Polyimide Surface Modification Using He-H2O Atmospheric Pressure Plasma Jet-Discharge Power Effect
Abstract
:1. Introduction
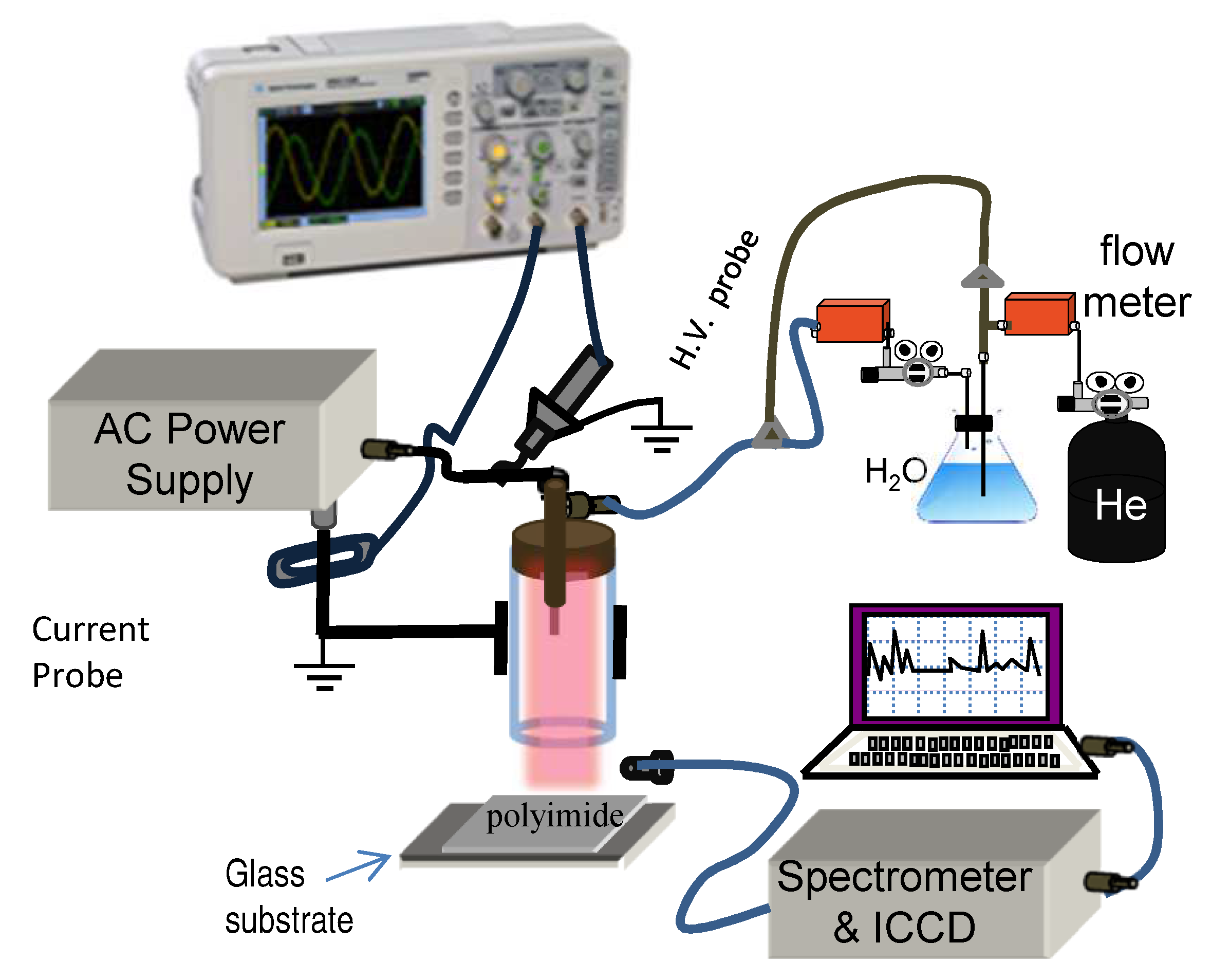
2. Materials and Methods
3. Results and Discussion
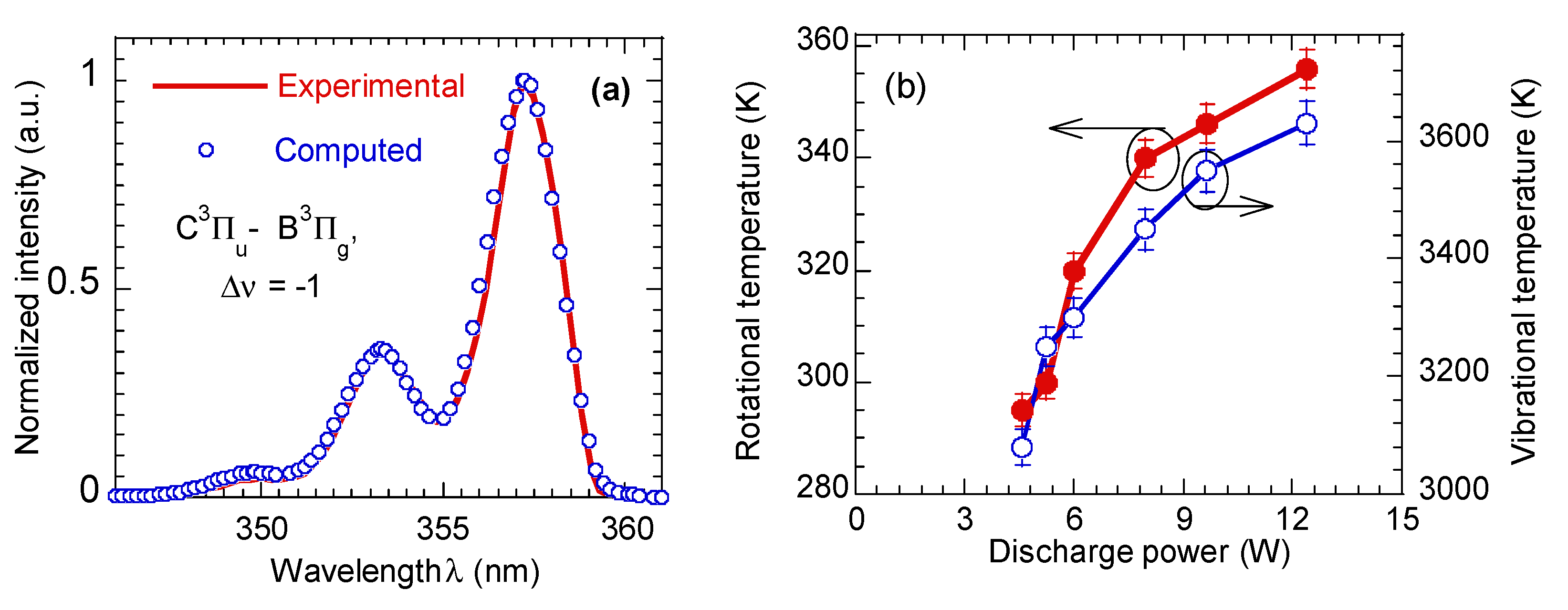
3.1. Electrical and Optical Characteristics of He- Plasma Jet
3.2. Surface Characterization
Hydrophilic Effect Induced by Plasma at Various Discharge Powers
3.3. AFM Surface Morphology
X-ray Photoelectron Spectra Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akinwande, D.; Petrone, N.; Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 2014, 5, 5678. [Google Scholar] [CrossRef] [PubMed]
- Chwang, A.; Lu, J.P.; Shih, C.; Tung, Y.-J.; Hewitt, R.; Hack, M.; Ho, J.; Brown, J. Cockpit and Future Displays for Defense and Security (Proceedings of SPIE); Society of Photo Optical: Orlando, FL, USA, 2005; pp. 234–248. [Google Scholar]
- Bauer, S. Flexible electronics: Sophisticated skin. Nat. Mater. 2013, 12, 871–872. [Google Scholar] [CrossRef]
- Kubo, Y.; Tanaka, H.; Saito, Y.; Mizoguchi, A. Fabrication of a bilayer structure of Cu and polyimide to realize circuit microminiaturization and high interfacial adhesion in flexible electronic devices. ACS Appl. Mater. Interfaces 2018, 10, 44589–44602. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Choi, S.-Y.; Jung, A.; Ko, S.H. Highly conductive aluminum textile and paper for flexible and wearable electronics. Angew. Chem. 2013, 125, 7872–7877. [Google Scholar] [CrossRef]
- Jin, H.; Zhou, J.; He, X.; Wang, W.; Guo, H.; Dong, S.; Wang, D.; Xu, Y.; Geng, J.; Luo, J.K.; et al. Flexible surface acoustic wave resonators built on disposable plastic film for electronics and lab-on-a-chip applications. Sci. Rep. 2013, 3, 2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isfahani, H.N.; Faghihi, K.; Hajibeygi, M.; Bokaei, M. New optically active poly(amide-imide)s from N’-(bicyclo[2,2,2]oct-7-ene-2,3,5,6-tetracarboxylic)bis-L-phenyl alanine and aromatic diamines: Synthesis and characterization. Polym. Bull. 2010, 64, 633–646. [Google Scholar] [CrossRef]
- Chao, M. Synthesis and characterization of semicrystalline polyimides containing bridged linkages. Int. J. Polym. Sci. 2018, 2018, 8590567. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Fattah, E. Surface activation of poly(Methyl Methacrylate) with atmospheric pressure Ar- H2O plasma. Coatings 2019, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-J.; Lee, E.-J.; Kwon, S.-H. Influence of surface treatment of polyimide film on adhesion enhancement between polyimide and metal films. Bull. Korean Chem. Soc. 2007, 28, 188. [Google Scholar]
- Park, Y.J.; Yu, D.M.; Ahn, J.H.; Choi, J.-H.; Hong, Y.T. Surface modification of polyimide films by an ethylenediamine treatment for a flexible copper clad laminate. Macromol. Res. 2012, 20, 168–173. [Google Scholar] [CrossRef]
- Dong, S.-S.; Shao, W.-Z.; Yang, L.; Yeb, H.-J.; Zhena, L. Surface characterization and degradation behavior of polyimide films induced by coupling irradiation treatment. RSC Adv. 2018, 8, 28152. [Google Scholar] [CrossRef] [Green Version]
- Castillo, F.J.A.S.; Rivadeneyra, A.; Albrecht, A.; Godoy, A.; Morales, D.P.; Rodriguez, N. In-depth study of laser diode ablation of kapton polyimide for flexible conductive substrates. Nanomaterials 2018, 8, 517. [Google Scholar]
- Velardi, L.; Lorusso, A.; Paladini, F.; Siciliano, M.V.; Giulio, M.; Raino, A.; Nassisi, V. Modification of polymer characteristics by laser and ion beam. Radiat. Effects Def. Solids 2010, 165, 637–642. [Google Scholar] [CrossRef]
- Nedela, O.; Slepicka, P.; Svorcik, V. Surface modification of polymer substrates for biomedical applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jeong, D.; Hwang, J.; Chae, H. Argon and nitrogen plasma surface treatments of polyimide films for electroless copper plating. J. Korean Phys. Soc. 2009, 54, 621–627. [Google Scholar] [CrossRef]
- Sava, I.; Kruth, A.; Kolb, J.F.; Miron, C. Optical properties of polyimides films treated by nanosecond pulsed electrical discharges in water. Jpn. J. Appl. Phys. 2018, 57, 0102BF. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Fattah, E. Surface and thermal characteristics relationship of atmospheric pressure plasma treated natural luffa fibers. Eur. Phys. J. D. 2019, 73, 71. [Google Scholar] [CrossRef]
- Bhusari, D.; Hayden, H.; Tanikell, R.; Allen, S.A.B.; Kohla, P.A. Plasma treatment and surface analysis of polyimide films for electroless copper buildup process. J. Electrochem. Soc. 2005, 152, F162–F170. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Fattah, E.; Yehia, A.; Bazavan, M.; Ishijima, T. Optical emission and surface characterization of stainless steel treated by pulsed microwave-atmospheric helium plasma jet. Eur. Phys. J. D. 2017, 71, 178. [Google Scholar] [CrossRef]
- Abdel-Fattah, E.; Bazavan, M.; Shindo, H. Temperature measurements in microwave argon plasma source by using overlapped molecular emission spectra. Phys. Plasma 2015, 22, 093509. [Google Scholar] [CrossRef]
- Laux, C.O. Radiation and nonequilibrium collisional-radiative models. In Physico-Chemical Modeling of High Enthalpy and Plasma Flows; van Karman Institute Lecture Series; Fletcher, D., Charbonnier, J.-M., Sarma, G.S.R., Magin, T., Eds.; Von Karman Institute: Rhode-Saint-Genese, Belgium, 2002. [Google Scholar]
- Available online: http://physics.nist.gov/cgi-bin/ATDdata/display.ksh (accessed on 10 May 2020).
- Kostova, K.G.; Nishime, T.M.C.; Castro, A.H.R.; Toth, A.; Hein, L.R.O. Surface modification of polymeric materials by cold atmospheric plasma jet. Appl. Surf. Sci. 2014, 314, 367. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, N.; Tasaka, S. and Hibi, K. Surface modification of Kapton film by plasma treatments. J. Polym. Sci. A Polym. Chem. 1992, 30, 1425–1431. [Google Scholar] [CrossRef]
- Abdel-Fattah, E.; Ogawa, D.; Nakamura, K. Oxygen functionalization of MWCNTs in RF-dielectric barrier discharge Ar/O2 plasma. J Phys. D Appl. Phys. 2017, 50, 265301. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers, the Scienta ESCA 300 Database; John Wiley: Chichester, UK; New York, NY, USA, 1992; pp. 54–75. [Google Scholar]
- Flitsch, R.; Shih, D.Y. An XPS study of argon ion beam and oxygen RIE modified BPDA-PDA polyimide as related to adhesion. J. Adhes. Sci. Technol. 1996, 10, 1241–1253. [Google Scholar] [CrossRef]





| Discharge Power (W) | C 1s (%) | O 1s (%) | N 1s (%) | O/C (%) | N/C (%) |
|---|---|---|---|---|---|
| 0 | 71.6 | 23.9 | 4.52 | 33.3 | 6.3 |
| 5.2 | 58.3 | 36 | 5.7 | 61.7 | 9.8 |
| 6.2 | 60.9 | 32.7 | 6.4 | 53.7 | 10.5 |
| 8.0 | 61.8 | 31.2 | 7 | 50.5 | 11.3 |
| 9.6 | 63.4 | 28.5 | 8.1 | 45.0 | 12.8 |
| 12.4 | 66.6 | 25.40 | 8 | 38.1 | 12.0 |
| Discharge Power (W) | C 1s | ||||
|---|---|---|---|---|---|
| C–C (%) | C–N (%) | C–O (%) | N–C=O (%) | (%) | |
| 0 | 47.8 | 12.1 | 3.7 | 6.6 | 0 |
| 5.2 | 23.3 | 15.5 | 4.9 | 12.1 | 0.5 |
| 6.2 | 26.2 | 16.0 | 3.8 | 13.3 | 0.75 |
| 8.0 | 26.6 | 16.7 | 3.5 | 13.94 | 1.2 |
| 9.6 | 29.0 | 18.3 | 2.4 | 12.26 | 1.5 |
| 12.4 | 30.3 | 21.9 | 1.73 | 11.5 | 1.5 |
| Discharge Power (W) | N 1s | O 1s | |||
| C–N (%) | N–C=O (%) | N- (%) | C=O (%) | C–O (%) | |
| 0 | 2.0 | 1.7 | 0.0 | 16.24 | 9.9 |
| 5.2 | 2.2 | 2.8 | 0.75 | 20.4 | 15.4 |
| 6.2 | 2.3 | 3.6 | 0.95 | 17.8 | 14.2 |
| 8.0 | 2.5 | 4.0 | 0.9 | 15.1 | 14.6 |
| 9.6 | 2.6 | 4.0 | 0.7 | 15.7 | 12.7 |
| 12.4 | 3.0 | 3.7 | 0.5 | 13.6 | 12.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel–Fattah, E.; Alshaer, M. Polyimide Surface Modification Using He-H2O Atmospheric Pressure Plasma Jet-Discharge Power Effect. Coatings 2020, 10, 662. https://doi.org/10.3390/coatings10070662
Abdel–Fattah E, Alshaer M. Polyimide Surface Modification Using He-H2O Atmospheric Pressure Plasma Jet-Discharge Power Effect. Coatings. 2020; 10(7):662. https://doi.org/10.3390/coatings10070662
Chicago/Turabian StyleAbdel–Fattah, Essam, and Mazen Alshaer. 2020. "Polyimide Surface Modification Using He-H2O Atmospheric Pressure Plasma Jet-Discharge Power Effect" Coatings 10, no. 7: 662. https://doi.org/10.3390/coatings10070662





