Preparation and Self-Repairing Properties of MF-Coated Shellac Water-Based Microcapsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
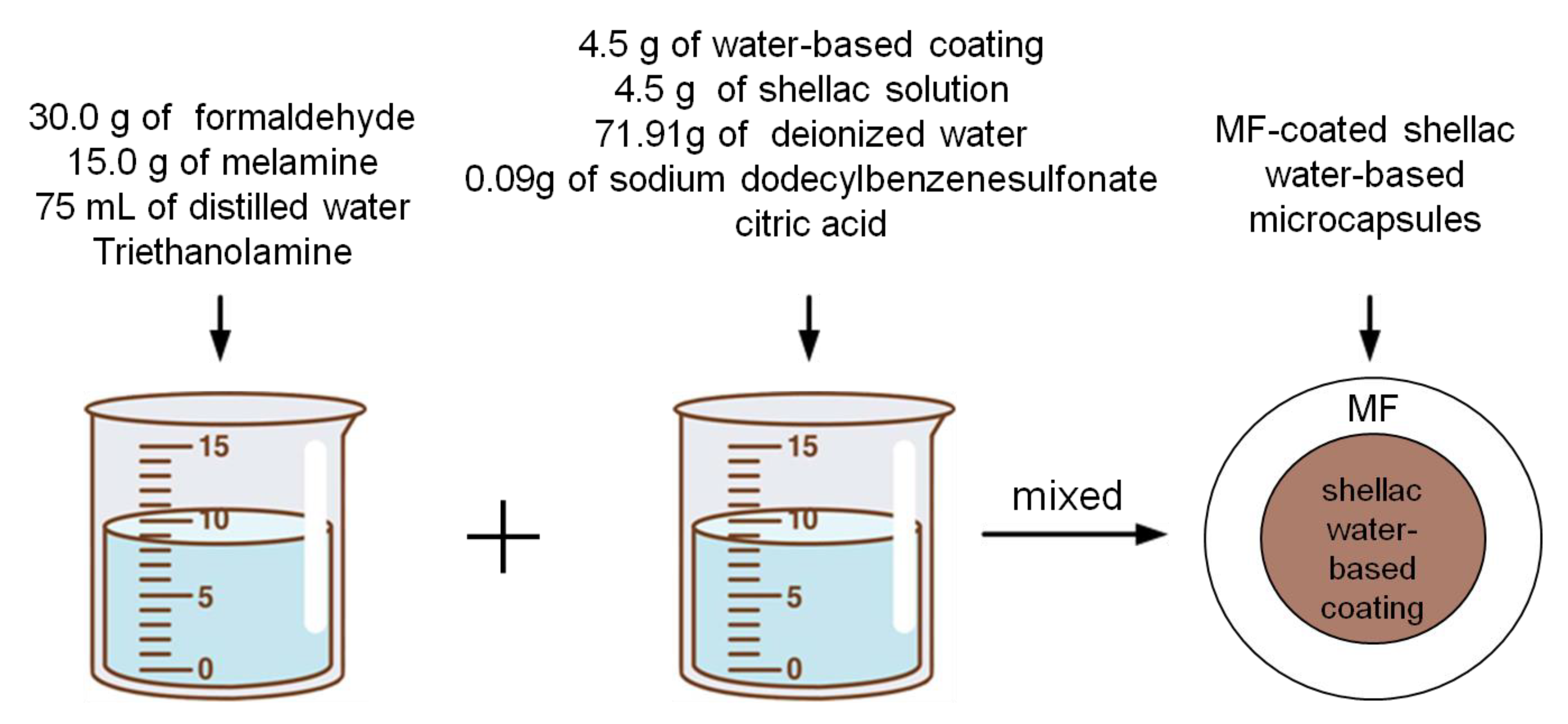
2.2. Preparation of Melamine Formaldehyde (MF)-Coated Shellac Water-Based Microcapsules
- Preparation of wall material: the 15.0 g of melamine, 30.0 g of formaldehyde and 30.0 g of distilled water were weighed. After mixing, the mixture was stirred evenly, and the pH was adjusted to 8.0–9.0 by dropping triethanolamine. It was stirred and heated in a 70 °C water bath until the wall material was transparent. Then, the wall material solution was obtained by adding 45.0 g distilled water and stirred persistently for 30 min. The wall material prepolymer was obtained after natural cooling at room temperature for 10 min.
- Preparation of core material emulsion: the mixture of shellac and anhydrous ethanol according to the ratio of 1:5 was dissolved and the impurities were separated in a centrifuge. Taking the microcapsule (No. 1 in Table 2) as an example, the 4.5 g of water-based coating and 4.5 g of shellac solution were weighed and put in a beaker and stirred continuously on a blender until they were completely mixed evenly. Then, the 71.91 g of distilled water and 0.09 g of sodium dodecyl benzene sulfonate powder (as emulsifier) were mixed and stirred into emulsifier solution. The emulsifier solution was dropped into the mixture of shellac and water-based paint, stirred at 70 °C in a water bath for 60 min at 600 rpm, and the core material emulsion was obtained. Other samples refer to the preparation method of Sample 1.
- Preparation of microcapsules: the cooled melamine wall material was slowly added to the core material emulsion at a certain speed of 400, 600, 800, and 1000 rpm, respectively, and then the citric acid was added to the mixed solution to adjust the pH value to 2.5–3.0. The mixture was slowly heated to 70 °C and stirred for 3 h. The resulting product was rinsed and filtered by deionized water and anhydrous ethanol many times after being aged for 7 days at room temperature. Finally, the remaining product was put into an oven at 40 °C for heating and drying for 48 h, and the white powder obtained was the MF-coated shellac water-based microcapsules (Figure 1). The output is 25.0–38.0 g. Based on orthogonal experiment, the single factor experiment was carried out (Nos. 17–20 in Table 2), whereas the mixing speed was different.
2.3. Preparation of Coating
2.4. Testing and Characterization
3. Results and Discussion
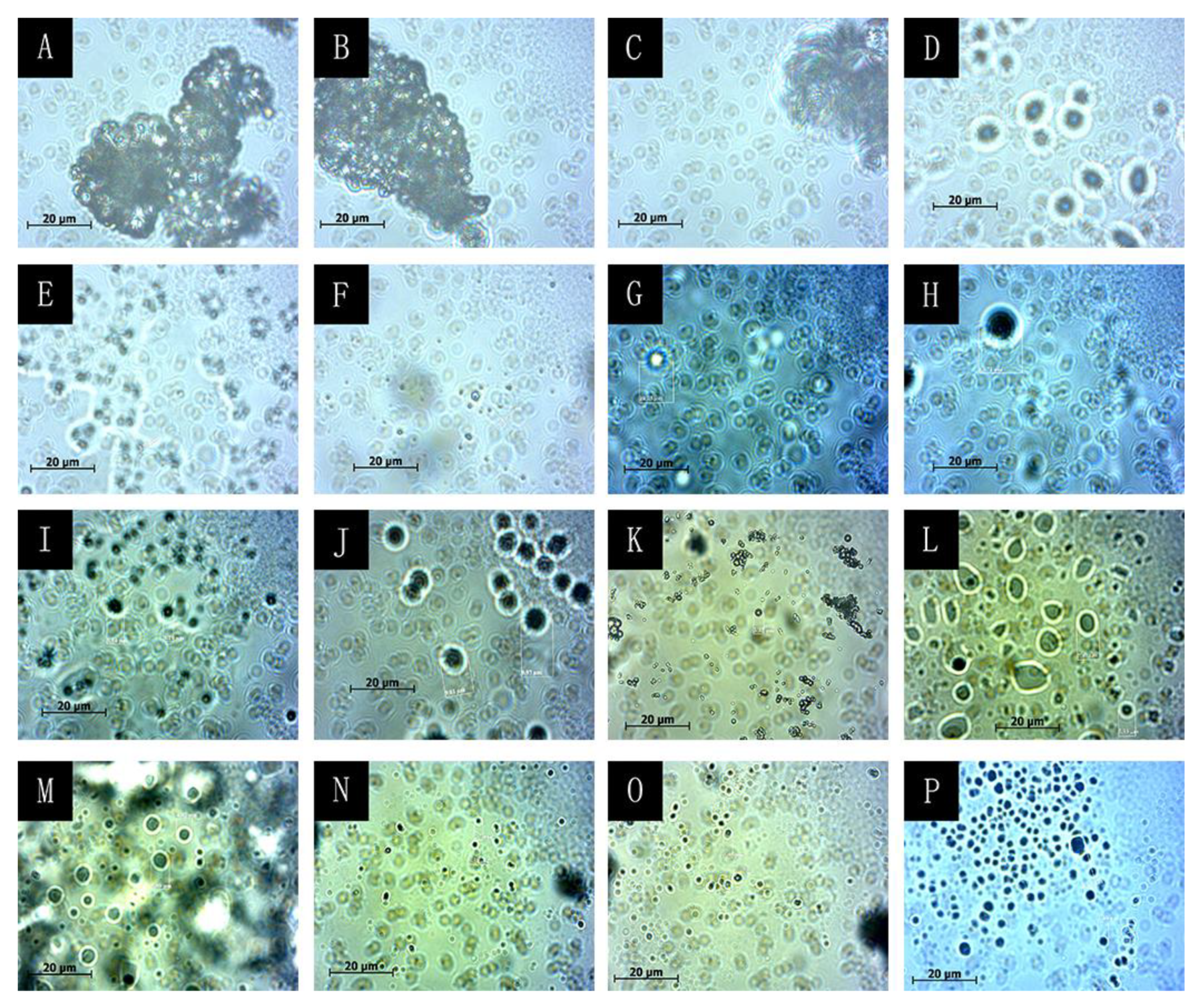
3.1. Morphological Analysis of Microcapsules for the Orthogonal Test
3.2. Analysis of Single Factor Test Results
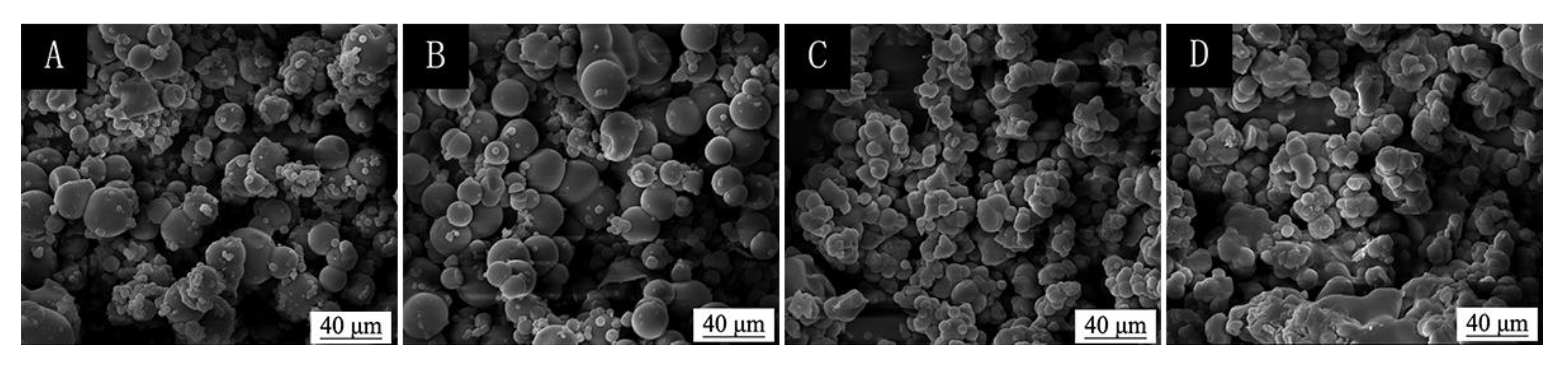
3.3. Analysis of Micro-Morphology of Microcapsules
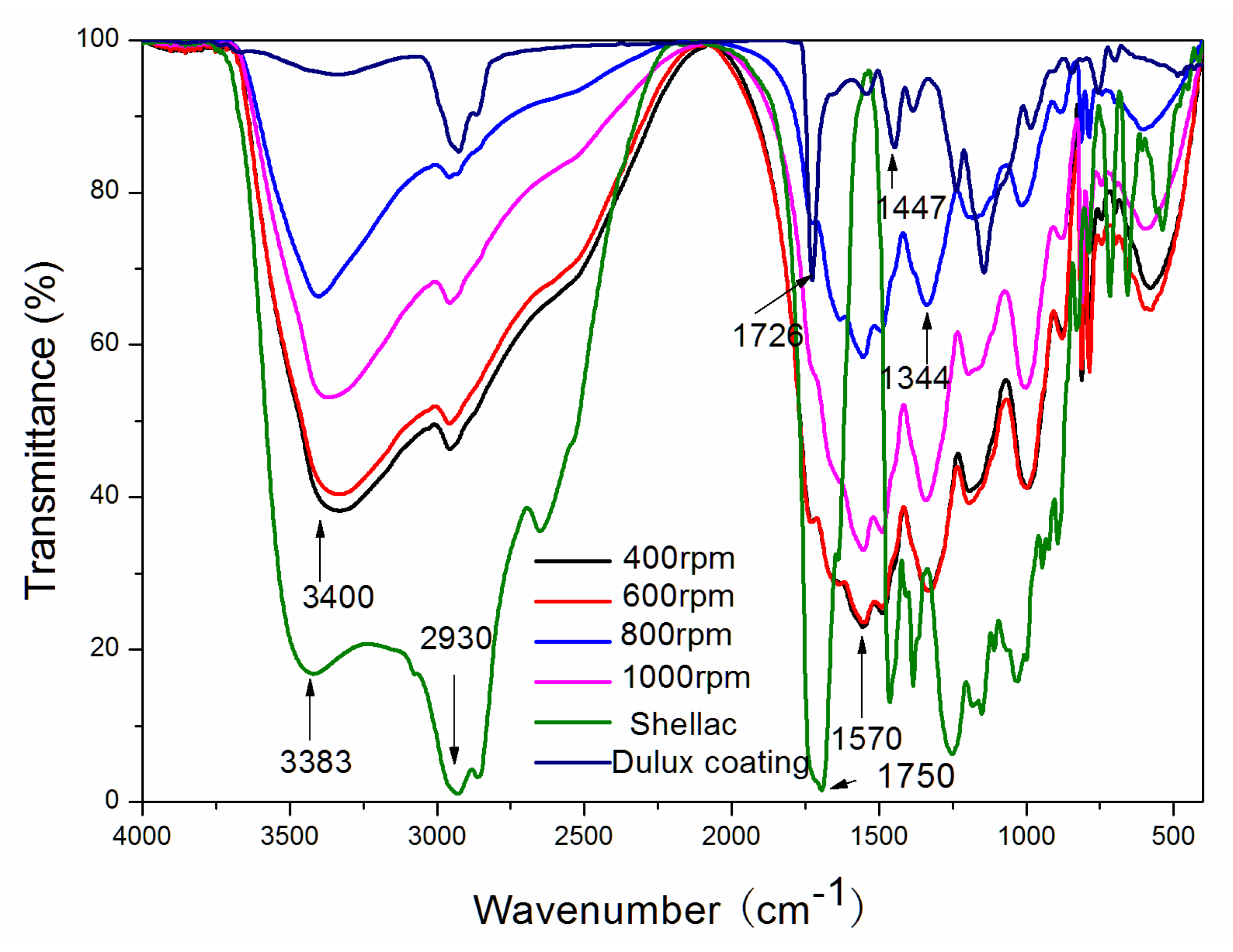
3.4. Analysis of the Chemical Composition of Microcapsules
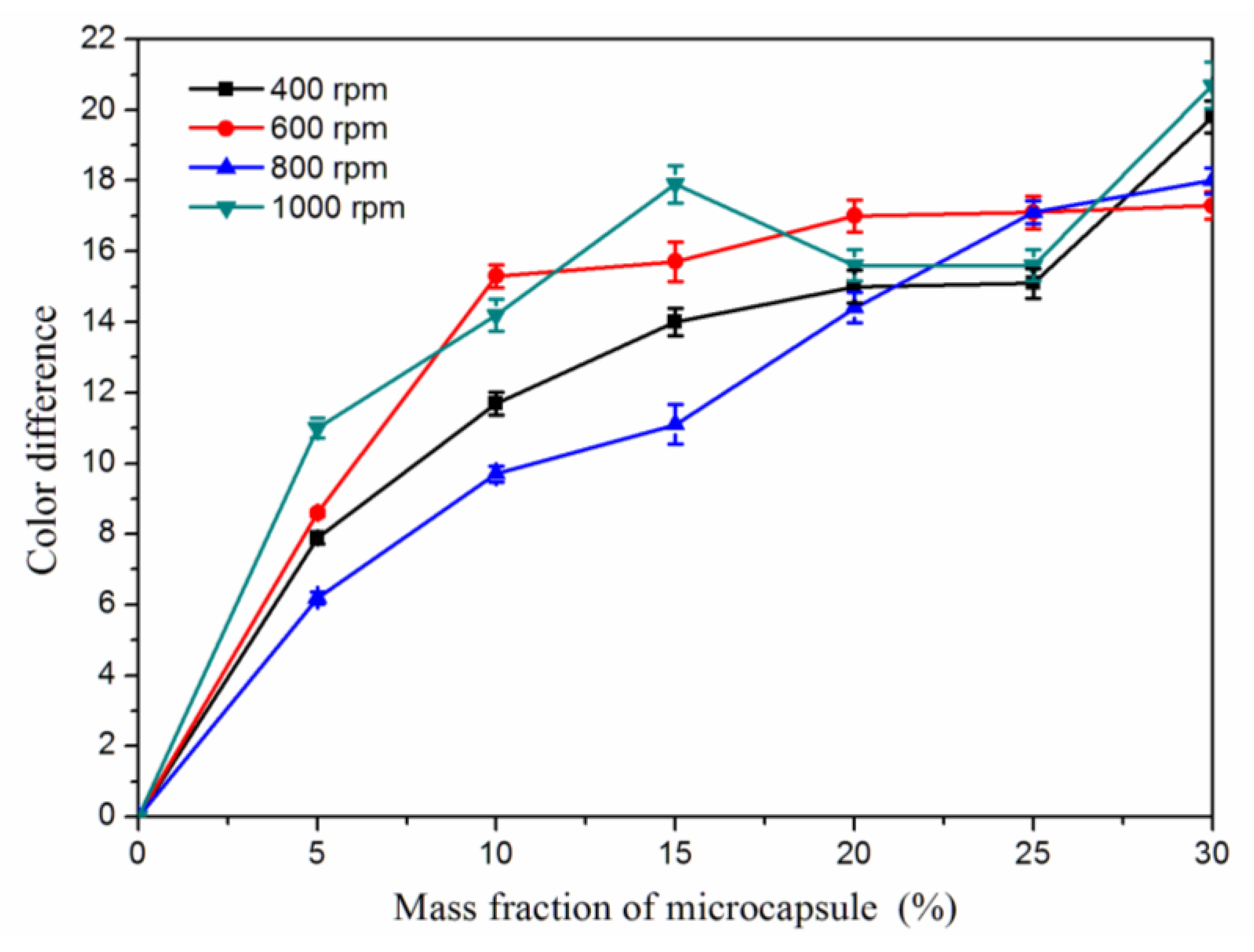
3.5. Effect of Microcapsule Mass Fraction on Color Difference
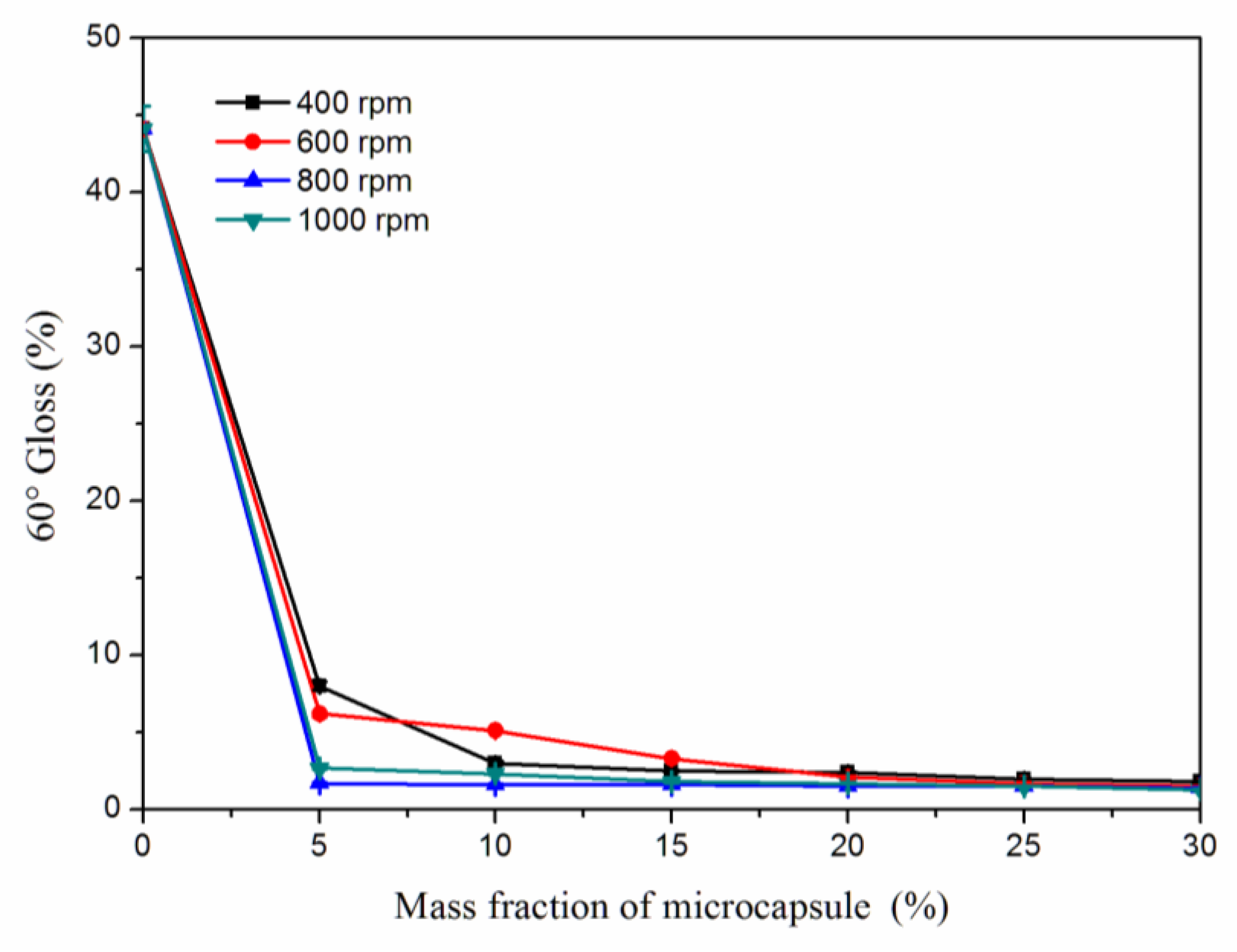
3.6. Effects of Microcapsule Mass Fractions on the Gloss and Roughness
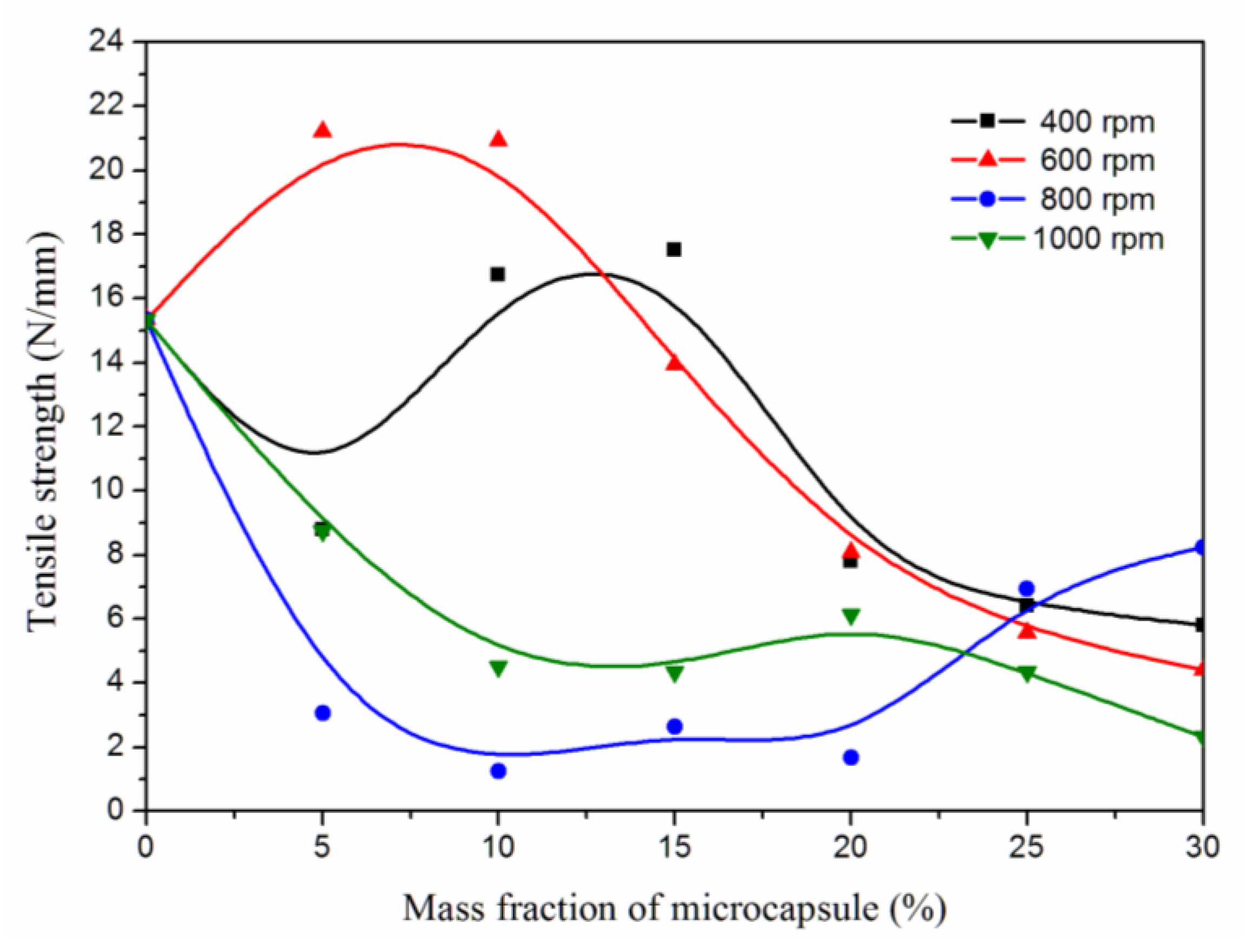
3.7. Effect of Microcapsule Mass Fractions at Different Stirring Rates on Tensile Strength
3.8. Repair Ability Effect
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, M.; Zhang, C.; Du, Z.; Zou, W. Fabrication of shape-stabilized phase change materials with melamine foam/multi-walled carbon nanotubes composite as container. Compos. Interfaces 2020, 1–17. [Google Scholar] [CrossRef]
- Morales, E.; Rubilar, M.; Burgos-Díaz, C.; Acevedo, F.; Penning, M.; Shene, C. Alginate/shellac beads developed by external gelation as a highly efficient model system for oil encapsulation with intestinal delivery. Food Hydrocoll. 2017, 70, 321–328. [Google Scholar] [CrossRef]
- Silva, M.; Tulini, F.; Ribas, M.M.; Penning, M.; Favaro-Trindade, C.S.; Poncelet, D. Microcapsules loaded with the probiotic lactobacillus paracasei BGP-1 produced by co-extrusion technology using alginate/shellac as wall material: Characterization and evaluation of drying processes. Food Res. Int. 2016, 89, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Shen, L.; Zhang, J.; Yao, J.; Lu, R.; Miyakoshi, T. Species and release characteristics of VOCs in furniture coating process. Environ. Pollut. 2019, 245, 810–819. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Liu, X.; Han, L.; Wu, Y.; Dai, X.; Zhu, J. Synthesis of bio-based unsaturated polyester resins and their application in waterborne UV-curable coatings. Prog. Org. Coat. 2015, 78, 49–54. [Google Scholar] [CrossRef]
- Herrera, R.; Muszyńska, M.; Krystofiak, T.; Labidi, J. Comparative evaluation of different thermally modified wood samples finishing with UV-curable and waterborne coatings. Appl. Surf. Sci. 2015, 357, 1444–1453. [Google Scholar] [CrossRef]
- Zhang, S.W.; Yu, A.X.; Song, X.Q.; Liu, X.Y. Synthesis and characterization of waterborne UV curable polyurethane nanocomposites based on the macromonomer surface modification of colloidal silica. Prog. Org. Coat. 2013, 76, 1032–1039. [Google Scholar] [CrossRef]
- Pepin, S.; Blanchet, P.; Landry, V. Interactions between a buffered amine oxide impregnation carrier and an acrylic resin, and their relationship with moisture. Coatings 2020, 10, 366. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.Q.; Yuan, Y.Y.; Niu, Y.T.; Zhang, L.T. Development of a cornstarch adhesive for laminated veneer lumber bonding for use in engineered wood flooring. Int. J. Adhes. Adhes. 2020, 98, 102534. [Google Scholar]
- Ye, C.; Combs, Z.A.; Calabrese, R.; Dai, H.; Kaplan, D.L.; Tsukruk, V.V. Robust microcapsules with controlled permeability from silk fibroin reinforced with graphene oxide. Small 2014, 10, 5087–5097. [Google Scholar] [CrossRef]
- Lv, L.; Yang, Z.; Chen, G.; Zhu, G.; Han, N.; Schlangen, E.; Xing, F. Synthesis and characterization of a new polymeric microcapsule and feasibility investigation in self-healing cementitious materials. Constr. Build. Mater. 2016, 105, 487–495. [Google Scholar] [CrossRef]
- Ahangari, M.G.; Fereidoon, A.; Jahanshahi, M.; Sharifi, N. Effect of nanoparticles on the micromechanical and surface properties of poly(urea–formaldehyde) composite microcapsules. Compos. Part. B Eng. 2014, 56, 450–455. [Google Scholar] [CrossRef]
- Zamani, M.; Nikafshar, S.; Mousa, A.; Behnia, A. Bacteria encapsulation using synthesized polyurea for self-healing of cement paste. Constr. Build. Mater. 2020, 249, 118556. [Google Scholar] [CrossRef]
- Kong, L.; Amstad, E.; Hai, M.; Ke, X.; Chen, D.; Zhao, C.X.; Weitz, D.A. Biocompatible microcapsules with a water core templated from single emulsions. Chin. Chem. Lett. 2017, 28, 1897–1900. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Chang, Y. Investigation of waterborne thermochromic topcoat film with color-changing microcapsules on Chinese fir surface. Prog. Org. Coat. 2019, 136, 105262. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.Q.; Zhang, Y.; Wang, S.Q.; Wang, X.Z.; Xu, D.L.; Liu, X. Effects of thermal treatment on the mechanical properties of larch (larix gmelinii) and red oak (quercus rubra) wood cell walls via nanoindentation. Bioresources 2019, 14, 8048–8057. [Google Scholar]
- Xiong, X.Q.; Niu, Y.T.; Ma, Q.R.; Pan, Y.T. Study on the warm-cool and dry-wet feeling of straw board surface. Wood Res. 2020, 65, 221–230. [Google Scholar] [CrossRef]
- Xu, W.; Fang, X.Y.; Han, J.T.; Wu, Z.H.; Zhang, J.L. Effect of coating thickness on sound absorption property of four wood species commonly used for piano soundboards. Wood Fiber Sci. 2020, 52, 28–43. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.Q.; Niu, Y.T.; Yuan, Y.Y.; Zhang, L.T. Study on dimensional stability of veneer rice straw particleboard. Coatings 2020, 10, 558. [Google Scholar] [CrossRef]
- GB/T 11186.3-1989. Method of Measurement of Coating Color. Part III: Calculation of Chromatic Aberration; Standardization Administration of the People’s Republic of China: Beijing, China, 1990. [Google Scholar]
- GB/T 4893.6-2013. Test of Surface Coatings of Furniture Part 6: Determination of Gloss Value; Standardization Administration of the People’s Republic of China: Beijing, China, 2013; pp. 1–6. (In Chinese) [Google Scholar]
- ASTMD 882-02 Standard. Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Kong, Q.M.; Wang, X.J.; Lou, T. Preparation of millimeter-sized chitosan/carboxymethyl cellulose hollow capsule and its dye adsorption properties. Carbohyd. Polymer. 2020, 244, 116481. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Yuan, Y.Y.; Niu, Y.T.; Zhang, L.T. Research on the effects of roughness on the tactile properties of rice straw particleboard surface. Sci. Adv. Mater. 2020, 12, 795–801. [Google Scholar] [CrossRef]
- Ueda, K.; Kanai, H.; Suzuki, T.; Amari, T. Effects of mechanical properties of paint film on the forming of pre-painted steel sheets. Prog. Org. Coat. 2001, 43, 233–242. [Google Scholar] [CrossRef]
- Jadhav, R.S.; Hundiwale, D.G.; Mahulikar, P.P. Synthesis and characterization of phenol-formaldehyde microcapsules containing linseed oil and its use in epoxy for self-healing and anticorrosive coating. J. Appl. Polym. Sci. 2010, 119, 2911–2916. [Google Scholar] [CrossRef]







| Level | A Wcore:Wwall | B Wemulsifier:Wcore | C Stirring Rate (rpm) | D Wshellac:Wcoating | E Wemulsifier solution:Wcore |
|---|---|---|---|---|---|
| 1 | 0.6:1 | 1:100 | 400 | 1:1.0 | 8:1 |
| 2 | 0.8:1 | 3:100 | 600 | 1:1.5 | 9:1 |
| 3 | 1.0:1 | 5:100 | 800 | 1:2.0 | 10:1 |
| 4 | 1.2:1 | 7:100 | 1000 | 1:2.5 | 11:1 |
| Sample No. | A Wcore:Wwall | B Wemulsifier:Wcore | C Stirring Rate (rpm) | D Wshellac:Wcoating | E Wemulsifier solution:Wcore |
|---|---|---|---|---|---|
| 1 | 0.6:1 | 1:100 | 400 | 1:1.0 | 8:1 |
| 2 | 0.6:1 | 3:100 | 600 | 1:1.5 | 9:1 |
| 3 | 0.6:1 | 5:100 | 800 | 1:2.0 | 10:1 |
| 4 | 0.6:1 | 7:100 | 1000 | 1:2.5 | 11:1 |
| 5 | 0.8:1 | 1:100 | 600 | 1:2.0 | 11:1 |
| 6 | 0.8:1 | 3:100 | 400 | 1:2.5 | 10:1 |
| 7 | 0.8:1 | 5:100 | 1000 | 1:1.0 | 9:1 |
| 8 | 0.8:1 | 7:100 | 800 | 1:1.5 | 8:1 |
| 9 | 1.0:1 | 1:100 | 800 | 1:2.5 | 9:1 |
| 10 | 1.0:1 | 3:100 | 1000 | 1:2.0 | 8:1 |
| 11 | 1.0:1 | 5:100 | 400 | 1:1.5 | 11:1 |
| 12 | 1.0:1 | 7:100 | 600 | 1:1.0 | 10:1 |
| 13 | 1.2:1 | 1:100 | 1000 | 1:1.5 | 10:1 |
| 14 | 1.2:1 | 3:100 | 800 | 1:1.0 | 11:1 |
| 15 | 1.2:1 | 5:100 | 600 | 1:2.5 | 8:1 |
| 16 | 1.2:1 | 7:100 | 400 | 1:2.0 | 9:1 |
| 17 | 0.8:1 | 3:100 | 400 | 1:1.0 | 9:1 |
| 18 | 0.8:1 | 3:100 | 600 | 1:1.0 | 9:1 |
| 19 | 0.8:1 | 3:100 | 800 | 1:1.0 | 9:1 |
| 20 | 0.8:1 | 3:100 | 1000 | 1:1.0 | 9:1 |
| Sample No. | Output (g) | Coverage Rate (%) |
|---|---|---|
| 1 | 29.81 | 30.0 |
| 2 | 28.39 | 23.0 |
| 3 | 25.51 | 21.0 |
| 4 | 30.05 | 13.0 |
| 5 | 27.65 | 15.0 |
| 6 | 29.42 | 30.0 |
| 7 | 28.83 | 30.0 |
| 8 | 29.84 | 18.0 |
| 9 | 29.32 | 23.0 |
| 10 | 28.54 | 24.0 |
| 11 | 28.70 | 21.0 |
| 12 | 28.59 | 21.0 |
| 13 | 36.50 | 22.0 |
| 14 | 30.29 | 23.0 |
| 15 | 31.56 | 18.0 |
| 16 | 37.25 | 24.0 |
| 17 | 28.89 | 9.0 |
| 18 | 30.56 | 24.0 |
| 19 | 35.21 | 15.0 |
| 20 | 40.25 | 43.0 |
| Horizontal Mean | A Wcore:Wwall | B Wemulsifier:Wcore | C Stirring Rate (rpm) | D Wshellac:Wcoating | E Wemulsifier solution:Wcore |
|---|---|---|---|---|---|
| Mean 1 | 28.44 | 30.82 | 31.30 | 29.38 | 29.94 |
| Mean 2 | 28.94 | 29.16 | 29.05 | 30.86 | 30.95 |
| Mean 3 | 28.79 | 28.65 | 28.74 | 29.74 | 30.01 |
| Mean 4 | 33.90 | 31.43 | 30.98 | 30.09 | 29.17 |
| Range | 5.46 | 2.78 | 2.56 | 1.48 | 1.78 |
| Variance | 80.988 | 21.006 | 20.525 | 4.781 | 6.342 |
| Horizontal Mean | A Wcore:Wwall | B Wemulsifier:Wcore | C Stirring Rate (rpm) | D Wshellac:Wcoating | E Wemulsifier solution:Wcore |
|---|---|---|---|---|---|
| Mean 1 | 21.750 | 22.500 | 26.250 | 26.000 | 22.500 |
| Mean 2 | 23.250 | 25.000 | 19.250 | 21.000 | 25.000 |
| Mean 3 | 22.250 | 22.500 | 21.250 | 21.000 | 23.500 |
| Mean 4 | 21.750 | 19.000 | 22.500 | 21.000 | 18.000 |
| Range | 1.500 | 6.000 | 7.000 | 5.000 | 7.000 |
| Variance | 6.000 | 73.000 | 104.000 | 75.000 | 109.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Yan, X. Preparation and Self-Repairing Properties of MF-Coated Shellac Water-Based Microcapsules. Coatings 2020, 10, 778. https://doi.org/10.3390/coatings10080778
Chang Y, Yan X. Preparation and Self-Repairing Properties of MF-Coated Shellac Water-Based Microcapsules. Coatings. 2020; 10(8):778. https://doi.org/10.3390/coatings10080778
Chicago/Turabian StyleChang, Yijuan, and Xiaoxing Yan. 2020. "Preparation and Self-Repairing Properties of MF-Coated Shellac Water-Based Microcapsules" Coatings 10, no. 8: 778. https://doi.org/10.3390/coatings10080778




