Enhancing Electrical and Thermal Properties of Al6061 Parts by Electrophoresis Deposition of Multi-Walled Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. SEM Analysis
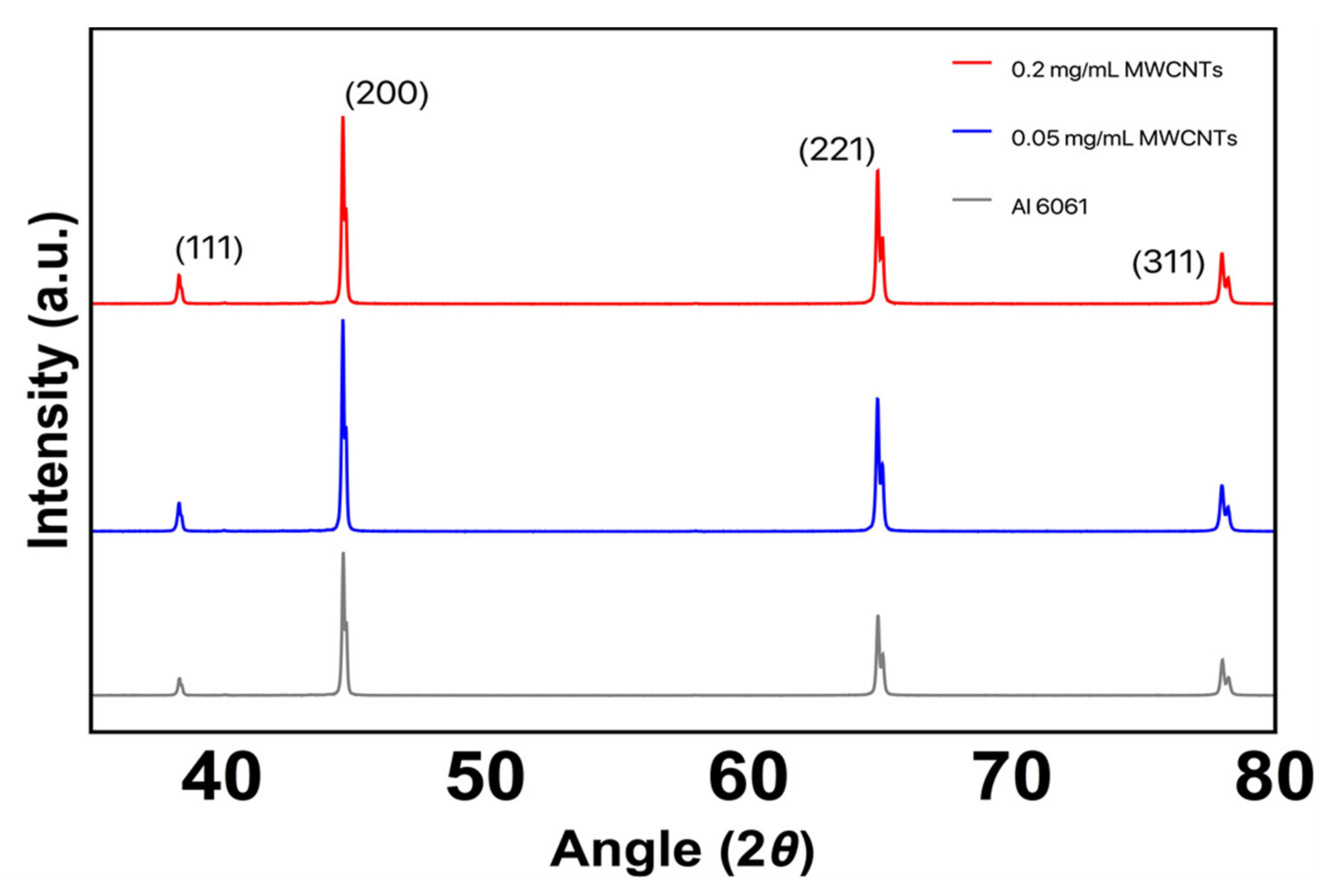
3.2. X-ray Diffraction (XRD)
3.3. Roughness Measurements: Surface Characterization
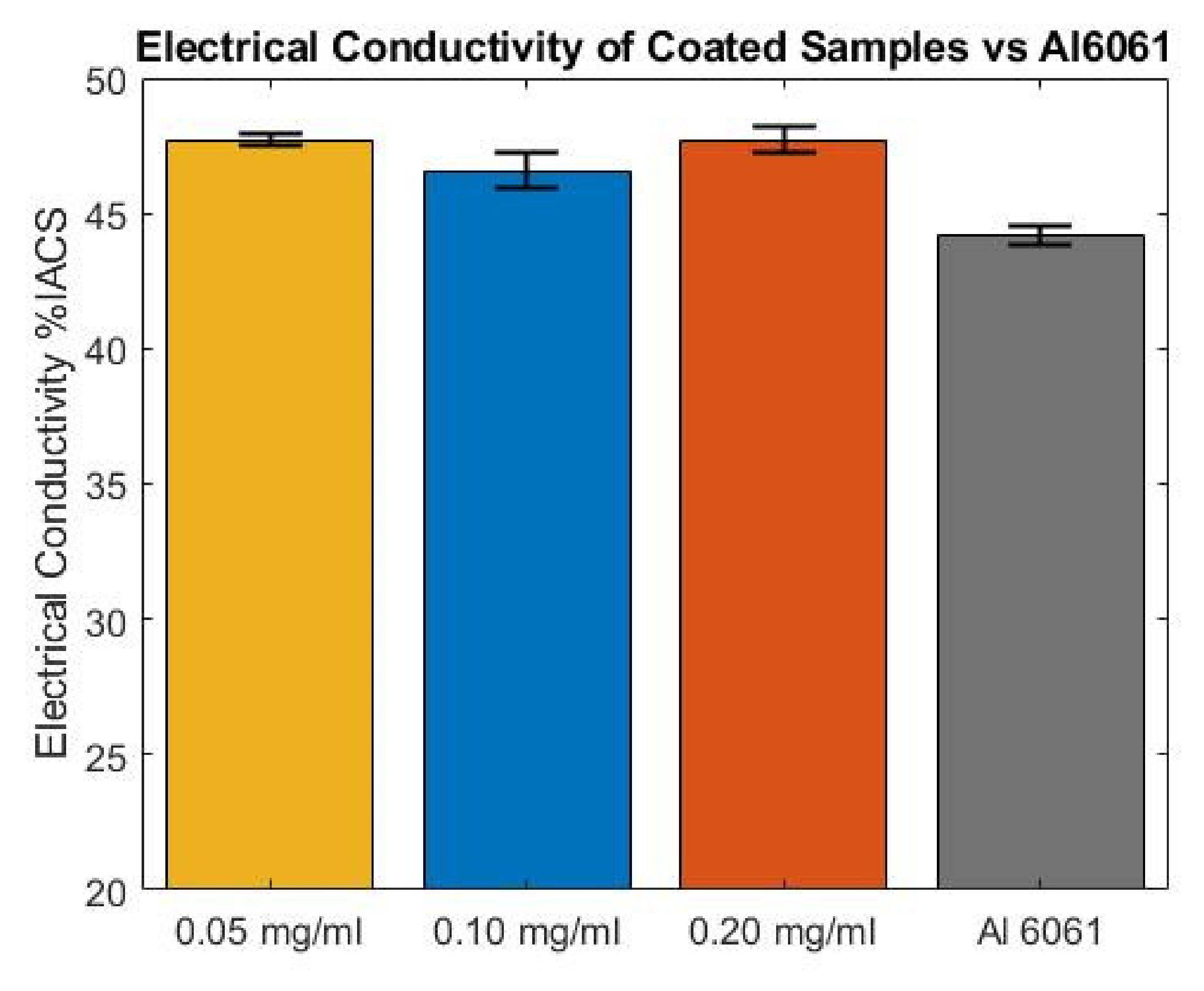
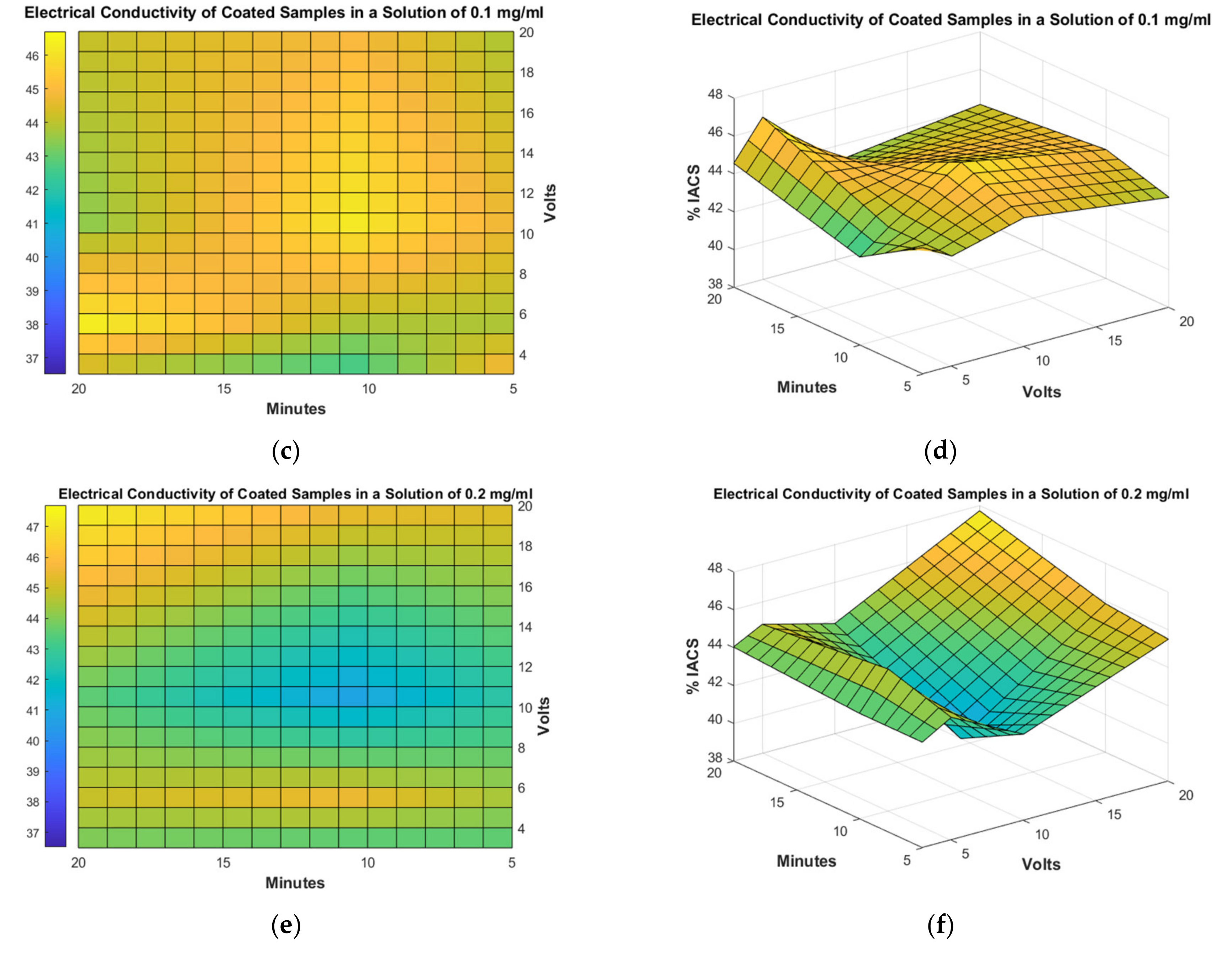
3.4. Electrical Conductivity
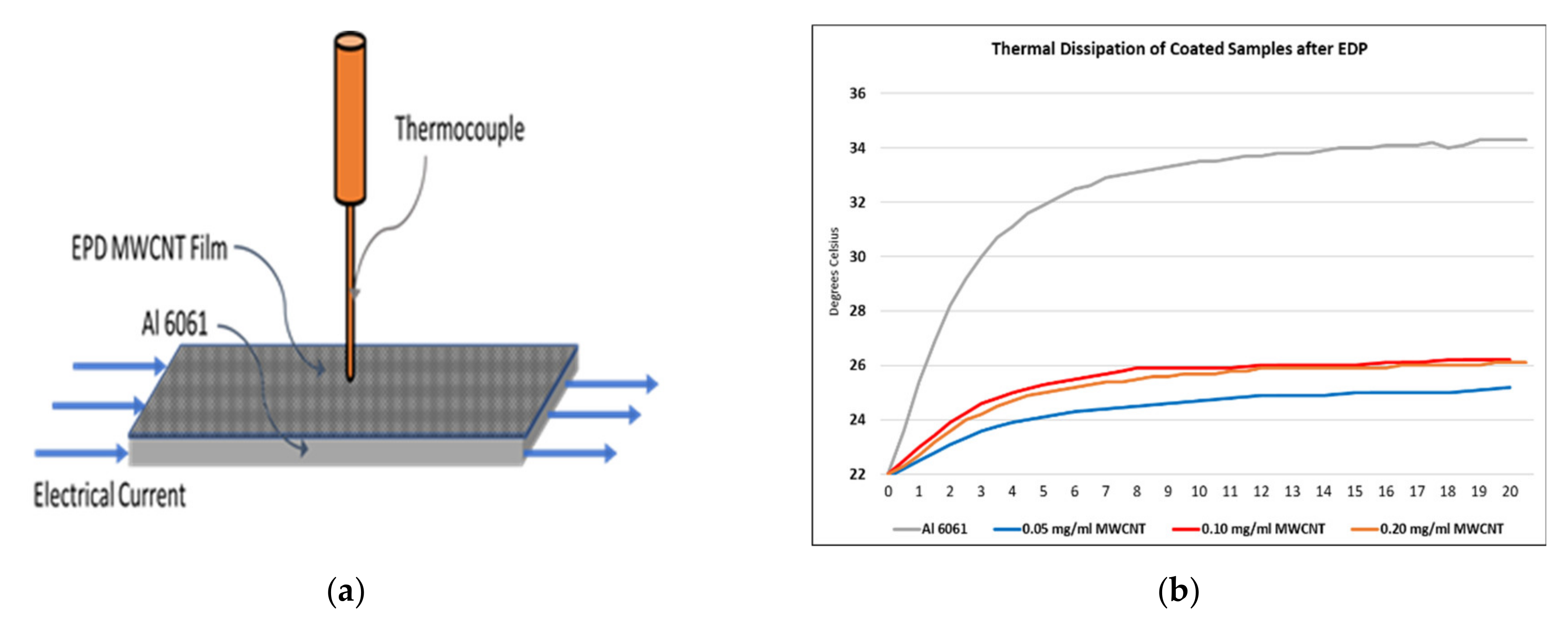
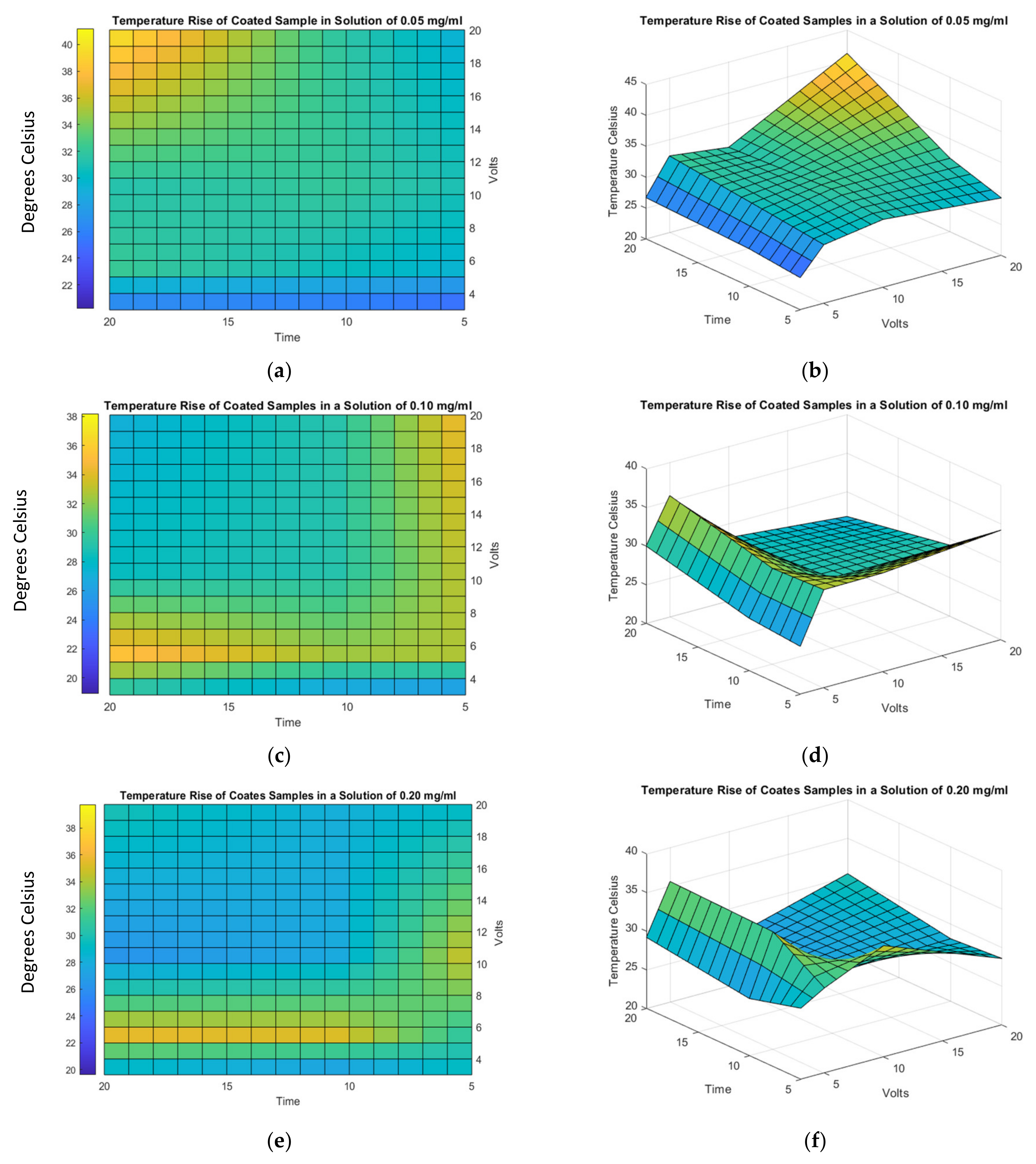
3.5. Thermal Results
4. Conclusions
- (a)
- The best electrical conductivity increment of 9.6% and thermal dissipation reduction of 36% were achieved by considering EDP parameters of 5 and 3 V, respectively, electrodeposition time of 5 min and a concentration solution of 0.05 mg/mL of MWCNTs.
- (b)
- For longer deposition times and for higher concentration solutions of MWCNT, the electrodeposited carbon nanotubes hinder heat exchange between the surrounding conditions and the coated surface, which reduces thermal dissipation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bradley, R. Electrical Conductivity-Enhancing and Protection Material. U.S. Patent US4578215A, 25 March 1986. [Google Scholar]
- Antonetti, V.W.; Yovanovich, M.M. Enhancement of thermal contact conductance by metallic coatings: Theory and experiment. J. Heat Transfer. 1985, 107, 513–519. [Google Scholar] [CrossRef]
- Hill, H.R.T. The Future of Gold and Silver Plating. Trans. IMF 1981, 59, 135–141. [Google Scholar] [CrossRef]
- Goodman, P. Current and future uses of gold in electronics. Gold Bull. 2002, 35, 21–26. [Google Scholar] [CrossRef]
- Du, C.; Heldbrant, D.; Pan, N. Preparation and preliminary property study of carbon nanotubes films by electrophoretic deposition. Mater. Lett. 2002, 57, 434–438. [Google Scholar] [CrossRef]
- Lim, J.; Jalali, M.; Campbell, S.A. Properties of electrophoretically deposited single wall carbon nanotube films. Thin Solid Film. 2015, 589, 278–285. [Google Scholar] [CrossRef]
- Castro, R.H.R.; Hidalgo, P.; Diniz, E.C. Enhanced electrical conduction in aluminum wires coated with carbon nanotubes. Mater. Lett. 2011, 65, 271–274. [Google Scholar] [CrossRef]
- Che, J.; Cagin, T.; Goddard, W.A. Thermal conductivity of carbon nanotubes. Nanotechnology 2000, 11, 65–69. [Google Scholar] [CrossRef]
- Hedges, A.R.; Iijima, S.; Tanaka, K. Carbon Nanotubes and Graphene, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 98. [Google Scholar]
- Dujardin, E.; Ebbesen, T.W.; Hiura, H.; Tanigaki, K. Capillarity and wetting of carbon nanotubes. Science 1994, 265, 1850–1852. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal matrix composites reinforced by nano-particles—A review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Lahiri, D.; Agarwal, A. Carbon nanotube reinforced metal matrix composites—A review. Int. Mater. Rev. 2010, 55, 41–64. [Google Scholar] [CrossRef]
- Silvestre, N. State-of-the-art review on carbon nanotube reinforced metal matrix composites. Int. J. Compos. Mater. 2013, 3, 28–44. [Google Scholar]
- Chen, B.; Kondoh, K.; Li, J.S.; Qian, M. Extraordinary reinforcing effect of carbon nanotubes in aluminium matrix composites assisted by in-situ alumina nanoparticles. Compos. Part. B Eng. 2020, 183. [Google Scholar] [CrossRef]
- George, R.; Kashyap, K.T.; Rahul, R.; Yamdagni, S. Strengthening in carbon nanotube/aluminum (CNT/Al) composites. Scr. Mater. 2005, 53, 1159–1163. [Google Scholar] [CrossRef]
- Vajtai, R. Handbook of Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2012; Volume 108. [Google Scholar]
- Mansoor, M.; Shahid, M. Carbon nanotube-reinforced aluminum composite produced by induction melting. J. Appl. Res. Technol. 2016, 14, 215–224. [Google Scholar] [CrossRef]
- Esawi, A.; Morsi, K. Dispersion of carbon nanotubes (CNTs) in aluminum powder. Compos. Part. A Appl. Sci. Manuf. 2007, 38, 646–650. [Google Scholar] [CrossRef]
- Guo, B.; Chen, Y.; Wang, Z.; Yi, J.; Ni, S.; Du, Y.; Li, W.; Song, M. Enhancement of strength and ductility by interfacial nano-decoration in carbon nanotube/aluminum matrix composites. Carbon 2020, 159, 201–212. [Google Scholar] [CrossRef]
- Esawi, A.M.K.; Morsi, K.; Sayed, A.; Gawad, A.A.; Borah, P. Fabrication and properties of dispersed carbon nanotube-aluminum composites. Mater. Sci. Eng. A 2009, 508, 167–173. [Google Scholar] [CrossRef]
- Saheb, N.; Iqbal, Z.; Khalil, A.; Hakeem, A.S.; Aqeeli, N.A.; Laoui, T.; Al-Qutub, A.; Kirchner, R. Spark plasma sintering of metals and metal matrix nanocomposites: A review. J. Nanomater. 2012, 2012, 13. [Google Scholar] [CrossRef]
- Groza, J.R.; Zavaliangos, A. Sintering activation by external electrical field. Mater. Sci. Eng. A 2000, 287, 171–177. [Google Scholar] [CrossRef]
- Uddin, S.M.; Mahmud, T.; Wolf, C.; Glanz, C.; Kolaric, I.; Volkmer, C.; Höller, H.; Wienecke, U.; Roth, S.; Fecht, H. Effect of size and shape of metal particles to improve hardness and electrical properties of carbon nanotube reinforced copper and copper alloy composites. Compos. Sci. Technol. 2010, 70, 2253–2257. [Google Scholar] [CrossRef]
- Tokutomi, J.; Uemura, T.; Sugiyama, S.; Shiomi, J.; Yanagimoto, J. Hot extrusion to manufacture the metal matrix composite of carbon nanotube and aluminum with excellent electrical conductivities and mechanical properties. CIRP Ann. Manuf. Technol. 2015, 64, 257–260. [Google Scholar] [CrossRef]
- Zhong, R.; Cong, H.; Hou, P. Fabrication of nano-Al based composites reinforced by single-walled carbon nanotubes. Carbon N. Y. 2003, 41, 2001–2004. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, G.; Xu, Q.; Xiong, Y.; Luo, C.; Wu, J. A new technique for dispersion of carbon nanotube in a metal melt. Mater. Sci. Eng. A 2010, 527, 5335–5340. [Google Scholar] [CrossRef]
- Kasim, S.H.; Hashim, A.H. Electrophoretic Deposition of multi-walled carbon nanotubes on stainless steel (SS) foils. J. Ind. Technol. 2010, 19, 139–148. [Google Scholar]
- Thomas, B.J.C.; Shaffer, M.S.P.; Freeman, S.; Koopman, M.; Chawla, K.K.; Boccaccini, A.R. Electrophoretic deposition of carbon nanotubes on metallic surfaces. Key Eng. Mater. 2006, 314, 141–146. [Google Scholar] [CrossRef]
- Merino, C.A.I.; Sillas, J.E.L.; Meza, J.M.; Ramirez, J.M.H. Metal matrix composites reinforced with carbon nanotubes by an alternative technique. J. Alloys Compd. 2017, 707, 257–263. [Google Scholar] [CrossRef]
- Thomas, B.J.C.; Boccaccini, A.R.; Shaffer, M.S.P. Multi-walled carbon nanotube coatings using Electrophoretic Deposition (EPD). J. Am. Ceram. Soc. 2005, 88, 980–982. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, J.; Liu, M.; Chen, Q. Mechanical strength of carbon nanotube-nickel nanocomposites. Nanotechnology 2007, 18, 505704. [Google Scholar] [CrossRef]
- Fraczek-Szczypta, A.; Dlugon, E.; Weselucha-Birczynska, A.; Nocun, M.; Blazewicz, M. Multi walled carbon nanotubes deposited on metal substrate using EPD technique. A spectroscopic study. J. Mol. Struct. 2013, 1040, 238–245. [Google Scholar] [CrossRef]
- Ramalingam, S.; Balakrishnan, K.; Shanmugasamy, S.; Subramania, A. Electrodeposition and characterization of Cu–MWCNTs nanocomposite coatings. Surf. Eng. 2017, 33, 369–374. [Google Scholar] [CrossRef]
- Selvakumar, A.; Perumalraj, R.; Sudagar, J.; Mohan, S. Nickel multiwalled carbon nanotube composite coating on aluminum alloy rotor for textile industries. J. Mater. Des. Appl. 2016, 230, 319–327. [Google Scholar] [CrossRef]
- Madhu, S.; Kumaran, P.; Kumar, A.A.; Raafiq, N.M.; Murali, G. Pulse electro co-deposited Ni/MWCNTS coatings on al 6061 substrate using pulse electroplating. Rasayan J. Chem. 2017, 10, 1472–1480. [Google Scholar]
- Xu, C.L.; Wei, B.Q.; Ma, R.Z.; Liang, J.; Ma, X.K.; Wu, D.H. Fabrication of aluminum/carbon nanotube composites and their electrical properties. Carbon N. Y. 1999, 37, 855–858. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Cho, J.; Roether, J.A.; Thomas, B.J.C.; Minay, E.J.; Shaffer, M.S.P. Electrophoretic deposition of carbon nanotubes. Carbon 2006, 44, 3149–3160. [Google Scholar] [CrossRef]
- Lee, S.H.; Woo, S.P.; Kakati, N.; Kim, D.J.; Yoon, Y.S. A comprehensive review of nanomaterials developed using electrophoresis process for high-efficiency energy conversion and storage systems. Energies 2018, 11, 3122. [Google Scholar] [CrossRef]
- Yatsushiro, T.; Koura, N.; Nakano, S.; Ui, K.; Takeuchi, K. Electrodeposition of aluminum-carbon nanotube composite from room—Temperature molten salt electrolyte. Electrochemistry 2006, 74, 233–236. [Google Scholar] [CrossRef][Green Version]
- Azam, M.A.; Talib, E.; Seman, R.N.A.R. Direct deposition of multi-walled carbon nanotubes onto stainless steel and YEF foils using a simple electrophoretic deposition for electrochemical capacitor electrode. Mater. Res. Express 2019, 6, 015501. [Google Scholar] [CrossRef]
- Hekmat, F.; Sohrabi, B.; Rahmanifar, M.S.; Jalali, A. Electrophoretic deposition of multi-walled carbon nanotubes on porous anodic aluminum oxide using ionic liquid as a dispersing agent. Appl. Surf. Sci. 2015, 341, 109–119. [Google Scholar] [CrossRef]
- Esumi, K.; Ishigami, M.; Nakajima, A.; Sawada, K.; Honda, H. Chemical treatment of carbon nanotubes. Carbon N. Y. 1996, 34, 279–281. [Google Scholar] [CrossRef]
- Arrigo, R.; Teresi, R.; Gambarotti, C.; Parisi, F.; Lazzara, G.; Dintcheva, N.T. Sonication-induced modification of carbon nanotubes: Effect on the rheological and thermo-oxidative behaviour of polymer-based nanocomposites. Materials 2018, 11, 383. [Google Scholar] [CrossRef]
- Sarkar, A.; Hah, D. Electrophoretic deposition of carbon nanotubes on silicon substrates. J. Electron. Mater. 2012, 41, 3130–3138. [Google Scholar] [CrossRef]
- Lau, K.-T.; Azam, M.A.; Seman, R. Influence of pulsed electrophoretic deposition of graphitic carbon nanotube on electrochemical capacitor performance. J. Eng. Sci. Technol. 2018, 13, 295–308. [Google Scholar]
- Kamat, P.; Thomas, K.; Barazzouk, S.; Girishkumar, G.; Vinodgopal, K.; Meisel, D. Self-assembled linear bundles of single wall carbon nanotubes and their alignment and deposition as a film in a dc field. J. Am. Chem. Soc. 2004, 126, 10757–10762. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.N.; Randall, C.A.; Mayo, M.J. Fabrication of dense zirconia electrolyte films for tubular solid oxide fuel cells by electrophoretic deposition. J. Am. Ceram. Soc. 2001, 84, 33–40. [Google Scholar] [CrossRef]
- Zare, Y.; Yop Rhee, K.; Park, S.J. Modeling the roles of carbon nanotubes and interphase dimensions in the conductivity of nanocomposites. Results Phys. 2019, 15, 102562. [Google Scholar] [CrossRef]
- Feng, C.; Jiang, L. Micromechanics modeling of the electrical conductivity of carbon nanotube (CNT)-polymer nanocomposites. Compos. Part. A Appl. Sci. Manuf. 2013, 47, 143–149. [Google Scholar] [CrossRef]
- Deng, F.; Zheng, Q.S. An analytical model of effective electrical conductivity of carbon nanotube composites. Appl. Phys. Lett. 2008, 92. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Leu, I.-C.; Hon, M.-H. Kinetics of electrophoretic deposition for nanocrystalline zinc oxide coatings. J. Am. Ceram. Soc. 2004, 87, 84–88. [Google Scholar] [CrossRef]
- Suryawanshi, C.N.; Lin, C.T. Radiative cooling: Lattice quantization and surface emissivity in thin coatings. ACS Appl. Mater. Interfaces 2009, 1, 1334–1338. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, S.; Zhang, H.; Yao, W.; Liu, C. Excellent heat dissipation properties of the super-aligned carbon nanotube films. RSC Adv. 2016, 6, 61686–61694. [Google Scholar] [CrossRef]
- Wang, S.; Liang, R.; Wang, B.; Zhang, C. Dispersion and thermal conductivity of carbon nanotube composites. Carbon 2009, 47, 53–57. [Google Scholar] [CrossRef]





| MWCNT Concentration | Voltage | EDP Elapsed Time | Electrical Resistivity | Standard Deviaton | Temperature Rise |
|---|---|---|---|---|---|
| (mg/mL) | (V) | (min) | (% IACS) | (°C) | |
| 0 | - | - | 43.2 | - | 34.3 |
| 0.05 1 | 3 V 1 | 5 min 1 | 43.58 | 0.2815 | 25.2 |
| 10 min | 43.58 | 0.2807 | 26.1 | ||
| 20 min | 44.03 | 0.3327 | 26.7 | ||
| 5 V 1 | 5 min 1 | 47.70 | 0.5993 | 29.5 | |
| 10 min | 44.14 | 2.220 | 30.5 | ||
| 20 min | 43.49 | 0.950 | 32.4 | ||
| 10 V | 5 min | 44.47 | 0.9805 | 31.0 | |
| 10 min | 42.86 | 1.218 | 32.1 | ||
| 20 min | 44.47 | 0.2973 | 31.2 | ||
| 20 V | 5 min | 40.51 | 1.571 | 29.4 | |
| 10 min | 41.95 | 1.089 | 31.9 | ||
| 20 min | 44.81 | 1.441 | 41.2 | ||
| 0.10 | 3 V | 5 min | 44.68 | 0.3606 | 26.2 |
| 10 min | 42.66 | 0.7299 | 26.8 | ||
| 20 min | 44.55 | 0.3051 | 30 | ||
| 5 V | 5 min | 43.81 | 1.932 | 32.7 | |
| 10 min | 43.81 | 0.1828 | 32.4 | ||
| 20 min | 46.57 | 3.017 | 35.7 | ||
| 10 V | 5 min | 44.81 | 1.003 | 32.8 | |
| 10 min | 46.21 | 0.9145 | 29.1 | ||
| 20 min | 43.17 | 0.7507 | 28.2 | ||
| 20 V | 5 min | 43.81 | 0.7299 | 34.2 | |
| 10 min | 44.81 | 0.7853 | 29.2 | ||
| 20 min | 44.14 | 0.5613 | 26.8 | ||
| 0.20 2 | 3 V | 5 min | 44.16 | 0.2346 | 29.2 |
| 10 min | 45.32 | 0.3564 | 27.4 | ||
| 20 min | 43.14 | 0.2907 | 29.2 | ||
| 5 V | 5 min | 44.47 | 0.9570 | 31.0 | |
| 10 min | 45.50 | 0.5123 | 35.2 | ||
| 20 min | 44.81 | 1.219 | 35.6 | ||
| 10 V | 5 min | 42.55 | 0.5101 | 34.3 | |
| 10 min | 40.25 | 1.313 | 27.7 | ||
| 20 min | 43.81 | 0.8857 | 26.1 | ||
| 20 V 2 | 5 min | 45.50 | 1.189 | 28.6 | |
| 10 min | 45.85 | 1.968 | 28.1 | ||
| 20 min 2 | 47.70 | 1.451 | 30.4 |
| Al 6061 | Ra | Rq | Rz |
|---|---|---|---|
| 0.05 mg/mL MWCNT | (µm) | (µm) | (µm) |
| 0 | 0.1335 | 0.1705 | 0.5847 |
| 5 V | 0.1190 | 0.1457 | 0.5342 |
| 20 V | 0.1215 | 0.151 | 0.5080 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Salinas, J.; Hernández, M.B.; Cruz, L.G.; Martínez-Romero, O.; Ulloa-Castillo, N.A.; Elías-Zúñiga, A. Enhancing Electrical and Thermal Properties of Al6061 Parts by Electrophoresis Deposition of Multi-Walled Carbon Nanotubes. Coatings 2020, 10, 656. https://doi.org/10.3390/coatings10070656
Rodríguez-Salinas J, Hernández MB, Cruz LG, Martínez-Romero O, Ulloa-Castillo NA, Elías-Zúñiga A. Enhancing Electrical and Thermal Properties of Al6061 Parts by Electrophoresis Deposition of Multi-Walled Carbon Nanotubes. Coatings. 2020; 10(7):656. https://doi.org/10.3390/coatings10070656
Chicago/Turabian StyleRodríguez-Salinas, Juan, Marla B. Hernández, Luis Gerardo Cruz, Oscar Martínez-Romero, Nicolás A. Ulloa-Castillo, and Alex Elías-Zúñiga. 2020. "Enhancing Electrical and Thermal Properties of Al6061 Parts by Electrophoresis Deposition of Multi-Walled Carbon Nanotubes" Coatings 10, no. 7: 656. https://doi.org/10.3390/coatings10070656
APA StyleRodríguez-Salinas, J., Hernández, M. B., Cruz, L. G., Martínez-Romero, O., Ulloa-Castillo, N. A., & Elías-Zúñiga, A. (2020). Enhancing Electrical and Thermal Properties of Al6061 Parts by Electrophoresis Deposition of Multi-Walled Carbon Nanotubes. Coatings, 10(7), 656. https://doi.org/10.3390/coatings10070656






