Advances in Antibacterial Functionalized Coatings on Mg and Its Alloys for Medical Use—A Review
Abstract
:1. Introduction
2. Medical Applications of Antibacterial Functionalized Magnesium-Based Materials
2.1. Antibacterial Functionalized Magnesium Alloys Used as Orthopedic Appliances
2.2. Antibacterial-Functionalized Magnesium Alloys Used as Cardiovascular Stents
3. Antibacterial Functionalized Coatings on Magnesium
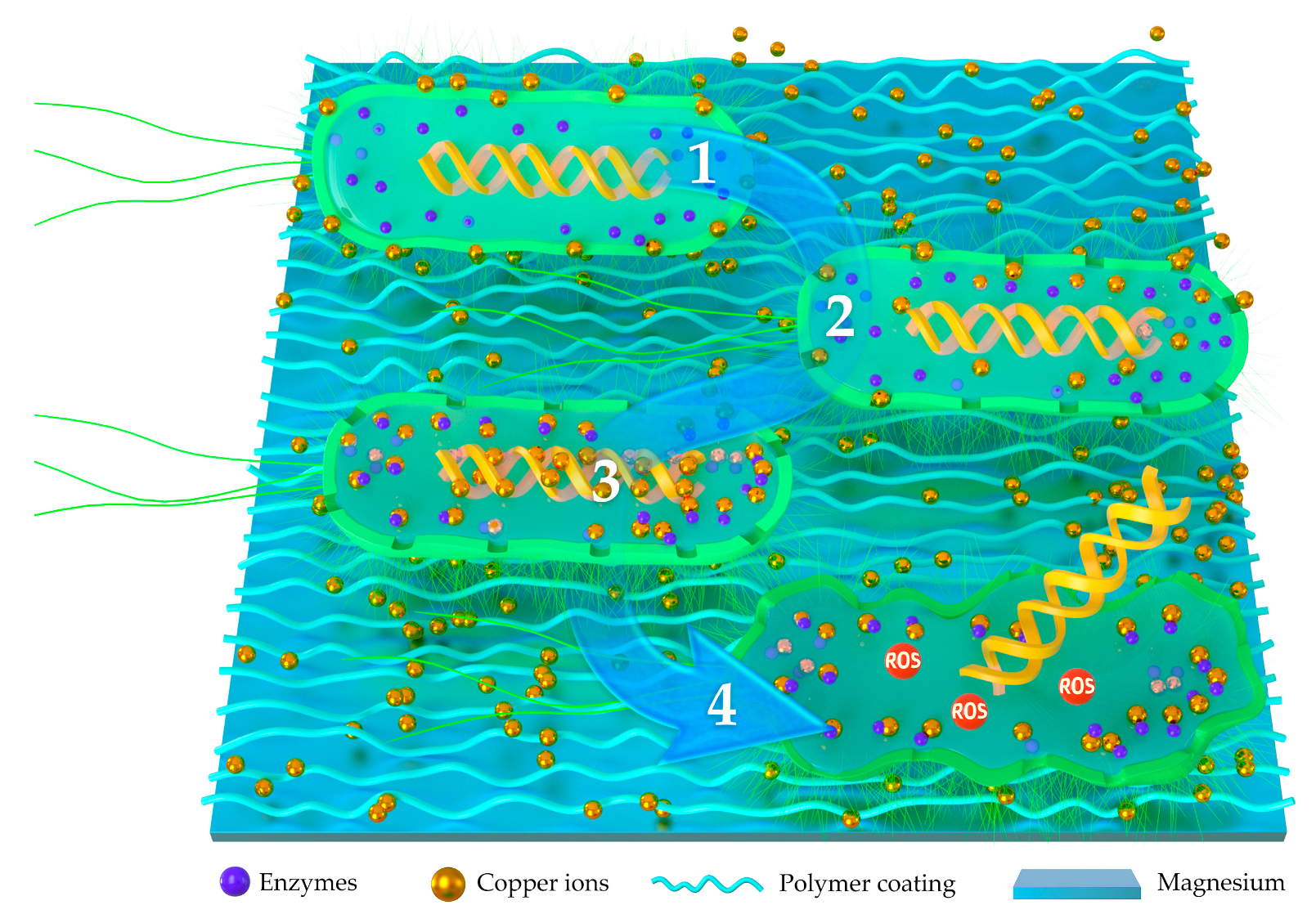
3.1. Polymer-Based Antibacterial Functionalized Coatings
3.1.1. Polymer-Based Coatings Combined with Antibiotics
3.1.2. Polymer-Based Coatings Combined with Antibacterial Ions
3.1.3. Antibacterial Activated Copolymers
3.1.4. Composite Polymer Coatings Based on Plasma Electrolytic Oxidation
3.2. CaP-Based Antibacterial Functionalized Coatings
4. Overall Performances of Antibacterial-Functionalized Coatings on Magnesium
4.1. An Overview on Antibacterial-Functionalized Coatings
4.2. Antibacterial Abilities of Different Antibacterial Coatings on Magnesium
4.3. In Vitro Cytocompatibility
4.4. Corrosion Resistance of Different Antibacterial Coatings on Magnesium
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.Y.; Normand, B.; Liao, H.L.; Zhao, G.F.; Mary, N.; Tang, J.L. SiCp/Al5056 Composite Coatings Applied to A Magnesium Substrate by Cold Gas Dynamic Spray Method for Corrosion Protection. Coatings 2020, 10, 325. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.L.; Yu, L.; Wan, P.; Yang, K. Biodegradable Materials for Bone Repairs: A Review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Gray-Munro, J.E.; Seguin, C.; Strong, M. Influence of surface modification on the in vitro corrosion rate of magnesium alloy AZ31. J. Biomed. Mater. Res. Part. A 2009, 91, 221–230. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, L.L.; Ni, D.R.; Chen, J.X.; Zhao, Y.C.; Liu, L.; Shuai, C.J.; Yang, K.; Atrens, A.; Zhao, M.C. Effect of grain refinement and crystallographic texture produced by friction stir processing on the biodegradation behavior of a Mg-Nd-Zn alloy. J. Mater. Sci. Technol. 2019, 35, 777–783. [Google Scholar] [CrossRef]
- Li, L.Y.; Cui, L.Y.; Zeng, R.C.; Li, S.Q.; Chen, X.B.; Zheng, Y.F.; Kannand, M.B. Advances in Functionalized Polymer Coatings on Biodegradable Magnesium Alloys—A Review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Cui, L.Y.; Wei, G.B.; Zeng, R.C.; Li, S.Q.; Zou, Y.H.; Han, E.H. Corrosion resistance of a novel SnO2-doped dicalcium phosphate coating on AZ31 magnesium alloy. Bioact. Mater. 2018, 3, 245–249. [Google Scholar] [CrossRef]
- Li, C.Y.; Fan, X.L.; Zeng, R.C.; Cui, L.Y.; Li, S.Q.; Zhang, F.; He, Q.K.; Kannan, M.B.; Jiang, H.W. (George); Chen, D.C.; et al. Corrosion resistance of in-situ growth of nano-sized Mg(OH)2 on micro-arc oxidized magnesium alloy AZ31—Influence of EDTA. J. Mater. Sci. Technol. 2019, 35, 1088–1098. [Google Scholar] [CrossRef]
- Robinson, D.A.; Griffith, R.W.; Shechtman, D.; Evans, R.B.; Conzemius, M.G. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Acta Biomater. 2010, 6, 1869–1877. [Google Scholar] [CrossRef]
- Feng, H.Q.; Wang, G.M.; Jin, W.H.; Zhang, X.M.; Huang, Y.F.; Gao, A.; Wu, H.; Wu, G.S.; Chu, P.K. Systematic Study of Inherent Antibacterial Properties of Magnesium-based Biomaterials. ACS Appl. Mater. Interfaces 2016, 8, 9662–9673. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.I.; Rohde, M.; Rais, B.; Seitz, J.M.; Mueller, P.P. Susceptibility of Metallic Magnesium Implants to Bacterial Biofilm Infections. J. Biomed. Mater. Res. A 2016, 104, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. The in Vitro Biological Properties of Mg-Zn-Sr Alloy and Superiority for Preparation of Biodegradable Intestinal Anastomosis Rings. Med. Sci. Monit. 2014, 20, 1056–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, L.; Lin, X.; Tan, L.L.; Yang, K. Effect of surface coating on antibacterial behavior of magnesium-based metals. Mater. Lett. 2011, 65, 3509–3511. [Google Scholar] [CrossRef]
- Zhou, W.C.; Hu, Z.R.; Wang, T.L.; Yang, G.Z.; Xi, W.H.; Gan, Y.Z.; Lu, W.; Hu, J.Z. Enhanced corrosion resistance and bioactivity of Mg alloy modified by Zn-doped nanowhisker hydroxyapatite coatings. Colloids Surf. B Biointerfaces 2019, 186, 110710. [Google Scholar] [CrossRef]
- Zou, Y.H.; Wang, J.; Cui, L.Y.; Zeng, R.C.; Wang, Q.Z.; Han, Q.X.; Qiu, J.; Chen, X.B.; Chen, D.C.; Guan, S.K.; et al. Corrosion resistance and antibacterial activity of zinc-loaded montmorillonite coatings on biodegradable magnesium alloy AZ31. Acta Biomater. 2019, 98, 196–214. [Google Scholar] [CrossRef]
- Ji, X.J.; Gao, L.; Liu, J.C.; Wang, J.; Cheng, Q.; Li, J.P.; Li, S.Q.; Zhi, K.Q.; Zeng, R.C.; Wang, Z.L. Corrosion resistance and antibacterial properties of hydroxyapatite coating induced by gentamicin-loaded polymeric multilayers on magnesium alloys. Colloids Surf. B Biointerfaces 2019, 179, 429–436. [Google Scholar] [CrossRef]
- Song, J.Q.; Jin, P.L.; Li, M.Q.; Liu, J.G.; Wu, D.M.; Yao, H.T.; Wang, J.Q. Antibacterial properties and biocompatibility in vivo and vitro of composite coating of pure magnesium ultrasonic micro-arc oxidation phytic acid copper loaded. J. Mater. Sci. Mater. Med. 2019, 30, 49–62. [Google Scholar] [CrossRef]
- Ji, X.J.; Cheng, Q.; Wang, J.; Zhao, Y.B.; Han, Z.Z.; Zhang, F.; Li, S.Q.; Zeng, R.C.; Wang, Z.L. Corrosion resistance and antibacterial effects of hydroxyapatite coating induced by polyacrylic acid and gentamicin sulfate on magnesium alloy. Front. Mater. Sci. 2019, 13, 87–98. [Google Scholar] [CrossRef]
- Zhuk, I.; Jarivala, F.; Attygalle, A.B.; Wu, Y.; Libera, M.R.; Sukhishvili, S.A. Self-Defensive Layer-by-Layer Films with Bacteria-Triggered Antibiotic Release. ACS Nano 2014, 8, 7733–7745. [Google Scholar] [CrossRef] [PubMed]
- Onuma, Y.; Serruys, P.W. Bioresorbable scaffold: The advent of a new era in percutaneous coronary and peripheral revascularization? Circulation 2011, 123, 779–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezk, A.I.; Sasikala, A.R.; Nejad, A.G.; Mousa, H.M.; Oh, Y.M.; Park, C.H.; Kim, C.S. Strategic design of a Musselinspired in situ reduced Ag/Au Nanoparticle Coated Magnesium Alloy for enhanced viability, antibacterial property and decelerated corrosion rates for degradable implant applications. Sci. Rep. 2019, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.C.; Liu, L.J.; Li, S.Q.; Zou, Y.H.; Zhang, F.; Yang, Y.N.; Cui, H.Z.; Han, E.H. Self-assembled silane film and silver nanoparticles coating on magnesium alloys for corrosion resistance and antibacterial applications. Acta Metall. Sin. 2013, 26, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Zeng, R.C.; Li, S.Q.; Cui, L.Y.; Zou, Y.H.; Guan, S.K.; Zheng, Y.F. Advance in Antibacterial Magnesium Alloys and Surface Coatings on Magnesium Alloys: A Review. Acta Metall. Sin. Engl. Lett. 2020, 33, 615–629. [Google Scholar] [CrossRef]
- Zeng, R.C.; Cui, L.Y.; Jiang, K.; Liu, R.; Zhao, B.D.; Zheng, Y.F. In Vitro Corrosion and Cytocompatibility of a Microarc Oxidation Coating and Poly (L-lactic acid) Composite Coating on Mg-1Li-1Ca Alloy for Orthopaedic Implants. Acs Appl. Mater. Interfaces 2016, 8, 10014–10028. [Google Scholar] [CrossRef]
- El-Kamel, R.S.; Ghoneim, A.A.; Fekry, A.M. Electrochemical, biodegradation and cytotoxicity of graphene oxide nanoparticles/polythreonine as a novel nano-coating on AZ91E Mg alloy staple in gastrectomy surgery. Materials Science Eng. C 2019, 103, 109780. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Liu, J.; Zhu, W.; Chen, J.; Duan, L.; Zhang, J.; Wang, D. Evaluation of the novel three-dimensional porous poly (L-lactic acid)/nano-hydroxyapatite composite scaffold. Bio-Med. Mater. Eng. 2015, 26, S197–S205. [Google Scholar] [CrossRef] [Green Version]
- Perkins, J.; Xu, Z.G.; Smith, C.; Roy, A.; Kumta, P.N.; Waterman, J.; Conklin, D.; Desai, S. Direct writing of polymeric coatings on magnesium alloy for tracheal stent applications. Ann. Biomed. Eng. 2015, 43, 1158–1165. [Google Scholar] [CrossRef]
- Zhao, C.; Hou, P.; Ni, J.; Han, P.; Chai, Y.; Zhang, X. Ag-incorporated FHA Coating on Pure Mg: Degradation and in Vitro Antibacterial Properties. ACS Appl. Mater. Interfaces 2016, 8, 5093–5103. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.H.; Shen, S.; Zhou, C.R.; Dang, X.L.; Jiao, Y.P.; Li, L.H.; Ding, S.; Li, H. Investigation of the antimicrobial activity and biocompatibility of magnesium alloy coated with HA and antimicrobial peptide. J. Mater. Sci. Mater. Med. 2015, 26, 66. [Google Scholar] [CrossRef] [PubMed]
- Plaass, C.; Ettinger, S.; Sonnow, L.; Koenneker, S.; Noll, Y.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Daniilidis, K.; Stukenborg-Colsman, C.; et al. Early Results Using a Biodegradable Magnesium Screw for Modified Chevron Osteotomies. J. Orthop. Res. 2016, 34, 2207–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces. Biomaterials 2013, 34, 8018–8029. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O. Treatment of Infections Associated with Surgical Implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Catena, A.; Borgnakke, W.S. Association between Diabetes Mellitus/Hyperglycemia and Peri-Implant Diseases: Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2017, 44, 636–648. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.G.F.P.; Bonfante, E.A.; Bergamo, E.T.P.; De Souza, S.L.S.; Riella, L.; Torroni, A.; Jalkh, E.B.B.; Witek, L.; Lopez, C.D.; Zambuzzi, W.F.; et al. Obesity/Metabolic Syndrome and Diabetes Mellitus on Peri-implantitis. Trends Endocrinol. Metab. 2020, 31, 596–610. [Google Scholar] [CrossRef]
- Waksman, R.; Erbel, R.; Mario, C.D.; Bartunek, J.; De Bruyne, B.; Eberli, F.R.; Erne, P.; Haude, M.; Horrigan, M.; Ilsley, C.; et al. Early and long-term intravascular ultrasound and angiographic findings after bioabsorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc. Interv. 2009, 2, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Hou, P.; Zhao, C.L.; Cheng, P.F.; Wu, H.L.; Ni, J.H.; Zhang, S.X.; Lou, T.F.; Wang, C.Y.; Han, P.; Zhang, X.N.; et al. Reduced antibacterial property of metallic magnesium in vivo. Biomed. Mater. 2017, 12, 015010. [Google Scholar] [CrossRef]
- Zhang, D.; Han, Q.; Yu, K.; Lu, X.P.; Liu, Y.; Lu, Z.; Wang, Q. Antibacterial activities against Porphyromonas gingivalis and biological characteristics of copper-bearing PEO coatings on magnesium. J. Mater. Sci. Technol. 2020, 61, 33–45. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Khoshnava, S.M.; Pagan, E.; Chen, M.B. Co-incorporation of graphene oxide/silver nanoparticle into poly-L-lactic acid fibrous: A route toward the development of cytocompatible and antibacterial coating layer on magnesium implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110812. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R. Bioresorbable scaffolds are here, please handle with care. Cardiovasc. Revasc. Med. 2016, 17, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Haude, M.; Erbel, R.; Erne, P.; Verheye, S.; Degen, H.; Bose, D.; Vermeersch, P.; Wijnbergen, I.; Weissman, N.; Prati, F.; et al. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicenter, first-in-man BIOSOLVE-I trial. Lancet 2013, 381, 836–844. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.; Birgelen, C.; Christiansen, E. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 6 month results of the prospective, multicenter, first-in-man BIOSOLVE-II trial. Lancet 2016, 387, 31–39. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, N.; Zhu, D.H. Sirolimus-eluting dextran and polyglutamic acid hybrid coatings on AZ31 for stent applications. J. Biomater. Appl. 2015, 30, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Kozerski, S.; Pawlowski, L.; Jaworski, R.; Roudet, F.; Petit, F. Two zones microstructure of suspension plasma sprayed hydroxyapatite coatings. Surf. Coat. Technol. 2010, 204, 1380–1387. [Google Scholar] [CrossRef]
- Yin, Z.Z.; Qi, W.C.; Zeng, R.C.; Chen., X.B.; Gu, C.D.; Guan, S.K.; Zheng, Y.F. Advances in coatings on biodegradable magnesium alloys. J. Magnes. Alloy 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Z.; Leng, Y. Biomimetic calcium phosphate coatings on nitric-acid-treated titanium surfaces. Mater. Sci Eng. 2007, 27, 700–708. [Google Scholar] [CrossRef]
- Griffith, L.G. Polymeric Biomaterials. Acta Mater. 2000, 48, 263–277. [Google Scholar] [CrossRef]
- Yang, Y.; Michalczyk, C.; Singer, F.; Virtanen, S.; Boccaccini, A. In Vitro Study of Polycaprolactone/bioactive Glass Composite Coatings on Corrosion and Bioactivity of Pure Mg. Appl. Surf. Sci. 2015, 355, 832–841. [Google Scholar] [CrossRef]
- Xu, L.; Yamamoto, A. Characteristics and Cytocompatibility of Biodegradable Polymer Film on Magnesium by Spin Coating. Colloids Surf. B. Biointerfaces 2012, 93, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Acharya, G.; Park, K. Mechanisms of controlled drug release from drug-eluting stents. Adv. Drug Deliv. Rev. 2006, 58, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Liang, H.F.; Chiu, Y.L.; Chang, Y.; Wei, H.J.; Sung, H.W. A novel drug-eluting stent spray-coated with multi-layers of collagen and sirolimus. J. Control. Release 2005, 108, 178–189. [Google Scholar] [CrossRef]
- Lee, S.J.; Oh, S.H.; Liu, J.; Soker, S.; Atala, A.; Yoo, J.J. The use of thermal treatments to enhance the mechanical properties of electrospun poly(ɛ-caprolactone) scaffolds. Biomaterials 2008, 29, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Walter, E.R. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Patra, S.N.; Easteal, A.J.; Bhattacharyya, D. Parametric study of manufacturing poly (lactic) acid nanofibrous mat by electrospinning. J. Mater. Sci. 2009, 44, 647–654. [Google Scholar] [CrossRef]
- Virto, M.R.; Frutos, P.; Torrado, S.; Frutos, G. Gentamicin release from modified acrylic bone cements with lactose and hydroxypropylmethylcellulose. Biomaterials 2003, 24, 79–87. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Chen, X.Y.; Li, S.Q.; Zeng, R.C.; Zhang, F.; Wang, Z.L.; Guan, S.K. Corrosion resistance and drug release profile of gentamicin-loaded polyelectrolyte multilayers on magnesium alloys: Effects of heat treatment. J. Colloid Interface Sci. 2019, 547, 309–317. [Google Scholar] [CrossRef]
- Janeesh, P.A.; Sami, H.; Dhanya, C.R.; Sivakumar, S.; Avraham, A. Biocompatibility and genotoxicity studies of polyallylamine hydrochloride nanocapsules in rats. RSC Adv. 2014, 4, 24484–24497. [Google Scholar] [CrossRef]
- Lei, L.; Hsieh, Y.L. Ultra-fine polyelectrolyte hydrogel fibres from poly (acrylic acid)/poly (vinyl alcohol). Nanotechnology 2005, 16, 2852–2860. [Google Scholar] [CrossRef]
- Xu, Q.W.; Li, X.; Jin, Y.Y.; Sun, L.; Ding, X.X.; Liang, L.; Wang, L.; Nan, K.H.; Ji, J.; Chen, H. Bacterial self-defense antibiotics release from organic-inorganic hybrid multilayer films for long-term anti-adhesion and biofilm inhibition properties. Nanoscale 2017, 9, 19245–19254. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Jiang, G.F.; Wang, D.; Wang, H.; Ding, L.; He, G. Porous magnesium loaded with gentamicin sulphate and in vitro release behavior. Mater. Sci. Eng. C Mater. 2016, 69, 154–159. [Google Scholar] [CrossRef]
- Xu, W.; Yagoshi, K.; Koga, Y.; Sasaki, M.; Niidome, T. Optimized polymer coating for magnesium alloy-based bioresorbable scaffolds for long-lasting drug release and corrosion resistance. Colloids Surf. B Biointerfaces 2018, 163, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.J.; Zhang, L.; Chen, J.H.; Zhang, J.; Yuan, F.; Shen, L.; Chen, C.X.; Pei, J. In vitro and in vivo degradation of rapamycin-eluting Mg-Nd-Zn-Zr alloy stents in porcine coronary arteries. Mater. Sci. Eng. C 2017, 80, 1–6. [Google Scholar] [CrossRef]
- Dayaghi, E.; Bakhsheshi-Rad, H.R.; Hamzah, E.; Akhavan-Farid, A.; Ismail, A.F.; Aziz, M.; Abdolahi, E. Magnesium-zinc scaffold loaded with tetracycline for tissue engineering application: In vitro cell biology and antibacterial activity assessment. Mater. Sci. Eng. C 2019, 102, 53–65. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Shi, L.Q.; Ji, X.J.; Li, J.C.; Han, Z.Z.; Li, S.Q.; Zeng, R.C.; Zhang, F.; Wang, Z.L. Corrosion resistance and antibacterial properties of polysiloxane modified layer-by-layer assembled self-healing coating on magnesium alloy. J. Colloid Interface Sci. 2018, 526, 43–50. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Zheng, K.; Liang, R.F.; Mainka, A.; Taccardi, N.; Roether, J.A.; Detsch, R.; Goldmann, W.H.; Virtanen, S.; Boccaccini, A.R. Cu-releasing BG/PCL Coating on Mg with Antibacterial and Anticorrosive Properties for Bone Tissue Engineering. Biomed. Mater. 2017, 13, 015001. [Google Scholar] [CrossRef]
- Rau, J.V.; Curcio, M.; Raucci, M.G.; Barbaro, K.; Fasolino, I.; Teghil, R.; Ambrosio, L.; Bonis, A.D.; Boccaccini, A.R. Cu-releasing bioactive glass coatings and their in vitro properties. ACS Appl. Mater. Interfaces 2019, 11, 5812–5820. [Google Scholar] [CrossRef]
- Geng, Z.; Cui, Z.D.; Li, Z.Y.; Zhu, S.L.; Liang, Y.Q.; Liu, Y.D.; Li, X.; He, X.; Yu, X.X.; Wang, R.F.; et al. Strontium Incorporation to Optimize the Antibacterial and Biological Characteristics of Silver-Substituted Hydroxyapatite Coating. Mater. Sci. Eng. C. 2016, 58, 467–477. [Google Scholar] [CrossRef]
- Cho, K.H.; Park, J.E.; Osaka, T.; Park, S.G. The Study of Antimicrobial Activity and Preservative Effects of Nanosilver Ingredient. Eletrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Jamuna-Thevi, K.; Bakar, S.A.; Ibrahim, S.; Shahab, N.; Toff, M.R.M. Quantification of silver ion release, in vitro cytotoxicity and antibacterial properties of nanostuctured Ag doped TiO2 coatings on stainless steel deposited by RF magnetron sputtering. Vacuum 2011, 86, 235–241. [Google Scholar] [CrossRef]
- Yang, W.J.; Shen, C.C.; Ji, Q.L.; An, H.J.; Wang, J.J.; Liu, Q.D.; Zhang, Z.Z. Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology 2009, 20, 085102. [Google Scholar] [CrossRef] [PubMed]
- DeVasConCellos, P.; Bose, S.; Beyenal, H.; Bandyopadhyay, A.; Zirkle, L.G. Antimicrobial Particulate Silver Coatings on Stainless Steel Implants for Fracture Management. Mater. Sci. Eng. C 2012, 32, 1112–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zille, A.; Fernandes, M.M.; Francesko, A.; Tzanov, T.; Fernandes, M.; Oliveira, F.R.; Almeida, L.; Amorim, T.; Almeida, L.; Amorim, T.; et al. Size and Aging Effects on Antimicrobial Efficiency of Silver Nanoparticles Coated on Polyamide Fabrics Activated by Atmospheric DBD Plasma. ACS Appl. Mater. Interfaces 2015, 7, 13731–13744. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, Q.; Lin, X.; Wan, P.; Zhang, G.; Zhang, Q.; Yang, K. Loss of mechanical properties in vivo and bone–implant interface strength of AZ31B magnesium alloy screws with Si-containing coating. Acta Biomater. 2014, 10, 2333–2340. [Google Scholar] [CrossRef]
- Hans, M.; Mathwes, S.; Mucklich, F.; Solioz, M. Physicochemical properties of copper important for its antibacterial activity and development of a unified model. Biointerphases 2015, 11, 018902. [Google Scholar] [CrossRef]
- Yang, G.Z.; Yang, H.W.; Shi, L.; Wang, T.L.; Zhou, W.C.; Zhou, T.; Han, W.; Zhang, Z.Y.; Lu, W.; Hu, J.Z. Enhancing corrosion resistance, osteoinduction, and antibacterial properties by Zn/Sr additional surface modification of magnesium alloy. ACS Biomater. Sci. Eng. 2018, 4, 4289–4298. [Google Scholar] [CrossRef]
- Guo, Y.T.; Jia, S.Q.; Qiao, L.; Su, Y.C.; Gu, R.; Li, G.Y.; Lian, J.S. A multifunctional polypyrrole/zinc oxide composite coating on biodegradable magnesium alloys for orthopedic implants. Colloids Surf. B Biointerfaces 2020, 194, 111186. [Google Scholar] [CrossRef]
- Salem, W.; Leitner, D.R.; Zingl, F.G.; Schratter, G.; Prassl, R.; Goessler, W.; Reidl, J.; Schild, S. Antibacterial activity of silver and zinc nanoparticles against Vibrio cholerae and enterotoxic Escherichia coli. Int. J. Med. Microbiol. 2015, 305, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Pati, R.; Mehta, R.K.; Mohanty, S.; Padhi, A.; Sengupta, M.; Vaseeharan, B.; Goswami, C.; Sonawane, A. Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages. Nanomedicine: Nanotechnology. Biol. Med. 2014, 10, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Su, P.L.; Chen, S.; Wang, N.; Ma, Y.P.; Liu, Y.R.; Wang, J.S.; Zhang, Z.T.; Li, H.Y.; Webster, T.J. Synthesis of TiO2 nanotubes with ZnO nanoparticles to achieve antibacterial properties and stem cell compatibility. Nanoscale 2014, 6, 9050–9062. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Fang, X.; Jia, H.M.; Liu, M.X.; Shi, X.T.; Xue, C.W.; Chen, T.T.; Wei, Z.P.; Fang, F.; Zhu, H.; et al. Zn or O? An Atomic Level Comparison on Antibacterial Activities of Zinc Oxides. Chemistry 2016, 22, 8053–8058. [Google Scholar] [CrossRef] [PubMed]
- Joe, A.; Park, S.H.; Kim, D.J.; Lee, Y.J.; Jhee, K.H.; Sohn, Y.K.; Jang, E.S.; Jang, E.S. Antimicrobial activity of ZnO nanoplates and its Ag nanocomposites: Insight into an ROS-mediated antibacterial mechanism under UV light. J. Solid. State. Chem. 2018, 267, 124–133. [Google Scholar] [CrossRef]
- Ding, X.; Yang, C.; Lim, T.P.; Hsu, L.Y.; Engler, A.C.; Hedrick, J.L.; Yang, Y.Y. Antibacterial and antifouling catheter coatings using surface grafted peg-b-cationic polycarbonate deblock copolymers. Biomaterials 2012, 33, 6593–6603. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Xin, Z.; Xu, S.; Shi, H.; Yang, H.; Song, L.; Yan, S.; Luan, S.; Yin, J.; Khan, A.F.; et al. Enzyme-mimicking polymer brush-functionalized surface for combating biomaterial-associated infections. Appl. Surf. Sci. 2017, 423, 869–880. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Mu, C.; Lin, W. A novel approach for synthesis of zwitterionic polyurethane coating with protein resistance. Langmuir 2014, 30, 12860–12867. [Google Scholar] [CrossRef]
- Luo, L.; Li, G.; Luan, D.; Yuan, Q.; Wei, Y.; Wang, X. Antibacterial adhesion of borneol-based polymer via surface chiral stereochemistry. ACS Appl. Mater. Interfaces 2014, 6, 19371–19377. [Google Scholar] [CrossRef]
- Wang, C.H.; Yi, Z.L.; Sheng, Y.F.; Tian, L.; Qin, L.; Ngai, T.; Lin, W. Development of a novel biodegradable and anti-bacterial polyurethane coating for biomedical magnesium rods. Mater. Sci. Eng. C 2019, 99, 344–356. [Google Scholar] [CrossRef]
- Sun, J.D.; Zhu, Y.; Meng, L.; Chen, P.; Shi, T.T.; Liu, X.Y.; Zheng, Y.F. Electrophoretic deposition of colloidal particles on Mg with cytocompatibility, antibacterial performance, and corrosion resistance. Acta Biomater. 2016, 45, 387–398. [Google Scholar] [CrossRef]
- Mai, L.M.; Lin, C.Y.; Chen, C.Y.; Tsai, Y.C. Synergistic effect of bismuth subgallate and borneol, the major components of sulbogin®, on the healing of skin wound. Biomaterials 2003, 24, 3005–3012. [Google Scholar] [CrossRef]
- Bertuola, M.; Miñán, A.; Grillo1, C.A.; Cortizo, M.C.; De Mele, M.A.F.L. Corrosion protection of AZ31 alloy and constrained bacterial adhesion mediated by a polymeric coating obtained from a phytocompound. Colloids Surf. B Biointerfaces 2018, 172, 187–196. [Google Scholar] [CrossRef]
- Yao, Z.; Li, L.; Jiang, Z. Adjustment of the ratio of Ca/P in the ceramic coating on Mg alloy by plasma electrolytic oxidation. Appl. Surf. Sci. 2009, 255, 6724–6728. [Google Scholar] [CrossRef]
- Liu, G.Y.; Hu, J.; Ding, Z.K.; Wang, C. Bioactive calcium phosphate coating formed on micro-arc oxidized magnesium by chemical deposition. Appl. Surf. Sci. 2011, 257, 2051–2057. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation—An Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Chen, J.X.; Zhang, Y.; Ibrahima, M.; Etim, I.P.; Tan, L.L.; Yang, K. In vitro degradation and antibacterial property of a copper-containing microarc oxidation coating on Mg-2Zn-1Gd-0.5Zr alloy. Colloids Surf. B Biointerfaces 2019, 179, 77–86. [Google Scholar] [CrossRef]
- Tian, P.; Xu, D.M.; Liu, X.Y. Mussel-inspired functionalization of PEO/PCL composite coating on a biodegradable AZ31 magnesium alloy. Colloids Surf. B Biointerfaces 2016, 141, 327–337. [Google Scholar] [CrossRef]
- Takagi, S.; Chow, L.C.; Ishikawa, K. Formation of hydroxyapatite in new calcium phosphate cements. Biomaterials 1998, 19, 1593–1599. [Google Scholar] [CrossRef]
- Ramselaar, M.M.A.; Driessens, F.C.M.; Kalk, W.; De Wijn, J.R.; Van Mullen, P.J. Biodegradation of four calcium phosphate ceramics: In vivo rates and tissue interactions. J. Mater. Sci. Mater. Med. 1991, 2, 63–70. [Google Scholar] [CrossRef]
- Coelho, P.G.; Assis, S.L.D.; Costa, I.; Van Thompson, P.V. Corrosion resistance evaluation of a Ca-and P-based bioceramic thin coating in Ti-6Al-4V. J. Mater. Sci. Mater. Med. 2009, 20, 215–222. [Google Scholar] [CrossRef]
- Sridhar, T.M.; Mudali, U.K.; Subbaiyan, M. Preparation and characterisation of electrophoretically deposited hydroxyapatite coatings on type 316 L stainless steel. Corros. Sci. 2003, 45, 237–252. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, X.X.; Li, Q. Fabrication of multifunctional CaP-TC composite coatings and the corrosion protection they provide for magnesium alloys. Biomed. Eng. 2017, 62, 375–381. [Google Scholar] [CrossRef]
- Tesavibul, P.; Chantaweroad, S.; Laohaprapanon, A.; Channasanon, S.; Uppanan, P.; Tanodekaew, S.; Chalermkarnnon, P.; Sitthiseripratip, K. Biocompatibility of hydroxyapatite scaffolds processed by lithography-based additive manufacturing. Bio-Med. Mater. Eng. 2015, 26, 31–38. [Google Scholar] [CrossRef]
- Ozeki, K.; Goto, T.; Aoki, H.; Masuzawa, T. Influence of the crystallinity of a sputtered hydroxyapatite film on its osteocompatibility. Bio-Med. Mater. Eng. 2015, 26, 139–147. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Buton, N.; Megier, C. Obtaining and Characterizing Thin Layers of Magnesium Doped Hydroxyapatite by Dip Coating Procedure. Coatings 2020, 10, 510. [Google Scholar] [CrossRef]
- Pang, K.M.; Lee, J.K.; Seo, Y.K.; Kim, S.M.; Kim, M.J.; Lee, J.H. Biologic properties of nano-hydroxyapatite: An in vivo study of calvarial defects, ectopic bone formation and bone implantation. Bio-Med. Mater. Eng. 2015, 25, 25–38. [Google Scholar] [CrossRef]
- Tan, F.; Naciri, M. Al-Rubeai, M. Osteoconductivity and growth factor production by MG63 osteoblastic cells on bioglass-coated orthopedic implants. Biotechnol. Bioeng. 2011, 108, 454–464. [Google Scholar] [CrossRef]
- Bai, N.N.; Tan, C.; Li, Q.; Xi, Z.X. Study on the corrosion resistance and anti-infection of modified magnesium alloy. Biomed. Mater. Eng. 2017, 28, 339–345. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, L.; Zhu, W.W.; Fang, L.M.; Ren, F.Z. Mussel-inspired nano-multilayered coating on magnesium alloys for enhanced corrosion resistance and antibacterial property. Colloids Surf. B Biointerfaces 2017, 157, 432–439. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- De Smet, K.; Contreras, R. Human antimicrobial peptides: Defensins, cathelicidins and histatins. Biotechnol. Lett. 2005, 27, 1337–1347. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Noordin, S.; Masri, B.A.; Garbuz, D.S.; Duncan, C.P.; Hancock, R.E.W.; Wang, R.Z. Drug release and bone growth studies of antimicrobial peptide-loaded calcium phosphate coating on titanium. J. Biomed. Mater. Res. Part. B 2012, 100, 1344–1352. [Google Scholar] [CrossRef]
- Janning, C.; Willbold, E.; Vogt, C.; Nellesen, J.; Meyer-Linderberg, A.; Windhagen, H.; Thorey, F.; Witte, F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodeling. Acta Biomater. 2010, 6, 1861–1868. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier Academic Press: New York, NY, USA, 2004; pp. 10–11. [Google Scholar]
- Poinern, G.E.J.; Brundavanam, S.; Fawcett, D. Biomedical Magnesium Alloys: A Review of Material Properties, Surface Modifications and Potential as a Biodegradable Orthopedic Implant. Am. J. Biomed. Eng. 2012, 2, 218–240. [Google Scholar] [CrossRef] [Green Version]
- Song, G.L.; Atren, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.; Shadanbaz, S.; Kirkland, N.T.; Stace, E.; Woodfield, T.; Staiger, M.P.; Dias, G.J. Magnesium alloys: Predicting in vivo corrosion with in vitro immersion testing. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2012, 100, 1134–1141. [Google Scholar] [CrossRef]


| Coatings | Substrate | Solution | Immersion Time | Temperature | Ref. | |
|---|---|---|---|---|---|---|
| 2015 | HA-PSI 10 coating | AZ91 | 7 mM Ca(NO3)2, 4.2 mM NaH2PO4 and 1 mM NaHCO3 | 24 h | Not mentioned | [32] |
| 2016 | Ag-FHA coating | High purity Mg (99.98%) | 0.042 M Ca(NO3)4·4H2O, 0.1 M NaNO3, 0.025 M NH4H2PO4, 6 vol.% H2O2 and 1 × 10−3 M NaF | 2 h | 60 | [31] |
| 2016 | Zn-doped nanowhisker HA coatings | ZK60 | Ca(NO3)2·4H2O (0.05 M) and NaH2PO4·2H2O (0.03 M) | 2 h | 140 | [17] |
| 2017 | HA/PFLX coating | AZ91 | 0.05 M EDTA-Na2, Ca(NO3)2, K2HPO4 | 6 h | 94 | [108] |
| Year | Coating Systems | Coating Base Material | Antibacterial Ingredient | Substrate | Methods | Coating Thickness | Ref. |
|---|---|---|---|---|---|---|---|
| 2015 | Sirolimus-PGA | PGA | Sirolimus | AZ31 | Dipping | NM | [53] |
| 2015 | HA-PSI 10 coating | HA | Antimicrobial peptides (AMPs) | AZ91 | biomimetic mineralization | NM | [32] |
| 2016 | CaP/TC composite coating | calcium phosphate (CaP) | Tetracycline (TC) | AZ91D | Dipping | ~8.0 μm | [102] |
| 2016 | Ag-FHA coating | Fluoridated hydroxyapatite (FHA) | Ag | magnesium | electrochemical deposition | NM | [31] |
| 2016 | ISA-co-DMA/TA | DMA/TA | isobornyl acrylate (ISA) | pure Mg | electrophoretic deposition | 80–125 nm | [90] |
| 2016 | PEO/PCL/PHMB composite coating | PEO/PCL composite coating | PHMB | AZ31 | PEO | NM | [97] |
| 2017 | HA/PFLX | HA | pefloxacin (PFLX) | AZ91 | hydrothermal and dip | NM | [108] |
| 2017 | chitosan-CAp@PDA-chitosan-Ag@PDA | chitosan-CAp@PDA | Ag | AZ31 | layer by layer | 100 nm | [109] |
| 2017 | Cu-releasing BG/PCL Coating | polycaprolactone (PCL) | Cu-BGNs | pure Mg | Spin coating | 8 ± 2 µm | [67] |
| 2017 | PDLLA/RAPA coating | PDLLA | rapamycin | Mg-Nd-Zn-Zr | ultrasonic spray-coating | 4–6 um | [64] |
| 2017 | SRL loaded PDLLA-PCL double layer | PDLLA-PCL double layer | sirolimus SRL | AZ 31 | ExactaCoat Ultrasonic Spraying | NM | [63] |
| 2018 | AgNPs-PMTMS | PMTMS | silver nanoparticles (AgNPs) | AZ 31 | layer-by-layer (LbL) assembly | NM | [58] |
| 2018 | polymeric layer (poly TOH) | Poly TOH | thymol (TOH) | AZ31 | potentiodynamic electrochemical | NM | [92] |
| 2019 | Ag and Au NPs enriched PD-HF coating | Polydopamine-Hydrofluoric acid | Ag/Cu Nanoparticle | AZ31 | Dipping | NM | [24] |
| 2019 | Copper-containing MAO coating | Nano hydroxyapatite | Copper | Mg-2Zn-1Gd-0.5Zr | Micro-arc oxidation | 8–11 μm | [96] |
| 2019 | GPU-ZPU | PU | PEG and zwitterions | Mg | Dipping | ~4–15 μm | [106] |
| Year | Coating Systems | Antibacterial Ability on G+ Bacteria | Antibacterial Ability on G- Bacteria | Ref. |
|---|---|---|---|---|
| 2015 | HA-PSI 10 coating | Staphylococcus aureus ATCC25923 | - | [32] |
| 2016 | Ag-FHA coating | Methicillin resistant Staphylococcus aureus ATCC 43300 | - | [31] |
| 2016 | CaP/TC composite coating | Staphylococcus aureus ATCC25923 | - | [102] |
| 2016 | ISA-co-DMA/TA | Staphylococcus aureus ATCC 6538 | Escherichia coli ATCC 8739 | [73] |
| 2016 | PEO/PCL/PHMB composite coating | Staphylococcus aureus ATCC25923 | Escherichia coli ATCC 52922 | [97] |
| 2017 | HA/PFLX | - | Escherichia coli (E. coli BL21). | [108] |
| 2017 | chitosan-Cap@PDA-chitosan-Ag@PDA | Staphylococcus aureus ATCC 29213 | Escherichia coli ATCC 52922 | [109] |
| 2017 | Cu-releasing BG/PCL Coating | Staphylococcus carnosus | Escherichia coli | [67] |
| 2018 | AgNPs-PMTMS | Staphylococcus aureus | - | [58] |
| 2018 | polymeric layer (poly TOH) | Staphylococcus aureus ATCC25923 | - | [92] |
| 2019 | Ag and Au NP-enriched PD-HF coating | Staphylococcus aureus ATCC 29231 | Escherichia coli ATCC 52922 | [24] |
| 2019 | Copper-containing MAO coating | Staphylococcus aureus ATCC25923 | - | [79] |
| 2019 | GPU-ZPU | - | E. coli and P. aeruginosa | [106] |
| Bacteria Assay | G+/G- | Anaerobic Bacteria | Aerobic Bacteria | Ref. |
|---|---|---|---|---|
| Staphylococcus aureus ATCC 29231 | G+ | + | [24,32,79,102] | |
| Staphylococcus aureus ATCC 25923 | G+ | + | [75] | |
| Staphylococcus aureus ATCC 6538 | G+ | + | [81] | |
| Staphylococcus aureus ATCC 29213 | G+ | + | [109] | |
| Methicillin-resistant Staphylococcus aureus (ATCC 43300) | G+ | + | [109] | |
| Escherichia coli ATCC 8739 | G- | + | [90] | |
| Escherichia coli (E. coli BL21). | G- | + | [108] | |
| P. aeruginosa | G- | + | [106] | |
| P. gingivalis | G- | + | [36] |
| Year | Antibacterial Ingredient | Bacterial Assay | CFU/mL | Immersion Time | Bacterial Counting Method | Agar | L/D | SEM | In Vivo | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | Sirolimus | E. coli | 1 × 108 | 4 h | Bacterial adhesion | [53] | ||||
| 2015 | AMPs | S. aureus | 1 × 105 | 6 h, 1–5 days | spread plate | + | + | [32] | ||
| 2016 | Ag | S. aureus | 1 × 106 | 24 and 72 h | spread plate | + | [31] | |||
| 2016 | Tetracycline | S. aureus | 1 × 106 | 48 h | disc diffusion | [102] | ||||
| 2016 | isobornyl acrylate | S. aureus E. coli | 1 × 106 | 72 h | spread plate | + | + | [90] | ||
| 2016 | PHMB | S. aureus; E. coli | + | [97] | ||||||
| 2017 | pefloxacin | E. coli | - | + | [108] | |||||
| 2017 | Ag | S. aureus; E. coli | 1 × 107 | 2 h, 4 h | spread plate | + | [109] | |||
| 2017 | Cu-BGNs | S. aureus | + | [67] | ||||||
| 2018 | AgNPs | S. aureus | 1 × 106 | - | spread plate | [58] | ||||
| 2018 | thymol | S. aureus | 1 × 106 | 2 h | spread plate | [92] | ||||
| 2019 | Ag/Cu Nanoparticle | S. aureus; E. coli | 1 × 106 | 24 h | spectrophotometric | [24] | ||||
| 2019 | PEG and zwitterions | P. aeruginosa | 1 × 108 | 4 h | Bacterial adhesion | [106] | ||||
| 2019 | Copper | S. aureus | 1 × 106 | 6, 12, and 24 h | spread plate | + | [79] |
| Year | Coating | Substrates | Cell Type | Cell Proliferation | Osteoblast Differentiation | Cell Adhesion | In Vivo | Refs. |
|---|---|---|---|---|---|---|---|---|
| 2015 | AMP-HA | AZ91 | rBMMSCs | improved | improved | NM | + | [32] |
| 2016 | MAO/PLLA coating | Mg-1Li-1Ca alloy | MC3T3-E1 | improved | improved | improved | [27] | |
| 2016 | Ag-FHA coating | Pure magnesium | MC3T3-E1 | improved | NM | improved | [31] | |
| 2016 | (ISA-co-DMA)/TA | Magnesium | L929 | improved | improved | improved | [90] | |
| 2017 | Cu-releasing BG/PCL coating | Magnesium | MG63 | improved | improved | NM | [67] | |
| 2019 | (PAA/GS)20/PAA-Hap coating | AZ31 | MC3T3-E1 | Slightly reduced | improved | improved | [19] | |
| 2019 | GONPs/PT | AZ91E | HGaEpC cell | improved | NM | NM | [28] | |
| 2019 | Zn-doped nanowhisker HA coatings | ZK60 | BMSCs | improved | improved | improved | [17] | |
| 2019 | Zinc-loaded montmorillonite coatings | AZ31 | MC3T3-E1 | improved | NM | NM | [18] | |
| 2019 | UMAO- phytic acid-Cu | Pure magnesium | MC3T3-E1 | improved | improved | improved | + | [20] |
| 2019 | Ag/Au Nanoparticle Coating | AZ31 | MC3T3-E1 | improved | NM | improved | [24] | |
| 2019 | Copper-bearing MAO coating | Mg-2Zn -1Gd-0.5Zr | MG63 | improved | NM | NM | [79] |
| Year | Coating Systems | Substrates | Electrolyte | pH Value | Ratio (cm2/mL) | Immersion Time | Refs. |
|---|---|---|---|---|---|---|---|
| 2015 | Sirolimus-PGA | AZ31 | HBSS | NM | 0.128 | 35 days | [53] |
| 2016 | CaP/TC composite coating | AZ91D | SBF | NM | NM | 5 days | [102] |
| 2016 | ISA-co-DMA/TA | Magnesium | SBF | 7.4 | NM | 40 days | [90] |
| 2016 | PEO/PCL/PHMB composite coating | AZ31 | SBF | NM | 1:2 | 28 days | [97] |
| 2017 | HA/PFLX | AZ91 | Hank’s solution | NM | NM | NM | [108] |
| 2017 | chitosan-Cap@PDA-chitosan-Ag@PDA | AZ31 | SBF | 8.3 | 1:50 | 14 days | [109] |
| 2017 | Cu-releasing BG/PCL Coating | pure Mg | DMEM | NM | NM | 7 days | [67] |
| 2019 | Ag and Au NPs enriched PD-HF coating | AZ31 | SBF | 7.4 | 1:30 | 7 days | [24] |
| 2019 | Copper-containing MAO coating | Mg-2Zn-1Gd-0.5Zr | Hank’s solution | 7.7 | 1.25 | 14 days | [79] |
| 2019 | Polyurethanes (PU) | MG | SBF | >7.5 | 0.0647 | 49 days | [106] |
| Year | Icorr (μA/cm2) | Ecorr (V) | CR (mm/y) | Refs. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | Inner | Composite | Substrate | Inner | Composite | Substrate | Inner | Composite | ||
| 2015 | 2.96 | NM | PGA30:6.75/PGA50:1.63 | −1.47 | NM | PGA30: −1.39/PGA50: −1.294 | 1.71 ± 0.21 | NM | PGA30:1.48 ± 0.16/PGA50:1.21 ± 0.48 | [53] |
| 2016 | 1.79 | NM | 5.53 × 10−2 | −1.59 | NM | −1.53 | NM | NM | NM | [102] |
| 2016 | 2100 | NM | MgCP30: 980/MgCP60:570 | −1.8 | NM | MgCP30: −1.51/MgCP60: −1.43 | 24.69 | NM | MgCP30: 13.21/MgCP60:7.03 | [90] |
| 2016 | 14.02 | 3.260 × 10−4 | 1.843 × 10−4 | −1.508 | −1.342 | −1.303 | NM | NM | NM | [97] |
| 2017 | 9.55 | 5.85 | 2.97 | −1.58 | −1.39 | −1.36 | 2.06 × 10−2 | 1.26 × 10−2 | 0.64 × 10−2 | [108] |
| 2017 | 120 | NM | 100 | −1.57 | NM | −1.5 | NM | NM | NM | [109] |
| 2019 | 25.137 | 3.189 | Ag:2.33/Au:2.47 | −1.48 | −1.45 | Ag:−1.33/Au:−1.17 | 82.24 | 10.43 | Ag:7.61/Au:8.07 | [24] |
| 2019 | 5.460 ± 0.990 | 0.273 ± 0.051 | 0.168 ± 0.042 | −1.510 ± 0.010 | −1.520 ± 0.010 | −1.510 ± 0.010 | 0.120 ± 0.023 | 0.006 ± 0.001 | 0.004 ± 0.001 | [79] |
| 2019 | 353.8 ± 14.5 | 80.38 ± 3.7 | 1.422 ± 0.07 | −2.140 ± 0.02 | −1.9176 ± 0.02 | −1.7007 ± 0.03 | NM | NM | NM | [106] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Liu, Y.; Liu, Z.; Wang, Q. Advances in Antibacterial Functionalized Coatings on Mg and Its Alloys for Medical Use—A Review. Coatings 2020, 10, 828. https://doi.org/10.3390/coatings10090828
Zhang D, Liu Y, Liu Z, Wang Q. Advances in Antibacterial Functionalized Coatings on Mg and Its Alloys for Medical Use—A Review. Coatings. 2020; 10(9):828. https://doi.org/10.3390/coatings10090828
Chicago/Turabian StyleZhang, Dan, Ying Liu, Zhaogang Liu, and Qiang Wang. 2020. "Advances in Antibacterial Functionalized Coatings on Mg and Its Alloys for Medical Use—A Review" Coatings 10, no. 9: 828. https://doi.org/10.3390/coatings10090828





