Interaction of Strontium Zirconate Plasma Sprayed Coating with Natural Silicate (CMAS) Dust—Origin of Luminescent Phases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
2.2.1. Phase Composition
2.2.2. Microstructure
2.2.3. Microhardness
3. Results and Discussion
3.1. Microstructure and Porosity
3.2. Phase Composition
3.3. Mechanical Properties
3.4. Optical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal Barrier Coatings for Gas-Turbine Engine Applications. Science 2002, 296, 280–298. [Google Scholar] [CrossRef]
- Evans, A.G.; Mumm, D.R.; Hutchinson, J.W.; Meier, G.H.; Pettit, F.S. Mechanisms Controlling, Durability of Thermal Barrier Coatings. Prog. Mater. Sci. 2001, 46, 505–532. [Google Scholar] [CrossRef]
- Levi, C.G. Emerging Materials and Processes for Thermal Barrier Systems. Curr. Opin. Solid State Mater. Sci. 2002, 8, 77–99. [Google Scholar] [CrossRef]
- Clarke, D.R.; Levi, C.G. Materials Design for the Next Generation Thermal Barrier Coatings. Annu. Rev. Mater. Sci. 2003, 33, 383–397. [Google Scholar] [CrossRef]
- Evans, A.G.; Clarke, D.R.; Levi, C.G. The Influence of Oxides on the Performance of Advanced Gas Turbines. J. Eur. Ceram. Soc. 2008, 28, 1405–1418. [Google Scholar] [CrossRef]
- Vassen, R.; Ophelia-Jarligo, M.; Steinke, T.; Emil-Mack, D.; Stöver, D. Overview on Advanced Thermal Barrier Coatings. Surf. Coat. Technol. 2010, 5, 938–949. [Google Scholar] [CrossRef]
- Clarke, D.R.; Philpot, S.R. Thermal Barrier Coating Materials. Mater. Today 2005, 8, 22–29. [Google Scholar] [CrossRef]
- Kulkarni, A.; Wang, Z.; Nakamura, T.; Sampath, S.; Goland, A.; Herman, H.; Allen, J.; Ilavsky, J.; Long, G.; Frahm, J.; et al. Comprehensive Microstructural Characterization and Predictive Property Modeling of Plasma-sprayed Zirconia Coatings. Acta Mater. 2003, 51, 2457–2475. [Google Scholar] [CrossRef]
- Wang, Z.; Kulkarni, A.; Deshpande, S.; Nakamura, T.; Herman, H. Effects of Pores and Interfaces on Effective Properties of Plasma Sprayed Zirconia Coatings. Acta Mater. 2003, 51, 5319–5334. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Jungen, W.; Schwartz, S.; Tietz, F.; Stöver, D. Thermal Stability of Lanthanum Zirconate Plasma-sprayed Coating. J. Am. Ceram. Soc. 2001, 84, 2086–2090. [Google Scholar] [CrossRef]
- Venkatesh, G.; Blessto, B.; Santhosh Kumar Rao, C.; Subramanian, R.; John Berchmans, L. Novel Perovskite Coating of Strontium Zirconate in Inconel Substrate. IOP Conf. Ser. Mater. Sci. Eng. 2018, 314, 012010. [Google Scholar] [CrossRef]
- Carlsson, L. High-temperature Phase Transitions in SrZrO3. Acta Crystallogr. 1967, 23, 901–905. [Google Scholar] [CrossRef]
- Li, W.; Zhao, H.; Zhong, X.; Wang, L.; Tao, S. Air Plasma-Sprayed Yttria and Yttria-Stabilized Zirconia Thermal Barrier Coatings Subjected to Calcium-Magnesium-Alumino-Silicate (CMAS). J. Thermal Spray Technol. 2014, 23, 975–983. [Google Scholar] [CrossRef]
- Berker Iyi, C.; Mecartney, M.; Mumm, D.R. On the CMAS Problem in Thermal Barrier Coatings: Benchmarking Thermochemical Resistance of Oxides Alternative to YSZ Through a Microscopic Standpoint. Uluslararasi Fen Arastirmalarinda Yenilikci Yaklasimlar Dergisi 2019, 3, 20–40. [Google Scholar]
- Kramer, S.; Yang, J.; Levi, C.G.; Johnson, C.A. Thermochemical Interaction of Thermal Barrier Coatings with Molten CaO-MgO-Al2O3-SiO2 (CMAS) Deposits. J. Am. Ceram. Soc. 2006, 89, 3167–3175. [Google Scholar] [CrossRef]
- Jing, W.; Hong-bo, G.; Yu-zhi, G.; Sheng-kai, G. Microstructure and Thermo-Physical Properties of Yttria Stabilized Zirconia Coatings with CMAS Deposits. J. Eur. Ceram. Soc. 2011, 31, 1881–1888. [Google Scholar]
- Kakuda, T.R.; Levi, C.G.; Bennett, T.D. The Thermal Behavior of CMAS-infiltrated Thermal Barrier Coatings. Surf. Coat. Technol. 2015, 272, 350–356. [Google Scholar] [CrossRef]
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier Coatings for More Efficient Gas-turbine Engines. MRS Bull. 2012, 37, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Ma, W.; Ma, B.; Guo, F.; Chen, W.; Dong, H.; Shuang, Y. Air Plasma-Sprayed La2Zr2O7-SrZrO3 Composite Thermal Barrier Coating Subjected to CaO-MgO-Al2O3-SiO2 (CMAS). J. Thermal Spray Technol. 2017, 26, 1076–1083. [Google Scholar] [CrossRef]
- Ctibor, P.; Nevrla, B.; Cizek, J.; Lukac, F. Strontium Zirconate TBC Sprayed by a High Feed-Rate Water-Stabilized Plasma Torch. J. Thermal Spray Technol. 2017, 26, 1804–1809. [Google Scholar] [CrossRef]
- Ctibor, P.; Sedlacek, J.; Janata, M. Dielectric Strontium Zirconate Sprayed by a Plasma Torch. Prog. Colol Colorants Coat. 2017, 10, 225–230. [Google Scholar]
- Ctibor, P. After-glow Luminescence of SrZrO3 Prepared by Plasma Spraying. Boletin Sociedad Espanola Ceramica Y Vidrio 2018, 57, 190–194. [Google Scholar] [CrossRef]
- Hrabovsky, M. Water-stabilized Plasma Generators. Pure Appl. Chem. 1998, 70, 1157–1162. [Google Scholar] [CrossRef] [Green Version]
- Mikulla, C.; Naraparaju, R.; Schulz, U.; Toma, F.-L.; Barbosa, M.; Leyens, C. Investigation of CMAS Resistance of Sacrificial Suspension Sprayed Alumina Topcoats on EB-PVD 7YSZ Layers. In Proceedings of the International Thermal Spray Conference and Exposition (ITSC 2019), Yokohama, Japan, 26–29 May 2019; pp. 79–85. [Google Scholar]
- Hasegawa, S.; Sugimoto, T.; Hashimoto, T. Investigation of Structural Phase Transition Behavior of SrZrO3 by Thermal Analyses and High-temperature X-ray Diffraction. Solid State Ionics 2010, 181, 1091–1097. [Google Scholar] [CrossRef]
- Mikhailov, M.M.; Verevkin, A.S. Photostability of Reflecting Coatings Based on the ZrO2 Powders Doped with SrSiO3. J. Spacecr. Rocket. 2005, 42, 716–722. [Google Scholar] [CrossRef]
- Kagomiya, I.; Suzuki, I.; Ohsato, H. Microwave Dielectric Properties of (Ca1−xSrx)SiO3 Ring Silicate Solid Solutions. Jpn. J. Appl. Phys. 2009, 48, 09KE02. [Google Scholar] [CrossRef]
- Thieme, C.; Russel, C. Thermal Expansion Behavior of SrSiO3 and Sr2SiO4 Determined by High-temperature X-ray Diffraction and Dilatometry. J. Mater. Sci. 2015, 50, 5533–5539. [Google Scholar] [CrossRef]
- Ctibor, P.; Pala, Z.; Nevrlá, B.; Neufuss, K. Plasma-sprayed Fine-grained Zirconium Silicate and Its Dielectric Properties. J. Mater. Eng. Perform. 2017, 26, 2388–2393. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, Y.; Li, E.; Qi, Y.; Liu, C.; Dong, H.; Jia, R.; Ma, W. Preparation and Characterization of SrZrO3–La2Ce2O7 Composite Ceramics as a Thermal Barrier Coating Material. Mater. Chem. Phys. 2020, 247, 122904. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, P.; Habibi, M.H.; Eldridge, J.I.; Guo, S.M. Infrared Radiative Properties of Plasma-sprayed Strontium Zirconate. Mater. Lett. 2014, 137, 5–8. [Google Scholar] [CrossRef]
- Limarga, A.M.; Clarke, D.R. Characterization of Electron Beam Physical Vapor-deposited Thermal Barrier Coatings Using Diffuse Optical Reflectance. Int. J. Appl. Ceram. Technol. 2009, 6, 400–409. [Google Scholar] [CrossRef]
- Makino, T.; Kunitomo, T.; Sakai, I.; Kinoshita, H. Thermal Radiation Properties of Ceramic Materials. Heat Transf. Jpn. Res. 1984, 13, 33–50. [Google Scholar]
- Baranowska, A.; Lesniak, M.; Kochanowicz, M.; Zmojda, J.; Miluski, P.; Dorosz, D. Crystallization Kinetics and Structural Properties of the 45S5 Bioactive Glass and Glass-Ceramic Fiber Doped with Eu3+. Materials 2020, 13, 1281. [Google Scholar] [CrossRef] [Green Version]
- Shawahni, A.M.; Abu-Jafar, M.S.; Jaradat, R.T.; Ouahrani, T.; Khenata, R.; Mousa, A.A.; Ilaiwi, K.F. Structural, Elastic, Electronic and Optical Properties of SrTMO3 (TM = Rh, Zr) Compounds: Insights from FP-LAPW Study. Materials 2018, 11, 2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Múnez, C.J.; Gómez-García, J.; Sevillano, F.; Poza, P.; Utrilla, M.V. Improving Thermal Barrier Coatings by Laser Remelting. J. Nanosci. Nanotechnol. 2011, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ctibor, P.; Kraus, L.; Tuominen, J.; Vuoristo, P.; Chráska, P. Improvement of Mechanical Properties of Alumina and Zirconia Plasma Sprayed Coatings Induced by Laser Post-treatment. Ceram. Silikáty 2007, 51, 181–189. [Google Scholar]
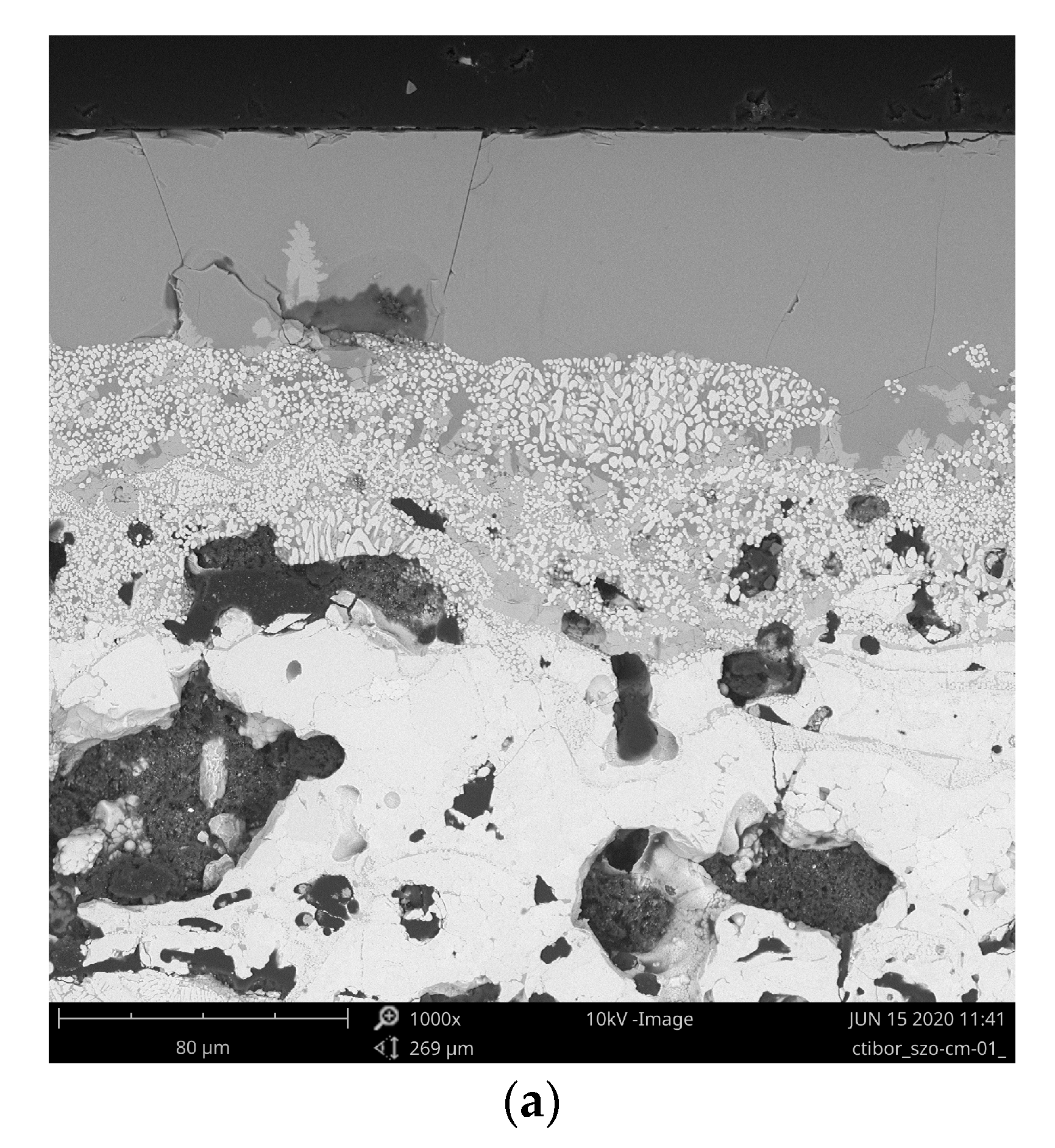



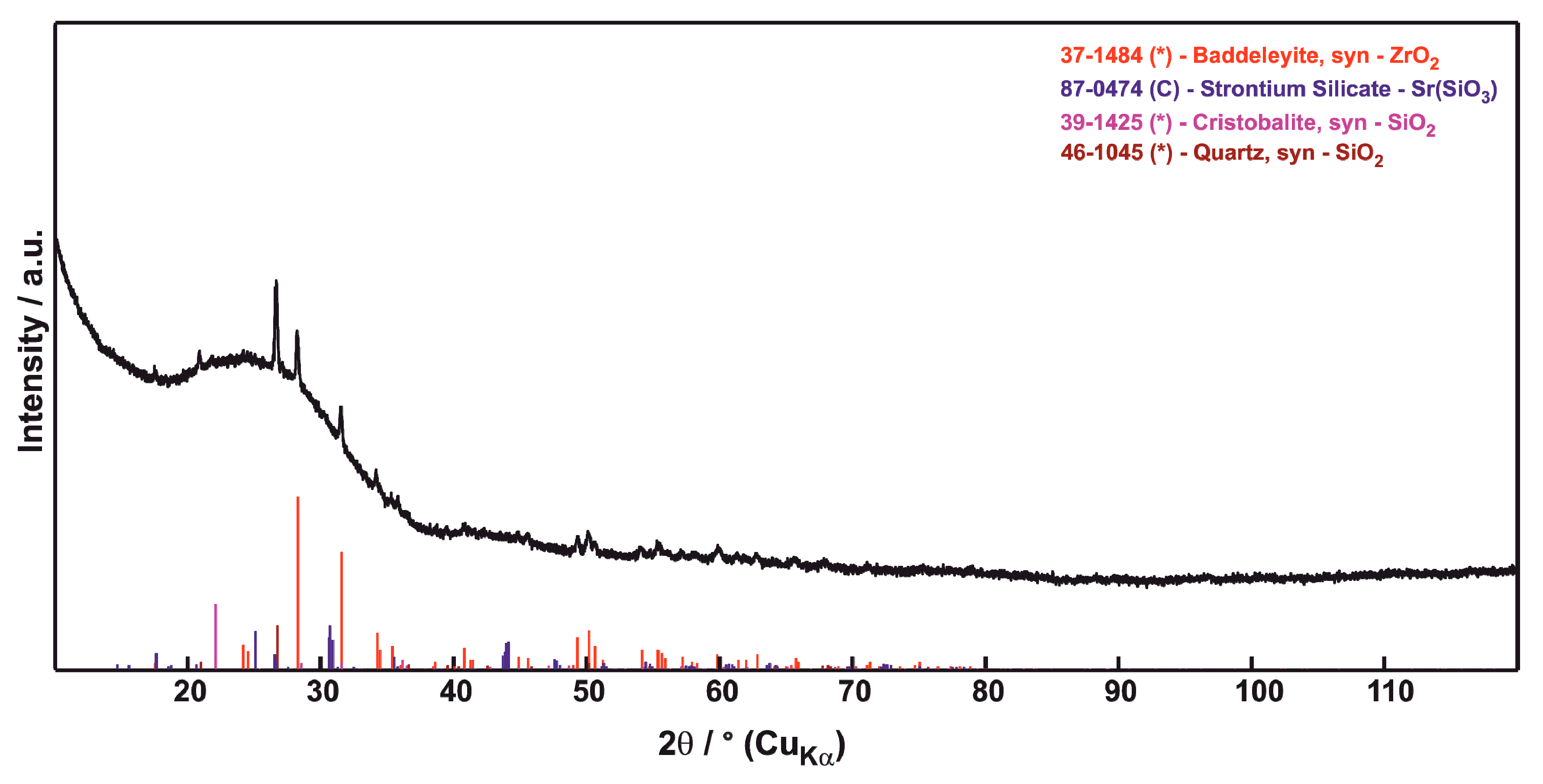

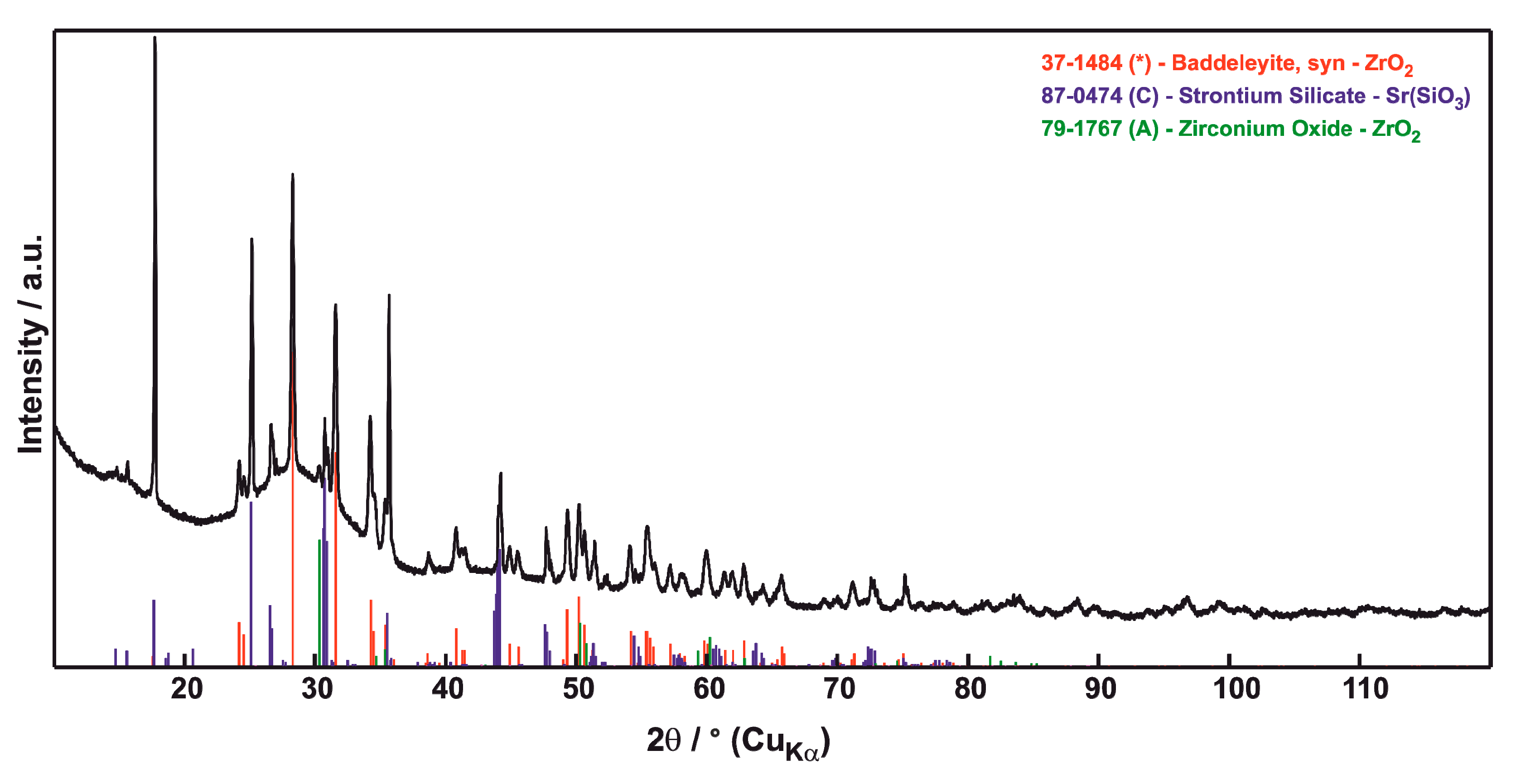


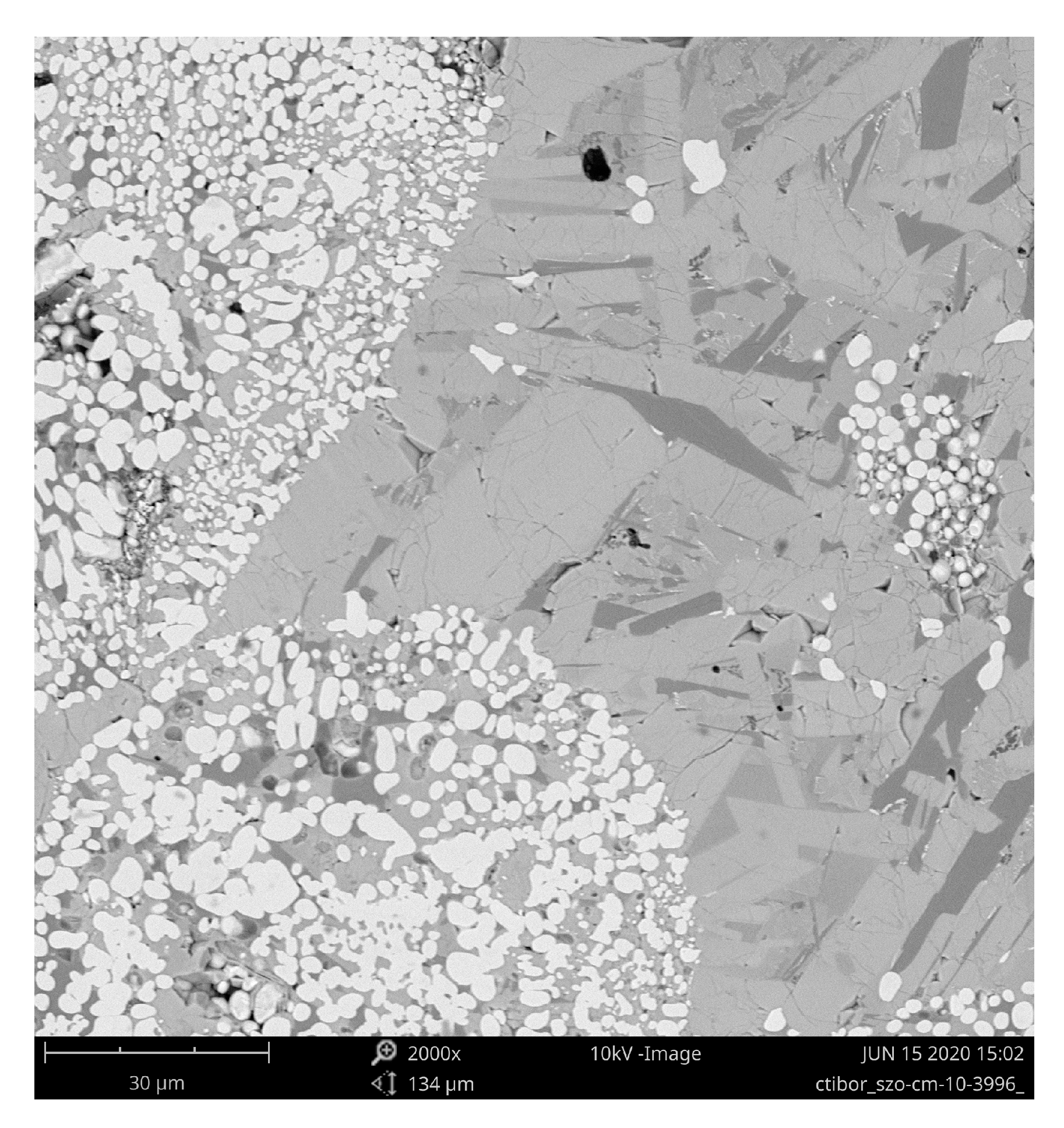
| Sample | Porosity (%) | N.V. per mm2 | E.D. (µm) | Circularity | Thick. (µm) |
|---|---|---|---|---|---|
| SrZrO3 coating | 23.8 | 5771 | 8.94 | 0.511 | 0 |
| 1h | 26.6 | 4341 | 9.36 | 0.695 | 222 |
| 4h | 32.1 | 5410 | 9.75 | 0.674 | 148 |
| 8h | 25.1 | 5137 | 8.63 | 0.607 | 333 |
| 10h | 27.7 | 4957 | 9.53 | 0.760 | 263 |
| CMAS Powder | SrZrO3 Powder | Sintered Mixed Powders |
|---|---|---|
| SiO2 (50) | SrZrO3 orthorhombic | ZrO2 monoclinic |
| Na(AlSi3O8) albite (32) | * impurities under 0.5% | ZrO2 tetragonal ** |
| CaMg(Si2O6) diopside (16) | Na4Ca4(Si6O18) combeite | |
| CaCO3 (2) | ||
| SrZrO3 coating | Coating+CMAS after 1 h | Coating+CMAS after 4 h |
| SrZrO3 orthorhombic (87) | ZrO2 monoclinic (2) | ZrO2 monoclinic (12) |
| ZrO2 tetragonal (13) | SrSiO3 (1) | SrSiO3 (20) |
| SiO2 cristobalite (2) | ZrO2 tetragonal ** (4) | |
| SiO2 quartz (3), AMORPH. 92 | AMORPH. 64 | |
| Coating+CMAS after 8 h | Coating+CMAS after 10 h | |
| ZrO2 monoclinic (28) | ZrO2 monoclinic (21) | |
| SrSiO3 (16) | SrSiO3 (42) | |
| ZrO2 tetragonal ** (2) | ZrO2 tetragonal ** (1) | |
| AMORPH. 54 | AMORPH. 36 |
| Sample | Microhardness (GPa) —Whole Coating | Microhardness (GPa) —Influenced Layer |
|---|---|---|
| SrZrO3 coating | 5.6 ± 2.6 | – |
| 1 h | 6.1 ± 1.6 | 6.5 ± 2.1 |
| 4 h | 6.0 ± 1.7 | 5.9 ± 1.2 |
| 8 h | 5.6 ± 1.8 | 5.6 ± 1.6 |
| 10 h | 6.8 ± 1.9 | 5.5 ± 0.9 |
| Sint. mix. powders | 5.5 * | – |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ctibor, P. Interaction of Strontium Zirconate Plasma Sprayed Coating with Natural Silicate (CMAS) Dust—Origin of Luminescent Phases. Coatings 2020, 10, 738. https://doi.org/10.3390/coatings10080738
Ctibor P. Interaction of Strontium Zirconate Plasma Sprayed Coating with Natural Silicate (CMAS) Dust—Origin of Luminescent Phases. Coatings. 2020; 10(8):738. https://doi.org/10.3390/coatings10080738
Chicago/Turabian StyleCtibor, Pavel. 2020. "Interaction of Strontium Zirconate Plasma Sprayed Coating with Natural Silicate (CMAS) Dust—Origin of Luminescent Phases" Coatings 10, no. 8: 738. https://doi.org/10.3390/coatings10080738
APA StyleCtibor, P. (2020). Interaction of Strontium Zirconate Plasma Sprayed Coating with Natural Silicate (CMAS) Dust—Origin of Luminescent Phases. Coatings, 10(8), 738. https://doi.org/10.3390/coatings10080738





