Influence of Different Electrolyte Additives and Structural Characteristics of Plasma Electrolytic Oxidation Coatings on AZ31 Magnesium Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
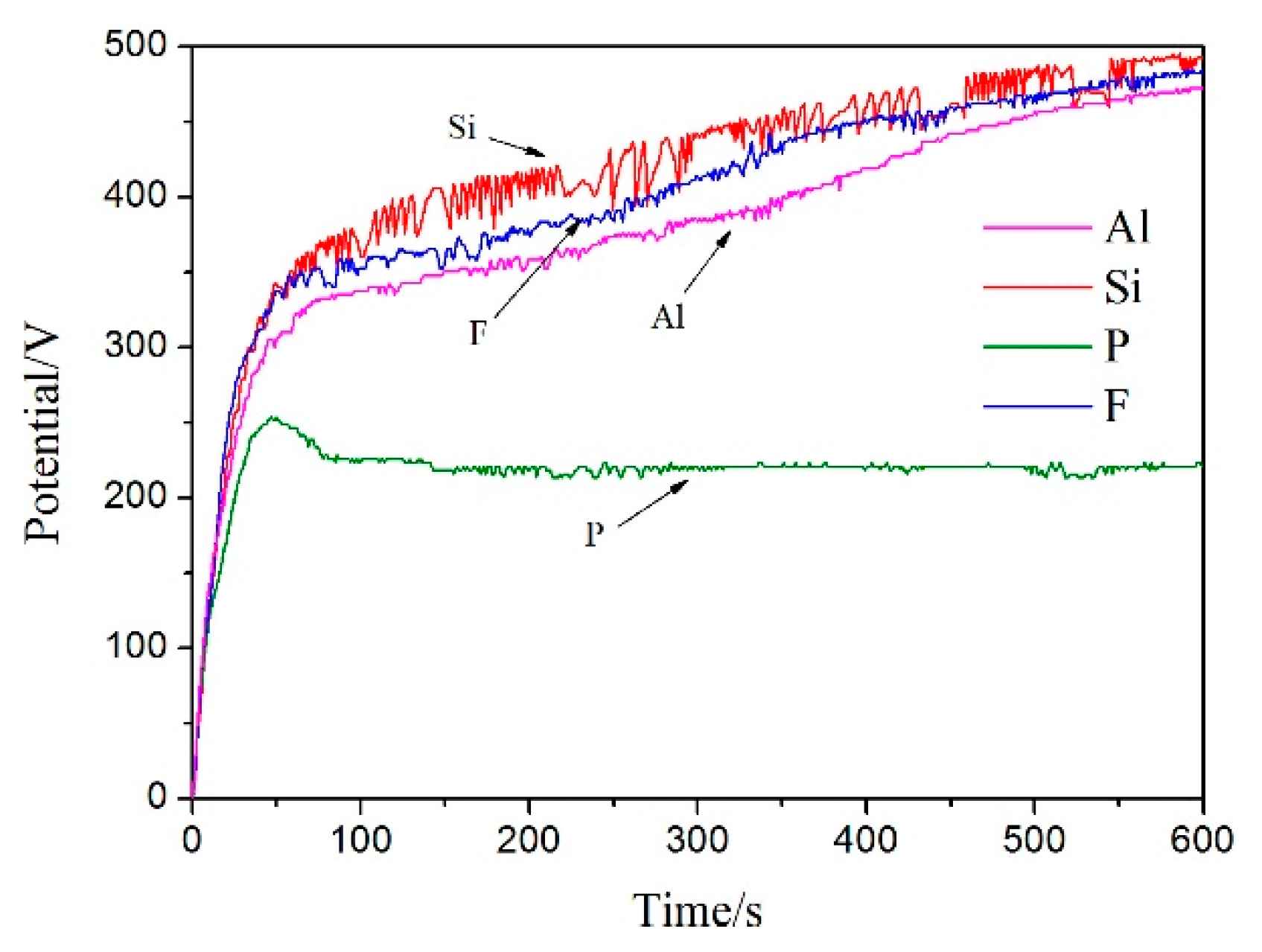
3.1. Effect of Different Electrolyte Components on PEO Voltage
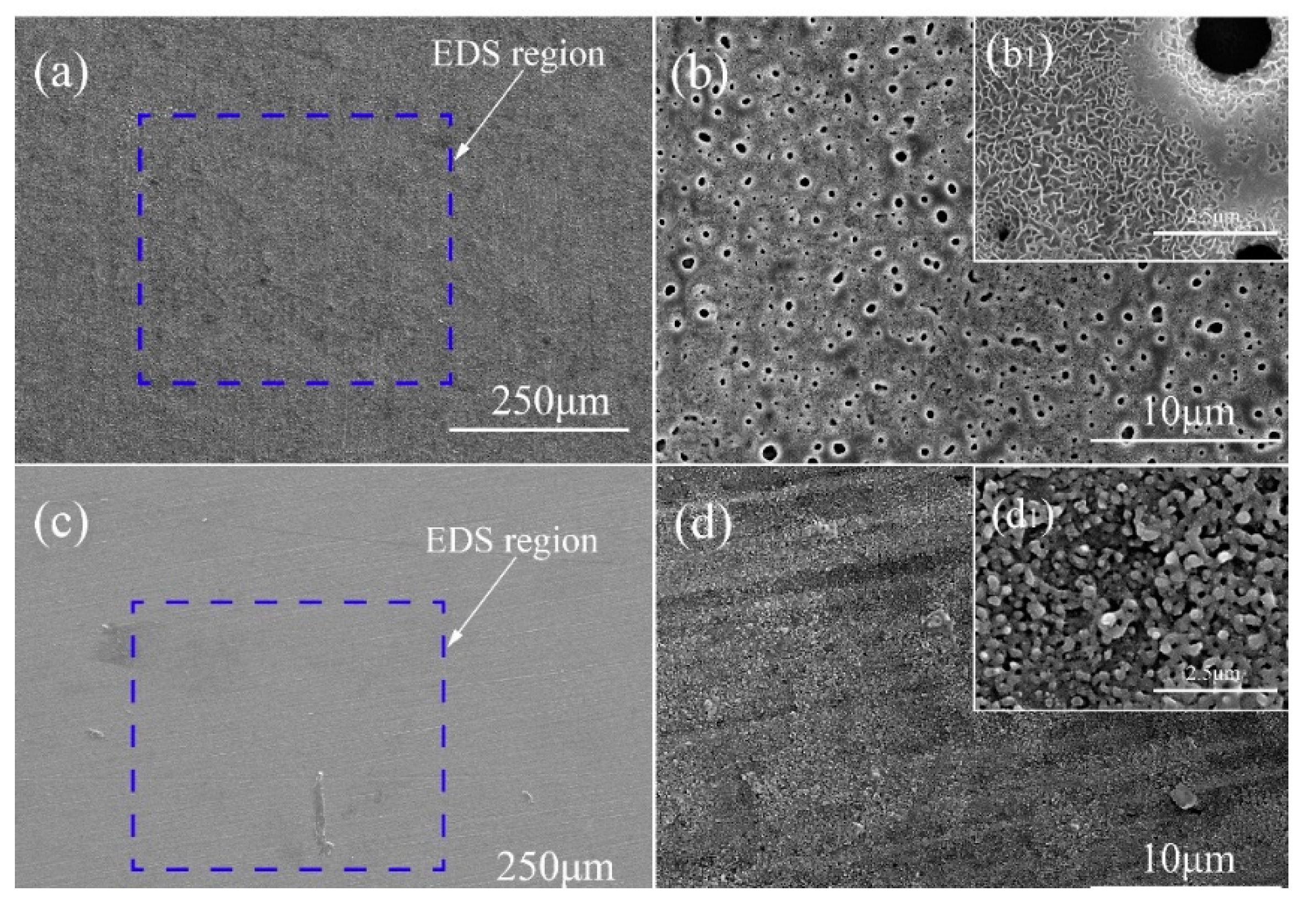
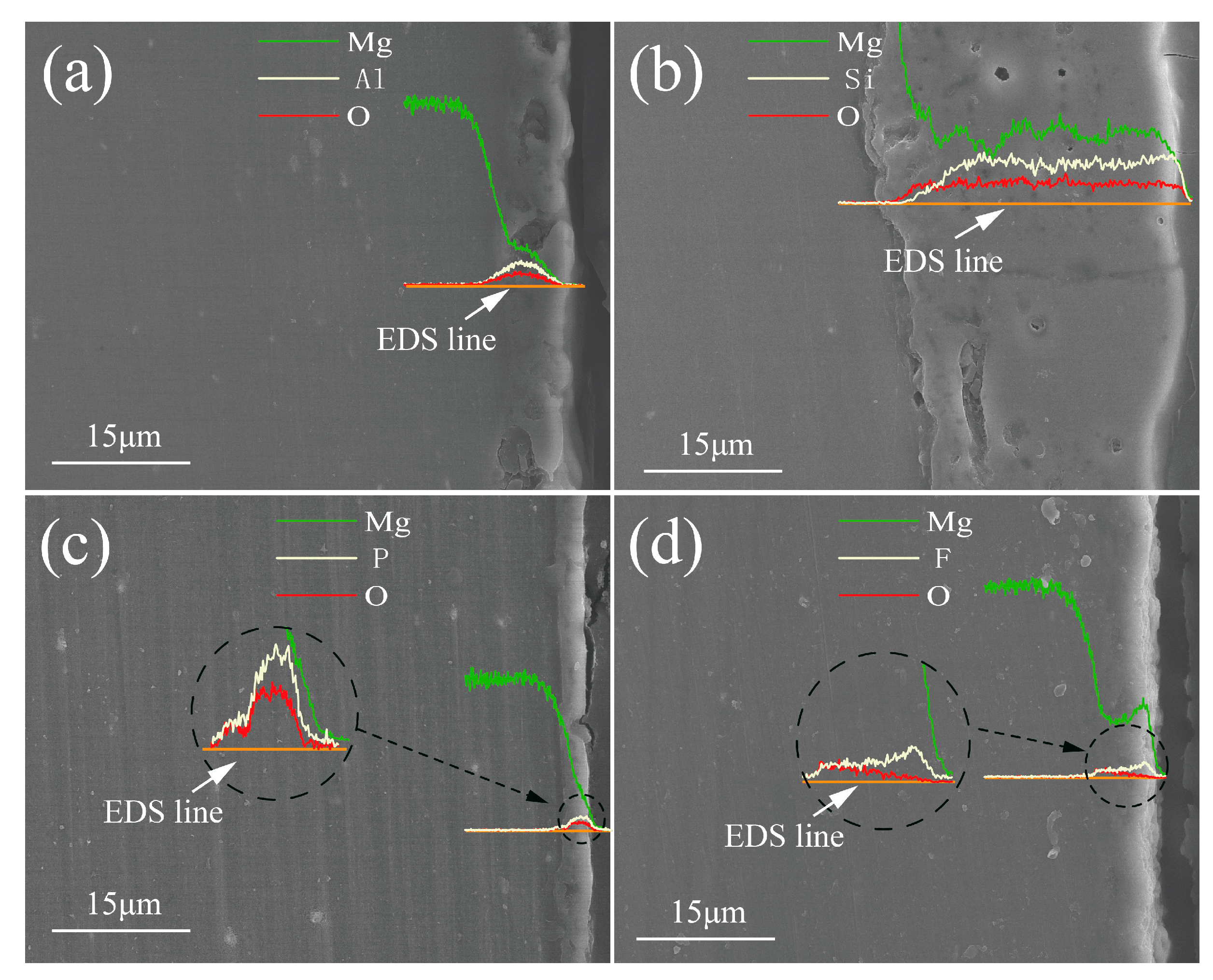
3.2. Morphologies and Elemental Composition of the PEO Coatings
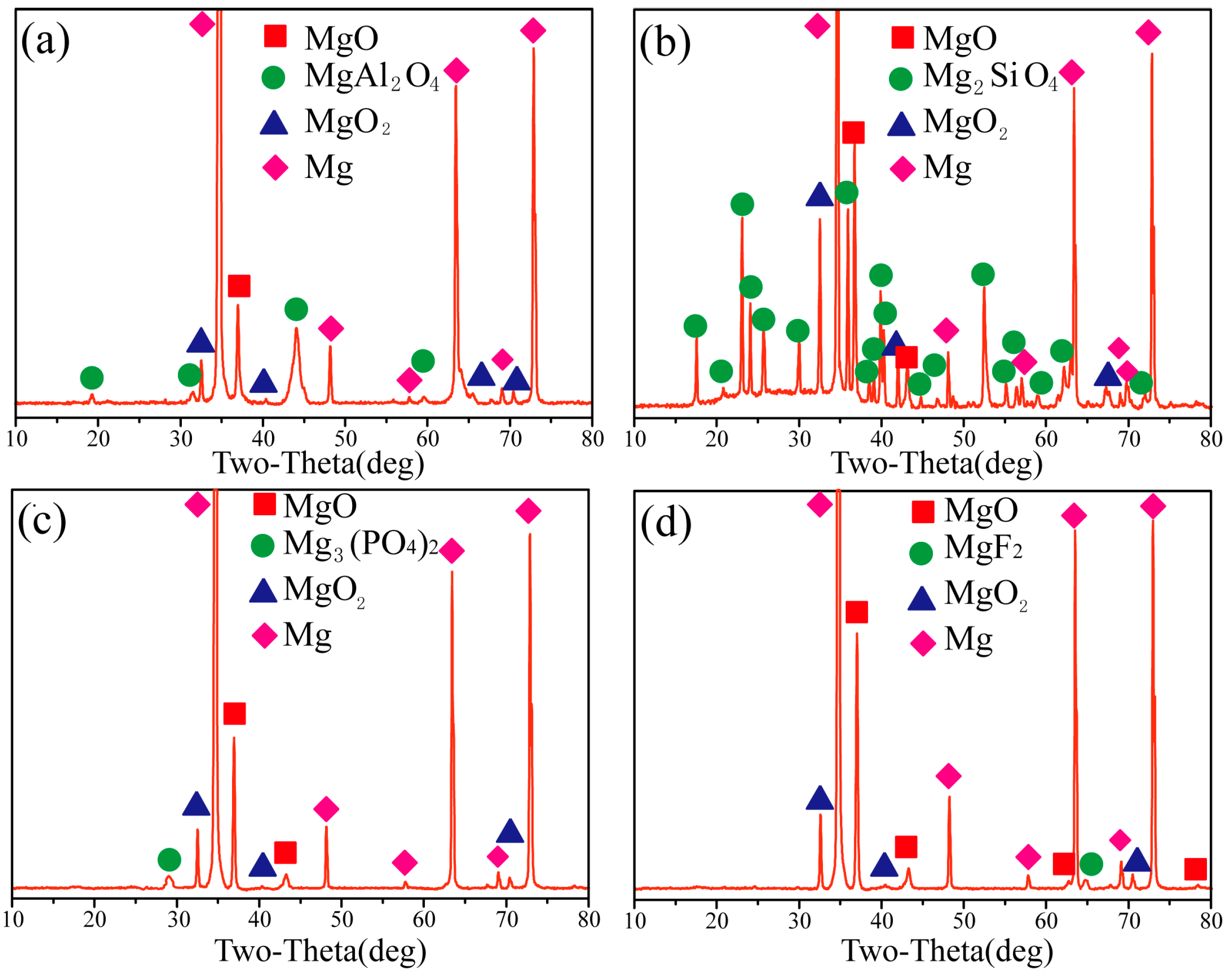
3.3. Phase Composition of the PEO Coatings
3.4. EIS Analysis of the PEO Coatings
4. Conclusions
- It can be seen that the Al-coating and Si-coating have the typical characteristics of PEO coating, such as discharge products, micropores and cracks. Filamentous and granular discharge products formed on the surface of Al-coating and Si-coating, respectively.
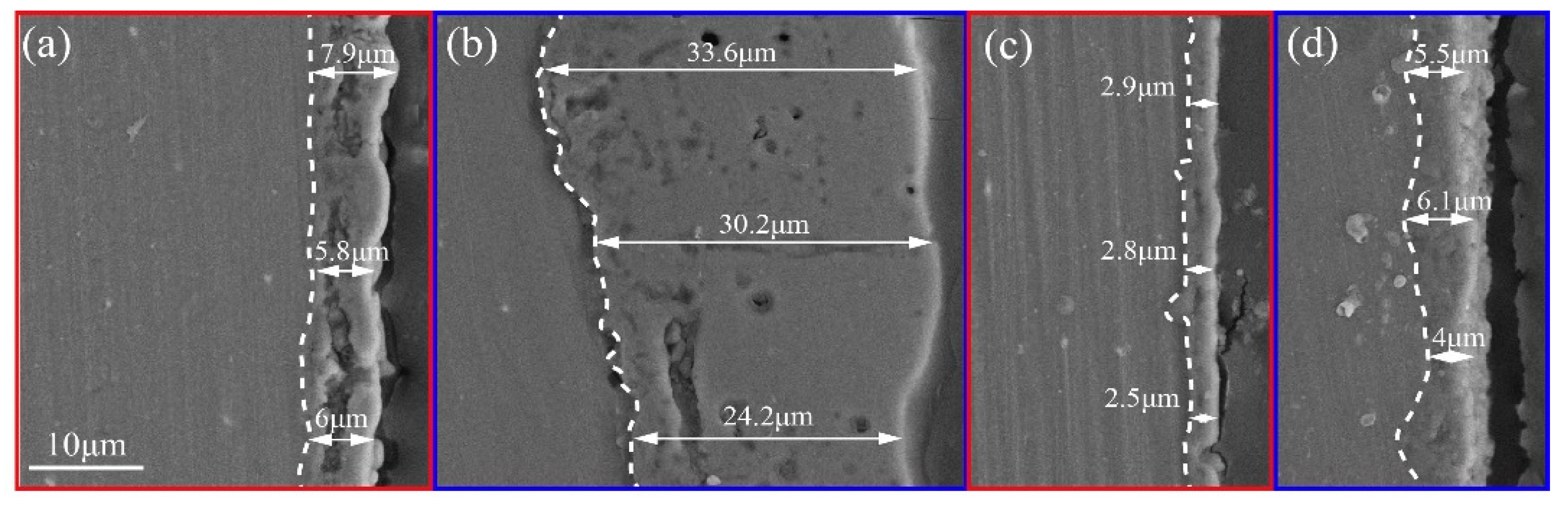
- The ceramic layer formed by AlO2− exhibits the typical double-layer structure and SiO32− is more likely to participate in the formation of the PEO ceramic layer. The P-coating and F-coating show thin thicknesses but with high density.
- The results showed that the additives directly affect the distribution of elements and the formation of phases. However, MgO and MgO2 constitute the common phases in the coatings; particularly, the phases MgAl2O4, Mg2SiO4, Mg3(PO4)2 and MgF2, resulted from the Al-coating, Si-coating, P-coating and F-coating, respectively. In addition, Mg2SiO4 and MgF2 are mainly distributed in the outer layer, while MgAl2O4 and Mg3(PO4)2 are mainly distributed in the inner layer of the coatings.
- Although F-coating has a lesser coating thickness compared to Al-coating and Si-coating, the higher resistance of the inner layer is favorable for improving the corrosion resistance. Moreover, the results of the outer layer and charge–transfer resistance indicated that the corrosion resistance of the P-coating is similarly outstanding. On the other hand, Si-coating and Al-coating, which have higher coating thickness, showed lower corrosion resistance than F-coating and P-coating, which can be attributed to their more severe discharge cracks and holes.
Author Contributions
Funding
Conflicts of Interest
References
- Mordike, B.; Ebert, T. Magnesium: Properties-applications-potential. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Calado, L.M.; Taryba, M.G.; Morozov, Y.; Carmezim, M.J.; Fátima Montemor, M. Novel smart and self-healing cerium phosphate-based corrosion inhibitor for AZ31 magnesium alloy. Corros. Sci. 2020, 170, 108648. [Google Scholar] [CrossRef]
- Chaharmahali, R.; Fattah-Alhosseini, A.; Esfahani, H. Increasing the in-vitro corrosion resistance of AZ31B-Mg alloy via coating with hydroxyapatite using plasma electrolytic oxidation. Asian Ceram. Soc. 2020, 8, 39–49. [Google Scholar] [CrossRef]
- Song, G.-L.; Xu, Z. Effect of microstructure evolution on corrosion of different crystal surfaces of AZ31 Mg alloy in a chloride containing solution. Corros. Sci. 2012, 54, 97–105. [Google Scholar] [CrossRef]
- Song, G.-L.; Xu, Z. The surface, microstructure and corrosion of magnesium alloy AZ31 sheet. Electrochim. Acta 2010, 55, 4148–4461. [Google Scholar] [CrossRef]
- Song, G.-L. Effect of tin modification on corrosion of AM70 magnesium alloy. Electrochim. Acta 2009, 51, 2063–2070. [Google Scholar] [CrossRef]
- Liu, L.J.; Schlesinger, M. Corrosion of magnesium and its alloys. Corros. Sci. 2009, 51, 1733–1737. [Google Scholar] [CrossRef]
- Asada, H.; Komori, S.; Aoki, K.; Aoyama, K. Study on the high purity and Iron-Free magnesium and its alloys. J. Jpn. Inst. Light Met. 1958, 8, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Inoue, M.; Iwai, M.; Matuzawa, K.; Kamado, S.; Kojima, Y. Effect of impurities on corrosion behavior of pure magnesium in salt water environment. J. Jpn. Inst. Light Met. 1998, 48, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Nam, N.D.; Bian, M.Z.; Forsyth, M.; Seter, M. Effect of calcium oxide on the corrosion behaviour of AZ91 magnesium alloy. Corros. Sci. 2012, 64, 263–271. [Google Scholar] [CrossRef]
- Pu, Z.; Song, G.-L.; Yang, S.; Outeiro, J.C.; Dillon, O.W., Jr. Grain refined and basal textured surface produced by burnishing for improved corrosion performance of AZ31B Mg alloy. Corros. Sci. 2012, 57, 192–201. [Google Scholar] [CrossRef]
- Krawiec, H.; Stanek, S.; Vignal, V.; Lelito, J.; Suchy, J.S. The use of microcapillary techniques to study the corrosion resistance of AZ91 magnesium alloy at the microscale. Corros. Sci. 2011, 53, 3108–3113. [Google Scholar] [CrossRef]
- Gray, J.; Luan, B. Protective coatings on magnesium and its alloys-a critical review. J. Alloy. Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Yerokhin, A.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Blawert, C.; Dietzel, W.; Ghali, E.; Song, G. Anodizing treatments for magnesium alloys and their effect on corrosion resistance in various environments. Adv. Eng. Mater. 2006, 8, 511–533. [Google Scholar] [CrossRef]
- Gupta, P.; Tenhundfeld, G.; Daigle, E.; Ryabkov, D. Electrolytic plasma technology: Science and engineering-An overview. Surf. Coat. Technol. 2007, 201, 8746–8760. [Google Scholar] [CrossRef]
- Steinbeck, C.; Han, Y.; Kuhn, S.; Horlacher, O.; Luttmann, E.; Willighagen, E. The Chemistry Development Kit (CDK): An open-source Java library for chemo-and bioinformatics. J. Chem. Inf. Comp. Sci. 2003, 43, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Li, D.; Liu, C.; Huang, Z.; He, D.; Yan, Q. An investigation of (NaPO3)6 effects and mechanisms during micro-arc oxidation of AZ31 magnesium alloy. Surf. Coat. Technol. 2015, 266, 151–159. [Google Scholar] [CrossRef]
- Zhang, P.; Nie, X.; Northwood, D. Influence of coating thickness on the galvanic corrosion properties of Mg oxide in an engine coolant. Surf. Coat. Technol. 2009, 203, 3271–3277. [Google Scholar] [CrossRef]
- Ghasemi, A.; Raja, V.; Blawert, C.; Dietzel, W.; Kainer, K. Study of the structure and corrosion behavior of PEO coatings on AM50 magnesium alloy by electrochemical impedance spectroscopy. Surf. Coat. Technol. 2008, 202, 3513–3518. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, A.; Raja, V.; Blawert, C.; Dietzel, W.; Kainer, K. The role of anions in the formation and corrosion resistance of the plasma electrolytic oxidation coatings. Surf. Coat. Technol. 2010, 204, 1469–1478. [Google Scholar] [CrossRef] [Green Version]
- Snizhko, L.; Yerokhin, A.; Pilkington, A.; Gurevina, N.; Misnyankin, D.; Leyland, A.; Matthews, A. Anodic processes in plasma electrolytic oxidation of aluminium in alkaline solutions. Electrochim. Acta 2004, 49, 2085–2095. [Google Scholar] [CrossRef]
- Wang, H.-M.; Chen, Z.-H.; Li, L.-L. Corrosion resistance and microstructure characteristics of plasma electrolytic oxidation coatings formed on AZ31 magnesium alloy. Surf. Eng. 2010, 26, 385–391. [Google Scholar] [CrossRef]
- Zhang, R.-F.; Zhang, S.-F.; Xiang, J.H. Influence of sodium silicate concentration on properties of micro arc oxidation coatings formed on AZ91HP magnesium alloys. Surf. Coat. Technol. 2012, 206, 5072–5079. [Google Scholar] [CrossRef]
- Li, X.-X.; Liu, X.-Y.; Luan, B.L. Corrosion and wear properties of PEO coatings formed on AM60B alloy in NaAlO2 electrolytes. Appl. Surf. Sci. 2011, 257, 9135–9141. [Google Scholar] [CrossRef]
- Cao, F.-H.; Cao, J.-L.; Zhang, Z. Plasma electrolytic oxidation of AZ91D magnesium alloy with different additives and its corrosion behavior. Mater. Corros. 2007, 58, 696–703. [Google Scholar] [CrossRef]
- Chen, D.; Wang, R.-Q.; Huang, Z.-Q. Evolution processes of the corrosion behavior and structural characteristics of plasma electrolytic oxidation coatings on AZ31 magnesium alloy. Appl. Surf. Sci. 2018, 434, 326–335. [Google Scholar] [CrossRef]
- Durdu, S.; Usta, M. Characterization and mechanical properties of coatings on magnesium by micro arc oxidation. Appl. Surf. Sci. 2012, 261, 774–782. [Google Scholar] [CrossRef]
- Da Forno, A.; Bestetti, M. Effect of the electrolytic solution composition on the performance of micro-arc anodic oxidation films formed on AM60B magnesium alloy. Surf. Coat. Technol. 2010, 205, 1783–1788. [Google Scholar] [CrossRef]
- Zhang, R.-F.; Xiong, G.-Y.; Hu, C.-Y. Comparison of coating properties obtained by MAO on magnesium alloys in silicate and phytic acid electrolytes. Curr. Appl. Phys. 2010, 10, 255–259. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yan, Z. Effect of potassium fluoride on structure and corrosion resistance of plasma electrolytic oxidation films formed on AZ31 magnesium alloy. J. Alloys Compd. 2009, 480, 469–474. [Google Scholar] [CrossRef]
- JCPDS-International Centre for Diffraction Data. Version 2.14. 1987–1994. Available online: http://www.icdd.com/ (accessed on 10 August 2020).
- Du, K.; Guo, X.; Guo, Q.; Wang, Y.; Wang, F.; Tian, Y. Effect of PEO coating microstructure on corrosion of Al 2024. J. Electrochem. Soc. 2012, 159, C597–C606. [Google Scholar] [CrossRef]
- Nocedal, J.; Wright, S.J. Springer series in operations research. In Numerical Optimization; Springer: New York, NY, USA, 1999. [Google Scholar] [CrossRef] [Green Version]
- Hussein, R.; Zhang, P.; Nie, X.; Xia, Y.; Northwood, D. The effect of current mode and discharge type on the corrosion resistance of plasma electrolytic oxidation (PEO) coated magnesium alloy AJ62. Surf. Coat. Technol. 2011, 206, 1990–1997. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yao, Z.; Jiang, Z.; Zhang, Y.; Liu, X. Investigation of the plasma electrolytic oxidation of Ti6Al4V under single-pulse power supply. Corros. Sci. 2011, 53, 2253–2262. [Google Scholar] [CrossRef]
- Yao, Z.; Jiang, Z.; Xin, S.; Sun, X.; Wu, X. Electrochemical impedance spectroscopy of ceramic coatings on Ti-6Al-4V by micro-plasma oxidation. Electrochim. Acta 2005, 50, 3273–3279. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Zeng, R.-C.; Lin, G.-G.; Wang, L.; Chen, X.-B.; Chen, D.-C. Corrosion resistance of self-cleaning silane/polypropylene composite coatings on magnesium alloy AZ31. J. Mater. Sci. Technol. 2020, 41, 43–55. [Google Scholar] [CrossRef]
- Chung, S.; Cheng, J.; Chiou, S.; Shih, H. EIS behavior of anodized zinc in chloride environments. Corros. Sci. 2000, 42, 1249–1268. [Google Scholar] [CrossRef]
- Xia, S.; Yue, R.; Rateick, R.; Birss, V. Electrochemical studies of AC/DC anodized Mg alloy in NaCl solution. J. Electrochem. Soc. 2004, 151, B179–B187. [Google Scholar] [CrossRef]



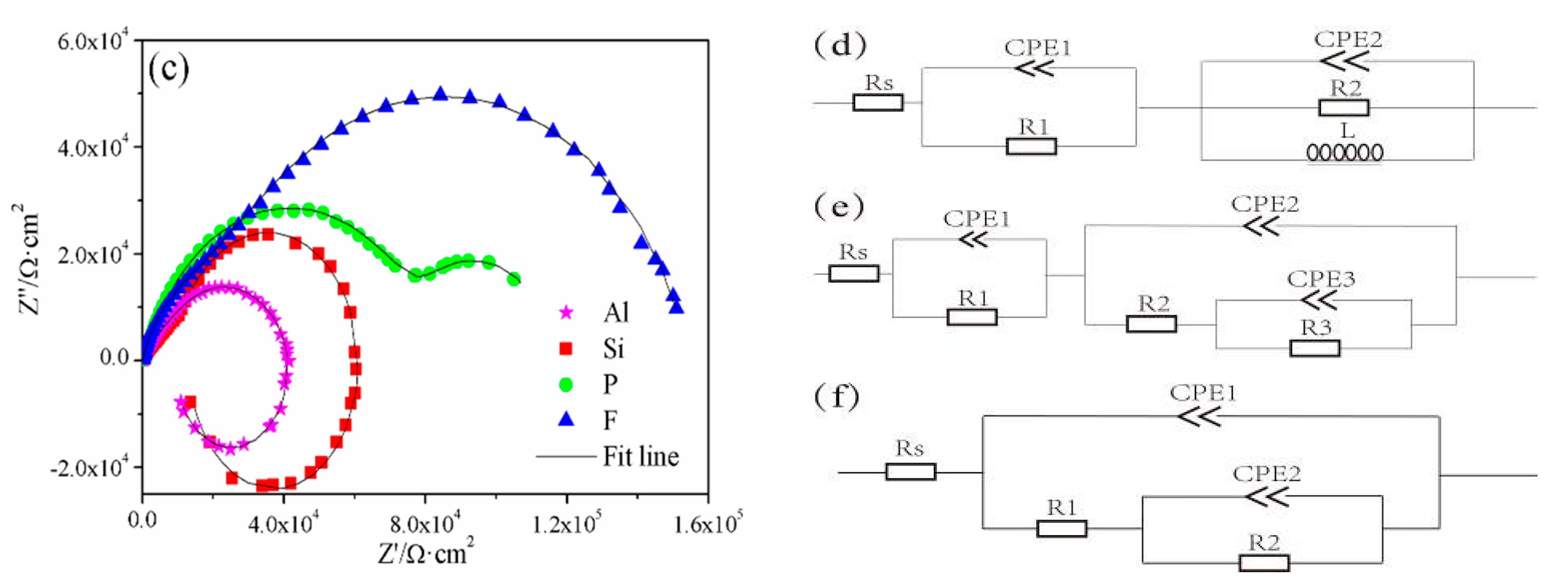
| Samples | R1 (kΩ·cm2) | CPE1 (S cm−2 s−n) | n1 | R2 (kΩ·cm2) | CPE2 (S cm−2 s−n) | n2 | L (×105 ·H·cm2) | R3 (kΩ·cm2) | CPE3 (S cm−2 s−n) | n3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Al-coating | 8.89 | 2.08 | 0.64 | 32.87 | 1.21 | 0.83 | 1.47 | -- | -- | -- |
| Si-coating | 12.98 | 1.84 | 0.62 | 48.39 | 1.34 | 0.96 | 1.45 | -- | -- | -- |
| P-coating | 79.42 | 0.39 | 0.78 | 0.93 | 4.83 | 0.54 | -- | 35.78 | 0.20 | 0.98 |
| F-coating | 38.08 | 0.14 | 0.82 | 121.9 | 0.56 | 0.74 | -- | -- | -- | -- |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Wang, R.; Liu, X.; Wang, D.; Zhang, H.; Shen, X.; Shen, D.; Li, D. Influence of Different Electrolyte Additives and Structural Characteristics of Plasma Electrolytic Oxidation Coatings on AZ31 Magnesium Alloy. Coatings 2020, 10, 817. https://doi.org/10.3390/coatings10090817
Huang Z, Wang R, Liu X, Wang D, Zhang H, Shen X, Shen D, Li D. Influence of Different Electrolyte Additives and Structural Characteristics of Plasma Electrolytic Oxidation Coatings on AZ31 Magnesium Alloy. Coatings. 2020; 10(9):817. https://doi.org/10.3390/coatings10090817
Chicago/Turabian StyleHuang, Zhiquan, Ruiqiang Wang, Xintong Liu, Dongdong Wang, Heng Zhang, Xiaojie Shen, Dejiu Shen, and Dalong Li. 2020. "Influence of Different Electrolyte Additives and Structural Characteristics of Plasma Electrolytic Oxidation Coatings on AZ31 Magnesium Alloy" Coatings 10, no. 9: 817. https://doi.org/10.3390/coatings10090817
APA StyleHuang, Z., Wang, R., Liu, X., Wang, D., Zhang, H., Shen, X., Shen, D., & Li, D. (2020). Influence of Different Electrolyte Additives and Structural Characteristics of Plasma Electrolytic Oxidation Coatings on AZ31 Magnesium Alloy. Coatings, 10(9), 817. https://doi.org/10.3390/coatings10090817




