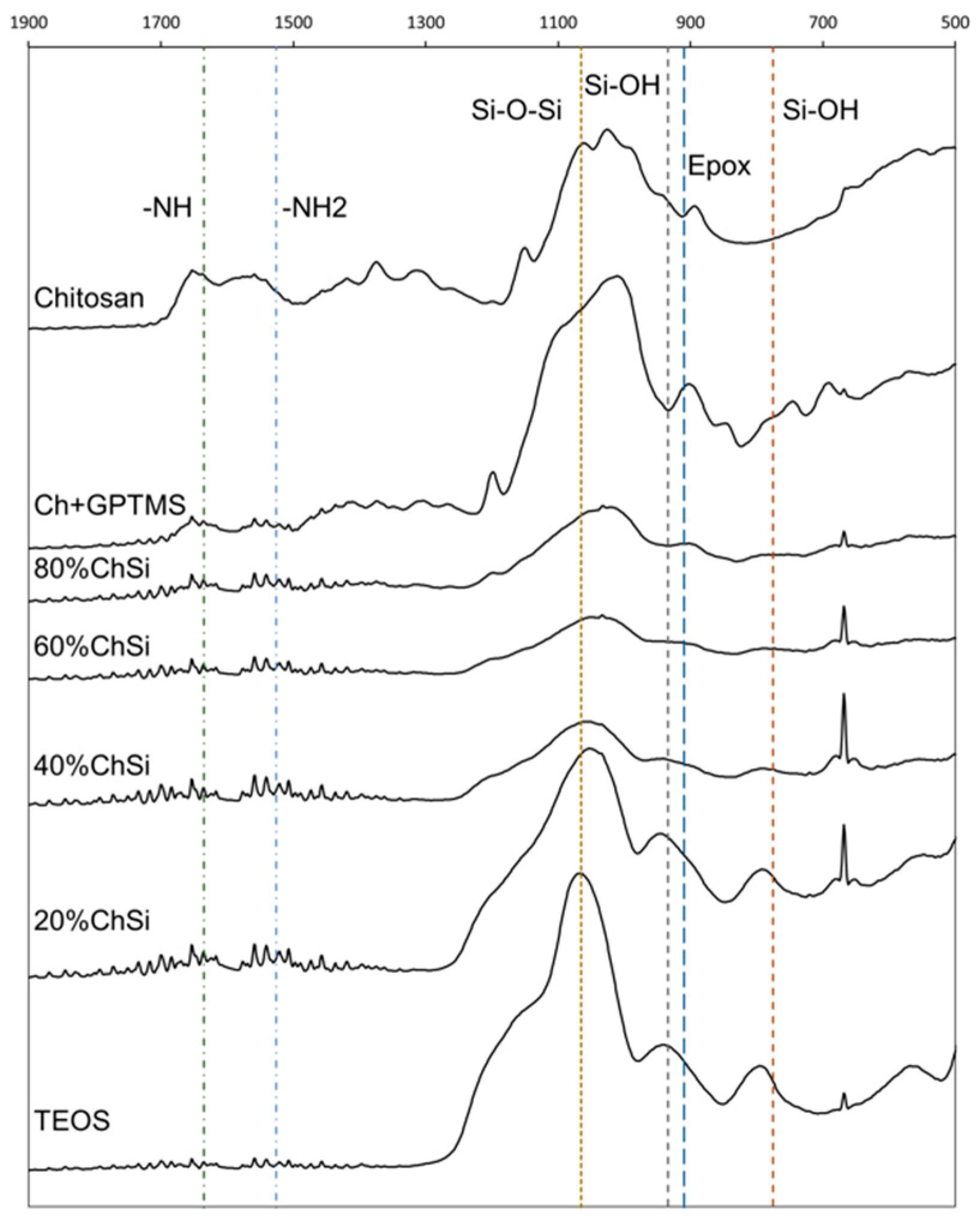
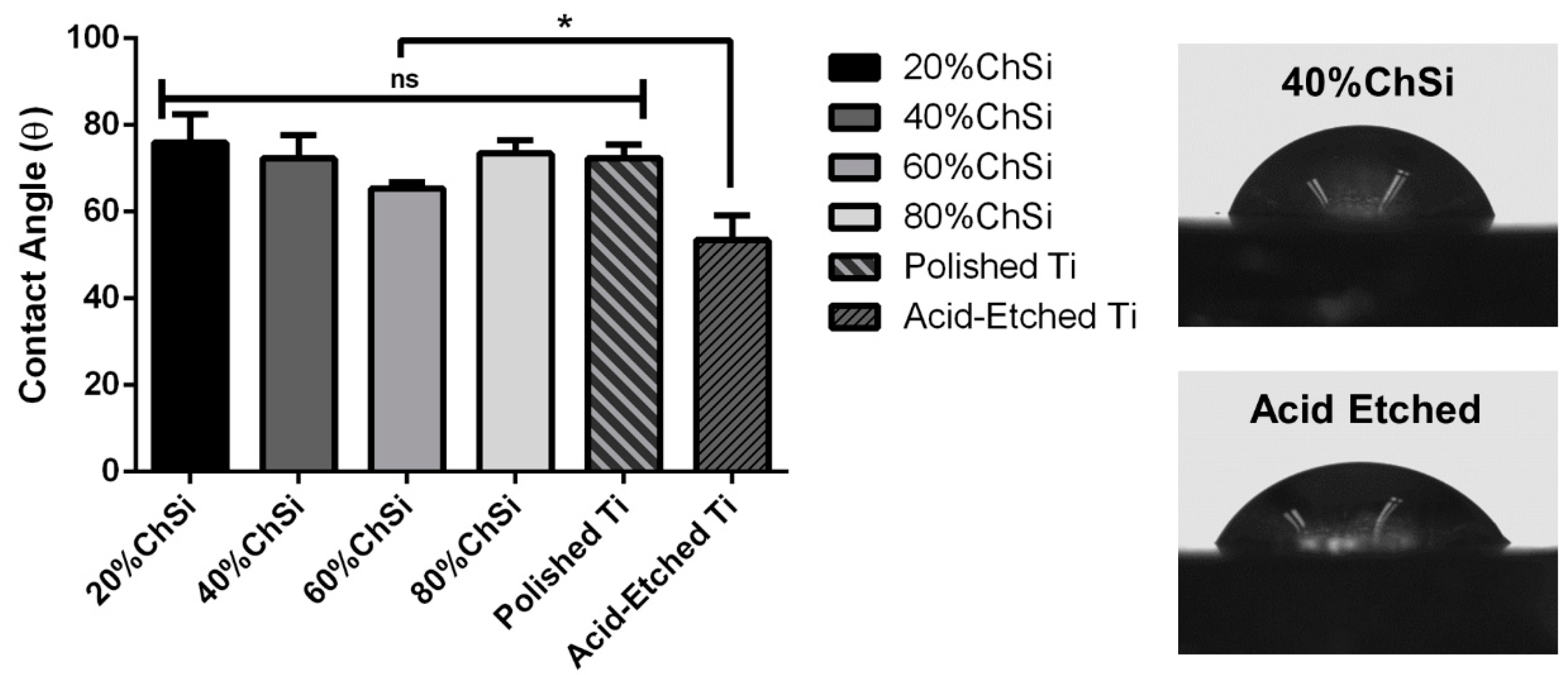
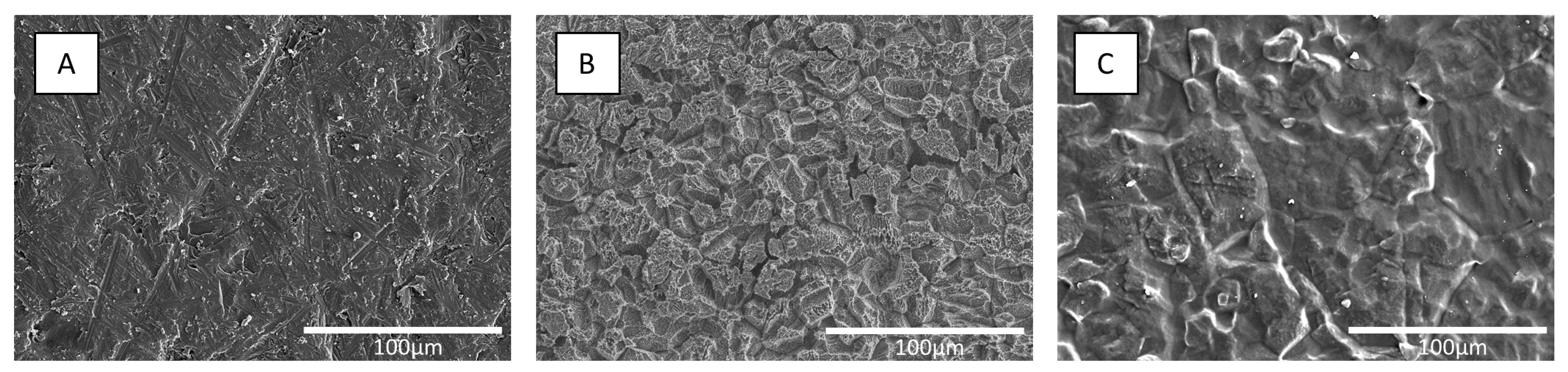
3.1. Characterization of Coating Materials
3.2. Mechanical Properties
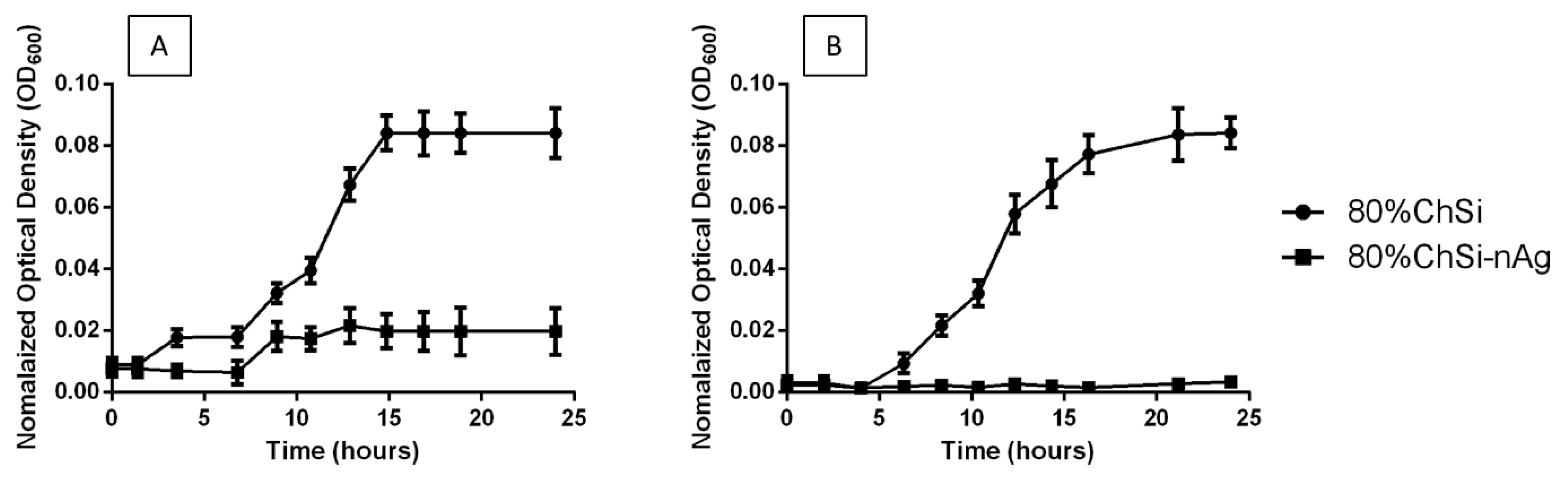
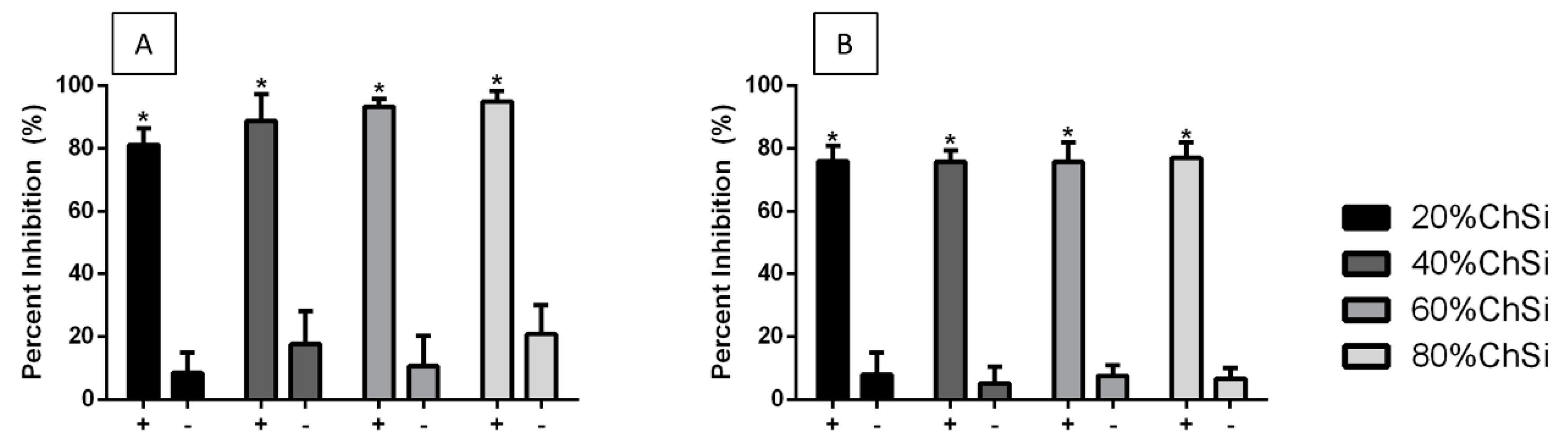
3.3. Antimicrobial Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Greger, M.; Widomská, M.; Snášel, V. Structure and properties of dental implants. In Proceedings of the METAL 2012—21st International Conference on Metallurgy and Materials, Brno, Czech Republic, 23–25 May 2012; Volume 120, pp. 434–439. [Google Scholar]
- Darouiche, R.O. Treatment of infections associated with surgical implants. Infect. Dis. Clin. Pract. 2004, 12, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Camps-Font, O.; Figueiredo, R.; Valmaseda-Castellón, E.; Gay-Escoda, C. Postoperative infections after dental implant placement: Prevalence, clinical features, and treatment. Implant. Dent. 2015, 24, 713–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mah, T.F.; Pitts, B.; Pellock, B.; Walker, G.C.; Stewart, P.S.; O’Toole, G.A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003, 426, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Yoda, I.; Koseki, H.; Tomita, M.; Shida, T.; Horiuchi, H.; Sakoda, H.; Osaki, M. Effect of surface roughness of biomaterials on Staphylococcus epidermidis adhesion. BMC Microbiol. 2014, 14, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Lo, J.C.Y.; Yan, M.; Yang, X.; Brooks, D.E.; Hancock, R.E.W.; Lange, D.; Kizhakkedathu, J.N. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 2017, 116, 69–81. [Google Scholar] [CrossRef]
- Swartjes, J.J.T.M.; Sharma, P.K.; Kooten, T.G.; Mei, H.C.; Mahmoudi, M.; Busscher, H.J.; Rochford, E.T.J. Current developments in antimicrobial surface coatings for biomedical applications. Curr. Med. Chem. 2014, 22, 2116–2129. [Google Scholar] [CrossRef] [Green Version]
- Radin, S.; Ducheyne, P. Controlled release of vancomycin from thin sol-gel films on titanium alloy fracture plate material. Biomaterials 2007, 28, 1721–1729. [Google Scholar] [CrossRef]
- Bračič, M.; Fras-Zemljič, L.; Pérez, L.; Kogej, K.; Stana-Kleinschek, K.; Kargl, R.; Mohan, T. Protein-repellent and antimicrobial nanoparticle coatings from hyaluronic acid and a lysine-derived biocompatible surfactant. J. Mater. Chem. B 2017, 5, 3888–3897. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chaimayo, W.; Yang, C.; Yao, J.; Miller, B.L.; Yates, M.Z. Silver-hydroxyapatite composite coatings with enhanced antimicrobial activities through heat treatment. Surf. Coat. Technol. 2017, 325, 39–45. [Google Scholar] [CrossRef]
- Tabesh, E.; Salimijazi, H.; Kharaziha, M.; Hejazi, M. Antibacterial chitosan-copper nanocomposite coatings for biomedical applications. Mater. Today Proc. 2018, 5, 15806–15812. [Google Scholar] [CrossRef]
- Stewart, P.S.; William Costerton, J. Antibiotic resistance of bacteria in biofilms. The Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-spectrum bioactivities of silver nanoparticles: The emerging trends and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Núñez, N.V.; del Turrent, L.C.I.; Padilla, C.R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Woo, K.J.; Hye, C.K.; Ki, W.K.; Shin, S.; So, H.K.; Yong, H.P. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [Green Version]
- Kvítek, L.; Panáček, A.; Soukupová, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial activity of nanosilver ions and particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.L.; Lucas, L.C. Post-deposition heat treatments for ion beam sputter deposited calcium phosphate coatings. Biomaterials 1994, 15, 337–341. [Google Scholar] [CrossRef]
- Li, T.; Lee, J.; Kobayashi, T.; Aoki, H. Hydroxyapatite coating by dipping method, and bone bonding strength. J. Mater. Sci. Mater. Med. 1996, 7, 355–357. [Google Scholar] [CrossRef]
- Wang, B.C.; Lee, T.M.; Chang, E.; Yang, C.Y. The shear strength and the failure mode of plasma-sprayed hydroxyapatite coating to bone: The effect of coating thickness. J. Biomed. Mater. Res. 1993, 27, 1315–1327. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan based self-assembled nanoparticles in drug delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Hou, J.; Su, C.; Zhao, L.; Shi, Y. Hyaluronic acid-coated chitosan nanoparticles induce ROS-mediated tumor cell apoptosis and enhance antitumor efficiency by targeted drug delivery via CD44. J. Nanobiotechnology 2017, 15, 7. [Google Scholar] [CrossRef] [Green Version]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104, 1003–1011. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Feng, G.; Gu, Z.; Sun, Y.; Bao, G.; Xu, G.; Lu, Y.; Chen, J.; Xu, L.; et al. Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: Potential roles for spinal cord injury therapy. Cell Tissue Res. 2016, 366, 129–142. [Google Scholar] [CrossRef]
- Saravanan, S.; Sareen, N.; Abu-El-Rub, E.; Ashour, H.; Sequiera, G.L.; Ammar, H.I.; Gopinath, V.; Shamaa, A.A.; Sayed, S.S.E.; Moudgil, M.; et al. Graphene oxide-gold nanosheets containing chitosan scaffold improves ventricular contractility and function after implantation into infarcted heart. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Vil’Danova, R.R.; Sigaeva, N.N.; Kukovinets, O.S.; Volodina, V.P.; Spirikhin, L.V.; Zaidullin, I.S.; Kolesov, S.V. Modification of hyaluronic acid and chitosan, aimed at developing hydrogels for ophthalmology. Russ. J. Appl. Chem. 2014, 87, 1547–1557. [Google Scholar] [CrossRef]
- De Oliveira Fulgêncio, G.; Viana, F.A.B.; Silva, R.O.S.; Lobato, F.C.F.; Ribeiro, R.R.; Fanca, J.R.; Byrro, R.M.D.; Faraco, A.A.G.; da Silva Cunha-Júnior, A. Mucoadhesive chitosan films as a potential ocular delivery system for ofloxacin: Preliminary in vitro studies. Vet. Ophthalmol. 2014, 17, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, D.; Zhu, X.; Li, L.; Li, H.; Guo, F.; Chen, X.; Tan, Y.; Xie, L. Evaluation of chitosan/aptamer targeting TGF-β receptor II thermo-sensitive gel for scarring in rat glaucoma filtration surgery. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5465–5476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Neto, A.I.; Cibrão, A.C.; Correia, C.R.; Carvalho, R.R.; Luz, G.M.; Ferrer, G.G.; Botelho, G.; Picart, C.; Alves, N.M.; Mano, J.F. Nanostructured polymeric coatings based on chitosan and dopamine-modified hyaluronic acid for biomedical applications. Small 2014, 10, 2459–2469. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Liu, Y.; Zhu, B.; Su, Y.; Zhu, X. Preparation of paclitaxel/chitosan co-assembled core-shell nanofibers for drug-eluting stent. Appl. Surf. Sci. 2017, 393, 299–308. [Google Scholar] [CrossRef]
- Tian, D.; Dubois, P.; Jérôme, R. Biodegradable and biocompatible inorganic-organic hybrid materials. I. Synthesis and characterization. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 2295–2309. [Google Scholar] [CrossRef]
- Valliant, E.M.; Jones, J.R. Softening bioactive glass for bone regeneration: Sol-gel hybrid materials. Soft Matter 2011, 7, 5083–5095. [Google Scholar] [CrossRef]
- Sachot, N.; Castaño, O.; Mateos-Timoneda, M.A.; Engel, E.; Planell, J.A. Hierarchically engineered fibrous scaffolds for bone regeneration. J. R. Soc. Interface 2013, 10, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Mahony, O.; Tsigkou, O.; Ionescu, C.; Minelli, C.; Ling, L.; Hanly, R.; Smith, M.E.; Stevens, M.M.; Jones, J.R. Silica-gelatin hybrids with tailorable degradation and mechanical properties for tissue regeneration. Adv. Funct. Mater. 2010, 20, 3835–3845. [Google Scholar] [CrossRef]
- Shirosaki, Y.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Lopes, M.A.; Santos, J.D.; Fernandes, M.H. In vitro cytocompatibility of MG63 cells on chitosan-organosiloxane hybrid membranes. Biomaterials 2005, 26, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Romer, F.; Connell, L.; Walter, C.; Saiz, E.; Yue, S.; Lee, P.D.; McPhail, D.S.; Hanna, J.V.; Jones, J.R. Highly flexible silica/chitosan hybrid scaffolds with oriented pores for tissue regeneration. J. Mater. Chem. B 2015, 3, 7560–7576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirosaki, Y.; Okamoto, K.; Hayakawa, S.; Osaka, A.; Asano, T. Preparation of porous chitosan-siloxane hybrids coated with hydroxyapatite particles. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Connell, L.S.; Romer, F.; Suárez, M.; Valliant, E.M.; Zhang, Z.; Lee, P.D.; Smith, M.E.; Hanna, J.V.; Jones, J.R. Chemical characterisation and fabrication of chitosan-silica hybrid scaffolds with 3-glycidoxypropyl trimethoxysilane. J. Mater. Chem. B 2014, 2, 668–680. [Google Scholar] [CrossRef]
- Palla-Rubio, B.; Araújo-Gomes, N.; Fernández-Gutiérrez, M.; Rojo, L.; Suay, J.; Gurruchaga, M.; Goñi, I. Synthesis and characterization of silica-chitosan hybrid materials as antibacterial coatings for titanium implants. Carbohydr. Polym. 2019, 203, 331–341. [Google Scholar] [CrossRef]
- Jun, S.H.; Lee, E.J.; Yook, S.W.; Kim, H.E.; Kim, H.W.; Koh, Y.H. A bioactive coating of a silica xerogel/chitosan hybrid on titanium by a room temperature sol-gel process. Acta Biomater. 2010, 6, 302–307. [Google Scholar] [CrossRef]
- Malina, D.; Sobczak-Kupiec, A.; Wzorek, Z.; Kowalski, Z. Silver nanoparticles synthesis with different concentrations of Polyvinylpyrrolidone. Dig. J. Nanomater. Biostructures 2012, 7, 1527–1534. [Google Scholar]
- Zahra, Q.; Fraz, A.; Anwar, A.; Awais, M.; Abbas, M. A mini review on the synthesis of Ag-nanoparticles by chemical reduction method and their biomedical applications. NUST J. Eng. Sci. 2016, 9, 1–7. [Google Scholar]
- Gakiya-teruya, M.; Palomino-marcelo, L.; Rodriguez-reyes, J.C.F. Synthesis of highly concentrated suspensions of silver nanoparticles by two versions of the chemical reduction method. Methods Protoc. 2019, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Brinker, C.J.; Frye, G.C.; Hurd, A.J.; Ashley, C.S. Fundamentals of sol-gel dip coating. Thin Solid Film. 1991, 201, 97–108. [Google Scholar] [CrossRef]
- Prochazkova, S.; Vårum, K.M.; Ostgaard, K. Quantitative determination of chitosans by ninhydrin. Carbohydr. Polym. 1999, 38, 115–122. [Google Scholar] [CrossRef]
- Shirosaki, Y.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Lopes, M.A.; Santos, J.D.; Costa, M.A.; Fernandes, M.H. Physical, chemical and in vitro biological profile of chitosan hybrid membrane as a function of organosiloxane concentration. Acta Biomater. 2009, 5, 346–355. [Google Scholar] [CrossRef] [PubMed]
- ASTM D3359-17 Standard Test Methods for Rating Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2017.
- Piveteau, L.D.; Gasser, B.; Schlapbach, L. Evaluating mechanical adhesion of sol-gel titanium dioxide coatings containing calcium phosphate for metal implant application. Biomaterials 2000, 21, 2193–2201. [Google Scholar] [CrossRef]
- Haas, K.-H. Hybrid inorganic-organic polymers based on organically modified Si-alkoxides. Adv. Eng. Mater. 2000, 2, 571–582. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Cipriani, E.; Gnavi, S.; Chiono, V.; Mattu, C.; Gentile, P.; Perroteau, I.; Zanetti, M.; Ciardelli, G. Crosslinked gelatin nanofibres: Preparation, characterisation and in vitro studies using glial-like cells. Mater. Sci. Eng. C 2013, 33, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Ramis, X.; Fernández-Francos, X. Epoxy sol-gel hybrid thermosets. Coatings 2016, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Zolkov, C.; Avnir, D.; Armon, R. Tissue-derived cell growth on hybrid sol-gel films. J. Mater. Chem. 2004, 14, 2200–2205. [Google Scholar] [CrossRef]
- Spirk, S.; Findenig, G.; Doliska, A.; Reichel, V.E.; Swanson, N.L.; Kargl, R.; Ribitsch, V.; Stana-Kleinschek, K. Chitosan-silane sol-gel hybrid thin films with controllable layer thickness and morphology. Carbohydr. Polym. 2013, 93, 285–290. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F. Characterization and biological properties of TiO2/PCL hybrid layers prepared via sol-gel dip coating for surface modification of titanium implants. J. Non. Cryst. Solids 2015, 415, 9–15. [Google Scholar] [CrossRef]
- Feller, L.; Jadwat, Y.; Khammissa, R.A.G.; Meyerov, R.; Schechter, I.; Lemmer, J. Cellular responses evoked by different surface characteristics of intraosseous titanium implants. BioMed Res. Int. 2015, 2015, 171945. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.; Tan, Y.; Ni, G.; Lan, G. Controlled oxidative nanopatterning of microrough titanium surfaces for improving osteogenic activity. J. Mater. Sci. Mater. Med. 2014, 25, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Boyan, B.D. Underlying Mechanisms at the Bone-Biomaterial Interface. J. Cell. Biochem. 1994, 56, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Chou, H.M.; Wu, J.D. Plasma-sprayed hydroxyapatite coating: Effect of different calcium phosphate ceramics. J. Mater. Sci. Mater. Med. 1994, 5, 147–153. [Google Scholar] [CrossRef]
- Wu, V.M.; Uskoković, V. Is there a relationship between solubility and resorbability of different calcium phosphate phases in vitro ? Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 2157–2168. [Google Scholar] [CrossRef] [Green Version]
- Usinskas, P.; Stankeviciute, Z.; Beganskiene, A.; Kareiva, A. Sol-gel derived porous and hydrophilic calcium hydroxyapatite coating on modified titanium substrate. Surf. Coat. Technol. 2016, 307, 935–940. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, E. Measurements of residual stresses in plasma-sprayed hydroxyapatite coatings on titanium alloy. Surf. Coat. Technol. 2005, 190, 122–131. [Google Scholar] [CrossRef]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [Green Version]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. J. Biomater. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef]
- Hou, N.; Perinpanayagam, H.; Mozumder, M.; Zhu, J. Novel development of biocompatible coatings for bone implants. Coatings 2015, 5, 737–757. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Sundfeldt, M.; Carlsson, L.V.; Johansson, C.B.; Thomsen, P.; Gretzer, C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Banaszkiewicz, P.A. Periprosthetic bone loss in total hip arthroplasty: Polyethylene wear debris and the concept of the effective joint space. Class. Pap. Orthop. 2014, 74, 85–87. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Neogi, S. Techniques for deposition of coatings with enhanced adhesion to bio-implants. In Adhesion in Pharmaceutical, Biomedical, and Dental Fields; Mittal, K.L., Etzler, F.M., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2017; pp. 235–254. ISBN 9781119323716. [Google Scholar]
- Chen, J.; Bull, S.J. Approaches to investigate delamination and interfacial toughness in coated systems: An overview. J. Phys. D Appl. Phys. 2011, 44, 034001. [Google Scholar] [CrossRef] [Green Version]
- van Dam, J.P.B.; Abrahami, S.T.; Yilmaz, A.; Gonzalez-Garcia, Y.; Terryn, H.; Mol, J.M.C. Effect of surface roughness and chemistry on the adhesion and durability of a steel-epoxy adhesive interface. Int. J. Adhes. Adhes. 2020, 96, 102450. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Hooton, R.D.; Zhang, X. Effects of interface roughness and interface adhesion on new-to-old concrete bonding. Constr. Build. Mater. 2017, 151, 582–590. [Google Scholar] [CrossRef]
- Geminger, T.; Jarka, S. Injection molding of multimaterial systems. In Specialized Injection Molding Techniques; Heim, H.-P., Ed.; William Andrew Publishing: Waltham, MA, USA, 2016; pp. 165–210. ISBN 9780323371216. [Google Scholar]
- Brown, S.R.; Turner, I.G.; Reiter, H. Residual stress measurement in thermal sprayed hydroxyapatite coatings. J. Mater. Sci. Mater. Med. 1994, 5, 756–759. [Google Scholar] [CrossRef]
- Yang, Y.C.; Chang, E. Influence of residual stress on bonding strength and fracture of plasma-sprayed hydroxyapatite coatings on Ti-6Al-4V substrate. Biomaterials 2001, 22, 1827–1836. [Google Scholar] [CrossRef]
- Zhang, Z.; Dunn, M.F.; Xiao, T.D.; Tomsia, A.P.; Saiz, E.M. Nanostructured hydroxyapatite coatings for improved adhesion and corrosion resistance for medical implants. Online Proc. Libr. Arch. 2001, 703. [Google Scholar] [CrossRef] [Green Version]
- Shockman, G.D.; Barren, J.F. Structure, function, and assembly of cell walls of gram-positive bacteria. Annu. Rev. Microbiol. 1983, 37, 501–527. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Ghotaslou, R. The in vitro effects of silver nanoparticles on bacterial biofilms. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1077–1080. [Google Scholar] [CrossRef] [Green Version]
- Flores, C.Y.; Min, A.G.; Grillo, C.A.; Salvarezza, R.C.; Vericat, C.; Schilardi, P.L. Citrate-capped silver nanoparticles showing good bactericidal e ff ect against both planktonic and sessile bacteria and a low cytotoxicity to osteoblastic cells. ACS Appl. Mater. Interfaces 2013, 5, 3149–3159. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Lee, J.H.; Chae, Y.J.; Kim, Y.S.; Kim, B.C.; Sang, B.I.; Gu, M.B. Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small 2008, 4, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef] [Green Version]
- Díaz, C.; Schilardi, P.; De Mele, M.F.L. Influence of surface sub-micropattern on the adhesion of pioneer bacteria on metals. Artif. Organs 2008, 32, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Senthamilselvi, S.; Lakshmipraba, A.; Premkumar, K.; Muthukumaran, R.; Visvanathan, P.; Ganeshkumar, R.S.; Govindaraju, M. Efficacy of bio-synthesized silver nanoparticles using Acanthophora spicifera to encumber biofilm formation. Dig. J. Nanomater. Biostructures 2012, 7, 511–522. [Google Scholar]






| Components | 80% Organic Blends | 60% Organic Blends | 40% Organic Blends | 20% Organic Blends | ||||
|---|---|---|---|---|---|---|---|---|
| 80% ChSi | 80% ChSi-nAg | 60% ChSi | 60% ChSi-nAg | 40% ChSi | 40% ChSi-nAg | 20% ChSi | 20% ChSi-nAg | |
| Ch-GPTMS a | 80 | 80 | 60 | 60 | 40 | 40 | 20 | 20 |
| TEOS b | 20 | 20 | 40 | 40 | 60 | 60 | 80 | 80 |
| AgNPs c | – | 0.023 | – | 0.023 | – | 0.023 | – | 0.023 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouveia, Z.; Perinpanayagam, H.; Zhu, J. Development of Robust Chitosan–Silica Class II Hybrid Coatings with Antimicrobial Properties for Titanium Implants. Coatings 2020, 10, 534. https://doi.org/10.3390/coatings10060534
Gouveia Z, Perinpanayagam H, Zhu J. Development of Robust Chitosan–Silica Class II Hybrid Coatings with Antimicrobial Properties for Titanium Implants. Coatings. 2020; 10(6):534. https://doi.org/10.3390/coatings10060534
Chicago/Turabian StyleGouveia, Zach, Hiran Perinpanayagam, and Jesse Zhu. 2020. "Development of Robust Chitosan–Silica Class II Hybrid Coatings with Antimicrobial Properties for Titanium Implants" Coatings 10, no. 6: 534. https://doi.org/10.3390/coatings10060534
APA StyleGouveia, Z., Perinpanayagam, H., & Zhu, J. (2020). Development of Robust Chitosan–Silica Class II Hybrid Coatings with Antimicrobial Properties for Titanium Implants. Coatings, 10(6), 534. https://doi.org/10.3390/coatings10060534






