Microstructure and Properties of the AlCrSi(O)N Tool Coatings by Arc Ion Plating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Deposition of the AlCrSi(O)N Coatings
2.2. Coating Characterization
2.3. Cutting Tests
3. Results and Discussion
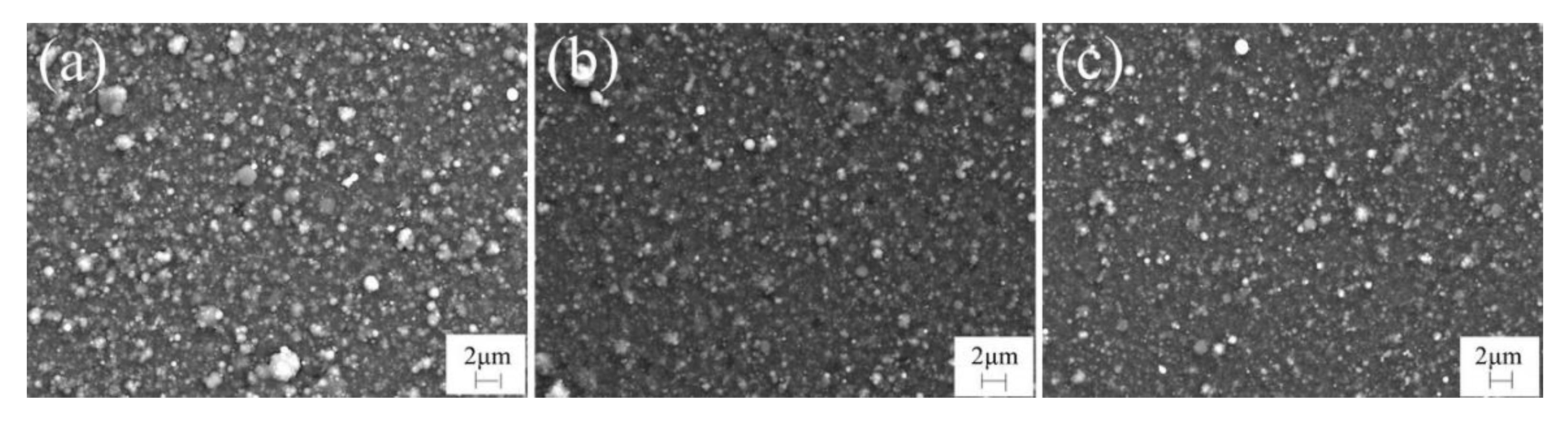
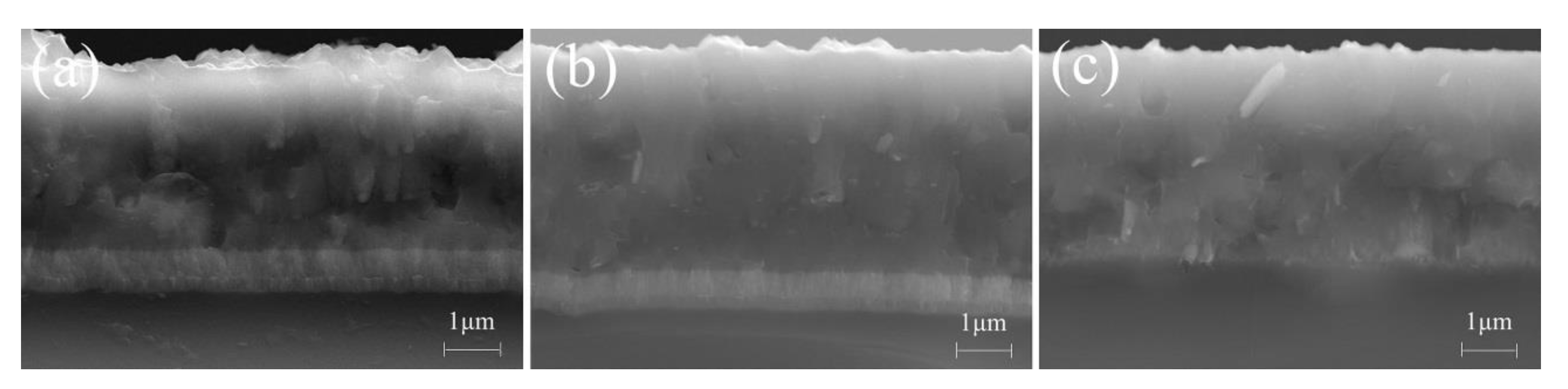
3.1. Deposition Rate and Microstructure
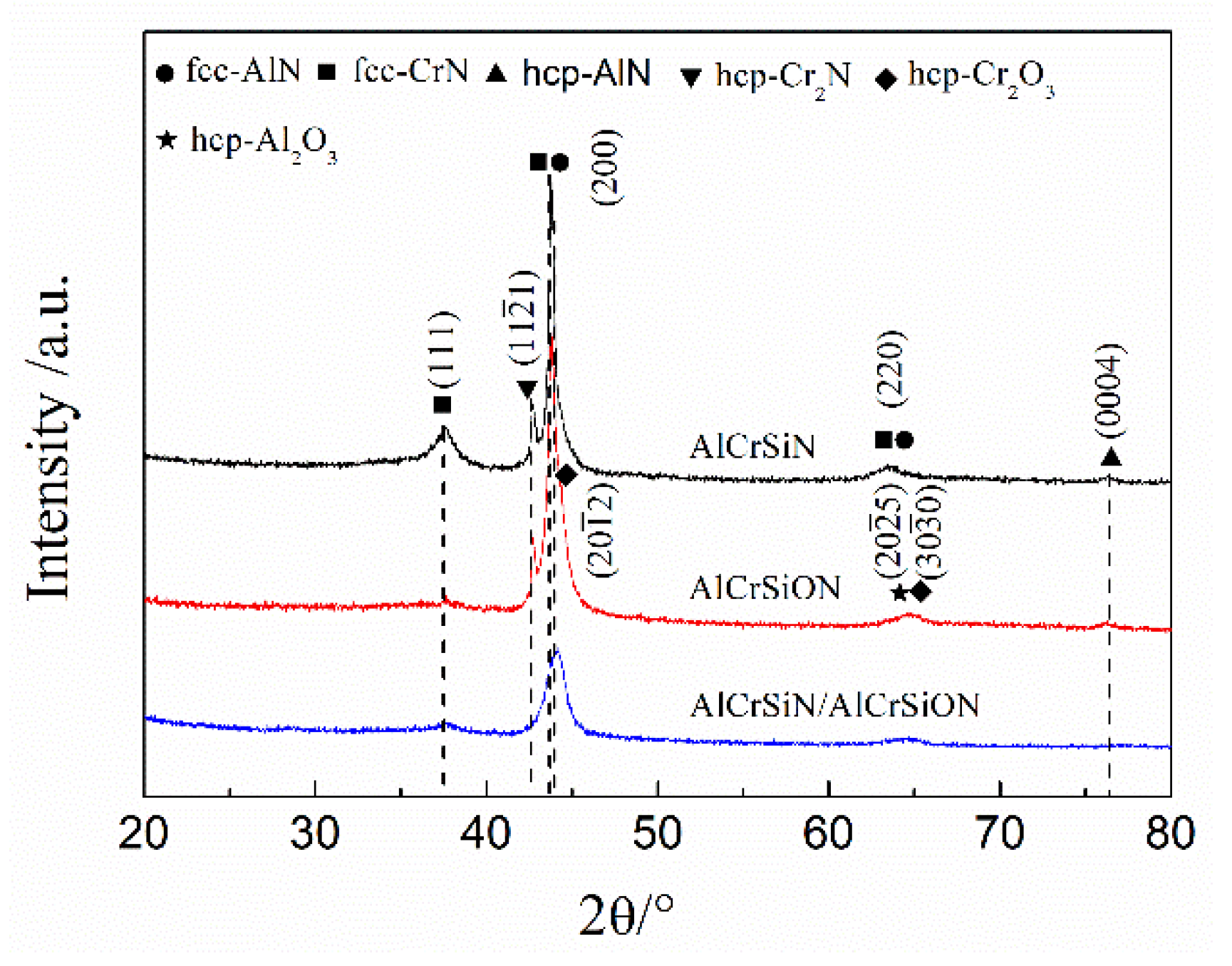
3.2. Composition and Phase Constituents
3.3. Mechanical Properties
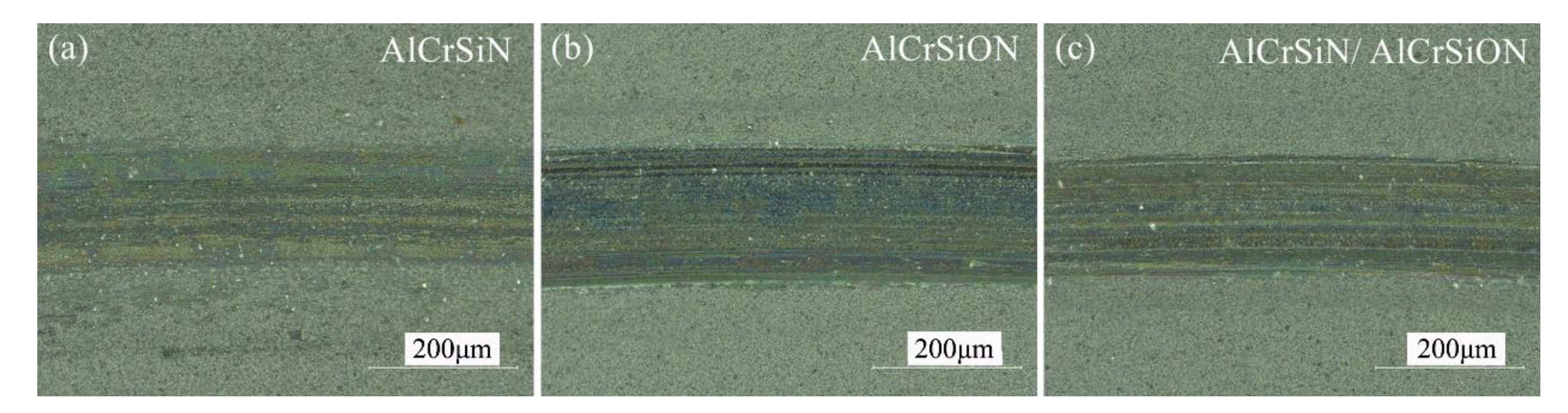
3.4. Tribological Behaviors
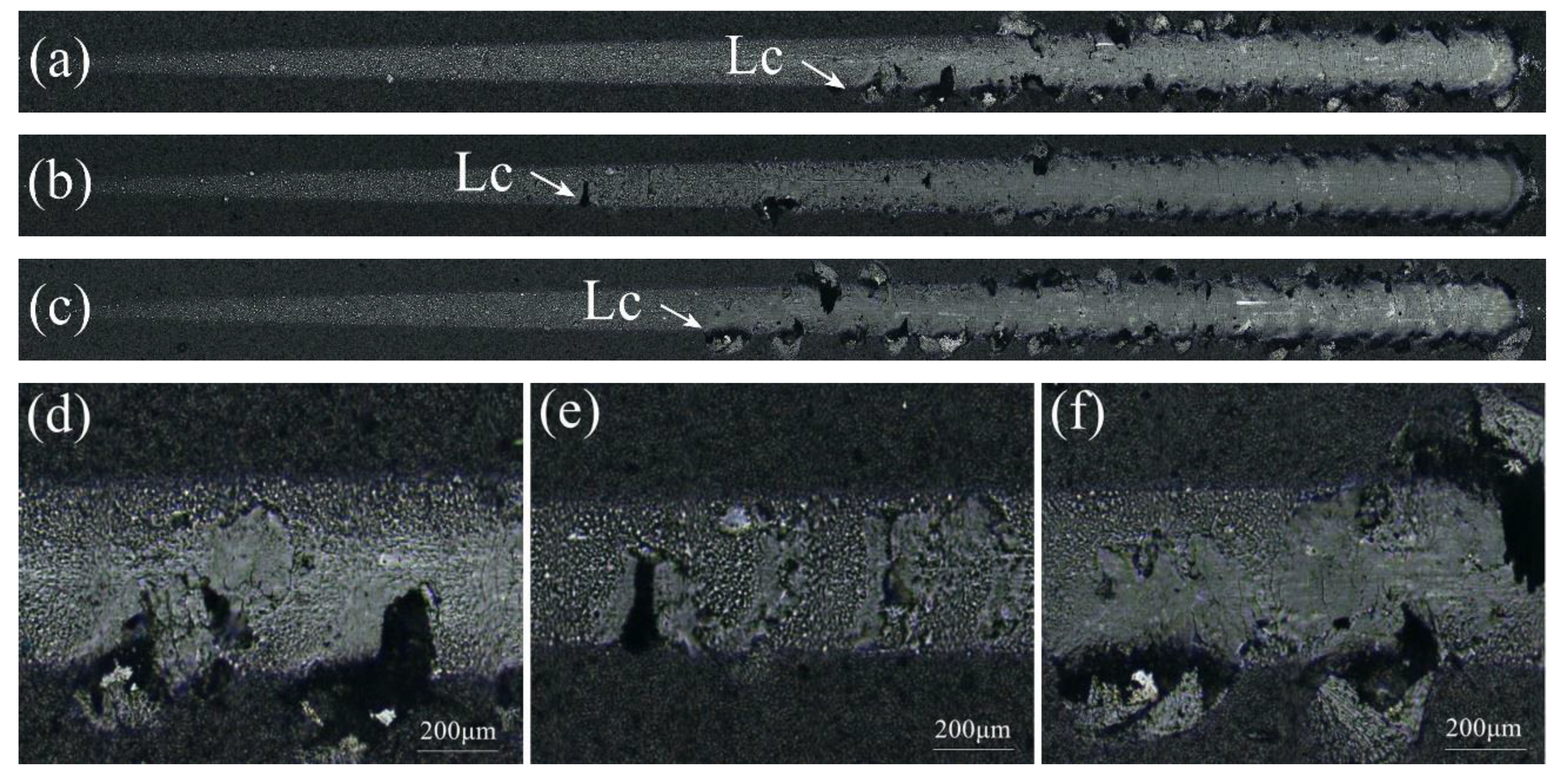
3.5. Cutting tests
4. Conclusions
- (1)
- All the coatings possessed the dense structure, and the face-centered cubic (Al,Cr)N crystallites preferentially grew along the (200) crystal plane. However, a hexagonal oxide phase appeared in the AlCrSiON coating.
- (2)
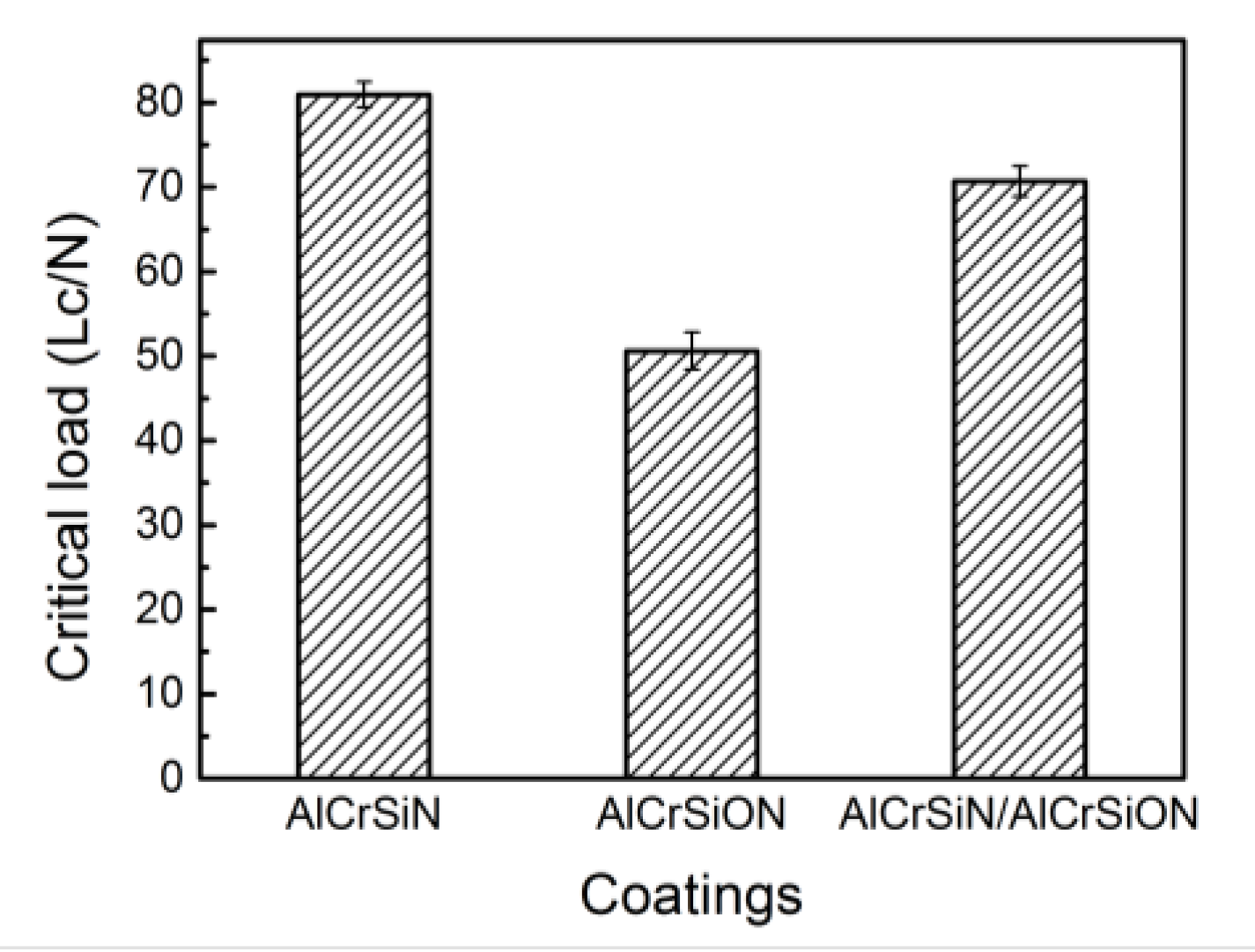
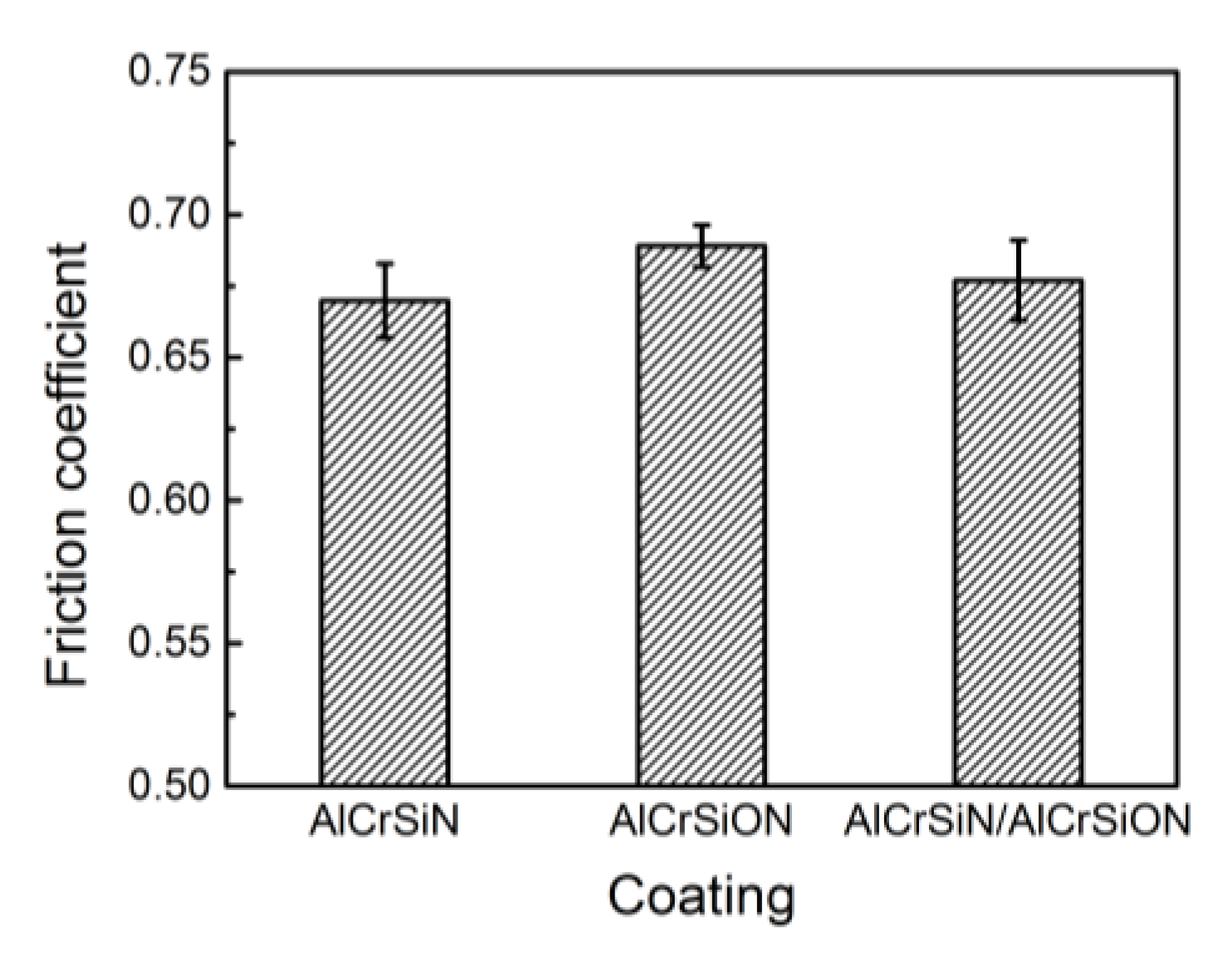
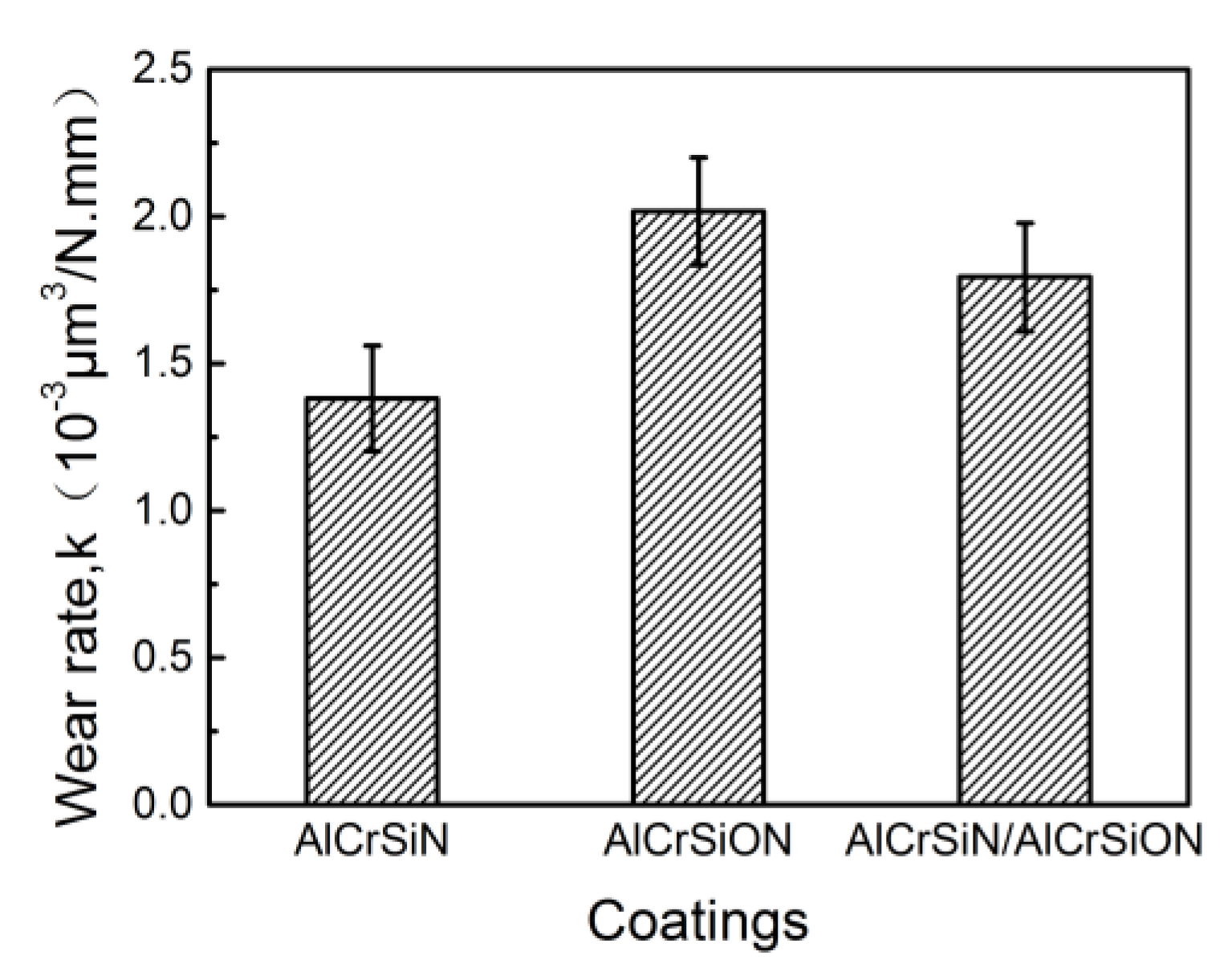
- The AlCrSiN coatings possessed the best mechanical properties, and the coating hardness and critical load reached 4000 HV and 81 N, respectively. Moreover, this coating showed the lowest friction coefficient and the best wear resistance.
- (3)
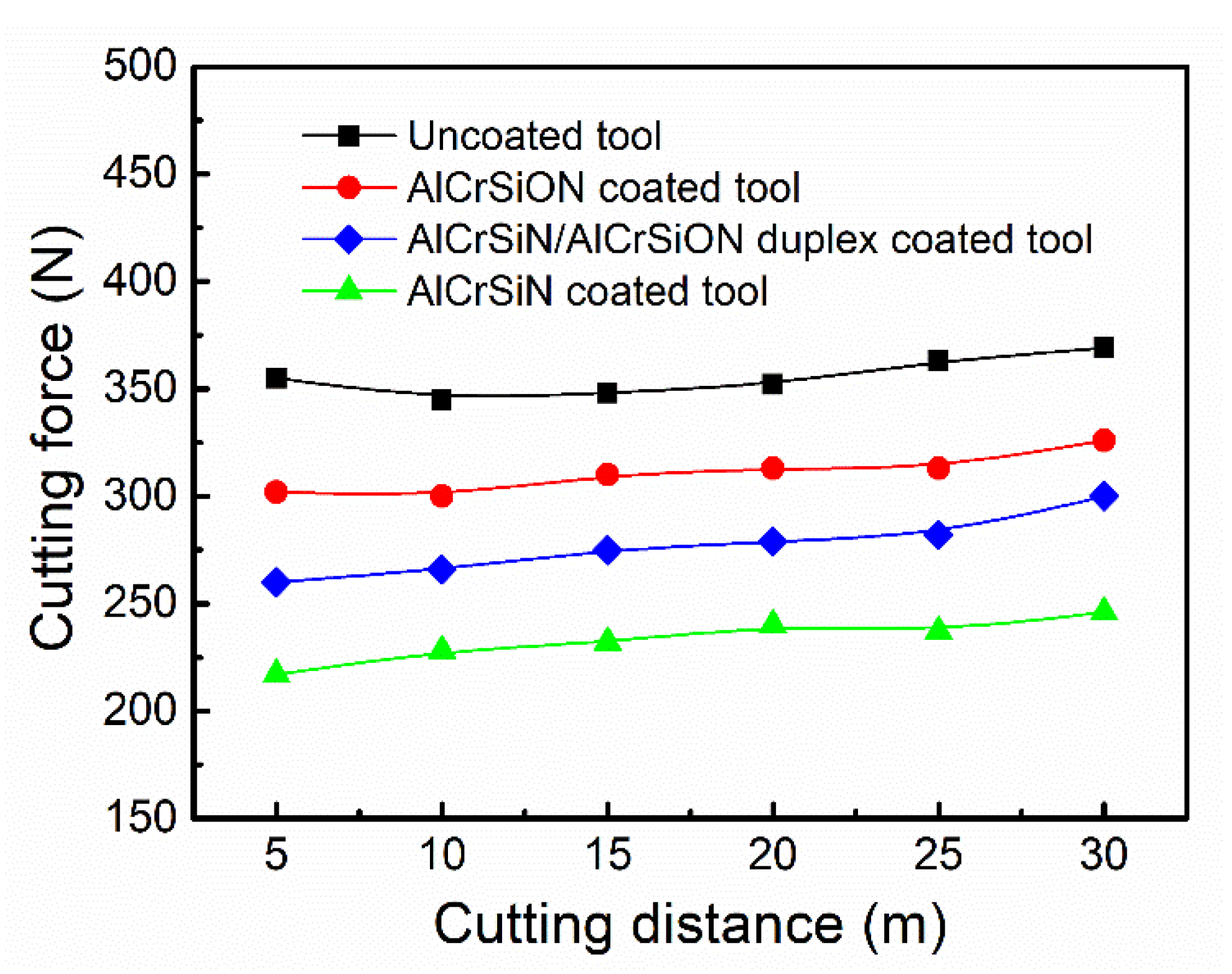
- By a comparison of cutting tests, the Al-Cr-Si-N coated tool presented the minimum cutting force. After 90 min of dry milling, the width of flank wear of the AlCrSiN coated tool reached 135 μm, which was much lower than that of the AlCrSiON coated tool and AlCrSiN/AlCrSiON duplex coated tool.
Author Contributions
Funding
Conflicts of Interest
References
- Bag, R.; Panda, A.; Sahoo, A.K.; Kumar, R. Cutting tools characteristics and coating depositions for hard part turning of AISI 4340 martensitic steel: A review study. Mater. Today Proc. 2020, 26, 2073–2078. [Google Scholar] [CrossRef]
- Vereschaka, A.; Tabakov, V.; Grigoriev, S.; Sitnikov, N.; Milovich, F.; Andreev, N.; Sotova, C.; Kutina, N. Investigation of the influence of the thickness of nanolayers in wear-resistant layers of Ti-TiN-(Ti,Cr,Al)N coating on destruction in the cutting and wear of carbide cutting tools. Surf. Coat. Technol. 2020, 385, 125402. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Z. Influences of coating thickness on cutting temperature for dry hard turning Inconel 718 with PVD TiAlN coated carbide tools in initial tool wear stage. J. Manuf. Process. 2020, 56, 1155–1165. [Google Scholar] [CrossRef]
- He, Q.; Paiva, J.M.; Kohlscheen, J.; Beake, B.; Veldhuis, S. An integrative approach to coating/carbide substrate design of CVD and PVD coated cutting tools during the machining of austenitic stainless steel. Ceram. Int. 2020, 46, 5149–5158. [Google Scholar] [CrossRef]
- Iram, S.; Cai, F.; Wang, J.; Zhang, J.; Liang, J.; Ahmad, F.; Zhang, S. Effect of Addition of Mo or V on the Structure and Cutting Performance of AlCrN-Based Coatings. Coatings 2020, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Hao, G.; Liu, Z.; Liang, X.; Zhao, J. Influences of TiAlN coating on cutting temperature during orthogonal machining H13 hardened steel. Coatings 2019, 9, 355. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.R.; Xu, Y.X.; Peng, B.; Wei, W.; Chen, L.; Wang, Q. Structure and property optimization of Ni-containing AlCrSiN coatings by nano-multilayer construction. J. Alloy. Compd. 2019, 808, 151630. [Google Scholar] [CrossRef]
- Cai, F.; Gao, Y.; Zhang, S.; Zhang, L.; Wang, Q. Gradient architecture of Si containing layer and improved cutting performance of AlCrSiN coated tools. Wear 2019, 193–202. [Google Scholar] [CrossRef]
- Bobzin, K.; Brögelmann, T.; Kruppe, N.; Carlet, M. Wear behavior and thermal stability of HPPMS (Al,Ti,Cr,Si)ON, (Al,Ti,Cr,Si)N and (Ti,Al,Cr,Si)N coatings for cutting tools. Surf. Coat. Technol. 2020, 385, 125370. [Google Scholar] [CrossRef]
- Bobzin, K.; Brögelmann, T.; Kruppe, N.; Carlet, M. Nanocomposite (Ti,Al,Cr,Si)N HPPMS coatings for high performance cutting tools. Surf. Coat. Technol. 2019, 378, 124857. [Google Scholar] [CrossRef]
- Liu, Z.R.; Peng, B.; Xu, Y.X.; Zhang, Q.; Wang, Q.; Chen, L. Influence of Ni-addition on mechanical, tribological properties and oxidation resistance of AlCrSiN coatings. Ceram. Int. 2019, 45, 3735–3742. [Google Scholar] [CrossRef]
- Tillmann, W.; Fehr, A.; Stangier, D. Microstructural and tribological properties of sputtered AlCrSiWN films deposited with segmented powder metallurgic target materials. Thin Solid Films 2019, 687, 137465. [Google Scholar] [CrossRef]
- Lukaszkowicz, K.; Kubacki, J.; Balin, K.; Sondor, J.; Pancielejko, M. Characteristics of CrAlSiN+MoS2 coating deposited by cathodic arc and magnetron sputtering process. Vacuum 2019, 163, 360–367. [Google Scholar] [CrossRef]
- Ahmad, F.; Zhang, L.; Zheng, J.; Sidra, I.; Cai, F.; Zhang, S. Structural evolution and high-temperature tribological properties of AlCrON coatings deposited by multi-arc ion plating. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Geng, D.; Li, H.; Zhang, Q.; Zhang, X.; Wang, C.; Wu, Z.; Wang, Q. Effect of incorporating oxygen on microstructure and mechanical properties of AlCrSiON coatings deposited by arc ion plating. Surf. Coat. Technol. 2017, 310, 223–230. [Google Scholar] [CrossRef]
- Chen, W.; Fang, B.; Zhang, D.; Meng, X.; Zhang, S. Thermal stability and mechanical properties of HVOF/PVD duplex ceramic coatings produced by HVOF and cathodic vacuum arc. Ceram. Int. 2017, 43, 7415–7423. [Google Scholar] [CrossRef]
- Li, W.; Tang, P.; Shang, L.; He, D.; Wang, L.; Zhang, G.; Jin, K. Tribological behaviors of CrN/Cr3C2-NiCr duplex coating at elevated temperatures. Surf. Coat. Technol. 2019, 378, 124926. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, J.; Zhang, P.; Wu, K.; Li, J.; Wang, Y.; Liu, G.; Zhang, G.; Sun, J. Modulation-dependent deformation behavior and strengthening response in nanostructured Ti/Zr multilayers. Appl. Surf. Sci. 2020, 502, 144118. [Google Scholar] [CrossRef]
- Li, B.S.; Wang, T.G.; Ding, J.; Cai, Y.; Shi, J.; Zhang, X. Influence of N2/Ar flow ratio on microstructure and properties of the AlCrSiN coatings deposited by high-power impulse magnetron sputtering. Coatings 2018, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.J.; Wang, T.G.; Yan, B.; Liu, Y.; Jiang, S.; Dong, Y. Influence of DC bias voltage on structure and performance of the Al-Cr-Si-N coatings prepared by arc ion plating technique. Rare Metal. Mat. Eng. 2018, 47, 2232–2239. [Google Scholar]
- Toubhans, B.; Fromentin, G.; Viprey, F.; Karaouni, H.; Dorlin, T. Machinability of inconel 718 during turning: Cutting force model considering tool wear, influence on surface integrity. J. Mater. Process. Technol. 2020, 285, 116809. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Cai, F.; Yang, Y.; Wang, Q.; Zhang, S. Characterization and corrosion behaviors of TiN/TiAlN multilayer coatings by ion source enhanced hybrid arc ion plating. Surf. Coat. Technol. 2019, 366, 355–365. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Chen, H. Corrosion mechanism investigation of TiAlN/CrN superlattice coating by multi-arc ion plating in 3.5 wt% NaCl solution. Surf. Coat. Technol. 2020, 391, 125660. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, J.; Bai, G.; Zhang, H. Microstructure and thermal conductivity of AlN coating on Cu substrate deposited by arc ion plating. Mater. Chem. Phys. 2020, 241, 122374. [Google Scholar] [CrossRef]
- Arif, M.; Eisenmenger-Sittner, C. In situ assessment of target poisoning evolution in magnetron sputtering. Surf. Coat. Technol. 2017, 324, 345–352. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, X.; Lin, L.; Zou, L. Effect of bias voltage on mechanical properties, milling performance and thermal crack propagation of cathodic arc ion-plated TiAlN coatings. Thin Solid Films 2020, 708, 138116. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Pei, Z.; Li, W.; Liu, J.; Gong, J.; Sun, C. Oxidation and hot corrosion behavior of NiCrAlYSi+NiAl/cBN abrasive coating. Corros. Sci. 2020, 167, 108486. [Google Scholar] [CrossRef]
- Liang, J.; Chen, S.; Zou, C.; Tian, C.; Wang, T.; Liao, S. Influence of oxygen contents on the microstructure, high temperature oxidation and corrosion resistance properties of Cr–Si–O–N coatings. Coatings 2018, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Karimi, A.; Morstein, M.; Cselle, T. Influence of oxygen content on structure and properties of multi-element AlCrSiON oxynitride thin films. Surf. Coat. Technol. 2010, 204, 2716–2722. [Google Scholar] [CrossRef]
- Tillmann, W.; Grisales, D.; Stangier, D.; Ben Jebara, I.; Kang, H. Influence of the etching processes on the adhesion of TiAlN coatings deposited by DCMS, HiPIMS and hybrid techniques on heat treated AISI H11. Surf. Coat. Technol. 2019, 378, 125075. [Google Scholar] [CrossRef]
- Wang, T.-G.; Zhao, S.-S.; Hua, W.-G.; Gong, J.; Sun, C. Design of a separation device used in detonation gun spraying system and its effects on the performance of WC–Co coatings. Surf. Coat. Technol. 2009, 203, 1637–1644. [Google Scholar] [CrossRef]
- Barthelmä, F.; Frank, H.; Mahr, P.; Reich, S. Oxygen-improved hard coatings for high performance cutting processes. Procedia CIRP 2012, 1, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Zhang, Y.; Wang, C.; Chen, Z.; Tang, N.; Chen, B. Effect of cutting parameters on cutting force and surface quality in cutting of articular cartilage. Procedia CIRP 2020, 89, 116–121. [Google Scholar] [CrossRef]




| Parameter | Values |
|---|---|
| Base pressure/Pa | 2.0 × 10−3 |
| Working pressure/Pa | 2.9 |
| Deposition temperature/°C | 450 |
| Arc current of AlCrSi cathode/A | 100 |
| Bias voltage/V | 80–200 |
| N2/Ar/O2 gas flow ratio/Sccm | 600/50/20 |
| Substrate rotation speed/(r·min−1) | 20 |
| Distance between the target and substrate/mm | 220 |
| Coating | Al | Cr | Si | N | O | Al:Cr | Al:Si |
|---|---|---|---|---|---|---|---|
| AlCrSiN | 26.16 | 16.69 | 4.04 | 53.11 | 0 | 1.57 | 6.48 |
| AlCrSiON | 24.62 | 14.42 | 3.79 | 22.72 | 34.46 | 1.71 | 6.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, T.-G.; Lin, W.; Zhu, Q.; Yan, B.; Hou, X. Microstructure and Properties of the AlCrSi(O)N Tool Coatings by Arc Ion Plating. Coatings 2020, 10, 841. https://doi.org/10.3390/coatings10090841
Liu Y, Wang T-G, Lin W, Zhu Q, Yan B, Hou X. Microstructure and Properties of the AlCrSi(O)N Tool Coatings by Arc Ion Plating. Coatings. 2020; 10(9):841. https://doi.org/10.3390/coatings10090841
Chicago/Turabian StyleLiu, Yanmei, Tie-Gang Wang, Wei Lin, Qiang Zhu, Bing Yan, and Xiang Hou. 2020. "Microstructure and Properties of the AlCrSi(O)N Tool Coatings by Arc Ion Plating" Coatings 10, no. 9: 841. https://doi.org/10.3390/coatings10090841





