Atomistic Simulations and Experimental Investigations of the Diffusion Behavior of Steel/ZCuPb20Sn5 Bimetals
Abstract
:1. Introduction
2. Methods
2.1. Simulation Methodology
2.2. Experimental Materials
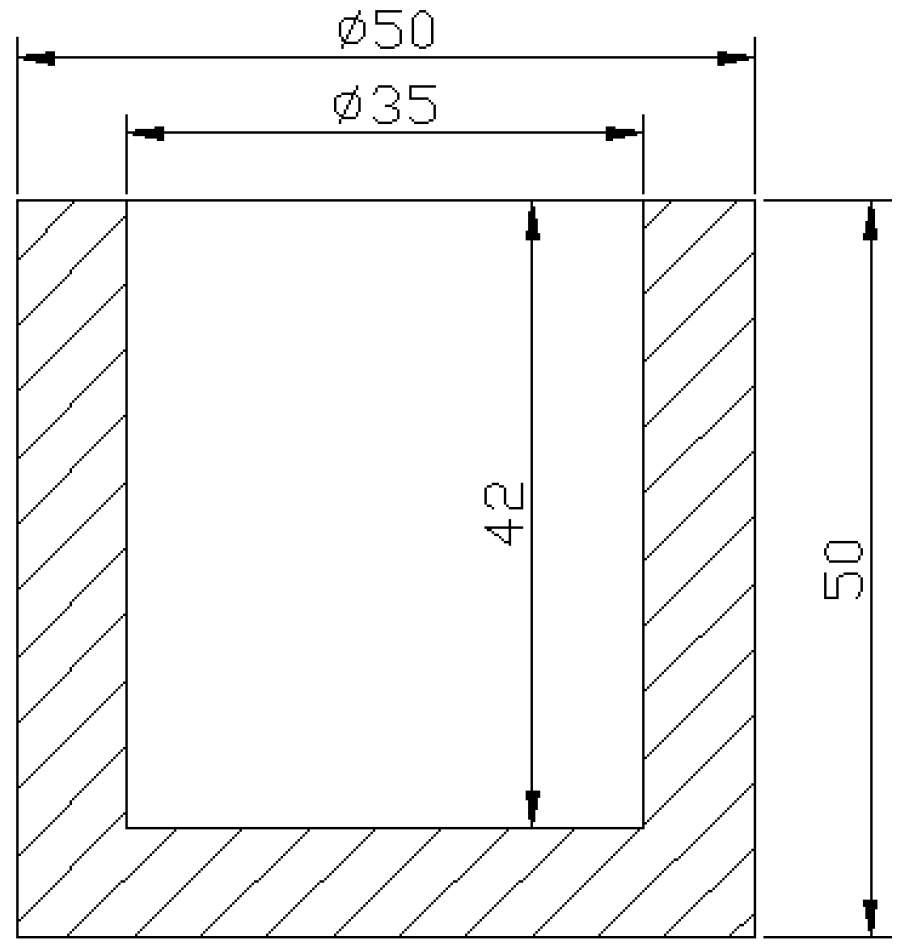
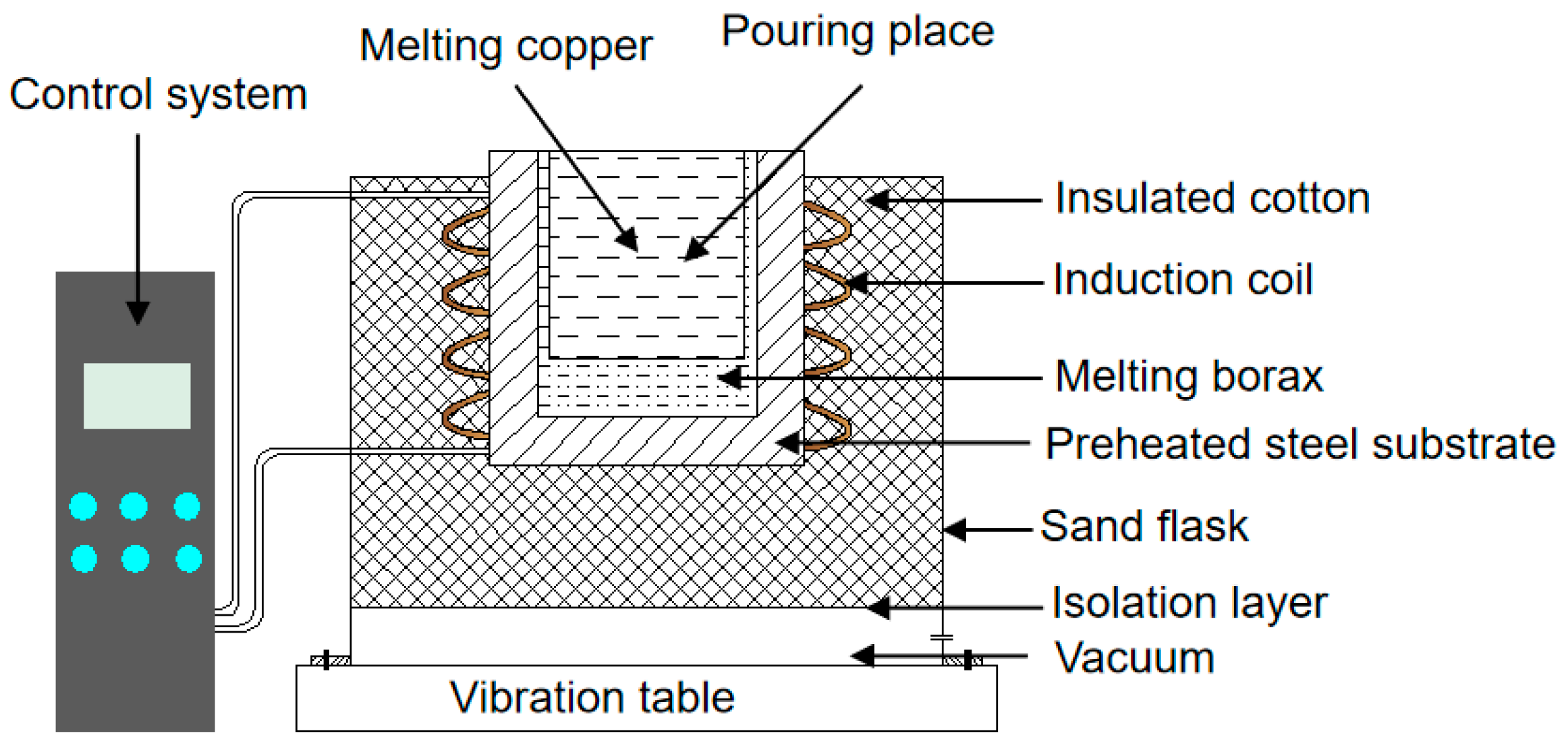
2.3. Casting Process
2.4. Microscopy Methodology
2.5. Mechanical Properties
3. Results and Discussion
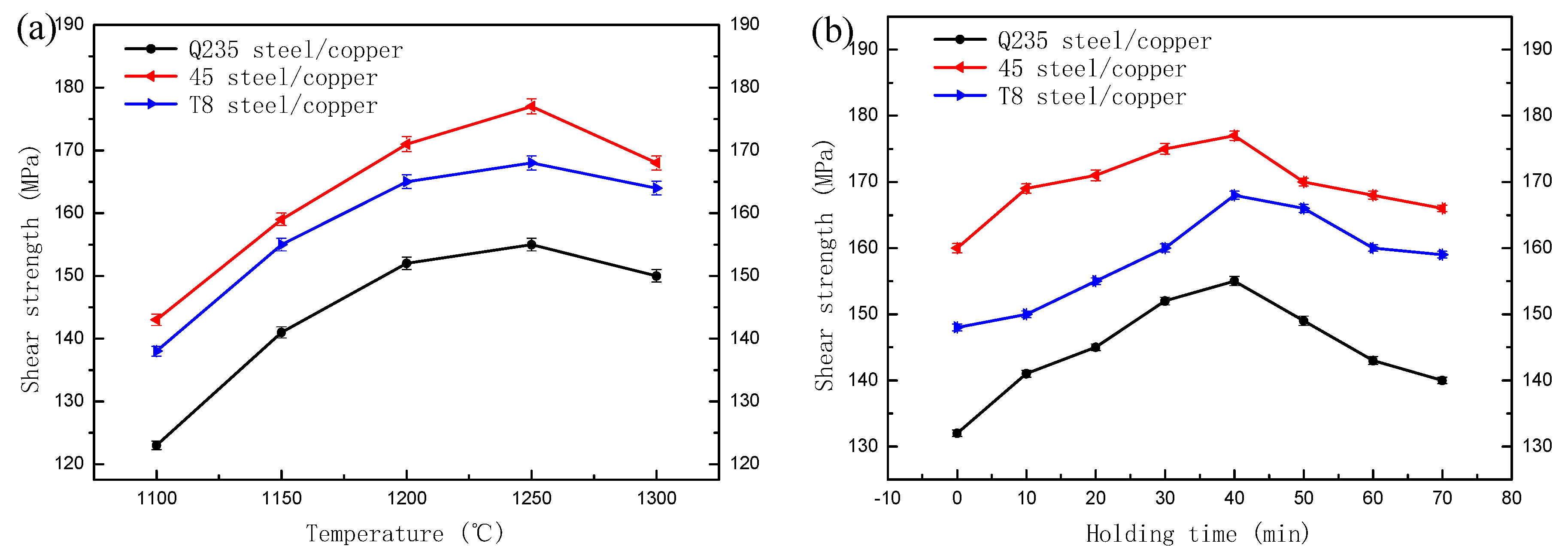
3.1. Mechanical Characterisation
3.2. Molecular Dynamics Simulation Result
3.3. Microstructure Characterization
3.4. Microhardness Characterisation
3.5. Discussion
4. Conclusions
- The pouring temperature and hold time had a considerable influence on the steel/copper bimetallic interface. A high bond strength bimetal was obtained at the pouring temperature of 1250 °C and the holding time of 40 min;
- The steel/copper bimetals were successfully fabricated by the rapid induction heating of a solid–liquid compound. The 45 steel/copper bimetal had the highest bonding strength of 177 MPa compared with the Q235 steel and T8 steel. The interface of the steel/copper exhibited preferable bonding and diffusion characteristics;
- The thicknesses of the diffusion distance of the copper atoms in ferrite, pearlite, and cementite were ranked in the following descending order: ferrite > pearlite > cementite. The order of the micro-hardness was the opposite;
- The diffusion distance of the copper atoms in the carbon steel matrix was smaller than that of the iron atoms in the ZCuPb20Sn5 matrix. The microstructure and the mechanical properties of the steel played an important role in the fabrication of the steel/copper bimetals with a higher bonding strength;
- The simulation results revealed that the diffusion coefficient of the Cu atoms at 1523 K was larger than that of the Fe atoms, but the diffusion distance of the Fe atoms in the Cu bulk was larger than that of the Cu atoms in the Fe bulk. These results agreed very well with the experimental results.
Author Contributions
Funding
Conflicts of Interest
References
- Rhee, K.; Han, W.; Park, H.; Kim, S. Fabrication of aluminum/copper clad composite using hot hydrostatic extrusion process and its material characteristics. Mater. Sci. Eng. A 2004, 384, 70–76. [Google Scholar] [CrossRef]
- Lee, J.; Bae, D.; Chung, W.; Kim, K.; Lee, J.; Cho, Y.-R. Effects of annealing on the mechanical and interface properties of stainless steel/aluminum/copper clad-metal sheets. J. Mater. Process. Technol. 2007, 187, 546–549. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Sun, B. Effect of Ag on the thermal stability of deformation processed Cu–Fe in situ composites. J. Alloy. Compd. 2009, 469, 580–586. [Google Scholar] [CrossRef]
- Ko, C.; Lee, K.; Kim, M.; Jo, H.; Jo, H. Evaluation of copper coating ratio in steel/copper clad wire drawing. J. Mater. Process. Technol. 2008, 186, 22–26. [Google Scholar] [CrossRef]
- Sarkar, T.; Bose, P.; Sutradhar, G. Mechanical and tribological characteristics of copper alloyed austempered gray cast iron (AGI). Mater. Today Proc. 2018, 5, 3664–3673. [Google Scholar] [CrossRef]
- Abbas, S.F.; Seo, S.-J.; Park, K.-T.; Kim, B.S.; Kim, T.-S. Effect of grain size on the electrical conductivity of copper–iron alloys. J. Alloy. Compd. 2017, 720, 8–16. [Google Scholar] [CrossRef]
- Tong, B.; Yang, W.; Liu, Q.; Ye, X.; Shi, L. Flowing and pressure-balancing characteristics of clearance field in helical grooved piston-copper sleeve pair of piston pump. Trans. Chin. Soc. Agric. Eng. 2018, 34, 55–63. [Google Scholar]
- Ramprabhu, T.; Sriram, S.S.; Boopathy, K.; Narasimhan, K.; Ramamurty, U. Effect of copper addition on the fatigue life of low alloy C-Mo powder metallurgy steel. Met. Powder Rep. 2011, 66, 28–34. [Google Scholar] [CrossRef]
- Silman, H.; Fry, M.F.E. The lead plating of bronze bearing surfaces for high pressure fuel pumps. Trans. IMF 1947, 23, 43–58. [Google Scholar] [CrossRef]
- Zou, J.; Li, S.; Wei, Y.; Liang, S. Research of the bonded interface of Cu9Al4Fe/1Cr18Ni9Ti stainless steel bimetallic composite. Vacuum 2017, 146, 266–273. [Google Scholar] [CrossRef]
- Hiroe, T.; Fujiwara, K.; Hata, H.; Takahashi, H. Deformation and fragmentation behaviour of exploded metal cylinders and the effects of wall materials, configuration, explosive energy and initiated locations. Int. J. Impact Eng. 2008, 35, 1578–1586. [Google Scholar] [CrossRef] [Green Version]
- Barlas, Z. Weldability of CuZn30 brass/DP600 steel couple by friction stir spot welding. Acta Phys. Pol. A 2017, 132, 991–993. [Google Scholar] [CrossRef]
- Kershenb, V.Y. Performance of steel-bronze bimetal produced by friction surfacing. Weld. Prod. 1972, 19, 48–50. [Google Scholar]
- Mai, T.; Spowage, A. Characterisation of dissimilar joints in laser welding of steel–kovar, copper–steel and copper–aluminium. Mater. Sci. Eng. A 2004, 374, 224–233. [Google Scholar] [CrossRef]
- Yao, C.; Xu, B.; Zhang, X.; Huang, J.; Fu, J.; Wu, Y. Interface microstructure and mechanical properties of laser welding copper–steel dissimilar joint. Opt. Lasers Eng. 2009, 47, 807–814. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Chen, W. Sintering procedure and properties of mining used bimetal shaft sleeve. Dev. Appl. Mater. 2004, 1, 24–26. [Google Scholar]
- Liu, R.-T.; Xiong, X.; Chen, F.-S.; Lu, J.; Hong, L.-L.; Zhang, Y.-Q. Tribological performance of graphite containing tin lead bronze–steel bimetal under reciprocal sliding test. Tribol. Int. 2011, 44, 101–105. [Google Scholar] [CrossRef]
- Yin, Y.; Tang, H.; Jiao, M.; Zhang, G.; Xue, L.; Tian, M. Tribological performance of lead-free Ni-contained copper-steel bimetal bearing materials. Chin. J. Nonferr. Met. 2017, 27, 1189–1198. [Google Scholar]
- Zhang, P.; Du, Y.; Liu, H.; Zeng, D.; Ba, L. The influence of preheat temperature of steel plate on steel-mushy Cu-graphite bonding. J. Wuhan Univ. Technol. Sci. Ed. 2006, 21, 12–14. [Google Scholar] [CrossRef]
- Tang, J.D.; Li, L.M.; Zhu, Z.F. Research of Induction Heating and Particle Centrifugal Melting Technique for Steel-backed Copper Lead Alloy Sliding Bearing. Locomot. Roll. Stock Technol. 2012, 5, 1–3. [Google Scholar]
- Du, Y.H.; Zhang, P.; Liu, H.W.; Zeng, D.B. Optimization of steel-mushy Cu-graphite press bonding. J. Tsinghua Univ. (Sci. Technol.) 2013, 2, 4–8. [Google Scholar]
- Luozzo, N.D.; Boudard, M.; Fontana, M.; Arcondo, B. Effective diffusion coefficient for Cu in steel joined by transient liquid phase bonding. Mater. Des. 2016, 92, 760–766. [Google Scholar] [CrossRef]
- Gale, W.F.; Butts, D.A. Transient liquid phase bonding. Sci. Technol. Weld. Join. 2004, 9, 283–300. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.X.; Pei, Y.; Niu, J. Properties of the joint bonded by transformation/diffusion brazing and diffusion behaviours of the major elements. Acta Metall. Sin. 2004, 40, 653–658. [Google Scholar]
- Meitei, R.K.B.; Maji, P.; Samadhiya, A.; Ghosh, S.K.; Roy, B.S.; Das, A.K.; Saha, S.C. A study on induction welding of mild steel and copper with flux under applied load condition. J. Manuf. Process. 2018, 34, 435–441. [Google Scholar] [CrossRef]
- Baglyuk, G.A.; Sosnovskii, L.A.; Volfman, V.I. Effect of carbon content on the properties of sintered steels doped with manganese and copper. Powder Met. Met. Ceram. 2011, 50, 189–193. [Google Scholar] [CrossRef]
- Gosh, R.C.; Syed, I.M.; Amina, Z.; Bhuiyan, G.M. A comparative study on temperature dependent diffusion coeffcient of liquid Fe. Phys. B Condens. Matter 2013, 426, 127–131. [Google Scholar] [CrossRef]
- Guo, M.H.; Enomoto, C.J. Simulation of bcc-Cu precipitation in ternary Fe-Cu-Mn alloys. Comput. Mater. Sci. 2018, 141, 101–113. [Google Scholar] [CrossRef]
- Shang, S.-L.; Zhou, B.-C.; Wang, W.Y.; Ross, A.J.; Liu, X.; Hu, Y.-J.; Fang, H.-Z.; Wang, Y.; Liu, Z.-K. A comprehensive first-principles study of pure elements: Vacancy formation and migration energies and self-diffusion coefficients. Acta Mater. 2016, 109, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Prokoshkina, D.; Esin, V.; Divinski, S.V. Experimental evidence for anomalous grain boundary diffusion of Fe in Cu and Cu-Fe alloys. Acta Mater. 2017, 133, 240–246. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Q.; Sui, Y.; Wang, Q.; Ding, W. An investigation into interface formation and mechanical properties of aluminum–copper bimetal by squeeze casting. Mater. Des. 2016, 89, 1137–1146. [Google Scholar] [CrossRef]
- Sun, C.; Li, L.; Fu, M.; Zhou, Q. Element diffusion model of bimetallic hot deformation in metallurgical bonding process. Mater. Des. 2016, 94, 433–443. [Google Scholar] [CrossRef]
- Tavassoli, S.; Abbasi, M.; Tahavvori, R. Controlling of IMCs layers formation sequence, bond strength and electrical resistance in Al Cu bimetal compound casting process. Mater. Des. 2016, 108, 343–353. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Li, C. Improved steel/aluminum bonding in bimetallic castings by a compound casting process. J. Mater. Process. Technol. 2015, 226, 25–31. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Bonny, G.; Pasianot, R.; Castin, N.; Malerba, L. Ternary Fe–Cu–Ni many-body potential to model reactor pressure vessel steels: First validation by simulated thermal annealing. Philos. Mag. 2009, 89, 3531–3546. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Xie, R.; Wang, W.; Zhou, J.; Cao, X.; Wei, Y. Molecular dynamics simulations and experimental investigations of atomic diffusion behavior at bonding interface in an explosively welded Al/Mg alloy composite plate. Acta Met. Sin. Engl. Lett. 2017, 30, 983–991. [Google Scholar] [CrossRef]
- Guo, Q.; Greer, J.R. Compressive properties of interface-containing Cu–Fe nano-pillars. Scr. Mater. 2011, 66, 272–275. [Google Scholar] [CrossRef]
- Edstrom, D.; SanGiovanni, D.; Hultman, L.; Petrov, I.; Greene, J.; Chirita, V. Effects of incident N atom kinetic energy on TiN/TiN(001) film growth dynamics: A molecular dynamics investigation. J. Appl. Phys. 2017, 121, 025302. [Google Scholar] [CrossRef] [Green Version]
- Edström, D.; SanGiovanni, D.; Hultman, L.; Chirita, V.; Petrov, I.; Greene, J. Ti and N adatom descent pathways to the terrace from atop two-dimensional TiN/TiN(001) islands. Thin Solid Films 2014, 558, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Ko, W.-S.; Grabowski, B.; Neugebauer, J. Development and application of a Ni-Ti interatomic potential with high predictive accuracy of the martensitic phase transition. Phys. Rev. B 2015, 92. [Google Scholar] [CrossRef] [Green Version]
- Maisel, S.B.; Ko, W.-S.; Zhang, J.-L.; Grabowski, B.; Neugebauer, J. Thermomechanical response of NiTi shape-memory nanoprecipitates in TiV alloys. Phys. Rev. Mater. 2017, 1. [Google Scholar] [CrossRef] [Green Version]
- SanGiovanni, D.; Mei, A.B.; Edstrom, D.; Hultman, L.; Chirita, V.; Petrov, I.; Greene, J. Effects of surface vibrations on interlayer mass transport: Ab initio molecular dynamics investigation of Ti adatom descent pathways and rates from TiN/TiN (001) islands. Phys. Rev. B 2018, 97, 035406. [Google Scholar] [CrossRef] [Green Version]
- SanGiovanni, D.; Edstrom, D.; Hultman, L.; Petrov, I.; Greene, J.; Chirita, V. Ti adatom diffusion on TiN(001): Ab initio and classical molecular dynamics simulations. Surf. Sci. 2014, 627, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Almyras, G.A.; Sangiovanni, D.G.; Sarakinos, K. Semi-Empirical Force-Field Model for the Ti1−xAlxN (0 ≤ x ≤ 1) System. Materials 2019, 12, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xiao, Y.; Luo, G.; Shen, Q.; Zhang, L. Effect of Ni interlayer on strength and microstructure of diffusion-bonded Mo/Cu joints. Mater. Lett. 2012, 66, 113–116. [Google Scholar] [CrossRef]
- Bu, H.; Yandouzi, M.; Lu, C.; Jodoin, B. Effect of heat treatment on the intermetallic layer of cold sprayed aluminum coatings on magnesium alloy. Surf. Coat. Technol. 2011, 205, 4665–4671. [Google Scholar] [CrossRef]









| Alloys | Elements (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | S | P | Ni | Cr | Fe | |
| Q235 steel | 0.18 | 0.02 | 0.45 | 0.035 | 0.035 | – | – | balance |
| 45 steel | 0.45 | 0.25 | 0.62 | 0.025 | ≤0.025 | 0.2 | 0.15 | balance |
| T8 steel | 0.81 | 0.30 | 0.30 | 0.015 | 0.02 | 0.15 | 0.15 | balance |
| Element | Cu | Pb | Zn | Sn | Ni | P |
|---|---|---|---|---|---|---|
| Content | balance | 20.4 | 1.8 | 5.0 | 2.0 | 0.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, G.; Xu, H.; Zhang, Y. Atomistic Simulations and Experimental Investigations of the Diffusion Behavior of Steel/ZCuPb20Sn5 Bimetals. Coatings 2020, 10, 549. https://doi.org/10.3390/coatings10060549
Wang M, Zhang G, Xu H, Zhang Y. Atomistic Simulations and Experimental Investigations of the Diffusion Behavior of Steel/ZCuPb20Sn5 Bimetals. Coatings. 2020; 10(6):549. https://doi.org/10.3390/coatings10060549
Chicago/Turabian StyleWang, Mingjie, Guowei Zhang, Hong Xu, and Yufei Zhang. 2020. "Atomistic Simulations and Experimental Investigations of the Diffusion Behavior of Steel/ZCuPb20Sn5 Bimetals" Coatings 10, no. 6: 549. https://doi.org/10.3390/coatings10060549
APA StyleWang, M., Zhang, G., Xu, H., & Zhang, Y. (2020). Atomistic Simulations and Experimental Investigations of the Diffusion Behavior of Steel/ZCuPb20Sn5 Bimetals. Coatings, 10(6), 549. https://doi.org/10.3390/coatings10060549




