Improving the Adsorption Performance of Loofah Sponge towards Methylene Blue by Coating Ca2+ Crosslinked Sodium Alginate Layers on Its Fiber Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LS@SA Adsorbents
2.3. Characterization
2.4. MB Sorption Studies
2.5. Evaluation of Reusability and Degradation of [email protected]
3. Results
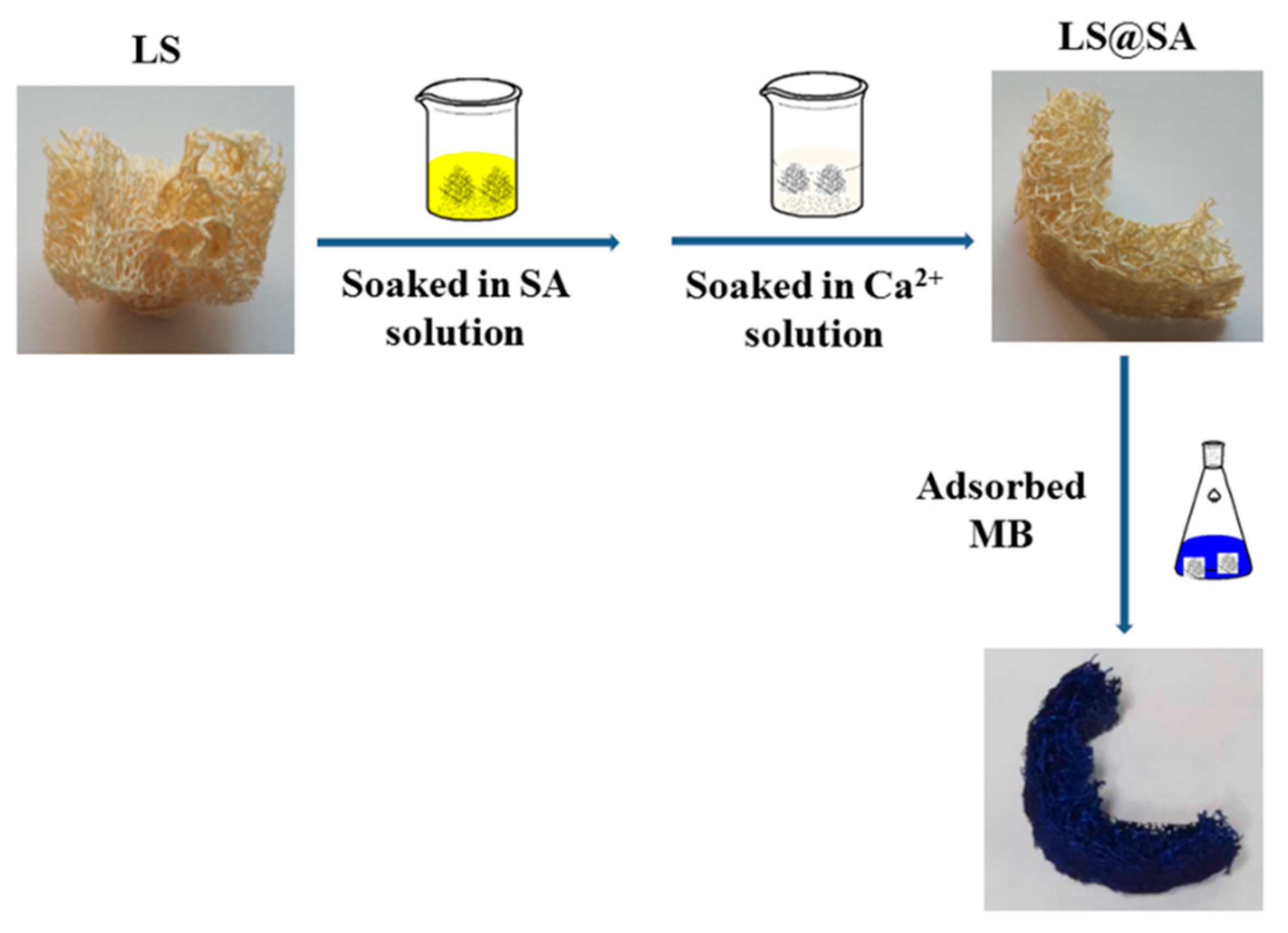
3.1. Preparation Process of LS@SA
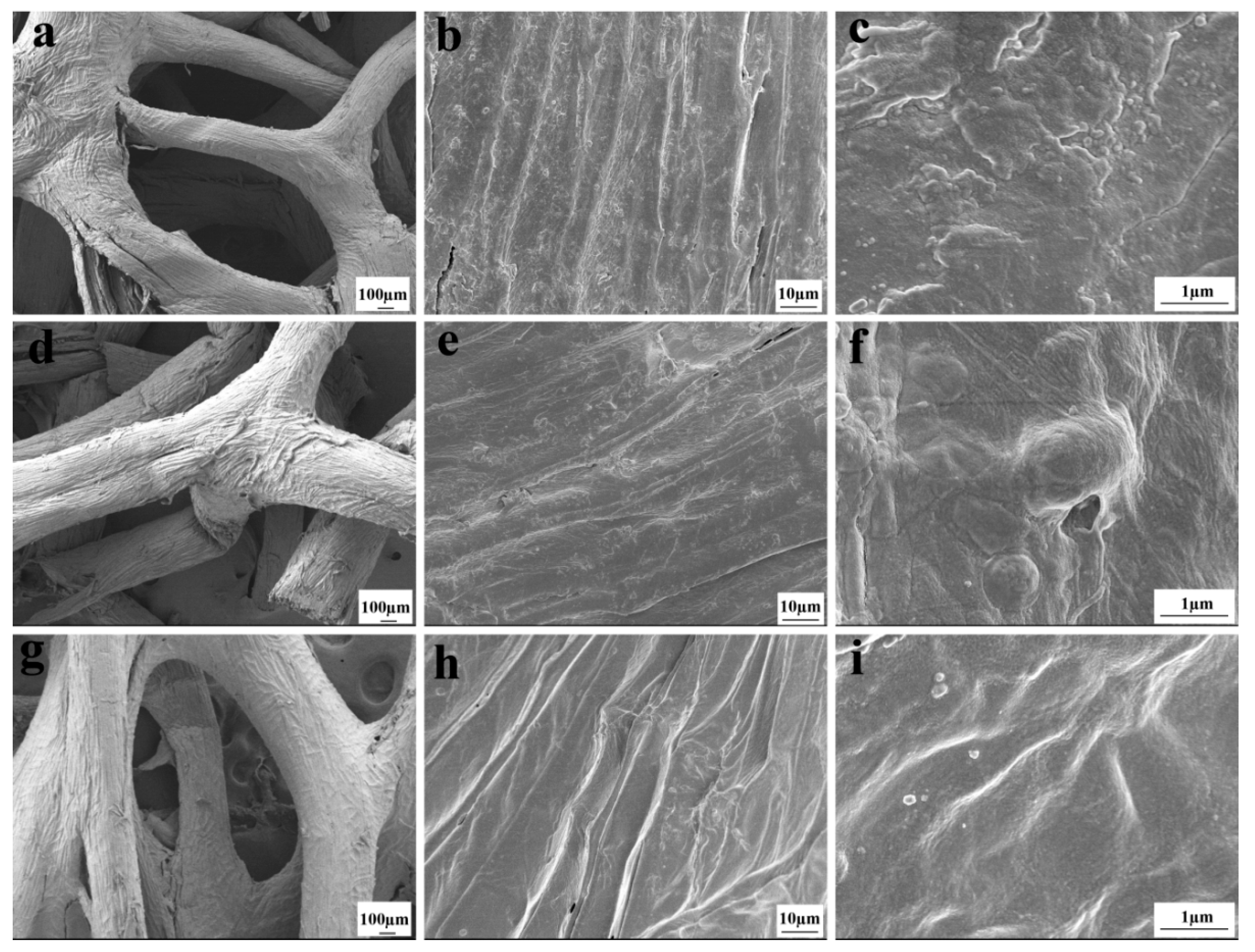
3.2. SEM Analysis
3.3. SA Loading Rate on LS
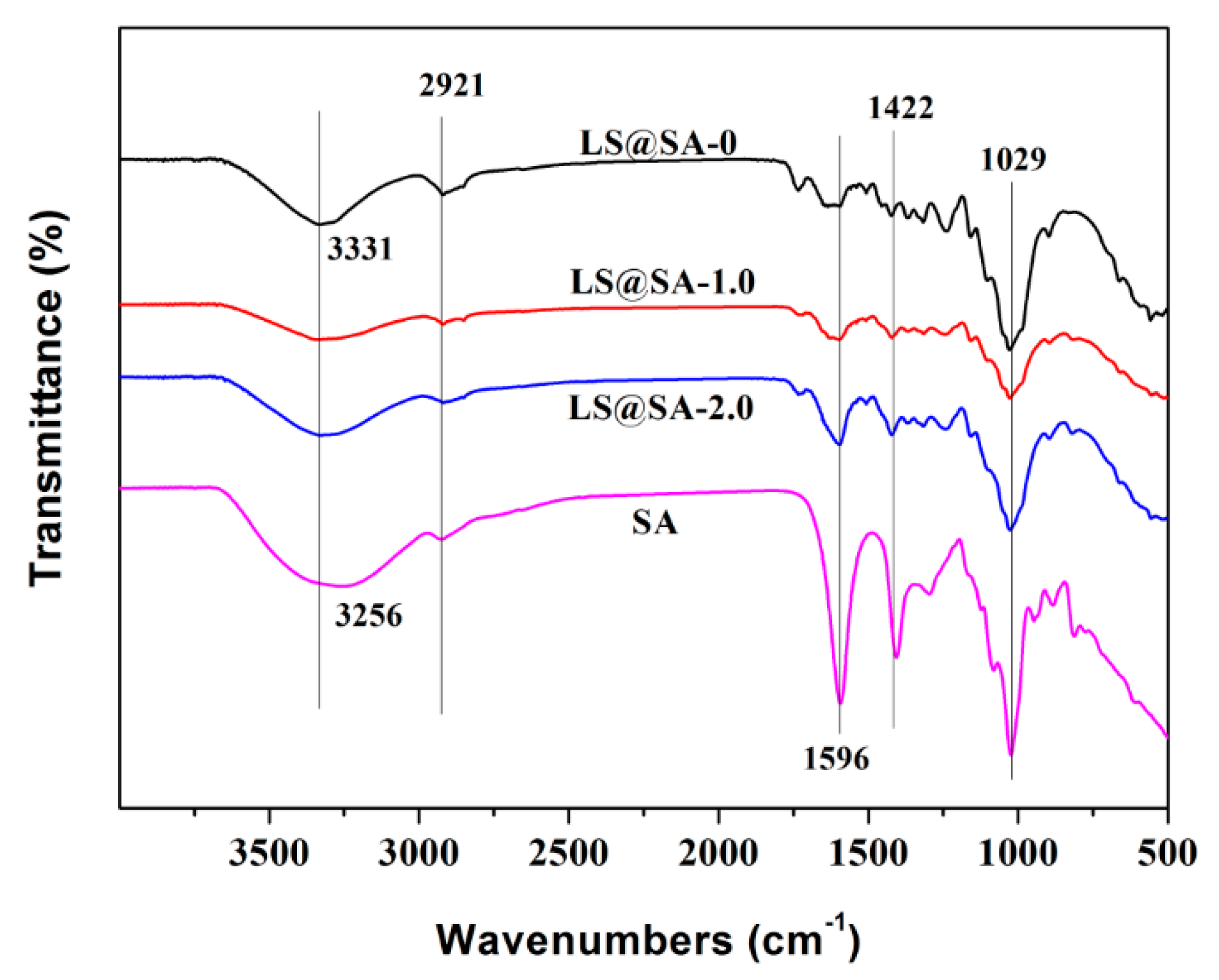
3.4. FTIR Analysis
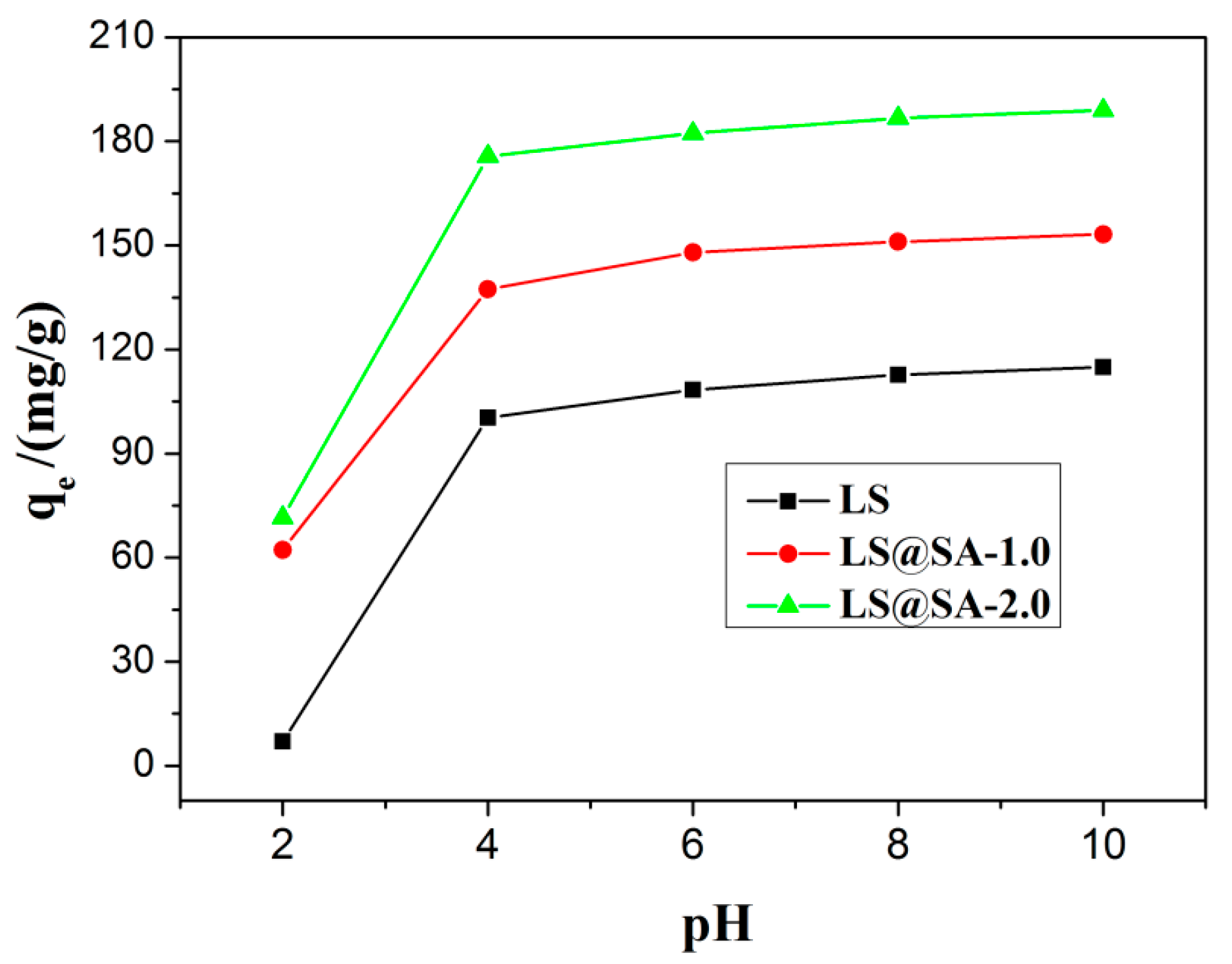
3.5. Effect of pH on MB Adsorption
3.6. Adsorption Kinetics
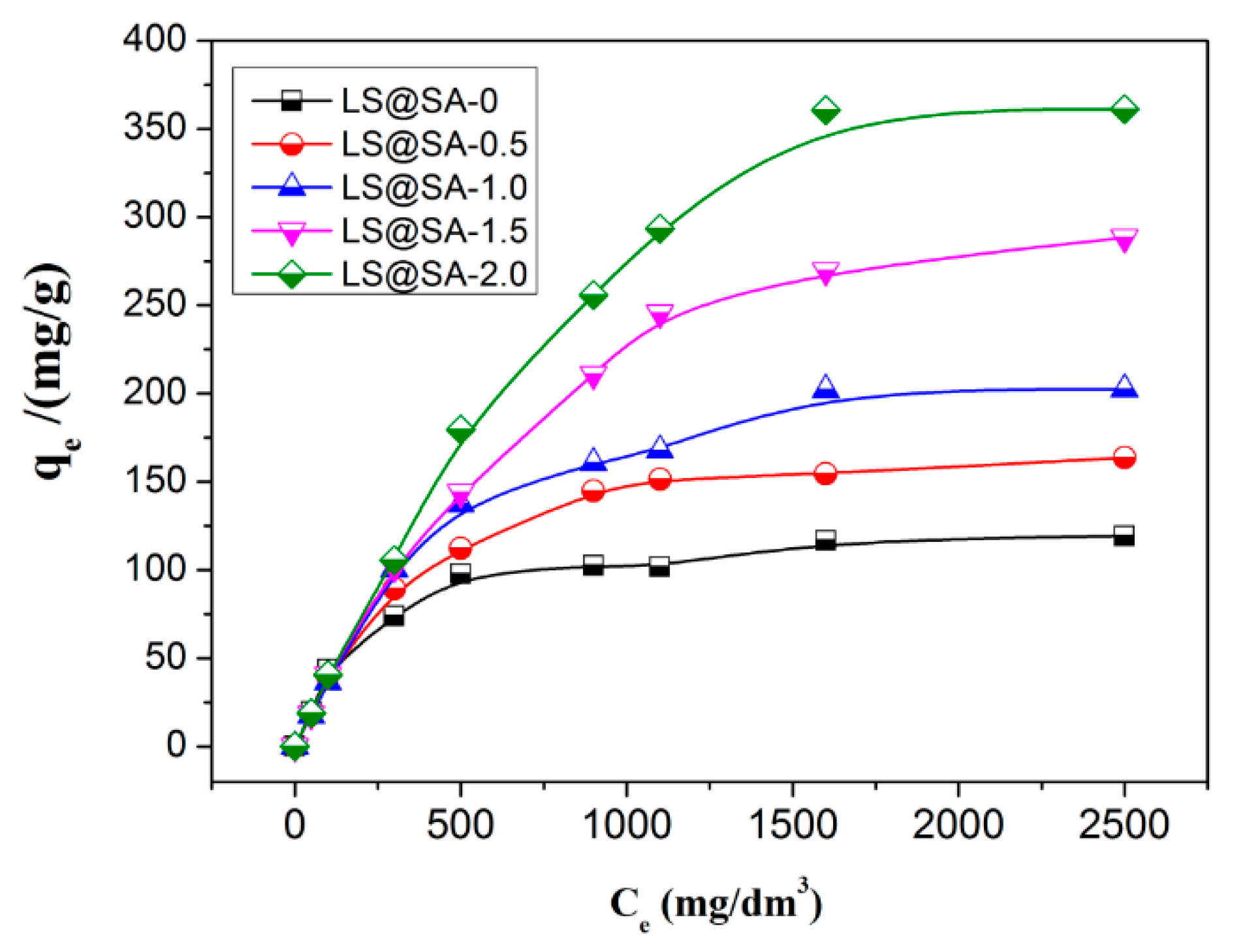
3.7. Adsorption Isotherm
3.8. Recycling of LS@SA adsorbent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 9, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Li, Q.; Luo, C.; Cheng, J.; Hou, X. Characterization of flake boron nitride prepared from the low temperature combustion synthesized precursor and its application for dye adsorption. Coatings 2018, 8, 214. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Krishnakumar, B.; Sobral, A.; Koh, J. Bio-based (chitosan/PVA/ZnO) nanocomposites film: Thermally stable and photoluminescence material for removal of organic dye. Carbohydr. Polym. 2019, 205, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Wang, S.; Wang, T.; Wu, J.; Xing, X.; Zhang, X. Effect of bifunctional acid on the porosity improvement of biomass-derived activated carbon for methylene blue adsorption. Environ. Sci. Pollut. Res. Int. 2019, 26, 30119–30129. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Omer, A.M.; Ouyang, X.K. Adsorptive removal of cationic methylene blue dye using carboxymethyl cellulose/k-carrageenan/activated montmorillonite composite beads: Isotherm and kinetic studies. Int. J. Biol. Macromol. 2018, 106, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Jodeh, S.; Hamed, O.; Melhem, A.; Salghi, R.; Jodeh, D.; Azzaoui, K.; Benmassaoud, Y.; Murtada, K. Magnetic nanocellulose from olive industry solid waste for the effective removal of methylene blue from wastewater. Environ. Sci. Pollut. Res. Int. 2018, 25, 22060–22074. [Google Scholar] [CrossRef] [PubMed]
- Makhado, E.; Pandey, S.; Nomngongo, P.N.; Ramontja, J. Preparation and characterization of xanthan gum-cl-poly(acrylic acid)/o-MWCNTs hydrogel nanocomposite as highly effective re-usable adsorbent for removal of methylene blue from aqueous solutions. J. Colloid Interface Sci. 2018, 513, 700–714. [Google Scholar] [CrossRef]
- Chen, B.; Cao, Y.; Zhao, H.; Long, F.; Feng, X.; Li, J.; Pan, X. A novel Fe(3+)-stabilized magnetic polydopamine composite for enhanced selective adsorption and separation of Methylene blue from complex wastewater. J. Hazard. Mater. 2020, 392, 122263. [Google Scholar] [CrossRef]
- Nasrullah, A.; Bhat, A.H.; Naeem, A.; Isa, M.H.; Danish, M. High surface area mesoporous activated carbon-alginate beads for efficient removal of methylene blue. Int. J. Biol. Macromol. 2018, 107, 1792–1799. [Google Scholar] [CrossRef]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.W.; Ok, Y.S.; Hou, D. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhan, C.; Wu, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.S. Highly efficient removal of methylene blue dye from an aqueous solution using cellulose acetate nanofibrous membranes modified by polydopamine. ACS Omega 2020, 5, 5389–5400. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Basniwal, R.K.; Suman; Srivastava, A.K.; Jain, V.K. Reusable nanomaterial and plant biomass composites for the removal of Methylene Blue from water. Environ. Technol. 2010, 31, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Sarat Chandra, T.; Mudliar, S.N.; Vidyashankar, S.; Mukherji, S.; Sarada, R.; Krishnamurthi, K.; Chauhan, V.S. Defatted algal biomass as a non-conventional low-cost adsorbent: Surface characterization and methylene blue adsorption characteristics. Bioresour. Technol. 2015, 184, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Comparative study for adsorption of methylene blue dye on biochar derived from orange peel and banana biomass in aqueous solutions. Environ. Monit. Assess. 2019, 191, 735. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, D.; Hu, J.; Shen, F.; Long, L.; Zhang, Y.; Yang, G.; Zeng, Y.; Zhang, J.; He, J.; et al. Functionalizing bottom ash from biomass power plant for removing methylene blue from aqueous solution. Sci. Total. Environ. 2018, 634, 760–768. [Google Scholar] [CrossRef]
- Bediako, J.K.; Sarkar, A.K.; Lin, S.; Zhao, Y.; Song, M.H.; Choi, J.W.; Cho, C.W.; Yun, Y.S. Characterization of the residual biochemical components of sequentially extracted banana peel biomasses and their environmental remediation applications. Waste. Manag. 2019, 89, 141–153. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Tian, Q.; Liang, X.; Zhu, Y.; Sand, W.; Li, F.; Ma, C.; Liu, Y.; Yang, B. Durability and performance of loofah sponge as carrier for wastewater treatment with high ammonium. Water Environ. Res. 2019, 91, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Saudagar, P.S.; Shaligram, N.S.; Singhal, R.S. Immobilization of Streptomyces clavuligerus on loofah sponge for the production of clavulanic acid. Bioresour. Technol. 2008, 99, 2250–2253. [Google Scholar] [CrossRef] [PubMed]
- Dzionek, A.; Wojcieszynska, D.; Adamczyk-Habrajska, M.; Guzik, U. Enhanced degradation of naproxen by immobilization of bacillus thuringiensis B1(2015b) on loofah sponge. Molecules 2020, 25, 872. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Tong, C.J.; Rehman, S.; Liu, L.M.; Hou, Y.; Zhang, S. Multifunctional nitrogen-doped loofah sponge carbon blocking layer for high-performance rechargeable lithium batteries. ACS Appl. Mater. Interfaces 2016, 8, 15991–16001. [Google Scholar] [CrossRef]
- Tuncel, U.; Turan, A.; Markoc, F.; Erkorkmaz, U.; Elmas, C.; Kostakoglu, N. Loofah sponge as an interface dressing material in negative pressure wound therapy: Results of an in vivo study. Ostomy Wound Manag. 2014, 60, 37–45. [Google Scholar] [PubMed]
- Tang, X.; Zhang, Q.; Liu, Z.; Pan, K.; Dong, Y.; Li, Y. Removal of Cu (II) by loofah fibers as a natural and low-cost adsorbent from aqueous solutions. J. Mol. Liq. 2014, 199, 401–407. [Google Scholar] [CrossRef]
- Demir, H.; Top, A.; Balkose, D.; Ulku, S. Dye adsorption behavior of Luffa cylindrica fibers. J. Hazard. Mater. 2008, 153, 389–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Yan, C.; Luo, W.; Li, X.; Ge, W.; Zhou, S. Simple preparation and enhanced adsorption properties of loofah fiber adsorbent by ultraviolet radiation graft. Mater. Lett. 2015, 157, 303–306. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J. Functionalization of loofah fibers via surface-initiated AGET ATRP for synergic adsorption of multiple pollutants from water. Mater. Lett. 2018, 210, 214–217. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, M.; Zhao, J.; Chen, J.; Zeng, G.; Huang, H.; Tian, J.; Wen, Y.; Zhang, X.; Wei, Y. Facile preparation of polyethylenimine-tannins coated SiO2 hybrid materials for Cu2+ removal. Appl. Surf. Sci. 2018, 427, 535–544. [Google Scholar] [CrossRef]
- Jawad, A.H.; Azharul Islam, M.; Hameed, B.H. Cross-linked chitosan thin film coated onto glass plate as an effective adsorbent for adsorption of reactive orange 16. Int. J. Biol. Macromol. 2017, 95, 743–749. [Google Scholar] [CrossRef]
- Boukhalfa, N.; Boutahala, M.; Djebri, N.; Idris, A. Kinetics, thermodynamics, equilibrium isotherms, and reusability studies of cationic dye adsorption by magnetic alginate/oxidized multiwalled carbon nanotubes composites. Int. J. Biol. Macromol. 2019, 123, 539–548. [Google Scholar] [CrossRef]
- Ohemeng-Boahen, G.; Sewu, D.D.; Woo, S.H. Preparation and characterization of alginate-kelp biochar composite hydrogel bead for dye removal. Environ. Sci. Pollut. Res. Int. 2019, 26, 33030–33042. [Google Scholar] [CrossRef]
- Vecino, X.; Devesa-Rey, R.; Cruz, J.M.; Moldes, A.B. Study of the physical properties of calcium alginate hydrogel beads containing vineyard pruning waste for dye removal. Carbohydr. Polym. 2015, 115, 129–138. [Google Scholar] [CrossRef]
- Asadi, S.; Eris, S.; Azizian, S. Alginate-based hydrogel beads as a biocompatible and efficient adsorbent for dye removal from aqueous solutions. ACS Omega 2018, 3, 15140–15148. [Google Scholar] [CrossRef] [PubMed]
- Pettignano, A.; Tanchoux, N.; Cacciaguerra, T.; Vincent, T.; Bernardi, L.; Guibal, E.; Quignard, F. Sodium and acidic alginate foams with hierarchical porosity: Preparation, characterization and efficiency as a dye adsorbent. Carbohydr. Polym. 2017, 178, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gao, B.; Wan, Y. Entrapment of ball-milled biochar in Ca-alginate beads for the removal of aqueous Cd (II). J. Ind. Eng. Chem. 2018, 61, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Wu, S.; Dong, L.; Wang, Q.; Liu, Q. Microfluidic synthesis of Ca-alginate microcapsules for self-healing of bituminous binder. Materials 2018, 11, 630. [Google Scholar] [CrossRef] [Green Version]
- Huq, T.; Salmieri, S.; Khan, A.; Khan, R.A.; Le Tien, C.; Riedl, B.; Fraschini, C.; Bouchard, J.; Uribe-Calderon, J.; Kamal, M.R.; et al. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Phisalaphong, M.; Suwanmajo, T.; Tammarate, P. Synthesis and characterization of bacterial cellulose/alginate blend membranes. J. Appl. Polym. Sci. 2008, 107, 3419–3424. [Google Scholar] [CrossRef]
- Gopalakannan, V.; Viswanathan, N. Synthesis of magnetic alginate hybrid beads for efficient chromium (VI) removal. Int. J. Biol. Macromol. 2015, 72, 862–867. [Google Scholar] [CrossRef]
- Mohammadi, A.; Daemi, H.; Barikani, M. Fast removal of malachite green dye using novel superparamagnetic sodium alginate-coated Fe3O4 nanoparticles. Int. J. Biol. Macromol. 2014, 69, 447–455. [Google Scholar] [CrossRef]
- Chiew, C.S.C.; Poh, P.E.; Pasbakhsh, P.; Tey, B.T.; Yeoh, H.K.; Chan, E.S. Physicochemical characterization of halloysite/alginate bionanocomposite hydrogel. Appl. Clay Sci. 2014, 101, 444–454. [Google Scholar] [CrossRef]
- Ren, H.; Gao, Z.; Wu, D.; Jiang, J.; Sun, Y.; Luo, C. Efficient Pb (II) removal using sodium alginate–carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydr. Polym. 2016, 137, 402–409. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Chen, S.; Huang, Y.; He, J. Adsorption of lysozyme by alginate/graphene oxide composite beads with enhanced stability and mechanical property. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, Y.; Yu, H.; Yan, L.; Du, B.; Pei, Z. Removal of Cu(2+), Cd(2+) and Pb(2+) from aqueous solutions by magnetic alginate microsphere based on Fe3O4/MgAl-layered double hydroxide. J. Colloid Interface Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Algothmi, W.M.; Bandaru, N.M.; Yu, Y.; Shapter, J.G.; Ellis, A.V. Alginate-graphene oxide hybrid gel beads: An efficient copper adsorbent material. J. Colloid Interface Sci. 2013, 397, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, Y.; Zhang, X.F.; Zhang, X.; Jiang, J.; Yao, J. Alginate-based attapulgite foams as efficient and recyclable adsorbents for the removal of heavy metals. J. Colloid Interface Sci. 2018, 514, 190–198. [Google Scholar] [CrossRef] [PubMed]

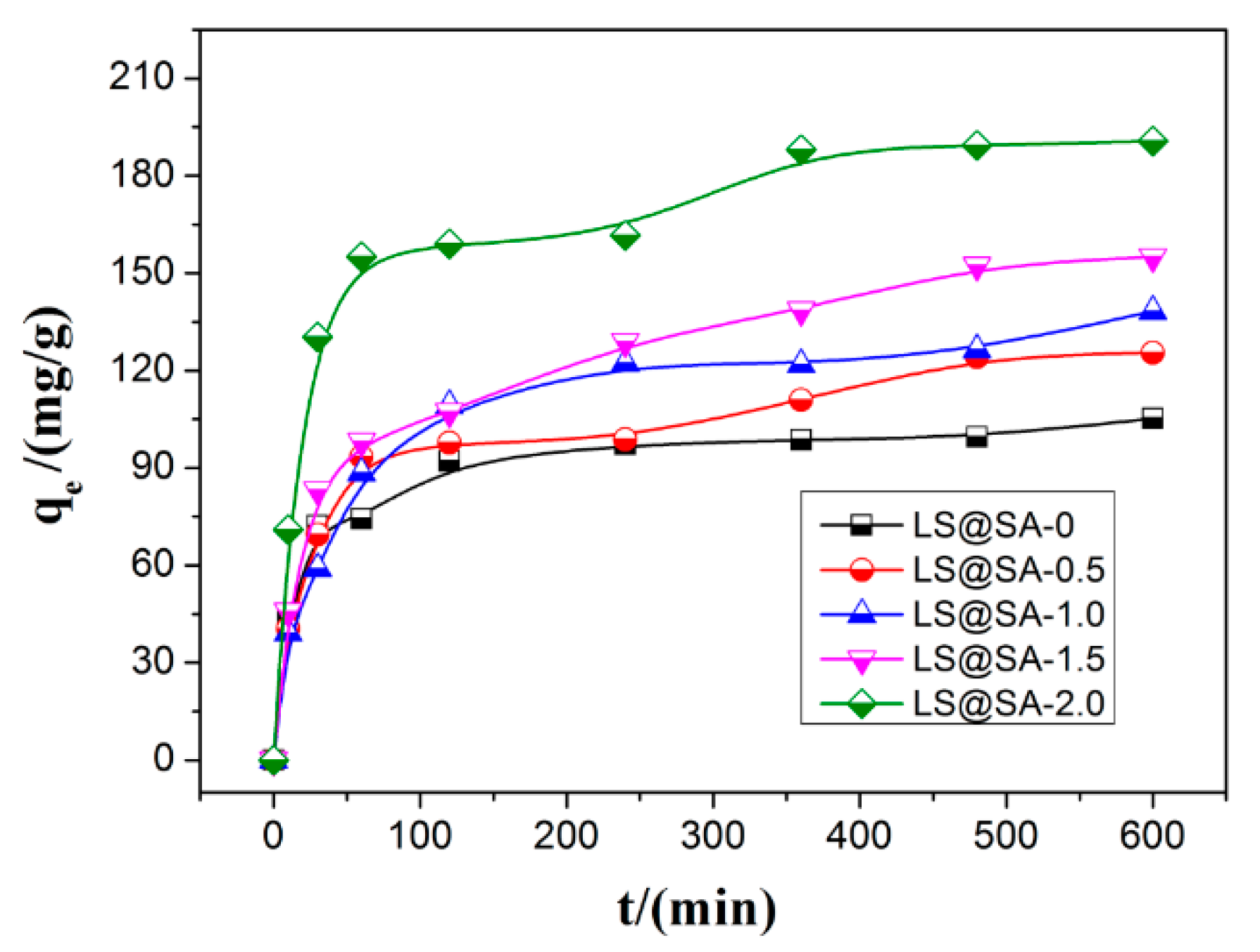

| Samples | Pseudo-First Order Model | |||
| qexp (mg/g) | q1e (mg/g) | k1 (min−1) | R2 | |
| LS@SA-0 | 105.23 | 100.81 | 12.54 | 0.9750 |
| [email protected] | 125.49 | 118.62 | 19.44 | 0.9852 |
| [email protected] | 138.27 | 126.90 | 23.57 | 0.9563 |
| [email protected] | 154.99 | 143.06 | 21.49 | 0.9814 |
| [email protected] | 190.66 | 191.20 | 16.66 | 0.9845 |
| Samples | Pseudo-Second Order Model | |||
| q2e mg/g | K2 × 104 g/(mg min) | h mg/(g min) | R2 | |
| LS@SA-0 | 106.04 | 4.81 | 5.41 | 0.9982 |
| [email protected] | 129.37 | 2.19 | 3.67 | 0.9898 |
| [email protected] | 141.04 | 1.89 | 3.76 | 0.9949 |
| [email protected] | 162.34 | 1.45 | 3.82 | 0.9935 |
| [email protected] | 196.46 | 2.23 | 8.59 | 0.9963 |
| Samples | Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|
| qe mg/g | qm mg/g | KL L/mg | R2 | KF (mg/g)(L/mg)1/n | n | R2 | |
| LS@SA-0 | 116.69 | 121.95 | 0.0123 | 0.9961 | 15.22 | 3.47 | 0.8788 |
| [email protected] | 163.66 | 169.49 | 0.0093 | 0.9992 | 9.70 | 2.48 | 0.8968 |
| [email protected] | 202.11 | 217.39 | 0.0066 | 0.9958 | 7.84 | 2.15 | 0.8848 |
| [email protected] | 288.42 | 322.58 | 0.0020 | 0.9970 | 7.00 | 1.87 | 0.9348 |
| [email protected] | 360.63 | 416.67 | 0.0048 | 0.9957 | 6.56 | 1.69 | 0.9327 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, X.; Guo, X.; Quan, Q.; Su, H.; Huang, D. Improving the Adsorption Performance of Loofah Sponge towards Methylene Blue by Coating Ca2+ Crosslinked Sodium Alginate Layers on Its Fiber Surface. Coatings 2020, 10, 814. https://doi.org/10.3390/coatings10090814
Qiang X, Guo X, Quan Q, Su H, Huang D. Improving the Adsorption Performance of Loofah Sponge towards Methylene Blue by Coating Ca2+ Crosslinked Sodium Alginate Layers on Its Fiber Surface. Coatings. 2020; 10(9):814. https://doi.org/10.3390/coatings10090814
Chicago/Turabian StyleQiang, Xiaohu, Xin Guo, Qiling Quan, Hongxi Su, and Dajian Huang. 2020. "Improving the Adsorption Performance of Loofah Sponge towards Methylene Blue by Coating Ca2+ Crosslinked Sodium Alginate Layers on Its Fiber Surface" Coatings 10, no. 9: 814. https://doi.org/10.3390/coatings10090814




