Abstract
The present review deals with the latest progress in the field of polysulfone composite membranes with carbon nanotubes, carbon fiber and graphene from both perspectives-synthesis and applications. These two fillers, extensively used in the last few years due to their remarkable properties, induce a high value character to the composite materials. On the other hand, polysulfone is one the most used polymers for preparing polymeric membranes due to its high versatility in a wide range of solvents and also to the properties of this remarkable polymer. All types of synthesis method were presented and also a large number of applications from industrial to biomedical were presented and discussed.
1. Introduction
In the last few decades, polymeric membranes reached a status of highly advanced materials due to their use in a variety of applications in various fields such as water purification [1], electronics [2], sensors [3], environmental decontamination techniques [4], separations [5], and biomedicine [6] like hemodialysis [7] or osseointegration [8,9].
The most well-known polymers for the synthesis of membranes include: polysulfone (PSF) [10], polyethersulfone [11], polyether ketone [12], polyphenylene oxide [13], polyphenylene sulphate [14], cellulose [15], nitrocellulose [16], and cellulose acetate [17]. The form, distribution and diameter of pores can be adjusted by the choice of the solvent, the non-solvent, the concentration of the polymer solution, the coagulation temperature or the addition of additives to the polymer solution [2].
One of the most used techniques for improving the properties of polymeric membranes; both structural and functional is represented by the preparation of composite membranes [18,19]. Between various used fillers, carbon nanotubes (CNTs) [20,21], respectively graphene and its derivatives [22,23] are extensively used due to their remarkable properties [24], like thermal [25], electrical [26], mechanical, and induced properties to composite materials [27,28,29].
The present review presents the latest approaches in the field of polysulfone composite membranes with carbon nanotubes or graphene from both perspectives—synthesis and applications.
2. Polysulfone-Carbon Nanotubes and Nanofiber Composite Membranes
2.1. Synthesis, Characterization and Properties
To overcome the disadvantages associated with acid treatment in order to increase multiwalled carbon nanotubes’ (MWNTs) hydrophilicity, they were covered with polydopamine (Pdop). Using various concentrations of Pdop-MWNT, between 0.1 and 0.5 wt.%, several PSF, PSF/Pdop-MWNT membranes were synthesized through the phase inversion method. The nanocomposite membranes obtained show an increase in water permeability compared to simple PSF, due to the increase in membrane hydrophilicity with the addition of Pdop-coated MWNT in the polymer matrix. At the same time, the increase in membrane hydrophilicity due to MWNT functionalization through the coating process has led to an increase in anticoagulation properties and membrane tear resistance. Also, morphological changes can be observed in cross-section of the membrane with a decrease of pore diameter correlated with the increase of CNT concentration (Figure 1).
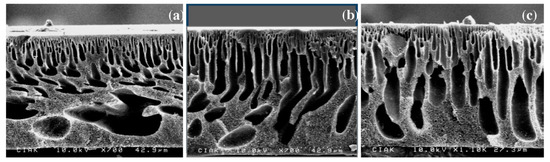
Figure 1.
Scanning electron microscope (SEM) images of the cross section of the (a) base membrane, (b) 0.1 wt.% and (c) 0.5 wt.% polydopamine-multiwalled carbon nanotube/polysulfone (Pdop-MWNT/PSF) membranes. (Reproduced with permission after [30]).
Due to superior mechanical properties these membranes could be used for ultrafiltration processes. It was observed that the optimal dose of Pdop for improving the hydrophilic properties was 0.1 wt.% Pdop [30].
The incorporation of several concentrations of oxidized MWNT (oMWNT) through treatment with a mixture of acids in the PSF matrix in order to obtain improved membranes has also been studied. The results of the membrane tests showed a similarity in the three PSF concentrations (16, 18 and 28 wt.% respectively), due to an initial increase in water flow followed by a gradual decrease with the increase of the oMWNT concentration.
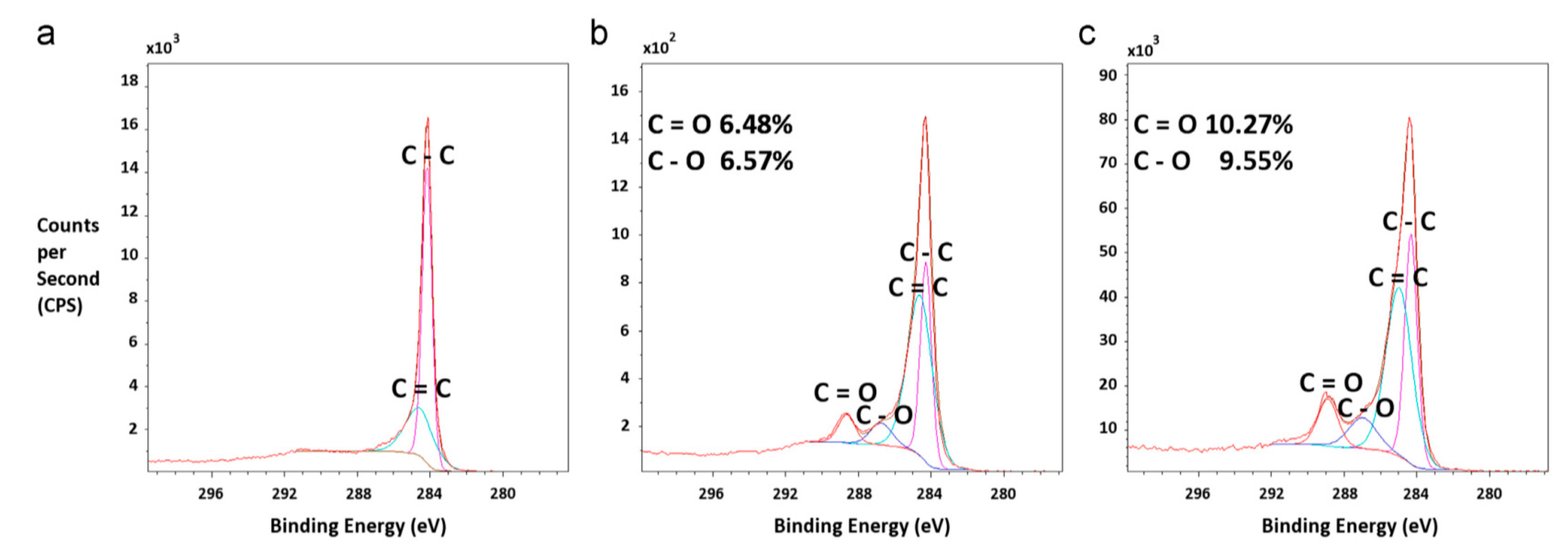
The analyses recorded a decrease of the contact angle with the increase in the concentration of the reinforcing agent due to the increased hydrophilicity of the membrane surface. The introduction of hydrophilic oMWNT into the polymer matrix led to a change in pore size which tends first to increase and then to decrease (Figure 2) [31]. On the other hand, the effect of carbon nanotubes carboxylation (MWNT-COOH) concentration on the PSF-based ultrafiltration membrane properties was studied and a change in hydrophilicity and its permeability was observed depending on the carboxylation ratio obtained. It seems that adding MWCNTs within a 3:1 sulfuric to nitric acid mixture does not lead to a 100% functionalization degree. This could be observed in the X-ray photoelectron spectroscopy (XPS) in which significant amounts of hydroxyl and carbonyl groups could also be noticed on the MWCNTs’ structure (Figure 3).
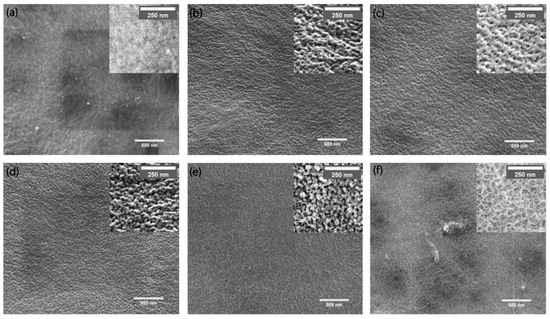
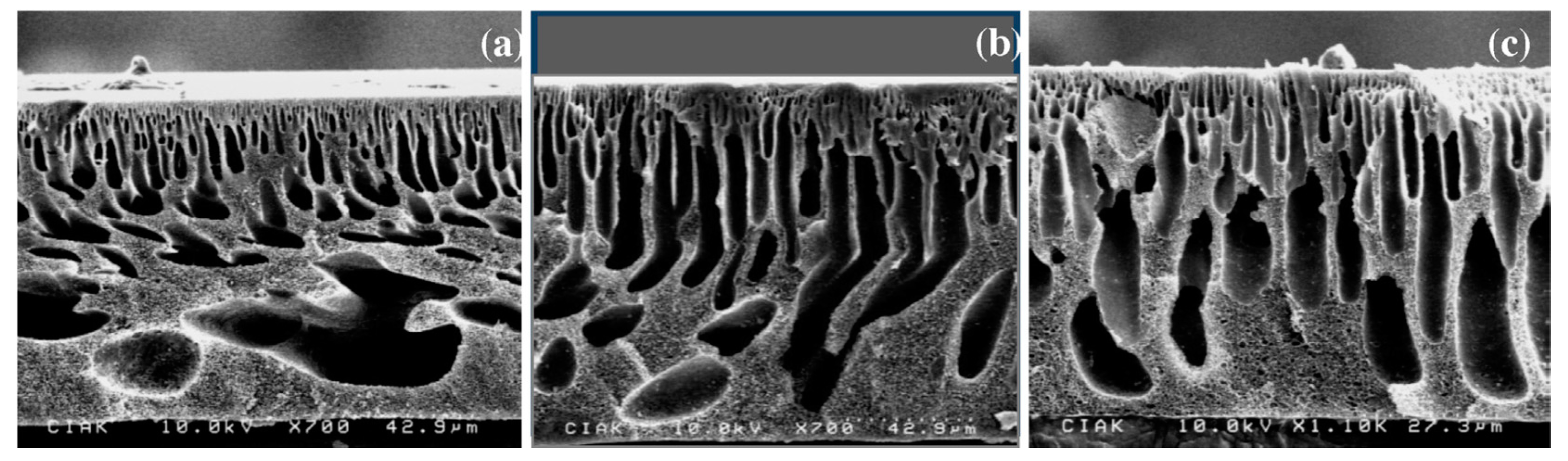
Figure 2.
Outside surface morphology of hallow fiber membrane containing 18 wt.% PSF (HFM18) with different oxidized MWNTs content: (a) 0 wt.%; (b) 0.1 wt.%; (c) 0.25 wt.%; (d) 0.5 wt.%; (e) 0.75 wt.% and (f) 1.0 wt.%. (Reproduced with permission after [31]).
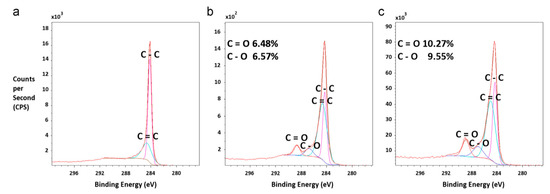
Figure 3.
X-ray photoelectron spectroscopy (XPS) data showing the main carbon bonding (C–C) peak and the small peaks representing the carboxyl, hydroxyl, and carbene groups formed on the carbon nanotubes (CNTs). (a) Pure CNTs with no carboxylation show a lack of secondary and tertiary peaks about the main C–C bonding peak. (b) CNTs carboxylated to 6.53% show a small peak about the main carbon peak indicating single-and doubly-bonded carbon to oxygen (C–O and C=O). (c) CNTs that have been carboxylated to 9.91% demonstrate more prominent peaks about the C–O and C=O bonds. (Reproduced with permission after [32]).
The presence of MWNT-COOH led to an increase in membrane tensile strength when the carboxylating ratio was less than 2.56 wt.%, followed by a decrease in membrane resistance for traction at a superior functionalization ratio compared to pure MWNT. It has been observed that a carboxylating ratio above this value has negative effect on membrane mechanical properties, resulting in a decrease in membrane retention degree and an increase in CNT leaching in the membranes due to the destruction of Π-Π bond in the CNT structure following the changing process [32].
At the same time, amino group-functionalized MWCNTs (f-MWCNT) were dispersed in 1,3-phenylenediamine solution in order to synthesis thin-film nanocomposite membrane (TFN) for forward osmosis (FO) through interfacial polymerization. A change in the membrane roughness (Figure 4) and an increase in hydrophilicity with the amount of f-MWCNT incorporated in the polymeric matrix (0.01, 0.05, 0.1 wt.%) was observed. Membrane performance was evaluated using 10 mM NaCl feed solution and 2 M NaCl filtration solution. An approximately 160% (95.7 L/m2·h) permeability increase for TFN with f-MWCNT compared to single TFN was observed [33]. Using another method, solvent casting, several MWCNT-PSF nanocomposites with different MWCNT ratios were synthesized. Thermogravimetric analyzes (TGA) showed an increase in nanocomposite thermostability following reinforcing agent addition. This increase was on one hand due to the high thermal stability of MWCNT and, on the other hand, due to the prevention of MWCNT nanodispersion composite thermal transfer. The synthesized nanocomposites were also studied in terms of electrical properties. Dielectric studies were performed at several frequencies (10 Hz–1 MHz) using an LCR (inductance, capacitance, resistance) meter and an increase in dielectric constant and in dielectric loss with MWCNT content added due to interfacial polarization between conductive MWCNT and PSF was observed. A decrease in dielectric constants (DC) resistivity in pure PSF was also observed, from 1014 Ω-cm to 104 Ώ-cm, after 1 wt.% MWCNT addition, followed by a decrease to 102 Ω-cm with the MWCNT content increase up to 7 wt.% [34].
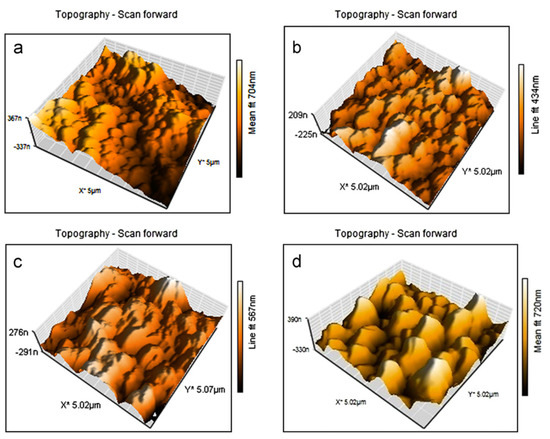
Figure 4.
Atomic force microscopy (AFM) analyses of (a) thin film composite (TFC), (b) thin-film nanocomposite membrane (TFN) 0.01, (c) TFN 0.05 and (d) TFN 0.1 membranes. (Reproduced with permission after [33]).
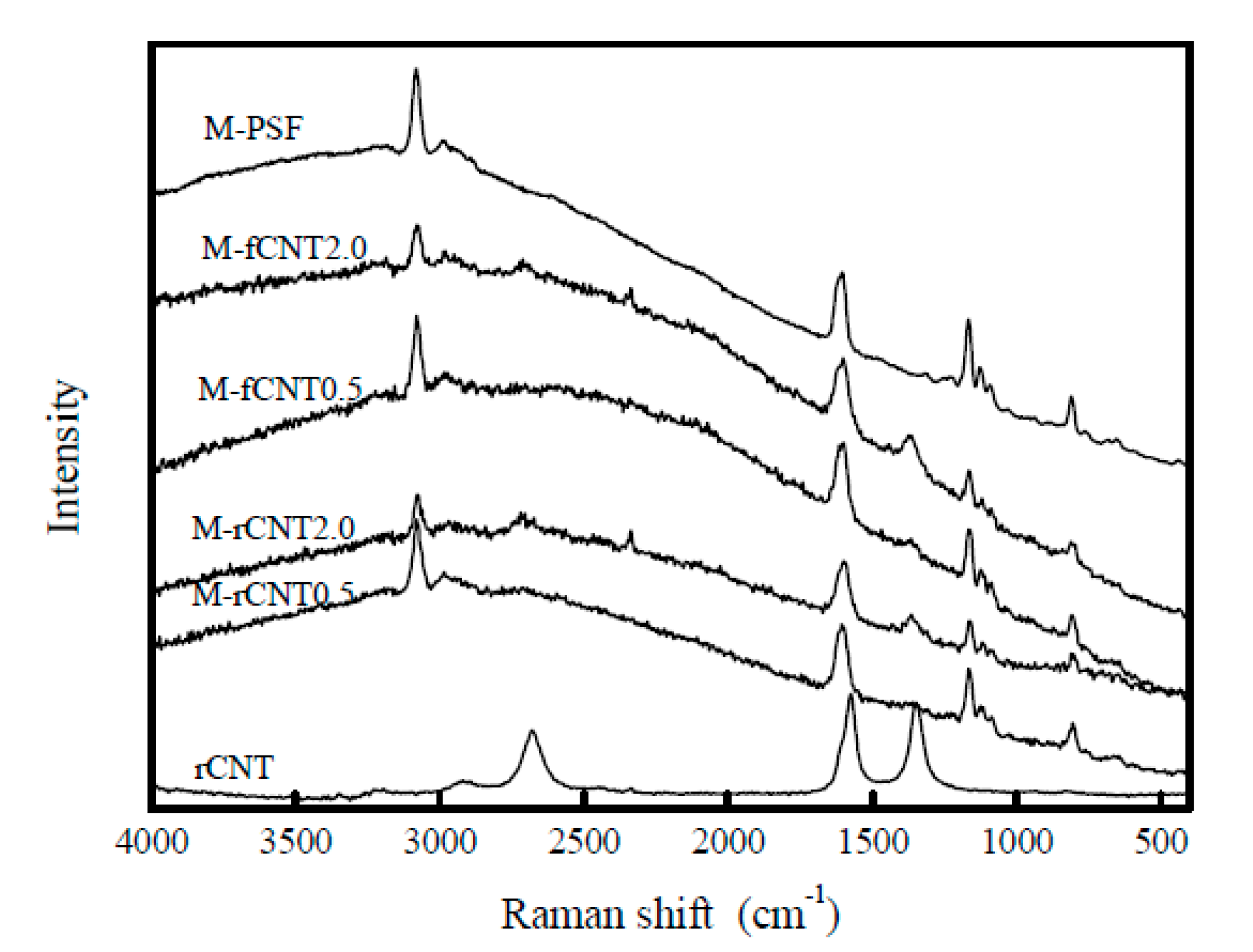
On the same research direction, the influence of two types of CNT (raw and oxidized) concentration on the physic-chemical properties and filtration performance of PSF/CNT type composite membranes was studied. The composite membranes were obtained through a phase inversion method using PSF as a polymer. During membrane fabrication, a leakage of the CNT has been observed, which seems to be higher in the case of oxidized CNT (fCNT) compared with raw CNT (rCNT). According to Raman spectroscopy, it seems that the dispersion of the CNT within the polymer matrix modify the microenvironments of the nanotubes (Figure 5). Porosity tests resulted in an increase in membrane porosity by 54% after addition of rCNT and 68% through addition of fCNT in a PSF matrix. At the same time, the presence of fCNT in the polymeric matrix also led to an improvement in membrane thermostability and its mechanical properties. There was a dramatic increase in water flow in composite membranes with 2 wt.% inorganic content compared to single membranes. These membranes were subsequently tested for 2-naphthol filtration and a two-fold enhancement in the rejection capacity was observed for composite membranes compared to single membranes [35].
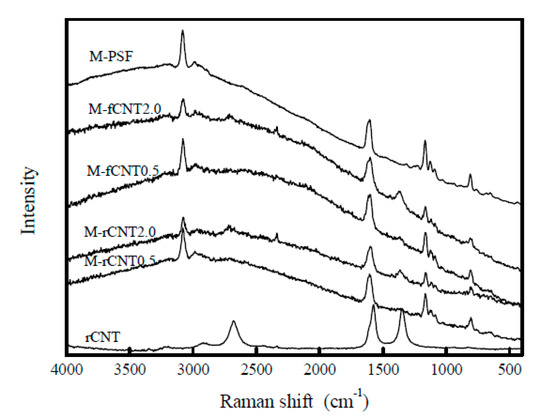
Figure 5.
Raman spectra of the CNT composite membranes. (Reproduced with permission after [35]).
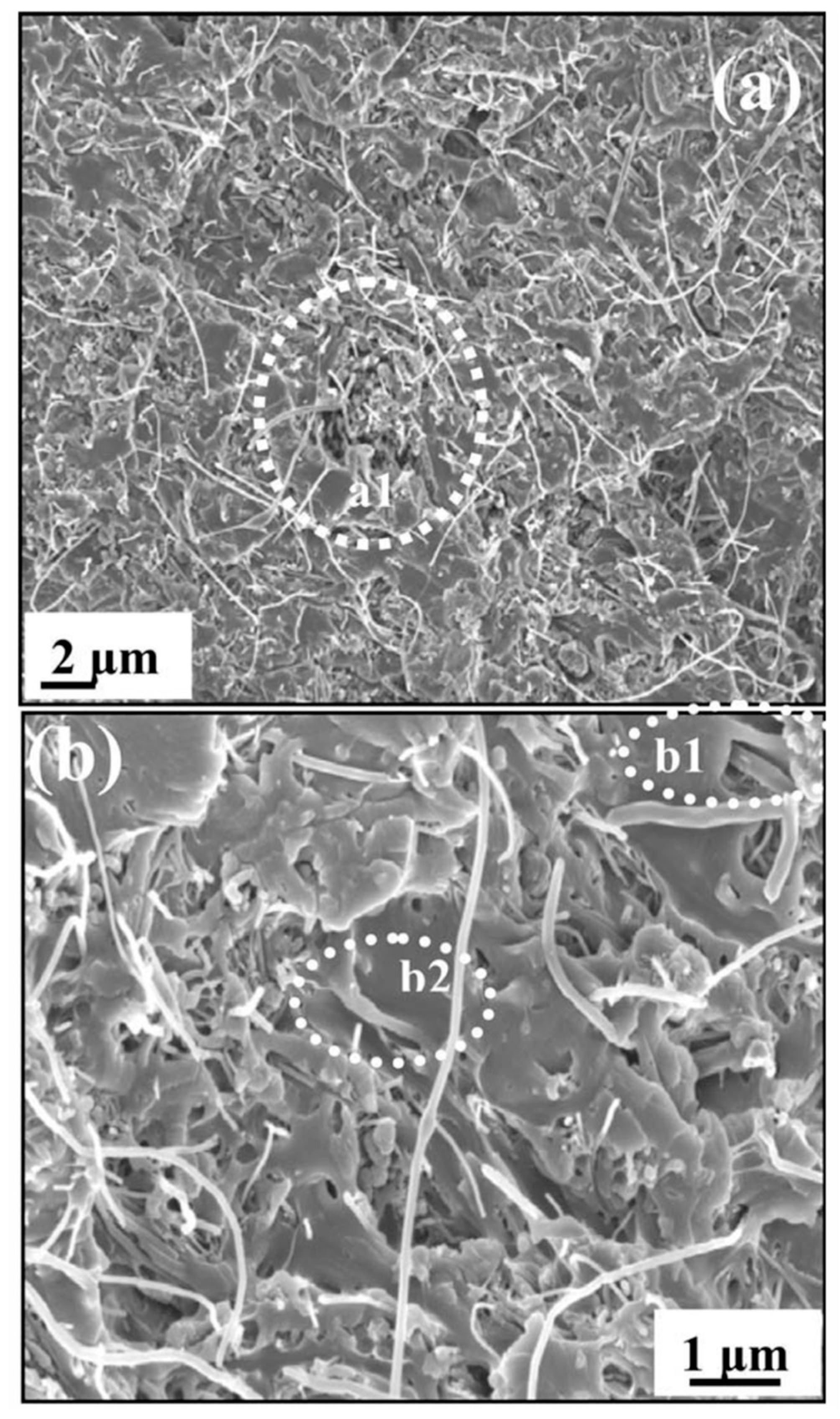
Using solvent evaporation method, several symmetric PSF- and CNT-type membranes were synthesized. In order to obtain symmetric membranes, amino groups functionalization on the CNT surface with crown ether derivatives was initially performed using the polymeric membrane as a polymerization reactor. The composite membranes have been characterized by various advanced characterization methods [36]. New PSF and CNT doped with N and P (fP-CNT and fN-CNT)-type polymeric membranes with high permeability and selectivity were successfully developed using a modified phase inversion process. These membranes were characterized morphologically, regarding their hydrophilicity, permeability and rejection properties and a dependence on the amount and type of functionalized CNT incorporated in the polymeric matrix was observed. Thus, the results obtained showed a significant improvement in hydrophilicity, thermo stability, and water uptake in the case of the composite membranes. At the same time, CNT doping gives the membrane a better flow permeation rate and a better selectivity, due to the membrane pore size increase and its hydrophilicity with CNT addition. A 30% fouling properties improvement was reported when small fractions of fN-CNT or fP-CNT were introduced into the PSF matrix. This improvement could be due to a combination of carboxyl functional groups steric limitations on membrane surface, which led to an electrostatic repulsion between the functional groups present on membrane surface and humic acid molecules (fouling agent) [37]. Moreover, new PSF-carbon nanofibre nanocomposites were synthesized by a solution mixing method and the effect of dispersion state and carbon nanofibers (CNFs) concentration added on PSF properties were studied. A field-emission scanning electron microscopy (FESEM) micrograph of nanocomposite with 5 wt.% CNFs shows some agglomerates, which may be due to the high loading of CNFs in the polyether sulfone PSU matrix. The adhesion of CNFs by PSU was also confirmed from b1 and b2 region of Figure 6. A number of microvoids were seen from Figure 6b, which may be due to presence of agglomerates of CNFs in the PSU matrix and shrinkage of PSU at the time of solvent evaporation. To improve the interfacial polarization between CNF and PSF, CNF have been functionalized through air oxidation. The presence of CNF in the polymeric matrix resulted in an improvement of material thermostability with increasing the inorganic agent concentration due to the impediment of CNF nanodispersion in the polymeric matrix thermal transfer and intermolecular hydrogen bonds between PSF and CNF. There was a direct current (DC) resistivity decrease from 1.08 × 1014 to 1.2 × 105 after adding a 1 wt.% CNF concentration. There was a dependence of the alternating current (AC) resistivity on frequency at low concentrations of CNF (0.5 and 1 wt.%), but at high concentrations (1, 3 and 5 wt.%) the resistivity was no longer affected by frequency, because a conductive CNF network formed. The synthesized materials were also characterized in terms of dielectric properties and a remarkable increase in dielectric constant was observed after CNF addition. Its highest value was 6.9 × 1011 and was obtained at 10 Hz for composites with the highest amount of CNF. Due to the remarkable improvement in the materials’ properties, they could be used in various industrial applications such as electromagnetic interference and electrostatic discharge (Figure 6) [38].
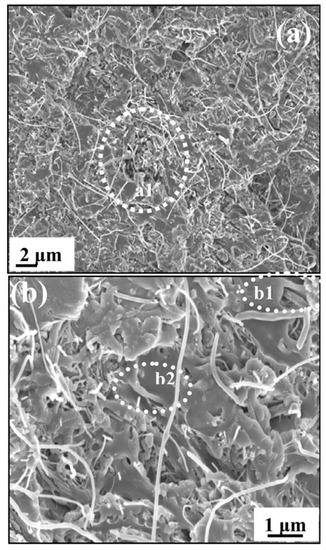
Figure 6.
Tensile fractured field-emission scanning electron microscope (FESEM) image of PSU/carbon nanofiber (CNF) nanocomposites (a) 5 wt.% CNFs and (b) magnified image of (a). (Reproduced with permission after [38]).
The influence of reinforcing agents on PSF-based composite membrane properties had also been studied. Subsequently, these membranes were used to support new thin film polyamide type membranes (TFMs), the performance of which was tested in forward osmosis (FO) using distilled water and NaCl solution 0.6 M as solvent. Pure MWCNT, functionalized MWCNT, graphene oxide (GO) and carbon-TiO2 composite were used as reinforcement agents. Compared to pure PSF, the presence of pure MWCNT in polymeric matrix favors a hydrophobic support formation, while functionalized MWCNT and GO lead to a hydrophilic material with high porosity and interconnected pores. After the addition of formation agent (polyvinylpyrrolidone (PVP)) and the carbon-TiO2 composite in PSF supports, an improvement of membrane porosity and hydrophilicity was observed. These support membranes had also been tested for permeability. The test results showed that PSF membranes which contains functionalized MWCNT and GO have the highest water permeation and solution rejection compared to those containing pure MWCNT or simple PSF. The presence of PVP in MWCNT hydrophilic membranes allows the passage of a high water stream compared to those containing GO and functionalized MWCNT because of the interconnected structure that allows a low selectivity to NaCl. A significant improvement was also seen with carbon-TiO2 and PVP composites. The best permeation was found on the membrane containing 0.5 wt.% GO-TiO2 and PVP (9.6–12.5 L·m−2·h−1). However, the best results were obtained when GO was replaced with functionalized MWCNT (8.4–20.3 L·m−2·h−1) [39].
Similarly, new nanostructured composite membranes based on PSF and different types of carbon nanotubes (single-walled carbon nanotube (SWCNT) and MWCNT) were obtained as reinforcing agents. For membrane synthesis, two types of solvents (N-dimethylformamide (DMF) and aniline) were used to solubilize the polymer powder. The covalent binding between MWCNT and polymeric matrix was also studied. For this, MWCNTs were functionalized with amino groups, and PSF was formulated. The PSF formulation was performed using the Vilsmeier–Haak method [40]. The performed analyzes showed a significant improvement in composite membrane properties compared to pure membrane [41]. On the other hand, the influence of treatment series for CNT functionalization such as cholesterol (octanamine (OCA) and octadecylamine (ODA)) and nitric acid were studied to improve their stability in three types of solvents (n-vinylpyrrolidone (NMP), trinitrofuran (THF) and DMF). Through a solution method, by varying the CNT concentration in the PSF matrix, 4 types of polymeric composites (0–5 wt.%) were synthesized. The performed analyzes showed an improvement of CNT stability after the modification processes, with a higher increase in NMP and DMF compared to THF. Both mechanical tests and thermal analysis confirmed the presence of a residual solvent in all samples as a plasticizer, resulting in a significant reduction in the glass transition temperature (Tg) [42]. Subsequently, symmetric polymeric membranes were obtained and characterized instead of asymmetric membranes by solvent evaporation method, using polysulfone as polymer and CNT-amino as additives. Obtaining symmetrical membranes was the result of CNT presence. After this, the polymeric membrane was used as a reactor for CNT functionalization with crown ether derivatives [36,43]. The symmetric character was given by the choosing synthesis method-solvent evaporation at 40 °C. Due to the low speed of the solvent at membrane formation and also the presence of CNT in the structure of polymer film, a symmetric structure was generated with the same pore diameter on both sides of the membrane.
Later, to remove the disadvantage of CNT agglomeration due to strong van der Walls interactions formed between CNT tubes, organic molecules were attached to their surface. New types of PSF, with different structural units in the polymeric structure, have been used as MWCNT dispersion media. Based on the analyzes, it has been observed that the presence of the sulfide-type groups in PSF favors MWCNT dispersion in the polymeric matrix due to the donor–acceptor interactions between MWCNT and sulfur groups and Π–Π interactions between the aromatic groups and MWCNT. The homogeneous MWCNT dispersion in polymer matrix resulted in a mechanical and thermal properties improvement of composite films compared to pure polymer films [44]. Similarly, using the casting solution method, several PSF-based composites were synthesized using different types of modified CNTs. Hildebrand solubility parameters were used to investigate the potential modification methods applied to increase interfacial interactions and improve CNT dispersion in solvent. The CNTs tested were: pure, chemically treated with a mixture of acids, physically linked to 1-octylamine (OCA) surfactant, covalently functionalized with OCA, pure CNT, treated with acid and subsequently modified with polymethyl methacrylate (PMMA). The studies showed a dramatic change in the carbon tube length and diameter for all applied treatments, except for the one involving surfactant physical absorption at the CNT surface. However, of all types of studied CNTs, the lowest percolation threshold (3 wt.%) and the best mechanical properties resulted for the composite containing CNT functionalized with OCA. The thermogravimetric (TG) analysis confirmed the presence of a high level of residual solvent for all applied treatments, the concentration of which is influenced by the type of treatment used. The smallest percentage of residual solvent was found in the pure CNT-containing composite, because of CNT presence as pores aiding solvent removal [45]. As a result of CNT concentration variation, through the solution growing method, new polycrystalline films polyurethane (PU)/PSF/CNT were obtained. These composite films were characterized by morphological, structural and thermal properties, and it was observed that the presence of CNT in the polymeric matrix led to an increase in materials crystallinity and their thermostability compared to PU and PSF films [46]. Subsequently, several PSF/CNT fouling resistant composite membranes were synthesized by the phase inversion method. Prior to composite membranes synthesis, they were doped with silver nanoparticles (Ag-CNT) through wet impregnation in order to increase the CNT hydrophilicity, resulting in a fouling tendency decrease of the composite membranes. Efficiency analyzes showed an improvement in fouling resistance during Bovine Serum Albumin BSA solution filtration, after Ag-CNT addition to polymer matrix compared to PSF membranes. PSF/Ag-CNT composite membranes showed a slight decrease in water flow but an increase in stability during compaction. For membranes with a 0.2 wt.% Ag-CNT concentration in a polymeric matrix, the highest flux recovery of about 80% and the lowest total flux loss of 56% with an irreversible reduction of fouling resistance of Rir = 21% was observed [47].
Aromatic electrophilic substitution in the presence of AlCl3 was used for direct coupling to a commercial PSF and C60 sample for new C60-polymer materials preparation. Subsequent studies were based on the hydrophobic filler effect on the phase inversion process, the morphological changes and the separation properties, and it was observed that a good filler dispersion in a polymer matrix induces an increase in casting mixture viscosity. The covalent interactions between C60 and the polymer have been demonstrated by nuclear magnetic resonance (1H-NMR), Fourier transform infrared (FT-IR) spectroscopy, and ultraviolet (UV) analyses. At the same time, it was observed that C60 was randomly distributed along the polymer chain. The presence of C60 in polymeric matrix in concentrations between 0.5 to 3.2 mol% has led to high thermal stability and interesting optics properties [48]. Last but not least, new PSF/fullerene and PSF/magnetic functionalized nanoparticles have been synthesized with applications in pre-evaporation of volatile amino compounds resulted from pharmaceutical and paint industry and wastewater Pb and Hg removal. PSF/C60-type membranes were obtained by phase inversion process using chloroform and DMF as solvent. Residual aniline solution pre-evaporation tests recorded the best results for C60 membranes using chloroform as solvent. The pre-evaporation flow value for PSF/C60 membranes was 9 × 10−6 g/s·cm2 greater than that obtained with simple PSF membranes 2.3 × 10−6 g/s·cm2. Composite membranes of PSF/magnetic nanoparticles-spacer-crown ether were synthesized by solvent evaporation method and tested for their Pb and Hg absorption capacity. Test results have shown outstanding performance with these composite membranes being suitable for heavy metal wastewater decontamination processes [49,50].
2.2. Applications
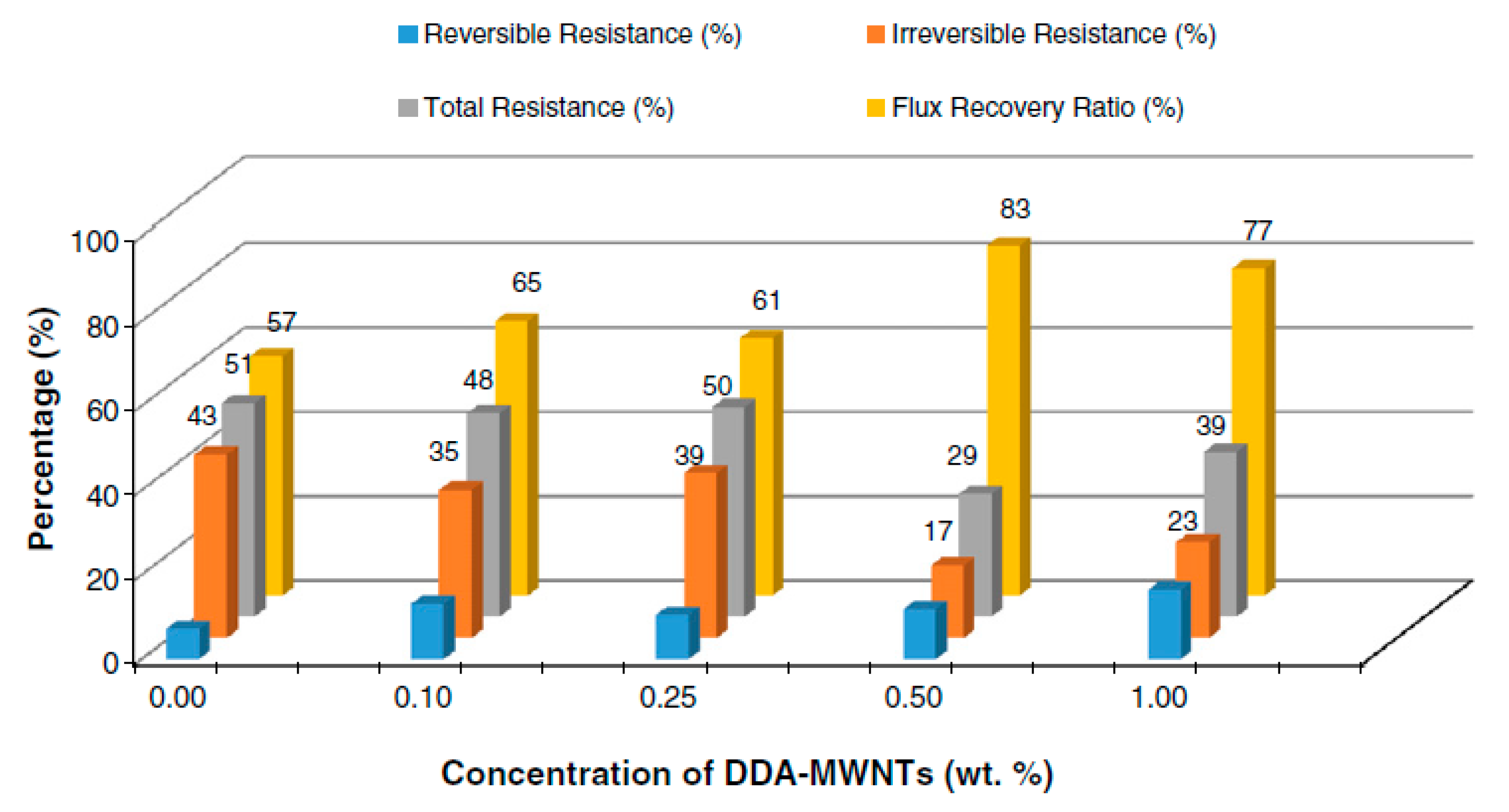
Using the phase inversion method, several nanocomposite membranes used for water desalination were synthesized, starting from PSF and MWNT functionalized with dodecylamine (DDA). The functionalization process was designed to increase the compatibility and interfacial adhesion between carbon nanotubes and polymer matrix by increasing MWNT dispersion in polymer solution. The presence of MWNT-DDA in the polymer matrix led to an increase in membrane surface hydrophilicity and a change in its morphology and roughness compared to the pure membrane. There was a significant improvement in fouling resistance and flux recovery, the best results being obtained for membranes with a MWNT-DDA content of 0.5 wt.% (Figure 7). Membrane anti-fouling properties testing were performed using BSA solution [51].
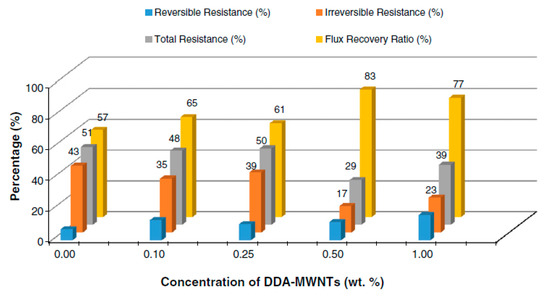
Figure 7.
Fouling resistance and flux recovery of nanocomposite membrane. (Reproduced with permission after [51]).
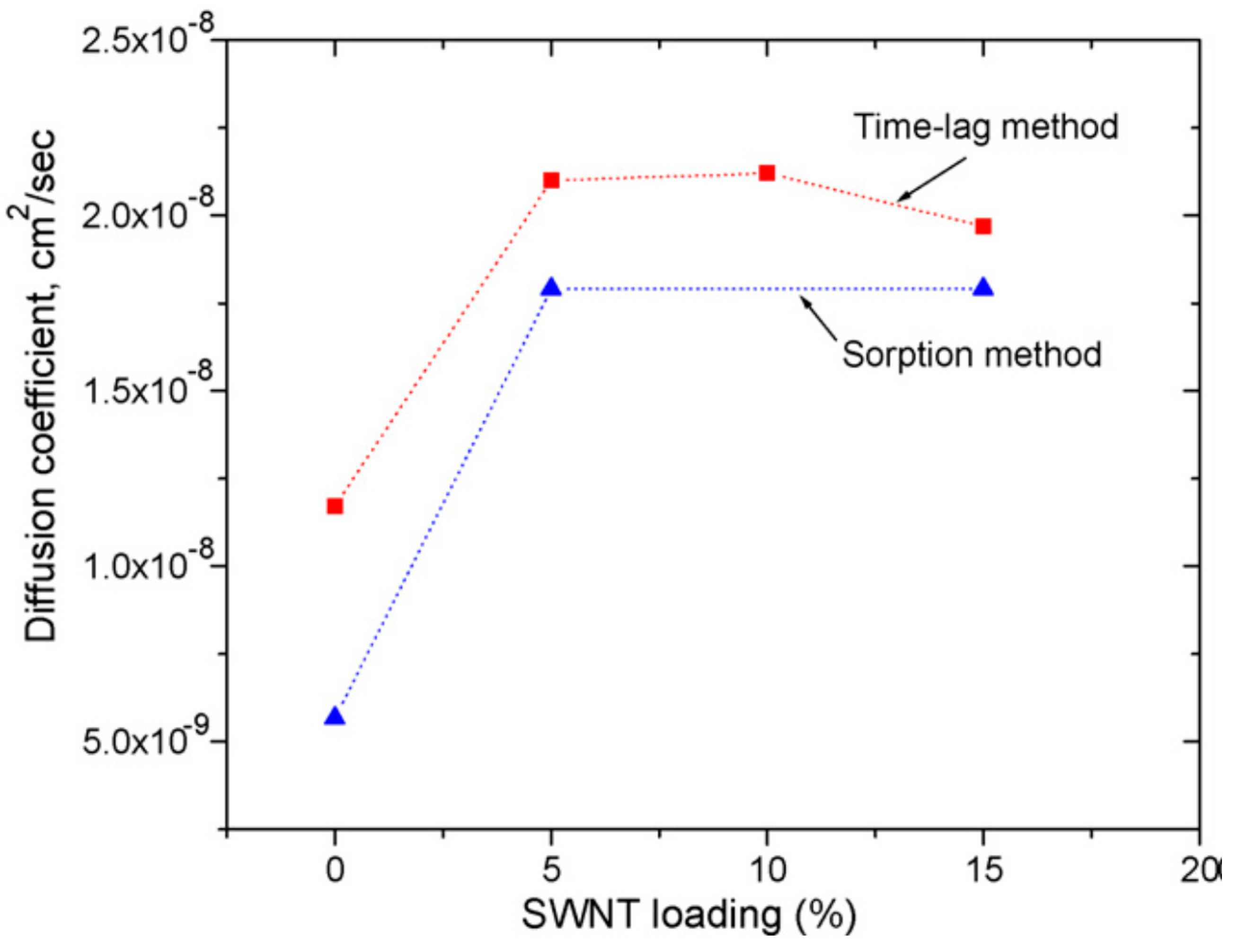
In addition, new composite membranes for Pb2+ and Hg2+ adsorption from blood or other physiological fluids based on PSF and different types of carbon nanotubes single- (SWCNT and double-walled carbon nanotubes (DWCNT) modified with NH2) have been synthesized. The composite membranes were obtained by two methods: a) physical incorporation/immobilization of SWCNT into the polymer matrix by simply dispersing CNT in DMF, followed by phase inversion process using water and i-propanol as non-solvent; b) chemical modification of PSF (chloromethyl and formyl) using p-formaldehyde and chlorotrimethylsilane as chloromethylation agent and Vilsmaier–Haak procedure with POCl2 in DMF to form PSF followed by covalent bonding of DWCNT-NH2 to the functionalized polymer. A linear dependence of membrane adsorption based on the added CNT concentration and an increase in maximum capacity of Pb2+ to 56.2 mg/dm2 for DWCNT-NH2 and 49.2 mg/dm2 respectively for SWCNT was observed. The resulting values were obtained at the maximum CNT concentration (5 wt.% relative to the polymer) [52]. On the other hand, mixed-matrix membranes (MMM) based on PSF and carbon nanoparticles (CNP) have been shown to be beneficial for adsorption of small-sized organic molecules such as benzene, toluene and phenol. Thus, MMM reinforced with CNPs whose concentration varies between 0 and 4 wt.% CNP by casting and wet-spinning method were synthesized. It has been observed that the presence of nanoparticles in the polymeric matrix leads to a membrane pores blocking resulting in a decrease in its porosity and permeability. Membranes with 4 wt.% CNP have a superior permeability value compared to the single membrane. A linear dependence on hydrophilicity (contact angle drops from 79° to 56°) and mechanical properties (breaking strength increases from 3.4 to 6.4 N/mm2) with increasing CNP concentration in polymer matrix were observed. The tests also showed an increase in benzene absorption capacity from 59 to 67 mg/g for the membrane with 4 wt.% CNP at acidic pH, a similar behavior for all three solutions [53]. A comparative study between the experimental tests and the atomic scale computational simulation on selectivity and permeability of some PSF and SWCNT functionalized membranes with alkylamino groups was carried out. The membranes obtained were used for gas separation. Experimental tests for adsorption isotherms for H2, O2 and CH4 were consistent with the predicted isotherms by atomistic scale simulation. The field-emission scanning microscope (FEMS) images showed a good dispersion of SWCNT in a polymeric matrix at a 5 wt.% concentration and the presence of two domains at a 15 wt.% concentration: a region with very well dispersed SWCNT and a dense region. Moreover, the diffusion coefficient for PSF and the composite films has been calculated by using both the kinetic sorption studies and the time-lag method. The results showed lower values obtained for the diffusion coefficients determinate by gravimetric sorption method compared with time-lag because the experimental method implies additional diffusion into the dead-end pores (Figure 8). Improvement of membrane performance after SWCNT addition was achieved when the diameter of the nanotubes was less than 10 Å [54].
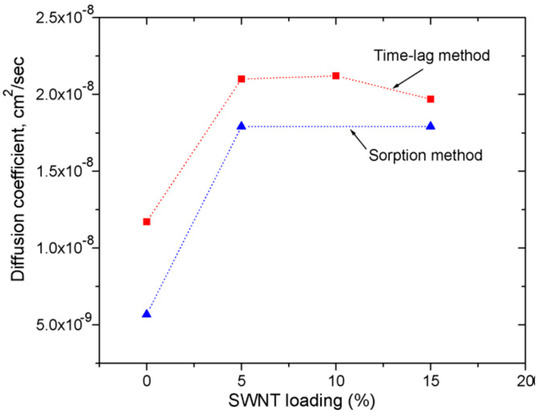
Figure 8.
CO2 diffusion coefficients in single-walled carbon nanotubes (SWCNT)/PSF mixed-matrix membranes (MMMs) at 308 K. (Reproduced with permission after [54]).
Similarly, the synthesis of PSF/MWCNT/TiO2 based ultrafiltration membranes through the phase inversion method (different MWCNT and TiO2 ratios so that the total concentration of filler material does not exceed 1 wt.%) has been studied. An increase in membrane porosity and pore size was observed by increasing the MWCNT or TiO2 content. The permeability properties were tested using crossflow filtration in the presence of humic acid (HA) and it was observed that the membrane with the highest MWCNT content showed the highest flow of pure water but the highest flux decrease due to humic acid addition in 700 ppm concentration. This was due to its particular affinity for being absorbed by MWCNT. The membrane with the highest TiO2 content recorded the highest HA recovery flux at 2 ppm. The membrane containing 0.5 wt.% TiO2 and 0.5 wt.% MWCNT has been shown to have an optimal balance between performance and synergism [55].
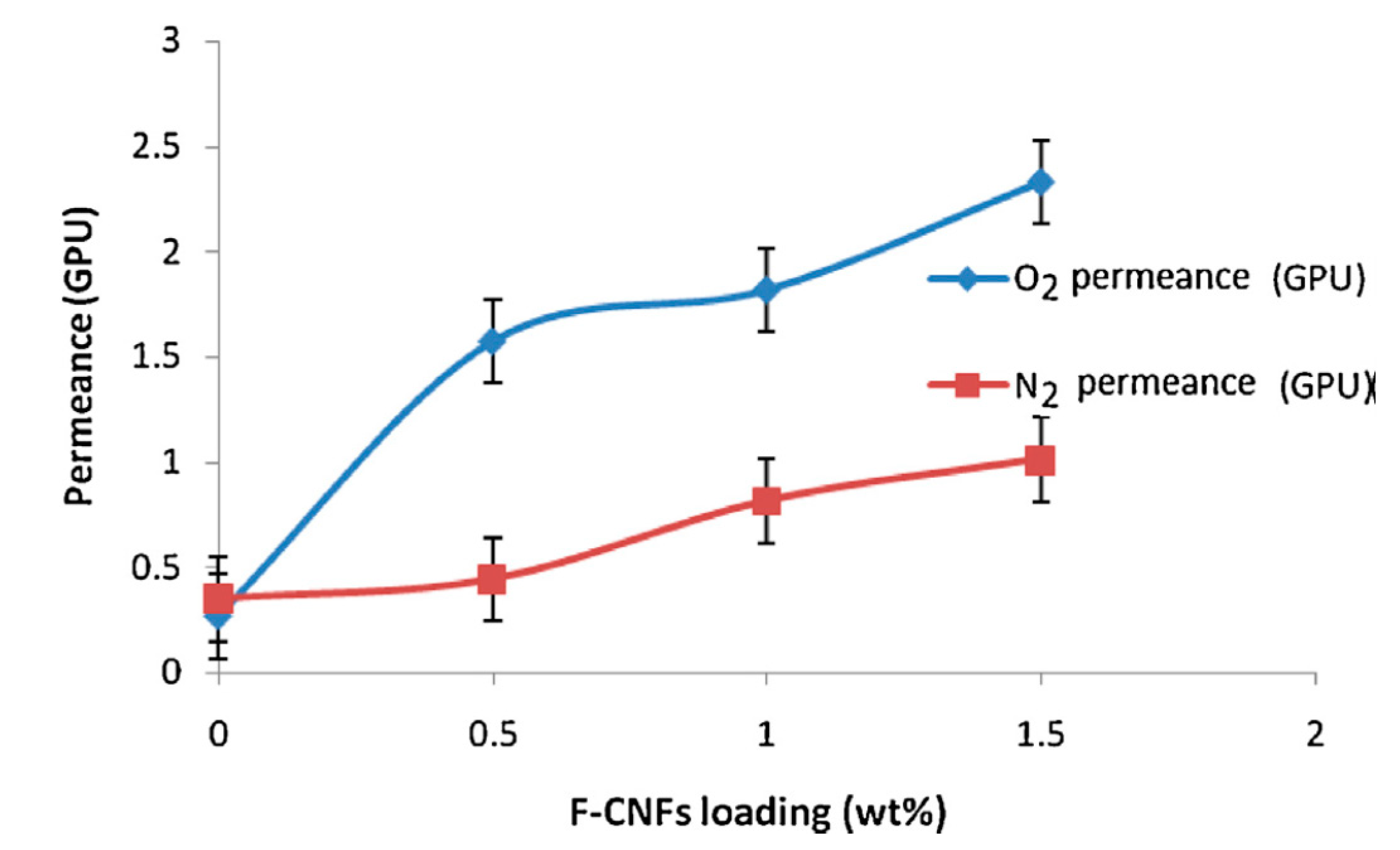
At the same time, new composite membranes with extraordinary separation capacity for CO2-CH4 and N2-O2 have been developed after the addition of various functional carbon nanofibres with amino (f-CNF) groups in PSF concentrations through a casting method. The permeability tests showed a membrane selectivity and permeability dependence of f-CNF content added to the polymer matrix for CO2/CH4 due to the high interactions between CNF surface amino groups and CO2. Thus, for a composite membrane with a 0.5 wt.% f-CNF content, a permeability value of 3.57 GPU for CO2 and 1.58 GPU for O2 and a selectivity value of 7.7 for CO2/CH4 and 3.5 for O2/N2 was obtained. Above this value, a decrease in N2 selectivity was observed [56].
Of all separation processes, membrane processes have gained a great significance. These have some limitations, of which bio-fouling has proven to be the most critical. To improve the membrane anti-biofouling properties, a solution would be to obtain composite membranes. Thus, new nanocomposite membranes containing both SWCNT and MWCNT were developed, and membrane performance was tested in terms of water permeability and solute rejection studies. The anti-biofouling properties were tested using E. coli cultures and it was observed that the best results were obtained for SWCNT reinforced membranes. Also, the analyzes results recorded a decrease of anti-biofouling properties on the membrane surface with the increase of CNT diameter added in the polymer matrix [57].
As a result of carbon nanotubes dispersion in PSF polymer solution and membranes acquisition through phase inversion-immersion precipitation technique, new porous composite membranes have been developed. Prior to incorporation into the polymer matrix, CNTs underwent a modification process with various enzymes including carbonic anhydrase, invertase and diastase using cyanuryl chloride as linker between enzyme and CNT. For better CNT dispersion, the composite membranes were used as a reactor. They have also facilitated the enzyme transport to CNT active sites (amino groups) for the functionalization process to take place more quickly. Covalently functionalized CNTs with various enzymes were isolated at the reaction end by dissolving the membrane and subsequently used for biosensors’ production [58]. In order to obtain stable high performance hydrothermal mixed membrane matrix (MMM) for CO2 separation from flue gas stream, various concentrations and ratios of MWCNT and zeolitic imidazole frameworks (ZIF-302) were incorporated together into a PSF matrix. The membranes performance has been tested both in dry and wet conditions. The two nanofillers presence in polymer matrix resulted in an improvement in CO2 permeability of composite membranes by three times as much as the simple membrane and an increase in ideal selectivity by 1.7. The composite containing 12 wt.% ZIF-302 and 8 wt.% CNT recorded an optimal separation performance by providing a CO2 permeability of 18 Barrer with a CO2/N2 selectivity of 35. There was a slight improvement in MMM permeability in wet conditions compared to dry conditions [59]. PSF/CNT-type functionalized with –COOH, –CONH2, –N3 groups composite membranes obtained through phase inversion method have been shown to have unique properties in terms of surface characteristics and metal ions selective separation from water. These membranes were characterized by a pore size decrease and increased permeability to ~600 L·m−2·h−1 (LMH) at reduced pressure due to the functional group type attached to the CNT surface. Also, depending on the functional group type, a better rejection of Cu (II) than Pb (II) was observed. Furthermore, membranes also exhibited an improvement in conductivity in the 1.0 × 10−2 S cm−1 area [60]. Through phase inversion method, conductive microporous PSF/MWCNT membranes were synthesized. The MWCNT dispersion in polymeric matrix was performed by a sonication method, this being not previously subjected to a functionalization process or surfactant’s use. This method allows good inorganic agent dispersion in polymeric matrix by developing electron percolation pathways across the membrane with a 3 wt.% MWCNT concentration. Taking into account the results obtained, these membranes proved to be perfect in chemical and biochemical separation and water treatment due to good polymer resistance, ease of processing and the possibility of filtering properties adjusting with external electric impulses [61].
Through a casting method, carbon nanofibre (CNF)-reinforced MMMs have been synthesized for gas separation. In MMM synthesis process the CNF concentration in polymeric matrix was varied from 0.1 to 1 wt.%. The synthesized membranes were morphologically characterized and regarding permeability and selectivity properties for CO2, and a change in membrane surface morphology was observed after the inorganic compound addition to the polymer matrix. At the same time, by increasing the CNF concentration in polymeric matrix, an increase in its permeability and selectivity was also observed (Figure 9). Thus, the analysis results recorded an increase in permeability from 12.134 to 12.04 Barres, and the highest selectivity recorded at 4 bar pressure was 12.17 for the composite with 1 wt.% CNF [62].
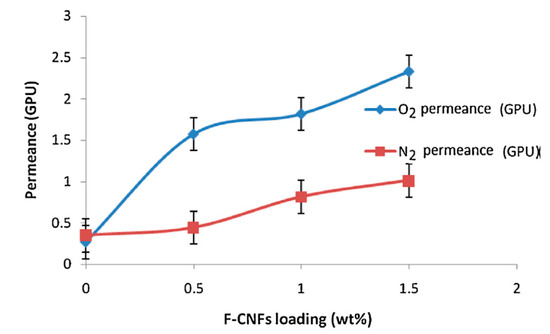
Figure 9.
O2/N2 separation performance of mixed matrix membranes as a function of carbon nanofiber (CNF) concentration (test conditions: 2 bar feed pressure gradient at ambient temperature).*All experimental data are reported as the average of at least three repeated measurements. (Reproduced with permission after [62]).
By means of a phase inversion method using DMF as solvent and a mixture of isopropyl alcohol/water as coagulation medium, several carbon nanotubes (fMWCNT)/PSF functionalized composite membranes were synthesized. MWCNTs were modified by grafting three types of functional groups including oxide, amide and azide and a reduction in membrane pore size at 20–30 nm was observed depending on the functionalization type obtained. Also, after performed analyzes, an increase in membrane hydrophilicity was observed with a reduction in flow and flow rate in case of composite membranes compared to pure membrane. These membranes were also tested for heavy metal retention and a linear dependence of metal absorbed percentage relative to the added fMWCNT concentration was recorded. Thus, the best results were obtained for fMWCNT membrane with amide groups for which retention of 94.2% for Cr (IV) and 78.2% for Cd (II) was recorded [63].
Also, new CNT-reinforced PSF composite membranes with a polyvinyl alcohol barrier layer (PVA) have been synthesized using phase inversion method for oil separation in wastewater decontamination. The obtained membranes were characterized in terms of mechanical properties and an increase in Young’s module of 77%, in tensile strength of 25.8% and in traction resistance of 11.9% in the composite with 7.5 wt.% CNT were observed. The composite membranes have also been tested for permeation properties and it has been observed that at the passage of the permeate through membranes an oil concentration below the accepted limit of 10 mL/L was recorded and an excellent discharge and rejection of over 95% [64].
Similarly, various PSF/CNT-type nanocomposite membranes were synthesized using phase inversion method induced by immersion precipitation technique. In order to improve the interactions between the two components, CNTs have been modified with amino and carboxyl polar groups. For this, CNTs were chemically treated with a mixture of strong acids (H2SO4: HNO3) and 1-4-diaminobenzene. The functionalized group’s effect on the composite membranes’ morphology and permeability properties was further studied. The analyzes results recorded a change in the membrane surface roughness, an increase in the pore size and the membrane hydrophilicity after 0.5 wt.% functionalized CNT addition in the polymeric matrix due to increased compatibility between the two components. By subsequent reinforcing agent concentration increase, a decrease in pore size and water flow probably occurred due to CNT agglomeration. The tests results showed an increase in water flow in CNT functionalized with amino (n-CNT) group-reinforced membranes and those containing CNT functionalized with carboxyl groups (cf-CNT) due to the presence of N2 atoms and the formation of hydrogen bonds between water molecules and amino groups existing on the membrane surface [65].
In the same research, a summary of literature studies has shown that CNT exhibits excellent rejection properties even at low pressure which has opened a series of opportunities in the use of CNT-based membranes, obtained through the phase inversion method, as membranes for ultrafiltration. The functional groups type attached to the CNT surface has a significant influence on pore size, membrane thermostability and membrane conductivity. Thus, PSF/CNT composite membranes recorded a water permeation of up to ~600 L·m−2·h−1 (LMH) and an improvement in conductivity in the range 1.0 × 10−2 S cm−1. The composite membranes were also characterized in terms of metal ions’ selectivity and it was observed that, depending on surface functionality, Cu (II) ions are better rejected than Pb (II) ions. At the same time, the percentage of rejected metal ions increases with increasing CNT concentration in the polymer matrix. Furthermore, CNT functionalized with azide groups showed a good selectivity for metal ions [60]. Thus, several interference materials for thermally stable electromagnetic shielding were synthesized starting from PSF reinforced with both MWCNT and CNF. The composite materials obtained were analyzed by studying the effect of reinforcement agent type used and its structural properties. It has been observed that CNF-reinforced composite materials exhibit mechanical and thermal properties superior to those MWCNT reinforced in the same concentration. The electromagnetic interference shielding efficiency is of greater value for CNF composites compared to those containing MWCNT. Moreover, both systems exhibit a low percolation threshold, i.e., φc = 0.0079 vol. fraction (0.9 wt.%) for PSF/CNF and φc = 0.014 vol. fraction (1.5 wt.%) for PSF/MWCNT [66].
Due to its exceptional properties, polymer composite materials have attracted a great deal of attention in various nuclear and space applications. Polymers, depending on their nature, react differently when exposed to certain high levels of radiation, some are degraded, while others preserve their stability. To increase their stability, several MWCNT concentrations were incorporated into the polymer matrix. Thus, through solution mixing method, PSF/MWCNT-type composite films were synthesized which were subsequently exposed to γ-radiation under an argon atmosphere. Performed analyses showed, in the case of simple polymeric films, a decrease in Tg and their stability after material exposure to different doses of radiation. Gel permeation chromatography (GPC) analysis recorded a molecular weight decrease due to polymer chain cleavage after radiation exposure. To increase Tg, MWCNT was introduced into the polymeric matrix, and an improvement could be seen with increased MWCNT and dose levels up to 1.5 MGy. This Tg increase was due to the Π–Π interactions between MWCNT and PSF aromatic ring. Mechanical tests recorded an increase in Young’s modulus value with the MWCNT increase in the polymer matrix, and a decrease in Young’s modulus value with the radiation dose increase. Film breakage resistance decreases with increasing MWCNT content and radiation dose [67].
At the same time, by varying MWCNT concentration in the polymer matrix, several nanocomposites were obtained through casting method. These composite materials have been studied regarding electrical properties by analyzing the alternative current impedance spectrum, and the dielectric response has been analyzed at several frequency values in the range of 10 Hz to 10−6 Hz. A significant increase in the dielectric constant for PSF/MWCNT nanocomposites from 2 × 1010 to 6 × 1010 at a frequency of 10 Hz could be observed due to the interfacial polarization between the polymer and the reinforcing agent [68].
Furthermore, soft composite membranes with gene IgG receptor PSF/MWCNT/RIgG type immune composites for amperometric immune sensing were obtained through phase inversion method. As it offers a large surface area, mechanical flexibility and hardness, PSF/MWCNT acts both as a reservoir for immune biological material and as a transducer. Compared with graphite-containing composites, in MWCNT the membrane selectivity is higher, and the roughness is doubled. To reduce the membrane roughness, an antibiotic is introduced to facilitate the MWCNT dispersion in a polymer matrix. The RIgG immune reagent was subsequently incorporated into the membrane by phase inversion using horseradish peroxidase enzyme as the label and hydroquinone as mediator. Following the analysis, a detection limit of 1.66 μg/mL, C50 of 3.65 μg/mL, a linear anti-Rigg range from 2 to 5 μg/mL and a 5-fold selectivity were obtained [69].
The PSF reinforced with 0.05–1 wt.% MWCNT-type nanoparticle films electrical and piezoresistive properties were also investigated. The best electrical conductivity was obtained for composite films with 0.2 wt.% MWCNT, over this value the films have excellent piezoresistive capacitance with a linear relationship between the electrical and mechanical resistance for a voltage below 1.3%. The highest average gage factor of 0.74 was obtained for composites with 0.5 wt.%, increasing SWCNT concentration; no appreciable increase in film sensitivity was observed [70]. In order to remove p-chlorophenol from aqueous solutions, polysulfone and CNT-type hybrid polymeric beads were used as adsorbents obtained through phase inversion method. From a morphological point of view, these hybrid beads have a smooth surface with a porous interior and CNT present in the form of worms between polymer layers, leading to a dramatic increase in polymer beads surface area. Based on the studies, it has been observed that CNT adsorbent hybrids have a superior adsorption capacity for p-chlorophenol compared to pure polymer or pure CNT as well as outstanding separation performance after adsorption. Kinetic adsorption studies of p-chlorophenol on hybride CNT adsorbent identified an exothermic process with a predominantly physical mechanism where the molecular form of p-chlorophenol proved to be the most effective form of adsorption [71]. To improve selective O2/N2 filtration a number of modified MMMs have been developed. These membranes are based on polyethersulfone, pure MWCNT and MWCNT functionalized with aminopropyltriethoxysilane (APTES). In the manufacture of composite membranes, the effect of several parameters such as MWCNT used type, concentration and size, solvent type, polymer concentration, synthesis method and pressure applied to both membrane manufacture and gas separation were studied. The investigation results showed an improvement in composite MMMs’ gas permeation and selectivity after MWCNT purification and functionalization with a mixture of acids and APTES. N-methyl-2-pyrrolidone (NMP) has been shown to be the most promising solvent for MWCNT dispersion, which has led to an increase in membrane selectivity for O2, the MWCNT homogeneous dispersion in solvent solution being also influenced by carbon tube diameter. A membrane superior was also obtained with a 29.5 wt.% polymer concentration compared to 25 wt.% and the optimal process pressure was found to be 4 bar [72]. On the other hand, PSF membranes for ultrafiltration with improved anti-fouling properties have been synthesized following three types of polyethylene glycol (PEG) derivatives immobilization on PSF surface. Two of the PEG derivatives contain amino groups at the ends, and the third one has at one end a methoxylated carboxyl group (MPEG550). For this purpose, the carboxyl groups were initially introduced on PSF through a Friedel–Craft reaction, the immobilization being carried out by forming an amide bond. It has been observed that a longer reaction time or an appropriate choice of immobilized PEG derivative on the PSF surface favors membrane hydrophilicity increasing, resulting in superior antifouling properties. Ultrafiltration experiments recorded an improvement in recycling properties and a better reliability of the PSF surface modification process [73].
The development of a desired chemical structure and exceptional properties of composite membranes involves a careful controlled follow-up of several steps. Therefore, the effect of introducing MWCNT into Kapton–PSF composite membranes with applications in different pairs of gases separation was investigated. At the same time, the effect of PSF percentage in the polymer blend on synthesized membrane properties was studied. Thus, the analysis results indicated that the presence of CNT in the polymer matrix, Kapton-PSF membranes composition and chemical structure significantly influence the resulting membranes properties. From the point of view of gas permeability, test results have shown that PSF percentage is an important factor in controlling permeability and ideal selectivity of precursors, a high percentage of PSF leading to a membrane with high permeability and selectivity for gases. The trend is PCO2 > PO2 > PCH4 > PN2. Furthermore, by CNT subsequent addition in polymer matrix, the gas permeability increases, and the gas pairs selectivity decreases especially for CO2/N2, CO2/CH4 and O2/N2. The permeability for CH4, CO2, N2 and O2 increases from 0.216, 4.462, 0.225 and 0.829 Barrer (values recorded for simple membranes at a pressure of 10 Bars) to 0.32, 5.434, 0.31 and 1.007 Barrer (composite recording values of 8% CNT) [74].
In order to increase polymeric membranes’ tolerance to various chlorine chemistry treatments, a solution would be to introduce different concentrations of CNT-COOH into a PVP–PSF matrix (polyvinyl-pyrrolidone-polysulfone). CNT-COOH introduction resulted in a smoothing of the membrane rusty surface, an increase in its hydrophilicity and an improvement in membrane performance for permeability and antifouling abilities. At the same time, the presence of CNT-COOH resulted in a residual chlorine compounds freed from the membrane concentration reduction and C-Cl (2p) was 2.8 times lower compared to PVA–PSF membranes. This explains the increase in membrane tolerance to Cl treatment due to its increased stability by forming hydrogen bonds between the polymer chain and CNT-COOH [75].
A series of nanocomposite membranes were synthesized using sol-gel method with reduced graphene oxide (RGO) and MWCNT as reinforcing agent, applicable to water electrolysis, the maximum reinforcing agent concentration being 0.5 wt.%. A comparative study between polysulfone sulphonate (SPSF)-type composite membranes with RGO and those containing MWCNT was realized in order to increase performances and improve electrical conductivity. The studies indicate higher conductivity and improved performances in terms of current density for SPSF-GO membranes compared to SPSF-MWCNT due to the exceptional properties of graphene including the high specific surface area, increased localized chargers, and the conductive paths of graphene. At a 60 °C temperature, in case of SPSF-GO composite membranes, a constant and uniform current density of 1.39 Å/cm2 was obtained [76].
A summary of all membranes is presented in Table 1.

Table 1.
Summarized applications for presented membranes.
3. Polysulfone–Graphene Composite Membranes
3.1. Synthesis, Characterization and Properties
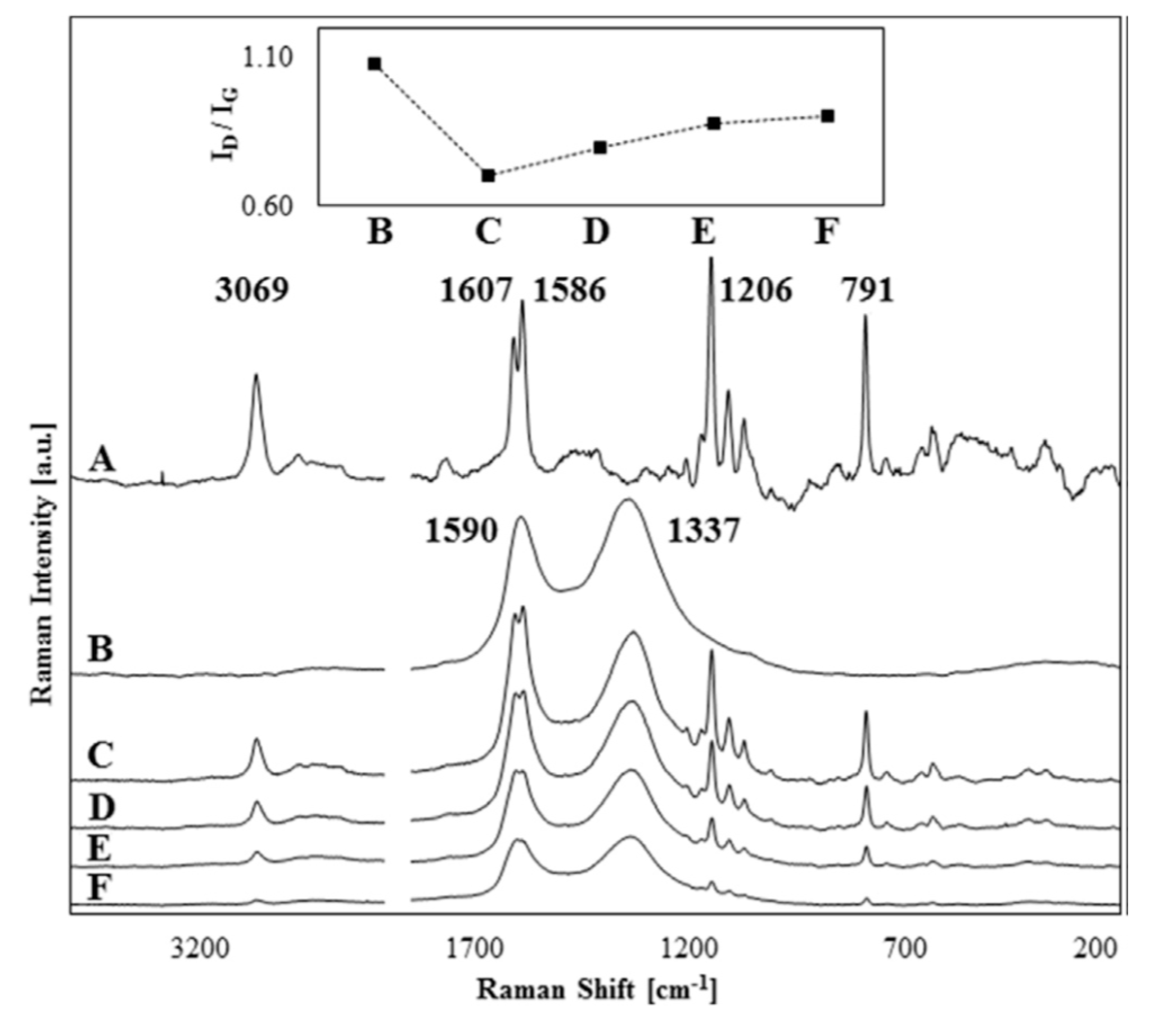
Using phase inversion method, a series of porous PSF/carboxylated graphene oxide (GO-COOH) composite films were synthesized. The films obtained were characterized morphologically, structurally and regarding thermal, mechanical and biological properties. The structural consistency as well as the presence of GO-COOH within the polymer matrix has been observed using Raman spectroscopy (Figure 10). The membrane’s hydrophilic-hydrophobic character has also been studied. The presence of GO-COOH in the polymeric matrix had a smoothing effect on the membrane surface on both sides, a surface roughness reduction of ~50% and an increase in membrane pores size. Moreover, a thermal and mechanical properties improvement for composite membranes compared to simple membranes has been observed. A good composite membrane biocompatibility and affinity for cells has been observed after performing the in vitro tests. However, after GO-COOH incorporation into polymer matrix, no significant changes in membrane hydrophilicity were recorded [77]. To improve reduced graphene oxide (RGO) dispersion in the PSF matrix, the increase in interfacial adhesion and the improvement of polymer mechanical properties at the ends and middle of RGO (PSF/GO end and PSF/GO mid) different PSF chains were grafted through two synthesis methods via nitrene chemistry. These methods involve 1) increasing the polymer chain directly on the RGO surface (grafting from) and 2) attaching a preformed polymer chain on the RGO (grafting to) surface. Prior to the grafting process, the PSF surface was functionalized with azide groups. The polymer chain grafting effect on graphene antibacterial behavior and graphene toxicity was further studied. Antibacterial tests were performed on planktonic Bacillus subtilis and Escherichia coli and it was observed that PSF/GO mid has superior antibacterial properties due to the shorter grafted polymer chain that facilitates the contact of microorganisms with the graphene surface [78]. Also, by varying the GO concentration in PSF a series of composite membranes were synthesized through a phase inversion method. These membranes were characterized morphologically, structurally and regarding thermal and mechanical properties. It has been observed that a low GO concentration in the polymer matrix has led to an improvement in composite membrane properties compared to pure membranes due to strong interactions between GO and the polymeric chain formation. By increasing the concentration above 1 wt.%, GO aggregates which lead to material defects were obtained which further decreases the membrane mechanical and thermal properties [79]. To obtain a uniform nanoparticle (NP) dispersion of Ag in the PSF matrix, the GO surface was enriched with AgNP by reducing Ag nitrite in the presence of an aqueous BrNa solution. The AgNP uniformly deposited on the GO surface was subsequently incorporated into a polymeric matrix and new PSF/AgNP-GO composite membranes were finally obtained through a phase inversion method. Synthesized composite membranes have been tested for their performance and an increase in their properties has been observed, optimal properties being obtained for membranes with 0.5 wt.% AgNP-GO added. At the same time, these composite membranes have also been shown to have excellent antibacterial properties, leading to prevention of biofouling surface formation [80].
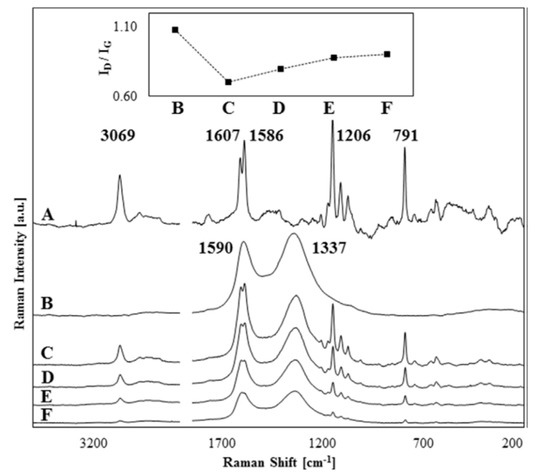
Figure 10.
Raman spectra of PSF (A), G-COOH (B) and PSF/G-COOH 0.25–2 wt.% (C-F). The variation of ID/IG ratio with G-COOH concentration is represented within the inset. (Reproduced with permission after [77]).
Similarly, new MMM membranes based on PSF and C60 have been developed and tested. The concentration of C60 in the polymer matrix went up to 5 wt.%. Two types of PSF and PSF/C60 membranes were studied, a dense one and a thin one obtained on porous hydrophobic fluorocarbon support. A mixture of ethyl acetate-water was used to study the transport properties of the synthesized membranes and it was observed that all samples were found to be selective for water, with the best results being obtained for membranes with a concentration of 0.5 wt.% C60 in a PSF matrix [81]. To increase PSF membranes hydrophilicity and selectivity for polycyclic aromatic hydrocarbons, initially, the polymeric matrix was introduced as a GO reinforcing agent followed by a change in the composite membrane surface by molecular impregnation (change by molecular impregnation - CIM). CIM membranes were obtained by phase inversion after mixing GO and PSF in N-methylpyrrolidone solution. Studies have shown that by combining GO with surface modification by molecular impregnation the membrane performance can be improved as the contact angle decreases from 72 ± 2.7% to 62.3 ± 2.1%, the water flow increases from 8.56 L·m−2·h−1to 15.3 L·m−2·h−1and the salt rejection increases from 57.2 ± 4.2% to 76 ± 4.5% [82].
On the other hand, following GO intercalation in a PSF matrix through a wet phase inversion method, new MMMs were synthesized. These membranes have been characterized morphologically and structurally and an improvement in membrane performance has been observed following the introduction of a reinforcing agent into the polymer matrix. Thus, the presence of GO in the polymeric matrix has led to increased membrane hydrophilicity due to functional groups on the GO surface. The composite membranes performance has also been tested for water flow and salt rejection, and it has been noticed that a doping concentration of 2000 ppm GO in PSF has been reported to have a maximum salt rejection of 72% for a solution of Na2SO4 with a concentration of 1000 ppm at an applied pressure of 4 bar [83].
3.2. Applications
A series of thermal curing composite membranes from PSF and RGO used as anion exchanger membranes (AEMs) were synthesized. RGO was introduced into the double-aspect polymer matrix: reinforcing agent and cross-linking agent. Prior to the introduction of RGO into the polymer matrix, azide groups were introduced on the PSF chain through a quaternizing reaction without affecting the ionic groups, the crosslinking reaction occurring between the introduced azide groups and RGO. The resulting studies showed a mechanical and thermal properties improvement of crosslinked membranes compared to a pure membrane. Furthermore, the crosslinked membrane exhibits better resistance to degradation in alkaline solutions and to free radicals action and good permeability to methanol. However, the main disadvantage of the as-synthesized membrane is the decrease in ionic conductivity due to the decrease in water uptake capacity after crosslinking process [84].
Following the graphene surface modification, a series of anion exchange composite membranes was synthesized through hydrothermal method. Changing the graphene surface involved a succession of chemical reactions that consisted in opening the epoxy ring, ultimately obtaining a quaternary graphene (QGs). This QGs was subsequently incorporated in various concentrations into chloroformated PSF (QPSF) and its influence on composite membranes properties was studied. The performed analyzes showed an improvement in traction resistance at 250 MPa and Young’s modulus at 5240 MPa compared to the simple membrane after intercalation of a 0.25 wt.% QGs amount within the polymer matrix. Furthermore, the highest value of bicarbonate conductivity, of 18.7 mS/cm recorded at 80 °C, was obtained with the 0.5 wt.% QGs composite membrane. This increase in membrane performance was due to excellent compatibility between the two components and formation of interconnected transfer channels due to the presence of QGs in the polymer matrix [85].
By varying GO concentration in PSF between 0 and 1 wt.%, several composite membranes were synthesized through a phase-inversion method. The composite membranes performance has been studied in terms of thermal and mechanical properties, permeability for both water and ethanol and biological behavior. The greatest increase in Young’s modulus and traction strength from 153 MPa to 330 MPa and from 3.5 MPa to 5.6 MPa was recorded for composite membranes with 0.25 wt.% GO. The presence of GO in the polymeric matrix indicates a decrease in water and ethanol flow for all composite membranes, from 4100 L/m2 h to 1500 L/m2 h, due to the porous structure’s increased stability of the composite membranes. Furthermore, the in vitro analyzes results shown that increasing the concentration of GO in the polymeric matrix has a positive effect on the proliferation of murine mesenchymal stem cells (MSCs) [86].
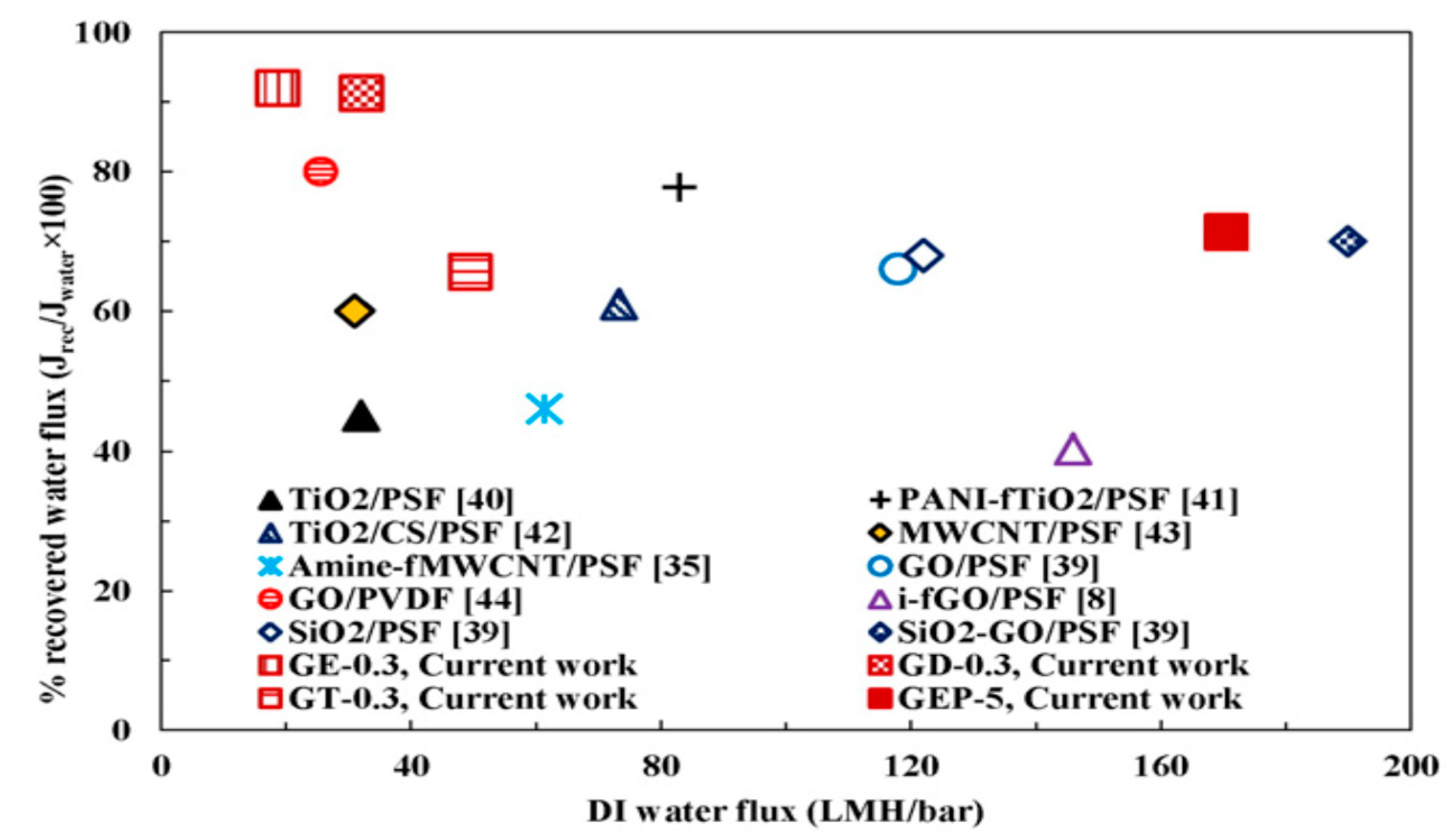
Intercalating functionalized graphene oxide (GOf) in PSF matrix MMM were synthesized. PSF functionalization was achieved by attaching three amines with different chain length (ethylenediamine, diethylenetriamine and triethylenethermine) to GO surface in order to increase the interbase distance between GO sites and to improve GOf dispersion in PSF-NMP solution. The presence of GOf in the polymeric matrix led to increased membrane permeability and hydrophilicity, improved structural, mechanical and anti-fouling properties, and a higher recovery flux after BSA solution filtration. MMM containing 1 wt.% GOf with ethylenediamine and 5 wt.% added PEG-600 showed a significant increase in water flow of 170.5 LMH/bar and 90.5% BSA rejection (Figure 11) [87].
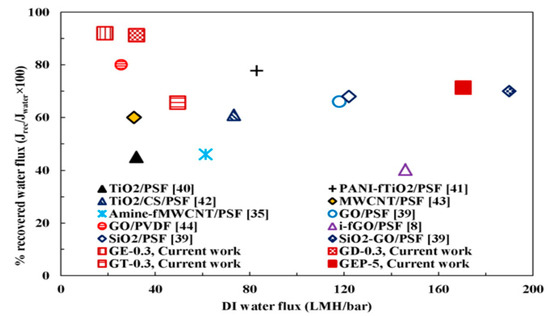
Figure 11.
Comparison of the performance of membranes for different fillers-polymer mixed matrix ultrafiltration membranes in terms of hydrophilicity and antifouling properties. (Reproduced with permission after [87]).
By varying the PSF concentration, two PSF/GOf composite membrane series were synthesized. The functionalization of GO consists of grafting the GRAFT (o-ethyl xanate) agent onto GO nanoplanes surface with help from bromopropionyl bromide linker followed by the growth of a polymeric chain (polydialildimethyl-ammonium chloride (PDADMAC)). The composite membranes were characterized and an improvement in water flow of 443.22 L·m−2·h−1was observed for membranes with 7 wt.% GO-PDADMAC/20% PSF and a 86.68% heavy metal rejection for Cu2+ in case of composites with 5 wt.% GOf/20 wt.% PSF and 88.68% for Cd2+ for composites with 3 wt.% GOf/23 wt.% PSF [88].
Thus, through a phase inversion method, new PSF/GO ultrafiltration membranes with improved antifouling properties have been developed. In order to modify the PSF membrane and generate membrane pores, in the PSF solution various polyvinylpyrrolidone (PVP) ratios were gradually added. The hydrophilicity of the PSF membrane has been improved by vacuum filtration of a GO layer prefiltered on previously obtained membranes. For evaluation of final membranes antifouling properties, the rate of water recovery (FRR) was compared before and after ultrafiltration to a protein solution. The analyzes result showed that FRR involving pure membranes (33.6%) was much lower than membranes containing PVP and GO (over 75%) [89].
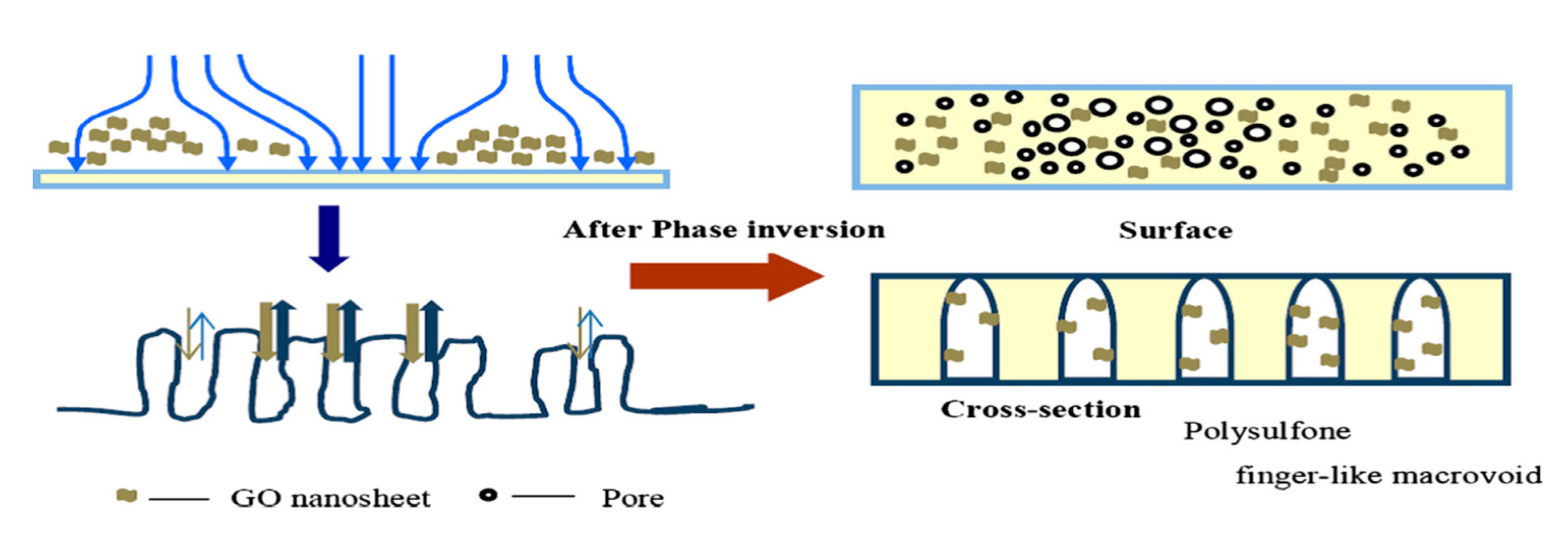
Subsequently, through a double-blade casting method, using different PSF concentrations (15% top and 7% bottom), new polymer membranes with a double membrane structure for advanced osmosis were prepared. This synthetic technique has resulted in a membrane with a very porous structure at the bottom and a dense and thin layer at the top. GO was incorporated into the polymer substrate to increase the final membrane hydrophilic character. Thus, due to the uniform dispersion of GO in the polymer, TFC-PSFdGO (dual layer PSF/GO) with high permeability for water and with increased ion selectivity was obtained. In permeability tests, deionizer water was used as the feed solution and NaCl as a draw solution. Following the addition of GO, the composite membranes exhibited the lowest specific backflow Js/Jv = 0.19 g·L−1, and the water flow increased from 30.3 L·m−2·h−1to 33.8 L·m−2·h−1 [90]. Also, a thin film composite forward osmosis membrane (TFC-FO) of the PSF/GO type was synthesized where GO was used as a modified substrate in different percentages, and the polyamide active layer (PA) on the membrane surface was obtained by interfacial polymerization. In order to obtain a TFC-FO with the desired structural properties, the added GO concentration was found to be 0.25%. This concentration is also favorable for efficient PA surface membrane formation and significant increase in membrane permeability. Thus, after GO addition in the polymeric matrix, the water flow increased from 6.08 L·m−2·h−1 to 19.77 L·m−2·h−1 due to increased membrane hydrophilicity, and the reserve flux selectivity increased from 3.36 L·g−1 to 5.75 L·g−1. By further increasing the GO concentration to 0.5 wt.%, because of the GO agglomeration, both the mechanical properties and its permeability decreased [91]. At the same time, a series of PSF/GO type composite membranes were synthesized through a phase inversion method using a GO solution in water at different concentrations as a coagulation bath. The presence of some GO traces in the coagulation bath could have an important effect on the properties of the as-synthesized membranes. This phenomenon could be observed in Figure 12. These membranes were characterized morphologically, structurally, mechanically and regarding membrane permeability, and it was observed that the presence of GO had a positive impact on membrane hydrophilicity and its mechanical properties. Traction resistance increased from 2.31 to 2.87 MPa and the modulus of elasticity from 237.86 to 258.47 MPa. However, compared to the membranes obtained in distilled water, the PSF/GO membranes show a higher layer thickness, which has resulted in a significant decrease in permeability. Thus, the water flow recorded a decrease of up to 75.1% for the composite membranes by 20 mg/L GO and the rejection rate was much lower because after GO intercalations the membrane pores’ size increase [92]. By combining dripping method with 90° processing for 24 hours on commercially available ultrafiltration membranes, GO nanoplanes have been deposited, providing improved mechanical strength. After the temperature process, intercalated water molecules are removed from GO layers and reduced. The GO membranes have been tested for liquid permeation, water and isopropyl alcohol (IPA), and it has been noticed that after the GO membrane temperature process, despite the increased IPA viscosity, membrane permeability has been improved 7 times. The water flow decreased by 82% compared to the initial flow and IPA flow, which was only 38% lower for the originally dried GO membrane. Furthermore, the rejection studies also registered a decrease in membrane pore size from 3.3 nm in a dry state to 1.3 nm in wet state for GO membrane [93].
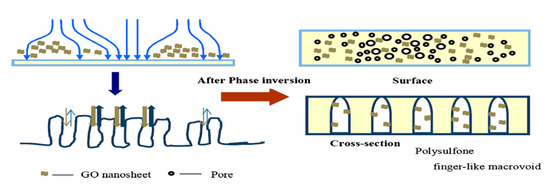
Figure 12.
Schematic of the influence of graphene oxide (GO) nanosheets on phase inversion process. (Reproduced with permission after [92]).
Three types of polyethersulfone (PES) ultrafiltration membranes with different pore sizes were coated with GO in a vacuum filtration process. As a result of coating commercial membranes with GO, the material hydrophilicity increased this, resulting in an increase water flow by approximately 20% compared to pure commercial membranes. Also, varying the pH value and the conductivity conditions, HA (humic acid) rejection through GO-coated membranes recorded a significant increase at 85.3%–93.9% compared to pure PES which had a value of 25.2–34.8%. After water washing, at different pH values and under certain conductivity conditions, the layer on the GO-coated membranes’ surface can be removed more easily compared to PES pure membranes, resulting in a high flux recovery and antifouling ability for membranes with GO [94]. An effective remedy for waste water treatment is the use of bioreactor membranes (MBRs). These membranes have a major disadvantage, namely fouling, which entails a high operating cost and a short membrane life. To combat this disadvantage, a solution would be to incorporate GO nanoplanes into PSF matrix. The presence of GO in the polymeric matrix has led to an increase in water hydrophilicity by increasing the pore size and modifying its structure resulting implicitly in an increase in composite membrane permeability. Due to the hydrophilic character and the electrostatic repulsion of GO, PSF/GO-type membranes exhibit mechanical properties and antifouling superior to single membranes [95].
Studies related to waste water treatment are considered nowadays very effective. Through phase inversion method, new composite membranes were synthesized by GO direct introduction as additive into PSF matrix. These composite membranes were subsequently tested for arsenate rejection in the aqueous solution. Due to the surface functional groups, following GO incorporation into polymer matrix, the composite membrane hydrophilicity and the water permeability have increased considerably. Moreover, the arsenate rejection increased with the increase in GO concentration in a polymeric matrix. Due to the negative membrane surface, because of GO, the predominant rejection mechanism has proven to be Donnan’s repulsion [96].
In applications such as seawater desalination and brackish water, the most used membranes are those containing ZnO nanoparticles (NP), as ZnO NP are known to improve composite membranes antifouling and antibacterial properties. However, ZnO NP shows a very high agglomeration trend that will be solved by decorating the GO surface with ZnO NP. Through phase inversion method, a series of PSF/ZnO and PSF/ZnO-GO type nanofibers were synthesized using different concentrations of reinforcing agents (1, 2, and 3 wt.% ZnO and 0.1, 0.3 and 0.6 wt.% ZnO-GO). The performed studies showed improved properties for all synthesized nanohybrid membranes, the best performances being recorded for membranes with 2 wt.% ZnO and 0.6 wt.% ZnO-GO. Thus, nanohybrid membranes show a decrease in contact angle from 65° to 39° due to a porosity increase from 75% to 95% and also a membrane permeability increase from 0.89 L·m−2·h−1·Bar−1 to 5.11 L·m−2·h−1·Ba−1. Nanohybrid membranes have also been tested for their antimicrobial properties using E. coli cultures, while obtaining a special control of antifouling properties and antibacterial properties for composite membranes [97].
New PSF/Fe3O4-GO composite membranes were successfully synthesized by a phase inversion method. For a good Fe3O4 dispersion in polymer matrix, GO surface was decorated with Fe3O4 nanoparticles in NH4OH presence through co-precipitation method. Following the incorporation of Fe3O4-GO into a PSF matrix, composite membranes permeability properties increased threefold due to increased membrane porosity, its hydrophilicity and pore size, which counterbalances decreasing humic acid retention [98].
At the same time, new PSF/TiO2-GO type hybrid membranes were synthesized by a phase inversion method. GO surface decoration with TiO2 was made in solution, by mixing, using Ti isopropoxide as precursor. The nanocomposites obtained were subsequently used as filler for the purpose of improving PSF membranes antifouling properties. The composite membranes were synthesized in varying TiO2-GO concentration in 0–5 wt.% range. A linear dependence of their properties was observed with the increase in reinforcing agent concentration in polymer matrix. These composite membranes have been tested for retention of several humic acid concentrations in aqueous solution and it has been observed that increasing TiO2-GO concentration to 5 wt.% in polymer matrix the irreversible HA antifouling has been substantially reduced, the lowest recorded value being 3.2%. At the same time, the highest HA concentration of 10 ppm was also recorded for membranes with 5 wt.% added TiO2-GO [99]. MMM membranes for ethanol/water mixtures dehydration were synthesized through a pervaporation process. These membranes were obtained after incorporation of both MWCNT and modified MWCNT into the membrane-selective PVA layer. The upper layer of PVA was deposited on polyester/polyester support obtained previously by a phase inversion process. MWCNT were obtained by thermal-chemical vapor deposition (T-CVD) and subsequently modified by attaching carboxyl functional groups and TiO2 nanocrystals to the surface. The analyzes showed that synthesized MWCNT purity was 95%, and the Attenuated Total Reflectance ATR-FT-IR analysis indicated the presence of carboxyl groups on MWCNT surface. The analyzes results showed that after modified CNT introduction into polymeric matrix, the membrane swelling rate decreased, and the network crosslinking density increased relative to the non-modified MWCNT membranes. On the other hand, the modified MWCNT dispersion in the membrane selective top layer was superior to unmodified MWCNT membranes. At the same time, the hydrophilicity and roughness of the membrane surface was significantly improved by MWCNT addition. Furthermore, the MWCNT presence in the membrane top layer results in an increase in its resistance and a decrease in total flow compared to pure membrane [99].
A summarization for all membranes is presented in Table 2.

Table 2.
Summarized applications for presented membranes.
4. Conclusions
The present review detailed the latest progress in the field of polysulfone composite membranes with carbon nanotubes, carbon fiber and graphene from both perspectives—synthesis and applications. These two fillers, extensively used in the last years due to their remarkable properties, induce a high-value character in the composite materials. On the other hand, polysulfone is one the most used polymers for preparing polymeric membranes due to its high versatility in a wide range of solvents and also to the properties of this remarkable polymer. All types of synthesis method were presented and also a large number of applications, from industrial to biomedical, were presented and discussed.
Author Contributions
Conceptualization, S.I.V. and A.M.P.; resources, S.I.V.; data curation, O.S.S.; writing-original draft preparation, A.M.P., O.S.S., and S.I.V.; project administration, S.I.V.; funding acquisition, S.I.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by UEFISCDI, grant number PN-III-P1-1.2-PCCDI-2017-0407.
Acknowledgments
This work was supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CNCS-UEFISCDI, project number PN-III-P1-1.2-PCCDI-2017-0407-Intelligent materials for medical applications, sub-project-New generation of hemodialysis composite membranes with derivatized graphene.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Miculescu, M.; Thakur, V.K.; Miculescu, F.; Voicu, S.I. Graphene-based polymer nanocomposite membranes: A review. Polym. Adv. Technol. 2016, 27, 844–859. [Google Scholar] [CrossRef]
- Voicu, S.I.; Sandru, M. Composite hybride membrane materials for artificial organs. In Handbook of Bioceramics and Biocomposites; Springer: Berlin, Germany, 2015; pp. 407–429. ISBN 978-3-319-12459-9. [Google Scholar]
- Corobea, M.C.; Muhulet, O.; Miculescu, F.; Antoniac, I.V.; Vuluga, Z.; Florea, D.; Vuluga, D.M.; Butnaru, M.; Ivanov, D.; Voicu, S.I.; et al. Novel Nanocomposite Membranes from Cellulose Acetate and Clay-Silica Nanowires. Polym. Adv. Technol. 2016, 27, 1586–1595. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.K.; Stan, G.; Ciocan, L.T. Progress in Hydroxyapatite-Starch Based Sustainable Biomaterials for Biomedical Bone Substitution Applications. ACS Sustain. Chem. Eng. 2017, 5, 8491–8512. [Google Scholar] [CrossRef]
- Pandele, A.M.; Comanici, F.E.; Carp, C.A.; Miculescu, F.; Voicu, S.I.; Thakur, V.K.; Serban, B.C. Synthesis and characterization of cellulose acetate-hydroxyapatite micro and nano composites membranes for water purification and biomedical applications. Vacuum 2017, 146, 599–605. [Google Scholar] [CrossRef]
- Dumitriu, C.; Voicu, S.I.; Muhulet, A.; Nechifor, G.; Popescu, S.; Ungureanu, C.; Carja, A.; Miculescu, F.; Trusca, R.; Pirvu, C. Cellulose acetate-titanium dioxide nanotubes membrane fraxiparinized throughpolydopamine. Carbohydr. Polym. 2018, 181, 215–223. [Google Scholar] [CrossRef]
- Voicu, S.I.; Condruz, R.M.; Mitran, V.; Cimpean, A.; Miculescu, F.; Andronescu, C.; Miculescu, M.; Thakur, V.K. Sericin Covalent Immobilization onto Cellulose Acetate Membranes. ACS Sustain. Chem. Eng. 2016, 4, 1765–1774. [Google Scholar] [CrossRef]
- Pandele, A.M.; Neacsu, P.; Cimpean, A.; Staras, A.I.; Miculescu, F.; Iordache, A.; Voicu, S.I.; Thakur, V.K.; Toader, O.D. Cellulose acetate membranes functionalized with resveratrol by covalent immobilization for improved Osseointegration. Appl. Surf. Sci. 2018, 438, 2–13. [Google Scholar] [CrossRef]
- Ouradi, A.; Nguyen, Q.T.; Benaboura, A. Polysulfone–AN69 blend membranes and its surface modification by polyelectrolyte-layer deposit—Preparation and characterization. J. Membr. Sci. 2014, 454, 20–35. [Google Scholar] [CrossRef]
- Li, S.; Rocha, D.J.; Zhou, S.J.; Meyer, H.S.; Bikson, B.; Ding, Y. Post-combustion CO2 capture using super-hydrophobic, polyether ether ketone, hollowfiber membrane contactors. J. Membr. Sci. 2013, 430, 79–86. [Google Scholar] [CrossRef]
- Lee, H.-J.; Suda, H.; Haraya, K.; Moona, S.-H. Gas permeation properties of carbon molecular sieving membranes derived from the polymer blend of polyphenylene oxide (PPO)/polyvinylpyrrolidone (PVP). J. Membr. Sci. 2007, 296, 139–146. [Google Scholar] [CrossRef]
- Caglar, B.; Fischer, P.; Kauranen, P.; Karttunen, M.; Elsner, P. Development of carbon nanotube and graphite filled polyphenylene sulfide based bipolar plates for all-vanadium redox flow batteries. J. Power Sources 2014, 256, 88–95. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Fabrication and characterization of cellulose nanofiber based thin-film nanofibrous composite membranes. J. Membr. Sci. 2014, 454, 272–282. [Google Scholar] [CrossRef]
- Soylak, M.; Cay, R.S. Separation/preconcentration of silver(I) and lead(II) in environmental samples on cellulose nitrate membrane filter prior to their flame atomic absorption spectrometric determinations. J. Hazard. Mater. 2007, 146, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Soyekwo, F.; Zhang, Q.G.; Deng, C.; Gong, Y.; Zhu, A.M.; Liu, Q.L. Highly permeable cellulose acetate nanofibrous composite membranes by freeze-extraction. J. Membr. Sci. 2014, 454, 339–345. [Google Scholar] [CrossRef]
- Soto Espinoza, S.L.; Arbeitman, C.R.; Clochard, M.C.; Grasselli, M. Functionalization of nanochannels by radio-induced grafting polymerization on PET track-etched membranes. Radiat. Phys. Chem. 2014, 94, 72–75. [Google Scholar] [CrossRef]
- Ionita, M.; Crica, L.E.; Voicu, S.I.; Pandele, A.M.; Iovu, H. Fabrication of Cellulose Triacetate/Graphene Oxide Porous Membrane. Polym. Adv. Technol. 2016, 27, 350–357. [Google Scholar] [CrossRef]
- Miculescu, F.; Mocanu, A.C.; Stan, G.E.; Miculescu, M.; Maidaniuc, A.; Cîmpean, A.; Mitran, V.; Voicu, S.I.; Machedon-Pisu, T. Influence of the modulated two-step synthesis of biogenic hydroxyapatite on biomimetic products’ surface. Appl. Surf. Sci. 2018, 438, 147–157. [Google Scholar] [CrossRef]
- Muhulet, A.; Miculescu, F.; Voicu, S.I.; Schütt, F.; Thakur, V.K.; Mishra, Y.K. Fundamentals and Scopes of Doped Carbon Nanotubes Towards Energy and Biosensing Applications. Mater. Today Energy 2018, 9, 154–186. [Google Scholar] [CrossRef]
- Rusen, E.; Mocanu, A.; Nistor, L.C.; Dinescu, A.; Călinescu, I.; Mustăţea, G.; Voicu, Ş.I.; Andronescu, C.; Diacon, A. New design of antimicrobial membranes based on polymers colloids/MWCNT hybrid materials and silver nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 17384–17393. [Google Scholar] [CrossRef]
- Ionita, M.; Vlasceanu, G.M.; Watzlawek, A.Z.; Voicu, S.I.; Burns, J.S.; Iovu, H. Graphene and functionalized graphene: Extraordinary prospects for nanobiocomposite materials. Compos. Part B Eng. 2017, 121, 34–57. [Google Scholar] [CrossRef]
- Ioniță, M.; Crica, L.E.; Voicu, S.I.; Dinescu, S.; Miculescu, F.; Costache, M.; Iovu, H. Synergistic effect of carbon nanotubes and graphene for high performance cellulose acetate membranes in biomedical applications. Carbohydr. Polym. 2018, 183, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.D.; Lourie, O.; Feldman, Y.; Tenne, R. Stress-induced fragmentation of multiwall carbon nanotubes in a polymer matrix. Appl. Phys. Lett. 1998, 72, 188–190. [Google Scholar] [CrossRef]
- Wagner, H.D.; Lourie, O.; Zhou, X.F. Macrofrag-mentation and microfragmentation phenomena in composite materials. Compos. Part A Appl. Sci. Manuf. 1998, 30A, 59–66. [Google Scholar]
- Wood, J.R.; Zhao, Q.; Wagner, H.D. Orientation of carbon nanotubes in polymers and its detection by Raman spectroscopy. Compos. Part A Appl. Sci. Manuf. 2001, 32A, 391–399. [Google Scholar] [CrossRef]
- Zhao, Q.; Wood, J.R.; Wagner, H.D. Using carbon nanotubes to detect polymer transitions. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 1492–1495. [Google Scholar] [CrossRef]
- Cochet, M.; Maser, W.K.; Benito, A.M.; Callejas, M.A.; Martinesz, M.T.; Benoit, J.M.; Schreiber, J.; Chauvet, O. Synthesis of a new polyaniline/nanotube composite: In-Situ polymerisation and charge transfer through site-selective interaction. Chem. Commun. 2001, 16, 1450–1451. [Google Scholar] [CrossRef]
- Qian, D.; Dickey, E.C.; Andrews, R.; Rantell, T. Load transfer and deformation mechanisms in carbon nanotube-polystyrene composites. Appl. Phys. Lett. 2000, 76, 2868–2870. [Google Scholar] [CrossRef]
- Sianipar, M.; Kim, S.H.; Min, C.; Tijing, L.D.; Shon, H.K. Potential and performance of a polydopamine-coated multiwalled carbon nanotube/polysulfone nanocomposite membrane for ultrafiltration application. J. Ind. Eng. Chem. 2016, 34, 364–373. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.; Deng, B. Multi-walled carbon nanotubes (MWNTs)/polysulfone (PSU) mixed matrix hollow fiber membranes for enhanced water treatment. J. Membr. Sci. 2013, 437, 237–248. [Google Scholar] [CrossRef]
- de Lannoy, C.F.; Soyerc, E.; Wiesner, M.R. Optimizing carbon nanotube-reinforced polysulfone ultrafiltration membranes through carboxylic acid functionalization. J. Membr. Sci. 2013, 447, 395–402. [Google Scholar] [CrossRef]
- Amini, M.; Jahanshahi, M.; Rahimpour, A. Synthesis of novel thin film nanocomposite (TFN) forward osmosis membranes using functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2013, 435, 233–241. [Google Scholar] [CrossRef]
- Nayak, L.; Rahaman, M.; Khastgir, D.; Chaki, T.K. Thermal and electrical properties of carbon nanotubes based polysulfone nanocomposites. Pollymer Bull. 2011, 67, 1029–1044. [Google Scholar] [CrossRef]
- Xu, L.; He, J.; Yu, Y.; Chen, J.P. Effect of CNT content on physicochemical properties and performance of CNT composite polysulfone membranes. Chem. Eng. Res. Des. 2017, 121, 92–98. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Panait, V.; Naftanaila, L.; Batalu, D.; Voicu, S.I. Symmetrical polysulfone membranes obtained by solvent evaporation using carbon nanotubes as additives. Synthesis, characterization and applications. Digers J. Nanomater. Biostruct. 2013, 8, 875–884. [Google Scholar]
- Yokwana, K.; Gumbi, N.; Adams, F.; Mhlanga, S.; Nxumalo, E.; Mamba, B. Development of funtionalized doped carbon nanotube/ polysulfone nanofiltration membranes for fouling control. J. Appl. Polym. Sci. 2015, 132, 41835. [Google Scholar] [CrossRef]
- Nayak, L.; Khastgir, D.; Chaki, T.K. Influence of Carbon nanofibers Reinforcement on Thermal and Electrical Behavior of Polysulfone Nanocomposites. Polym. Eng. Sci. 2012, 52, 2424–2434. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Esteves, C.M.P.; Figueiredo, J.L.; Silva, A.M.T. Thin-film composite forward osmosis membranes based on polysulfone supports blended with nanostructured carbon materials. J. Membr. Sci. 2016, 520, 326–336. [Google Scholar] [CrossRef]
- Kikukawa, K.; Totoki, T.; Wada, F.; Matsuda, W. Reaction of diazonium salts with transition metals: X. Formylation of arenediazonium salts with carbon monoxide and silyl hydrides under palladium, catalysis. J. Organomet. Chem. 1984, 270, 283–287. [Google Scholar] [CrossRef]
- Voicu, S.I.; Aldea, F.; Nechifor, A.C. Polysulfone-carbon Nanotubes Composite Membranes Synthesis and characterization. Rev. De Chim. 2010, 61, 817–821. [Google Scholar]
- Dooher, T.; Dixon, D.; McIlhagger, A. Multiwalled Carbon Nanotube/Polysulfone Composites. J. Thermoplast. Compos. Mater. 2011, 24, 499–515. [Google Scholar] [CrossRef]
- Voicu, Ş.I.; Pandele, A.; Tanasă, E.; Rughinis, R.; Crica, L.; Pilan, L.; Ionita, M. The impact of sonication time through polysulfone-graphene oxide composite films properties. Dig. J. Nanomater. Biostruct. 2013, 8, 1389–1394. [Google Scholar]
- Matsumoto, K.; Takahashi, T.; Ishii, S.; Jikei, M. Investigation of Dispersibility of Multi-Walled Carbon Nanotubes Using Polysulfones with Various Structures. Int. J. Soc. Mater. Eng. Resour. 2014, 20, 77–81. [Google Scholar] [CrossRef]
- Dooher, T.; Dixon, D. Multiwalled Carbon Nanotube/Polysulfone Composites: Using the Hildebrand Solubility Parameter to Predict Dispersion. Polym. Compos. 2011, 32, 1895–1903. [Google Scholar] [CrossRef]
- Gaur, M.S.; Singh, R.; Tiwari, R.K. Study of structural morphology, thermal degradation and surface charge decay in PU þ PSF þ CNTs polymer hybrid nanocomposite. J. Electrost. 2014, 72, 242–251. [Google Scholar] [CrossRef]
- Khalid, A.; Ibrahim, A.; Al-Hamouz, O.C.S.; Laoui, T.; Benamor, A.; Atieh, M.A. Fabrication of polysulfone nanocomposite membranes with silver-doped carbon nanotubes and their antifouling performance. J. Appl. Polym. Sci. 2016, 134, 44688–44700. [Google Scholar] [CrossRef]
- Panahian, S.; Raisi, A.; Aroujalian, A. Multilayer mixed matrix membranes containing modified-MWCNTs for dehydration of alcohol by pervaporation process. Desalination 2015, 355, 45–55. [Google Scholar] [CrossRef]
- Celli, A.; Marchese, P.; Vannini, M.; Berti, C.; Fortunati, I.; Signorini, R.; Bozio, R. Synthesis of novel fullerene-functionalized polysulfones for optical limiting applications. React. Funct. Polym. 2011, 71, 641–647. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Sava, S.; Voicu, S.I.; Nechifor, G. Polysulfone-C60 and polysulfone-magnetic particles composite membranes for decontamination techniques. Int. J. Energy Environ. 2011, 5, 645–652. [Google Scholar]
- Khalid, A.; Al-Juhani, A.A.; Al-Hamouz, O.C.; Laoui, T.; Khan, Z.; Atieh, M.A. Preparation and properties of nanocomposite polysulfone/multi-walled carbon nanotubes membranes for desalination. Desalination 2015, 367, 134–144. [Google Scholar] [CrossRef]
- Nechifor, G.; Voicu, S.I.; Nechifor, A.C.; Garea, S. Nanostructured hybrid membrane polysulfone-carbon nanotubes for hemodialysis. Desalination 2009, 241, 342–348. [Google Scholar] [CrossRef]
- Mukherjee, R.; De, S. Novel carbon-nanoparticle polysulfone hollow fiber mixed matrix ultrafiltration membrane: Adsorptive removal of benzene, phenol and toluene from aqueous solution. Sep. Purif. Technol. 2016, 157, 229–240. [Google Scholar] [CrossRef]
- Kim, S.; Chen, L.; Johnson, J.K.; Marand, E. Polysulfone and functionalized carbon nanotube mixed matrix membranes for gas separation: Theory and experiment. J. Membr. Sci. 2007, 294, 147–158. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Tyler, J.L.; Stretz, H.A.; Wells, M.J.L. Effects of a dual nanofiller, nano-TiO2 andMWCNT, for polysulfone-based nanocomposite membranes for water purification. Desalination 2015, 372, 47–56. [Google Scholar] [CrossRef]
- Kiadehi, A.D.; Jahanshahi, M.; Rahimpour, A.; Ghoreyshi, S.A.A. The effect of functionalized carbon nano-fiber (CNF) on gas separation performance of polysulfone (PSf) membranes. Chem. Eng. Process. 2015, 90, 41–48. [Google Scholar] [CrossRef]
- Kar, S.; Subramanian, M.; Pal, A.; Ghosh, A.K.; Bindal, R.C.; Prabhakar, S.; Nuwad, J.; Pillai, C.G.S.; Chattopadhyayand, S.; Tewari, P.K. Preparation, Characterisation and Performance Evaluation of Anti-biofouling Property of Carbon Nanotube Polysulfone Nanocomposite Membranes. Carbon Mater. 2013, 181–185. [Google Scholar] [CrossRef]
- Voicu, S.I.; Nechifor, A.C.; Gales, O.; Nechifor, G. Covalent enzyme immobilization onto carbon nanotubes using a membrane reactor. In Proceedings of the SPIE Microtechnologies, Prague, Czech Republic, 18–20 April 2011; Volume 8068, p. 80680Y. [Google Scholar]
- Sarfraz, M.; Ba-Shammakh, M. Combined effect of CNT with ZIF-302 into polysulfone to fabricate MMM for enhanced CO2 separation from flue gases. J. Ind. Eng. Chem. 2016, 36, 154–162. [Google Scholar] [CrossRef]
- Mangukiya, S.; Prajapati, S.; Kumar, S.; Aswal, V.K.; Murthy, C.N. Polysulfone-based composite membranes with functionalized carbon nanotubes show controlled porosity and enhanced electrical conductivity. J. Appl. Polym. Sci. 2016, 133, 43778–43786. [Google Scholar] [CrossRef]
- Medina-Gonzalez, Y.; Remigy, J.C. Sonication-assisted preparation of pristine MWCNT—polysulfone conductive microporous membranes. Mater. Lett. 2011, 65, 229–232. [Google Scholar] [CrossRef]
- Kiadehi, A.D.; Rahimpour, A.; Jahanshahi, M.; Ghoreyshi, A.A. Novel carbon nano-fibers (CNF)/polysulfone (PSf) mixed matrix membranes for gas separation. J. Ind. Eng. Chem. 2015, 22, 199–207. [Google Scholar] [CrossRef]
- Shah, P.; Murthy, C.N. Studies on the porosity control of MWCNT/polysulfone composite membrane and its effect on metal removal. J. Membr. Sci. 2013, 437, 90–98. [Google Scholar] [CrossRef]
- Maphutha, S.; Moothi, K.; Meyyappan, M.; Iyuke, S.E. A carbon nanotube-infused polysulfone membrane with polyvinyl alcohol layer for treating oil-containing waste water. Sci. Rep. 2013, 3, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Khoshrou1, S.; Moghbeli, M.R.; Ghasemi, E. Polysulfone/Carbon Nanotubes Asymmetric Nanocomposite Membranes: Effect of Nanotubes Surface Modification on Morphology and Water Permeability. Iran. J. Chem. Eng. 2015, 12, 69–83. [Google Scholar]
- Nayak, L.; Pradhan, R.R.; Khastgir, D.; Chaki, T.K. Thermally Stable Electromagnetic Interference Shielding Material from Polysulfone Nanocomposites: Comparison on Carbon Nanotube and Nanofiber Reinforcement. Polym. Compos. 2014, 36, 566–575. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Chaki, T.K.; Khastgir, D.; Pinto, R. γ Irradiation effects on optical, thermal, and mechanical properties of polysulfone/MWCNT nanocomposites in argon atmosphere. J. Appl. Polym. Sci. 2015, 132, 42017–42025. [Google Scholar] [CrossRef]
- Nayak, L.; Khastgir, D.; Chaki, T.K. Study of Alternating Current Impedance Analysis and Dielectric Properties of Carbon Nanotube-Based Polysulfone Nanocomposites. Polym. Compos. 2012, 33, 85–91. [Google Scholar] [CrossRef]
- Sanchez, S.; Pumera, M.; Fabregas, E. Carbon nanotube/polysulfone screen-printed electrochemical immunosensor. Biosens. Bioelectron. 2007, 23, 332–340. [Google Scholar] [CrossRef]
- Bautista-Quijano, J.R.; Avilés, F.; Aguilar, J.O.; Tapia Gonzales, J.A. Strain sensing capabilities of a piezoresistive MWCNT-polysulfone film. Sens. Actuators A Phys. 2010, 159, 135–140. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Z.; Ye, S.; Sui, X. Removal of p-chlorophenol from aqueous solutions by carbon nanotube hybrid polymer adsorbents. Chem. Eng. Res. Des. 2017, 123, 76–83. [Google Scholar] [CrossRef]
- Zeinali, S.; Aryaeinezhad, M. Improving O2/N2 Selective Filtration Using Carbon Nanotube-Modified Mixed-Matrix Membranes. Chem. Eng. Technol. 2015, 38, 2079–2086. [Google Scholar] [CrossRef]
- Yu, H.; Cao, Y.; Kang, G.; Liu, Z.; Kuang, W.; Liu, J.; Zhou, M. Enhancing the antifouling properties of polysulfone ultrafiltration membranes by the grafting of poly(ethylene glycol) derivatives via surface amidation reactions. J. Appl. Polym. Sci. 2015, 132, 41870. [Google Scholar] [CrossRef]
- Soleymanipour, S.F.; Dehaghani, A.H.S.; Pirouzfar, V.; Alihossein, A. The morphology and gas-separation performance of membranes comprising multiwalled carbon nanotubes/polysulfone–Kapton. J. Appl. Polym. Sci. 2016, 133, 43839–43847. [Google Scholar] [CrossRef]
- Xie, P.; de Lannoy, C.F.; Ma, J.; Wang, Z.; Wang, S.; Li, J.; Wiesner, M.R. Improved chlorine tolerance of a polyvinyl pyrrolidone-polysulfone membrane enabled by carboxylated carbon nanotubes. Water Res. 2016, 104, 497–506. [Google Scholar] [CrossRef]
- Seetharaman, S.; Raghu, S.C.; Velan, M.; Ramya, K.; Mahabadi, K.A. Comparison of the Performance of Reduced Graphene Oxide and Multiwalled Carbon Nanotubes Based Sulfonated Polysulfone Membranes for Electrolysis Application. Polym. Compos. 2014, 36, 475–481. [Google Scholar] [CrossRef]
- Ionita, M.; Crica, L.E.; Vasile, E.; Dinescu, S.; Pandele, M.A.; Costache, M.; Haugen, H.J.; Iovu, H. Effect of carboxylic acid functionalized graphene on physical-chemical and biological performances of polysulfone porous films. Polymer 2016, 92, 1–12. [Google Scholar] [CrossRef]
- Pena-Bahamonde, J.; San Miguel, V.; Nguyen, H.N.; Ozisik, R.; Rodrigues, D.F.; Cabanelas, J.C. Functionalization of reduced graphene oxide with polysulfone brushes enhance antibacterial properties and reduce human cytotoxicity. Carbon 2017, 111, 258–268. [Google Scholar] [CrossRef]
- Ionita, M.; Pandele, A.M.; Crica, L.; Pilan, L. Improving the thermal and mechanical properties of polysulfone by incorporation of graphene oxide. Compos. Part B Eng. 2014, 59, 133–139. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ng, L.Y.; Ba-Abbad, M.L.; Mohammad, A.W. Novel nanohybrid polysulfone membrane embedded with silver nanoparticles on graphene oxide nanoplates. Chem. Eng. J. 2015, 277, 1–10. [Google Scholar] [CrossRef]
- Penkova, A.V.; Dmitrenko, M.E.; Sokolova, M.P.; Chen, B.; Plisko, T.V.; Markelov, D.A.; Ermakov, S.S. Impact of fullerene loading on the structure and transport properties of polysulfone mixed-matrix membranes. J. Mater. Sci. 2016, 51, 7652–7659. [Google Scholar] [CrossRef]
- Kibechu, R.W.; Ndinteh, D.T.; Msagati, T.A.M.; Mamba, B.B.; Sampath, S. Effect of incorporating graphene oxide and surface imprinting on polysulfone membranes on flux, hydrophilicity and rejection of salt and polycyclic aromatic hydrocarbons from water. Phys. Chem. Earthparts A/B/C 2017, 100, 126–134. [Google Scholar] [CrossRef]
- Ganesh, B.M.; Isloor, A.M.; Ismail, A.F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 2013, 313, 199–207. [Google Scholar] [CrossRef]
- Hu, B.; Miao, L.; Zhao, Y.; Lü, C. Azide-assisted crosslinked quaternized polysulfone with reduced graphene oxide for highly stable anion exchange membranes. J. Membr. Sci. 2017, 530, 84–94. [Google Scholar] [CrossRef]
- Liu, L.; Tong, C.; He, Y.; Zhao, Y.; Lü, C. Enhanced properties of quaternized graphenes reinforced polysulfone based composite anion exchange membranes for alkaline fuel cell. J. Membr. Sci. 2015, 487, 99–108. [Google Scholar] [CrossRef]
- Ionita, M.; Vasile, E.; Crica, L.E.; Voicu, S.I.; Pandele, A.M.; Dinescu, S.; Predoiu, L.; Galateanu, B.; Hermenean, A.; Costache, M. Synthesis, characterization and In Vitro studies of polysulfone/graphene oxide composite membranes. Compos. Part B Eng. 2015, 72, 108–115. [Google Scholar] [CrossRef]
- Zambare, R.S.; Dhopte, K.B.; Patwardhan, A.V.; Nemade, P.R. Polyamine functionalized graphene oxide polysulfone mixed matrix membranes with improved hydrophilicity and anti-fouling properties. Desalination 2017, 403, 24–35. [Google Scholar] [CrossRef]
- Kochameshki, M.G.; Marjani, A.; Mahmoudian, M.; Farhadi, K. Grafting of diallyldimethylammonium chloride on graphene oxide by RAFT polymerization for modification of nanocomposite polysulfone membranes using in water treatment. Chem. Eng. J. 2017, 309, 206–221. [Google Scholar] [CrossRef]
- Hwang, T.; Oh, J.S.; Yim, W.; Nam, J.D.; Bae, C.; Kim, H.; Kim, K.J. Ultrafiltration using graphene oxide surface-embedded polysulfone membranes. Sep. Purif. Technol. 2016, 166, 41–47. [Google Scholar] [CrossRef]
- Lim, S.; Park, M.J.; Phuntsho, S.; Tijing, L.D.; Nisola, G.M.; Shim, W.G.; Chung, W.J.; Shon, H.K. Dual-layered nanocomposite substrate membrane based on polysulfone/graphene oxide for mitigating internal concentration polarization in forward osmosis. Polymer 2017, 110, 36–48. [Google Scholar] [CrossRef]
- Park, M.J.; Phuntsho, S.; He, T.; Nisola, G.M.; Tijing, L.D.; Li, X.M.; Chen, G.; Chung, W.J.; Shon, H.K. Graphene oxide incorporated polysulfone substrate for the fabrication of flat-sheet thin-film composite forward osmosis membranes. J. Membr. Sci. 2015, 493, 496–507. [Google Scholar] [CrossRef]
- Meng, N.; Wang, Z.; Low, Z.X.; Zhang, Y.; Wang, H.; Zhang, X. Impact of trace graphene oxide in coagulation bath on morphology and performance of polysulfone ultrafiltration membrane. Sep. Purif. Technol. 2015, 147, 364–371. [Google Scholar] [CrossRef]
- Aher, A.; Cai, Y.; Majumder, M.; Bhattacharyya, D. Synthesis of graphene oxide membranes and their behavior in water and isopropanol. Carbon 2017, 116, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.H.; Huang, Y.; Yu, M.; Heo, J.; Flora, J.R.V.; Jang, A.; Jang, M.; Jung, C.; Park, C.M.; Kim, D.H.; et al. Evaluation of graphene oxide-coated ultrafiltration membranes for humic acid removal at different pH and conductivity conditions. Sep. Purif. Technol. 2017, 181, 139–147. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.R.; Won, Y.J.; Lee, K.; Lee, C.H.; Lee, H.H.; Kim, I.C.; Lee, J. Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Rezaee, R.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Mousavi, S.A.; Rashidi, A.; Jafari, A.; Nazmara, S. Fabrication and characterization of a polysulfone-graphene oxide nanocomposite membrane for arsenate rejection from water. J. Environ. Health Sci. Eng. 2015, 13, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.T.; Mahmoudi, E.; Mohammad, A.W.; Benamor, A.; Johnson, D.; Hilal, N. Development of polysulfone-nanohybrid membranes using ZnO-GO composite for enhanced antifouling and antibacterial control. Desalination 2017, 402, 123–132. [Google Scholar] [CrossRef]
- Chai, P.V.; Mahmoudi, E.; Teow, Y.H.; Mohammad, A.W. Preparation of novel polysulfone-Fe3O4/GO mixed-matrix membrane for humic acid rejection. J. Water Process. Eng. 2017, 15, 83–88. [Google Scholar] [CrossRef]
- Kumar, M.; Gholamvand, Z.; Morrissey, A.; Nolan, K.; Ulbricht, M.; Lawler, J. Preparation and characterization of low fouling novel hybrid ultrafiltration membranes based on the blends of GOTiO2 nanocomposite and polysulfone for humic acid removal. J. Membr. Sci. 2016, 506, 38–49. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).