Influence of Laser Cladding Parameters on Microstructure, Microhardness, Chemical Composition, Wear and Corrosion Resistance of Fe–B Composite Coatings Reinforced with B4C and Si Particles
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
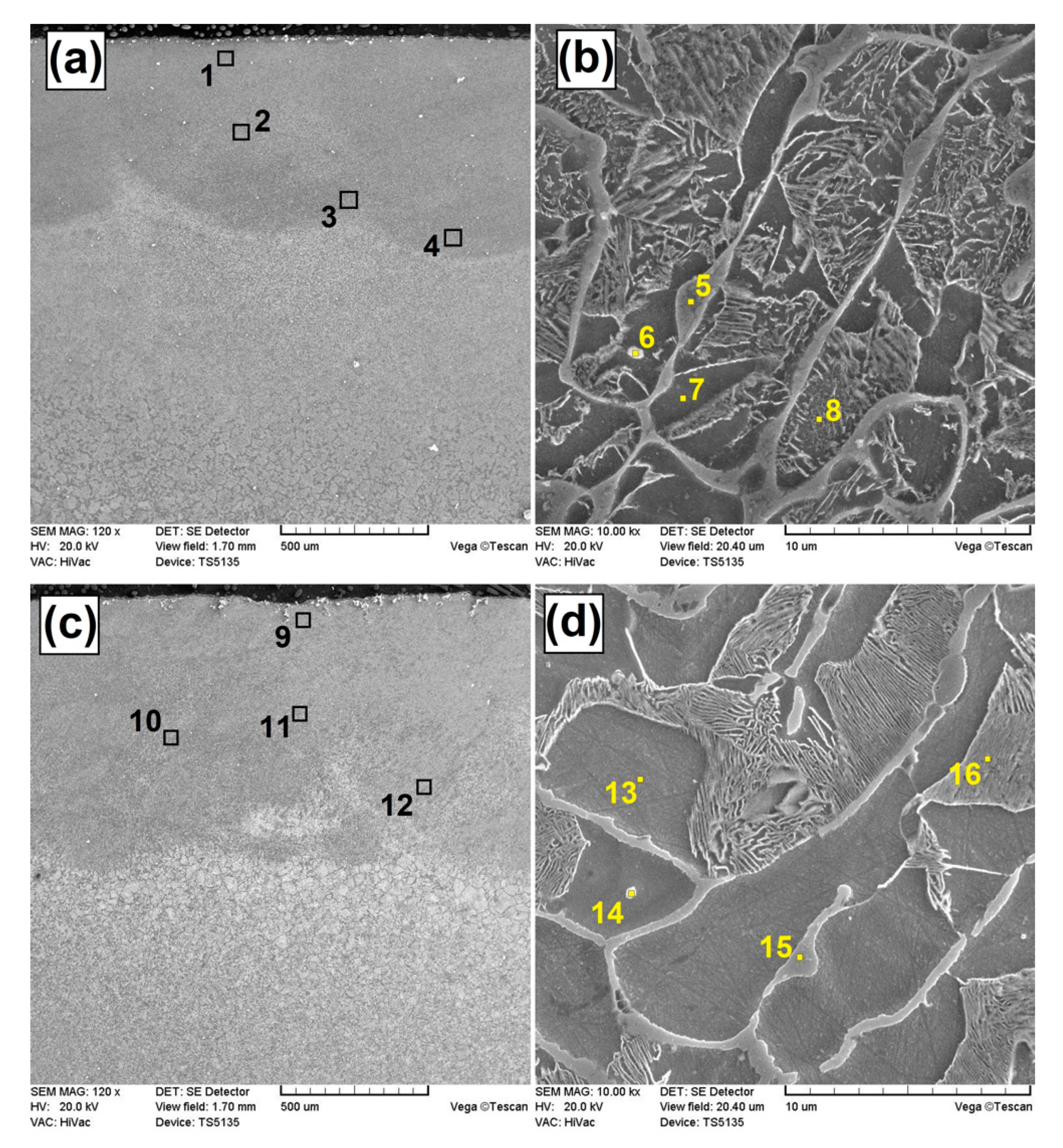
3.1. Microstructure Evaluation
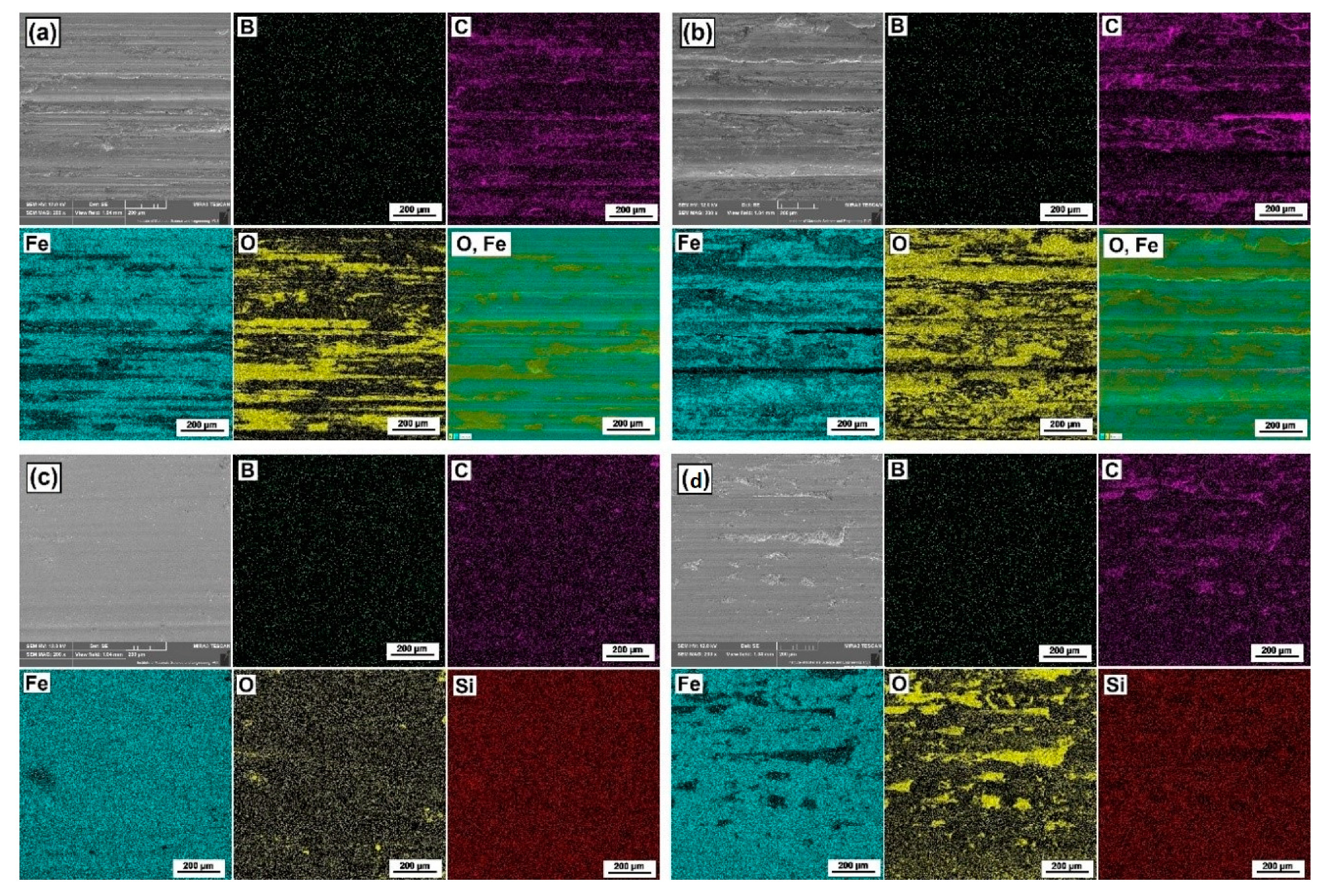
3.2. Phase Composition Analysis
3.3. Chemical Composition Analysis
3.4. Microhardness Profiles
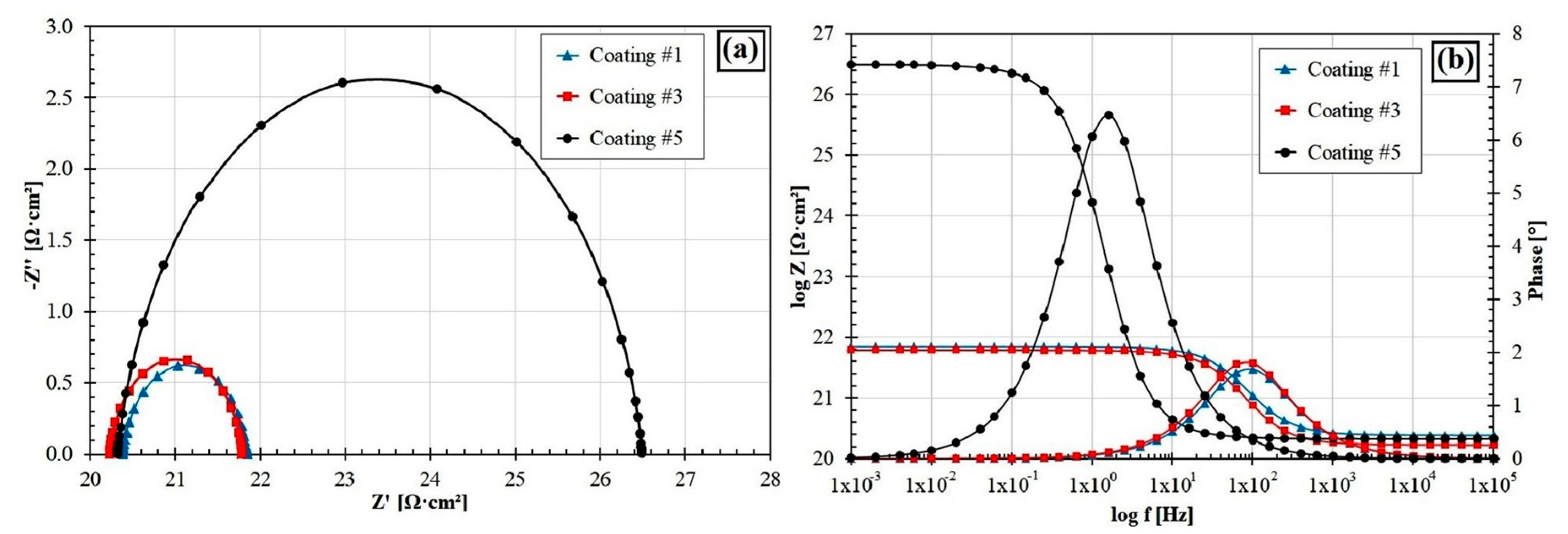
3.5. Corrosion Resistance
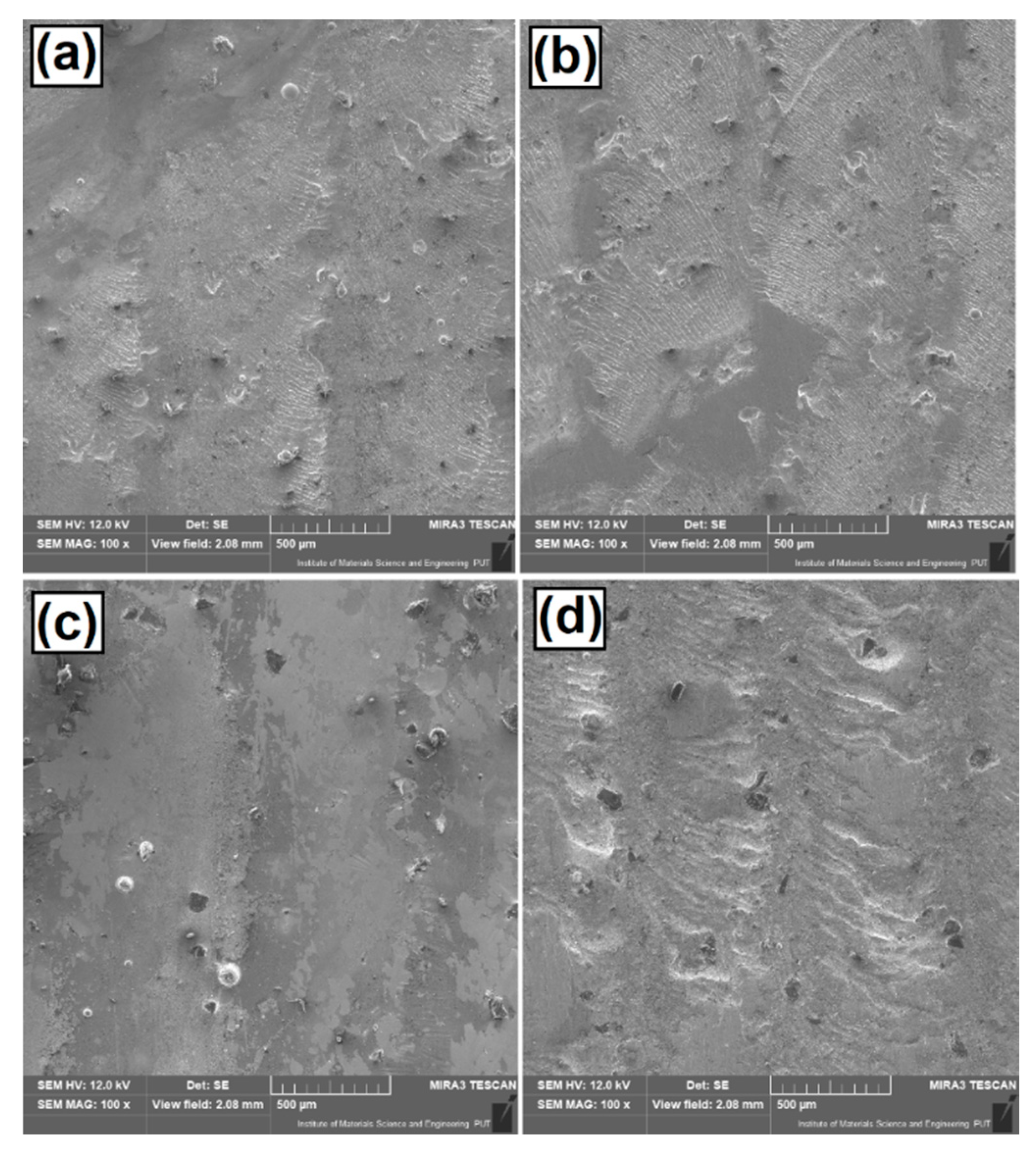
3.6. Wear Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Toyserkani, E.; Khajepour, A.; Corbin, S. Laser Cladding, 1st ed.; Taylor & Francis Group: London, UK, 2005. [Google Scholar]
- Steen, W.M.; Mazumder, J. Laser Material Processing, 4th ed.; Springer: London, UK, 2010. [Google Scholar]
- Farahmand, P.; Liu, S.; Zhang, Z.; Kovacevic, R. Laser cladding assisted by induction heating of Ni–WC composite enhanced by nano-WC and La2O3. Ceram. Int. 2014, 40, 15421–15438. [Google Scholar] [CrossRef]
- Bartkowski, D.; Młynarczak, A.; Piasecki, A.; Dudziak, B.; Gościański, M.; Bartkowska, A. Microstructure, microhardness and corrosion resistance of Stellite-6 coatings reinforced with WC particles using laser cladding. Opt. Laser Technol. 2015, 68, 191–201. [Google Scholar] [CrossRef]
- Smurov, I. Laser cladding and laser assisted direct manufacturing. Surf. Coat. Technol. 2008, 202, 4496–4502. [Google Scholar] [CrossRef]
- Paul, C.P.; Alemohammad, H.; Toyserkani, E.; Khajepour, A.; Corbin, S. Cladding of WC–12Co on low carbon steel using a pulsed Nd:YAG laser. Mater. Sci. Eng. A 2007, 464, 170–176. [Google Scholar] [CrossRef]
- Fomin, V.M.; Golyshev, A.A.; Malikov, A.G.; Orishich, A.M.; Filippov, A.A.; Ryashin, N.S. Optimization of laser cladding of cold spray coatings with B4C and Ni powders. In Proceedings of the AIP Conference 1909, Tomsk, Russia, 9–13 October 2017; p. 020054. [Google Scholar]
- Davydova, A.; Domashenkov, A.; Sova, A.; Movtchan, I.; Bertrand, P.; Desplanques, N.; Peillon, N.; Saunier, S.; Desrayaud, C.; Bucher, S.; et al. Selective laser melting of boron carbide particles coated by a cobalt-based metal layer. J. Mater. Process. Technol. 2016, 229, 361–366. [Google Scholar] [CrossRef]
- Saravanan, M.; Senthilkumar, P.; Channankaiah, D. Wear resistance improvement of a laser cladded B4C–NiCr on mild steel. IJIRST 2016, 2, 157–160. [Google Scholar]
- Guo, C.; Zhou, J.; Chen, J.; Zhao, J.; Yu, Y.; Zhou, H. High temperature wear resistance of laser cladding NiCrBSi and NiCrBSi/WC–Ni composite coating. Wear 2011, 270, 492–498. [Google Scholar] [CrossRef]
- Lin, W.C.; Chen, C. Characteristics of thin surface layers of cobalt-based alloys deposited by laser cladding. Surf. Coat. Technol. 2006, 200, 4557–4563. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Gao, W.; Zhou, C.; Liu, F.; Lin, X. Microstructure and properties of laser cladding FeCrBSi composite powder coatings with higher Cr content. J. Mater. Process. Technol. 2014, 214, 899–905. [Google Scholar] [CrossRef]
- Verwimp, J.; Rombouts, M.; Geerinckx, E.; Motmans, F. Applications of laser cladded WC-based wear resistant coatings. Phys. Procedia 2011, 12, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.R. Surface Engineering for Corrosion and Wear Resistance, 1st ed.; ASM International: Geauga County, OH, USA, 2001. [Google Scholar]
- Bartkowska, A.; Pertek, A. Laser production of B–Ni complex layers. Surf. Coat. Technol. 2014, 248, 23–29. [Google Scholar] [CrossRef]
- Bartkowska, A.; Jurči, P.; Hudáková, M.; Bartkowski, D.; Kusý, M.; Przestacki, D. The influence of the laser beam fluence on change in microstructure, microhardness and phase composition of FeB–Fe2B surface layers produced on Vanadis-6 steel. Arch. Metall. Mater. 2018, 63, 791–800. [Google Scholar]
- Safonov, A.N. Special features of boronizing iron and steel using a continuous-wave CO2 laser. Metal. Sci. Heat Treat. 1998, 40, 6–10. [Google Scholar] [CrossRef]
- Safonov, A.N. Microstructure of nickel-chromium-boron-silicon alloys deposited by laser radiation. J. Weld. Int. 1992, 4, 304–307. [Google Scholar] [CrossRef]
- Manna, I.; Dutta Majumdar, J.; Ramesh Chandra, B.; Nayak, S.; Dahotre, N.B. Laser surface cladding of Fe–B–C, Fe–B–Si and Fe–BC–Si–Al–C on plain carbon steel. Surf. Coat. Technol. 2006, 201, 434–440. [Google Scholar] [CrossRef]
- Riveiro, A.; Mejías, A.; Lusquiños, F.; del Val, J.; Coomesaña, R.; Pardo, J.; Pou, J. Laser cladding of aluminium on AISI 304 stainless steel with high-power diode lasers. Surf. Coat. Technol. 2014, 253, 214–220. [Google Scholar] [CrossRef]
- Bartkowski, D.; Kinal, G. Microstructure and wear resistance of Stellite-6/WC MMC coatings produced by laser cladding using Yb:YAG disk laser. Int. J. Refract. Met. Hard Mater. 2016, 58, 157–164. [Google Scholar] [CrossRef]
- Bartkowski, D.; Bartkowska, A.; Popławski, M.; Przestacki, D. Microstructure, microhardness, corrosion and wear resistance of B, Si and B–Si coatings produced on C45 steel using laser processing. Metals 2020, 10, 792. [Google Scholar] [CrossRef]
- PN-EN ISO 17475: 2010. Polish Standardization Committee Website. Available online: https://sklep.pkn.pl/pn-en-iso-17475-2010p.html (accessed on 20 August 2020).









| C | Mn | Si | P | S | Cr | Ni | Mo | Cu |
|---|---|---|---|---|---|---|---|---|
| 0.46 | 0.71 | 0.21 | 0.011 | 0.009 | 0.07 | 0.08 | 0.02 | 0.24 |
| Specimen Number | Type of Coating | Laser Beam Power (W) | Laser Beam Scanning Speed (mm/min) | Laser Beam Power Density (W/mm2) |
|---|---|---|---|---|
| #1 | Fe–B | 600 | 600 | 298.6 |
| #2 | Fe–B | 800 | 600 | 398.0 |
| #3 | Fe–B–B4C–Si | 600 | 600 | 298.6 |
| #4 | Fe–B–B4C–Si | 800 | 600 | 398.0 |
| #5 | Fe–B–B4C–Si | 600 | 800 | 298.6 |
| #6 | Fe–B–B4C–Si | 600 | 1000 | 298.6 |
| Coating | Place of Measurement | Fe [±0.1 wt.%] | B [±0.1 wt.%] | Si [±0.1 wt.%] | C [±0.1 wt.%] |
|---|---|---|---|---|---|
| FeB 600 W 600 mm/min (#1) | 1 | 94.8 | 0.6 | - | 4.6 |
| 2 | 95.1 | 0.5 | - | 4.4 | |
| 3 | 95.3 | 0.3 | - | 4.4 | |
| 4 | 94.9 | 0.4 | - | 4.7 | |
| 5 | 94.1 | 1.1 | - | 4.8 | |
| 6 | 96.4 | 0.0 | - | 3.6 | |
| 7 | 96.0 | 0.0 | - | 4.0 | |
| 8 | 97.2 | 0.5 | - | 2.2 | |
| FeB 800 W 600 mm/min (#2) | 9 | 95.6 | 0.1 | - | 4.4 |
| 10 | 95.3 | 0.3 | - | 4.5 | |
| 11 | 95.1 | 0.5 | - | 4.4 | |
| 12 | 95.4 | 0.1 | - | 4.6 | |
| 13 | 96.8 | 0.0 | - | 3.2 | |
| 14 | 96.7 | 0.0 | - | 3.3 | |
| 15 | 96.2 | 0.0 | - | 3.8 | |
| 16 | 95.5 | 0.0 | - | 4.5 | |
| Fe–B–B4C–Si 600 W 600 mm/min (#3) | 17 | 90.1 | 4.2 | 0.5 | 5.3 |
| 18 | 90.1 | 4.3 | 0.5 | 5.1 | |
| 19 | 92.4 | 1.9 | 0.5 | 5.2 | |
| 20 | 92.5 | 1.7 | 0.4 | 5.5 | |
| 21 | 92.8 | 0.7 | 1.4 | 5.1 | |
| 22 | 93.7 | 0.0 | 1.2 | 5.0 | |
| 23 | 89.9 | 4.9 | 0.4 | 4.9 | |
| 24 | 89.7 | 4.8 | 0.6 | 4.9 | |
| Fe–B–B4C–Si 800 W 600 mm/min (#4) | 25 | 92.1 | 2.8 | 0.5 | 4.6 |
| 26 | 91.9 | 3.0 | 0.5 | 4.6 | |
| 27 | 93.2 | 1.6 | 0.5 | 4.7 | |
| 28 | 91.2 | 2.9 | 0.6 | 5.3 | |
| 29 | 94.2 | 0.0 | 0.9 | 4.9 | |
| 30 | 88.7 | 5.5 | 0.3 | 5.5 | |
| 31 | 91.1 | 0.2 | 0.7 | 8.0 | |
| 32 | 89.2 | 4.9 | 0.8 | 5.1 | |
| Fe–B–B4C–Si 600 W 800 mm/min (#5) | 33 | 92.9 | 1.8 | 0.4 | 5.0 |
| 34 | 89.5 | 5.1 | 0.4 | 5.0 | |
| 35 | 90.0 | 4.7 | 0.4 | 4.8 | |
| 36 | 90.6 | 4.3 | 0.4 | 4.8 | |
| 37 | 90.2 | 4.7 | 0.4 | 4.7 | |
| 38 | 95.6 | 0.1 | 1.1 | 3.2 | |
| 39 | 95.5 | 0.1 | 1.2 | 3.2 | |
| 40 | 90.1 | 4.8 | 0.4 | 4.6 | |
| Fe–B–B4C–Si 600 W 1000 mm/min (#6) | 41 | 93.7 | 1.0 | 0.3 | 5.0 |
| 42 | 89.6 | 4.8 | 0.3 | 5.3 | |
| 43 | 88.4 | 5.4 | 0.3 | 5.8 | |
| 44 | 90.0 | 4.8 | 0.3 | 5.0 | |
| 45 | 88.7 | 5.2 | 0.3 | 5.8 | |
| 46 | 93.0 | 0.9 | 0.7 | 5.3 | |
| 47 | 88.8 | 5.1 | 0.3 | 5.8 | |
| 48 | 93.5 | 0.5 | 0.6 | 5.4 |
| Coating Type | Current Icorr (A·cm2) | Potential Ecorr (V) |
|---|---|---|
| Fe–B (600 W, 600 mm/min) (#1) | 8.47 × 10−6 | −1.04 × 100 |
| Fe–B–B4C–Si (600 W, 600 mm/min) (#3) | 7.56 × 10−6 | −1.03 × 100 |
| Fe–B–B4C–Si (600 W, 800 mm/min) (#5) | 8.84 × 10−7 | −9.39 × 10-1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkowski, D.; Bartkowska, A.; Piasecki, A.; Jurči, P. Influence of Laser Cladding Parameters on Microstructure, Microhardness, Chemical Composition, Wear and Corrosion Resistance of Fe–B Composite Coatings Reinforced with B4C and Si Particles. Coatings 2020, 10, 809. https://doi.org/10.3390/coatings10090809
Bartkowski D, Bartkowska A, Piasecki A, Jurči P. Influence of Laser Cladding Parameters on Microstructure, Microhardness, Chemical Composition, Wear and Corrosion Resistance of Fe–B Composite Coatings Reinforced with B4C and Si Particles. Coatings. 2020; 10(9):809. https://doi.org/10.3390/coatings10090809
Chicago/Turabian StyleBartkowski, Dariusz, Aneta Bartkowska, Adam Piasecki, and Peter Jurči. 2020. "Influence of Laser Cladding Parameters on Microstructure, Microhardness, Chemical Composition, Wear and Corrosion Resistance of Fe–B Composite Coatings Reinforced with B4C and Si Particles" Coatings 10, no. 9: 809. https://doi.org/10.3390/coatings10090809





