Highly Transparent Conducting Electrodes Based on a Grid Structure of Silver Nanowires
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Substrates
2.2. Patterning of the Substrates
2.3. Fabrication of AgNW Films
2.4. Characterization of AgNW Films
3. Results and Discussion
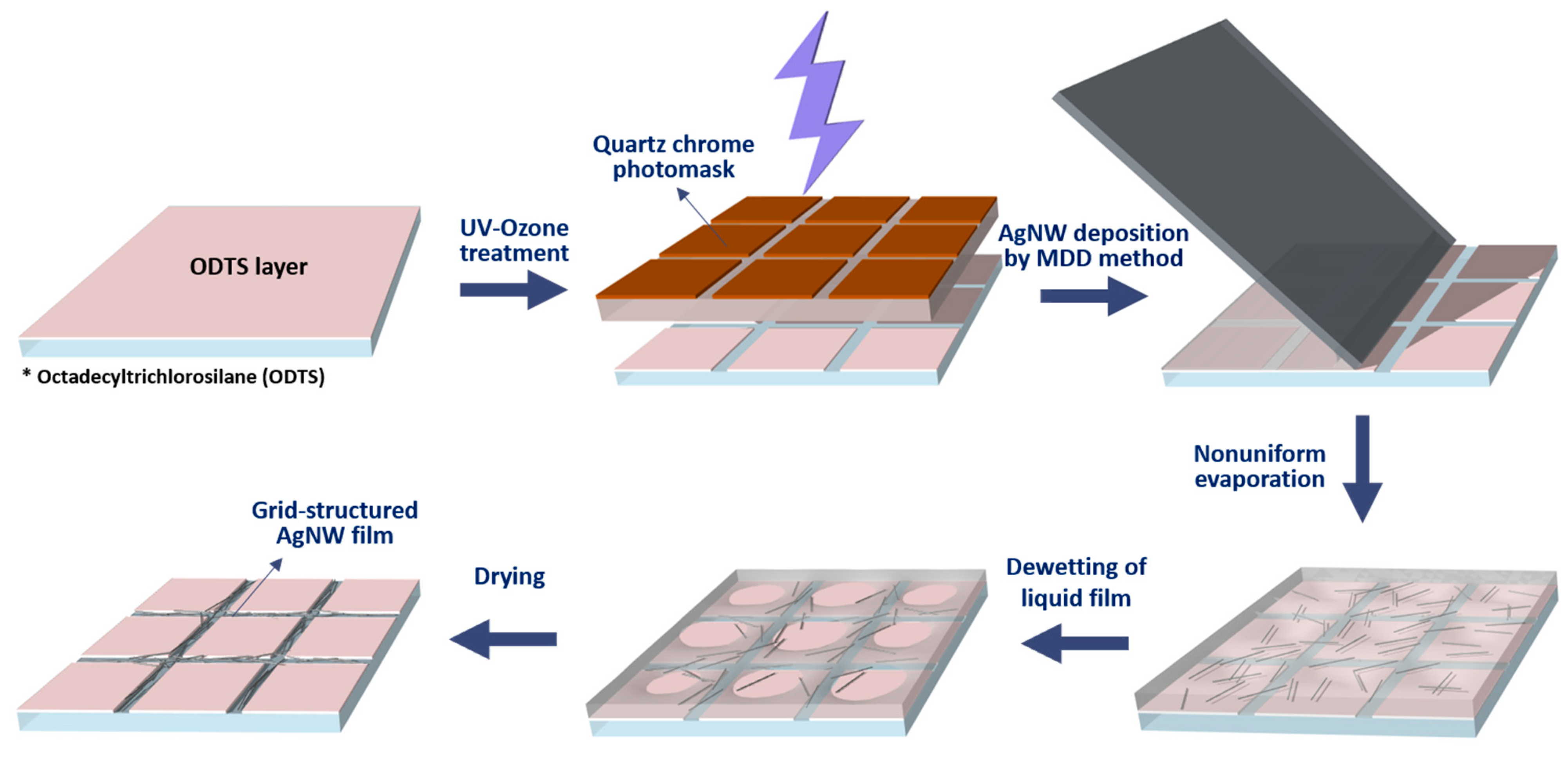
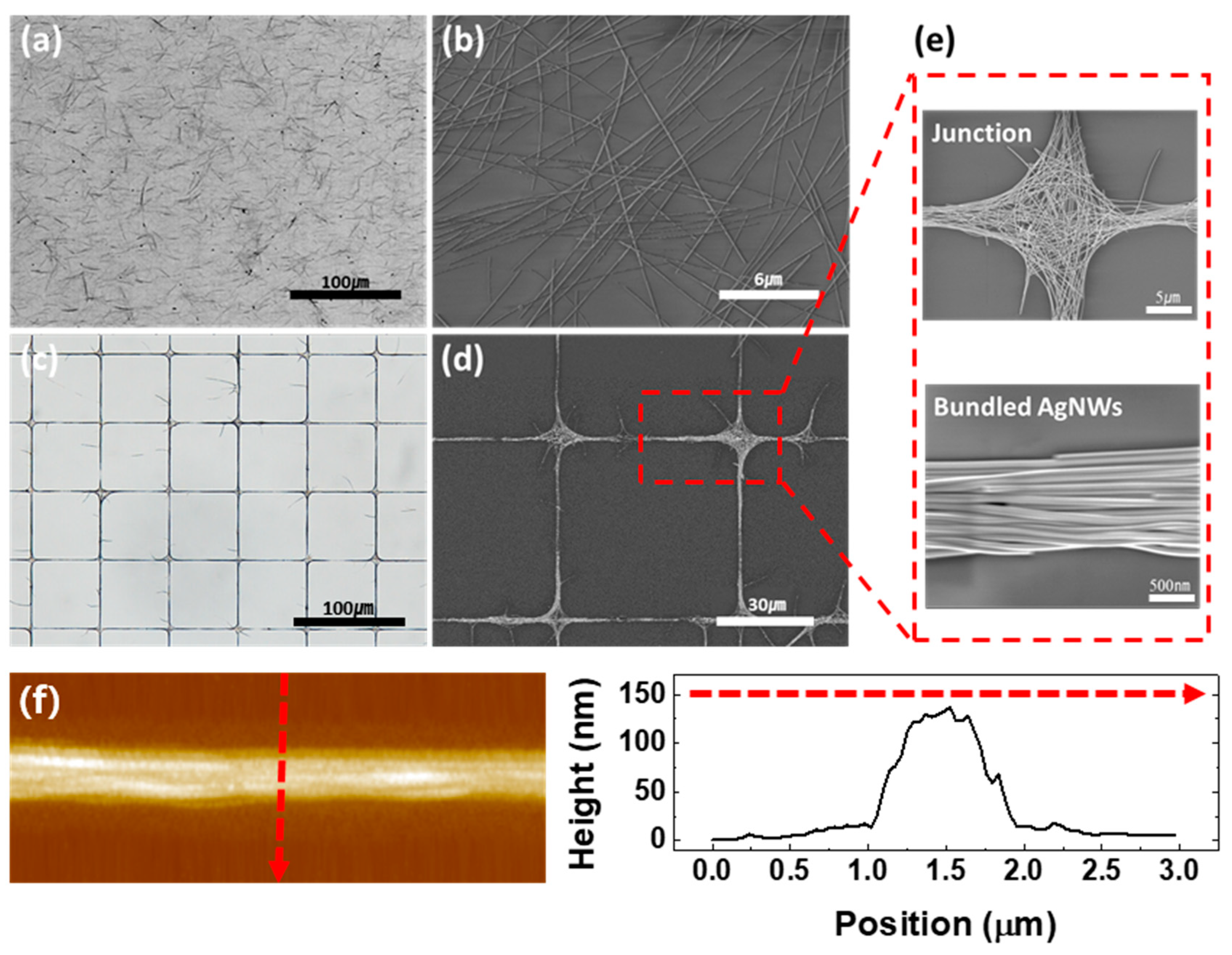
3.1. Fabrication of TCEs Based on Grid-Patterned AgNWs
3.2. Principle of Pattern Formation
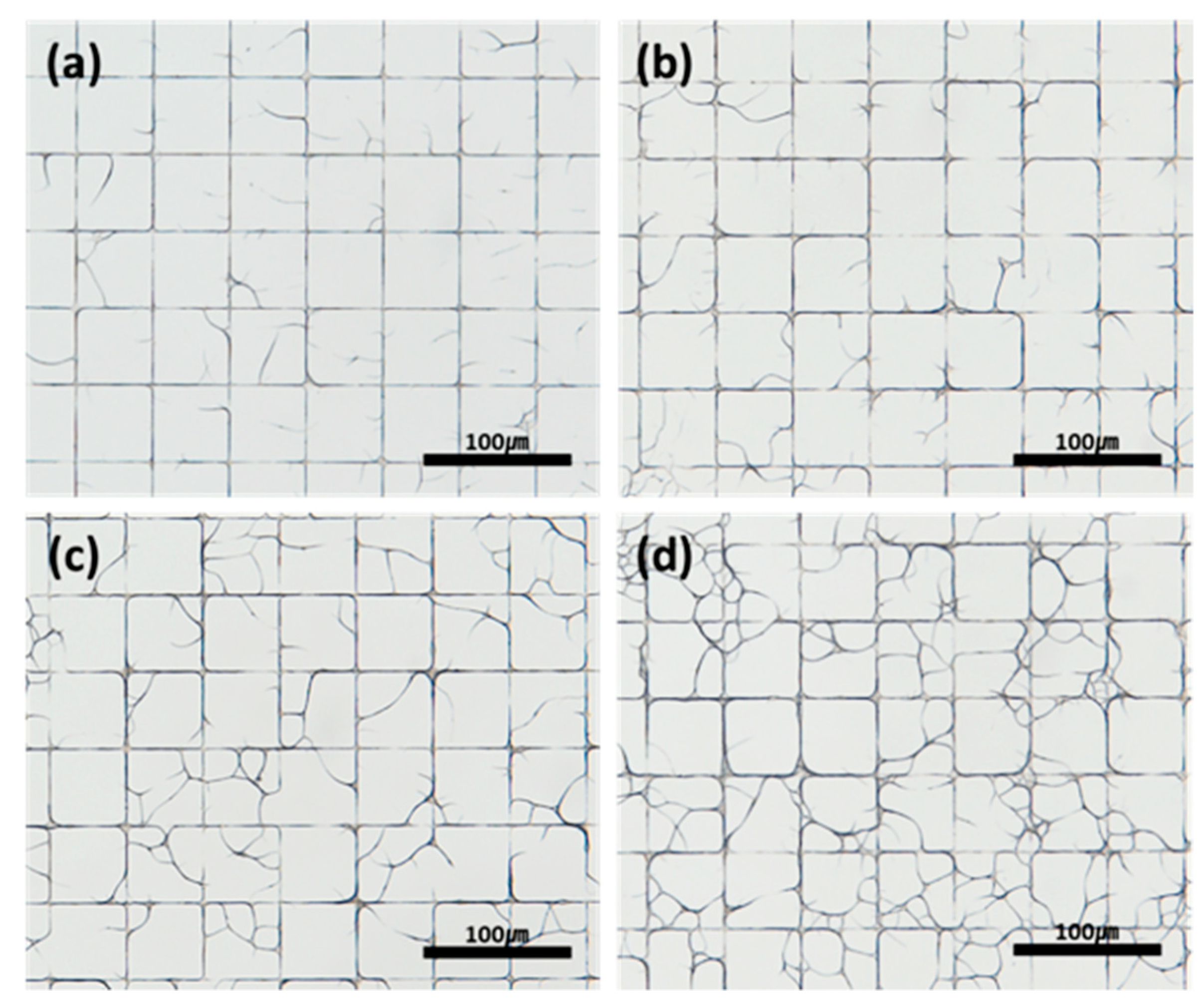
3.3. Effect of Pattern Design
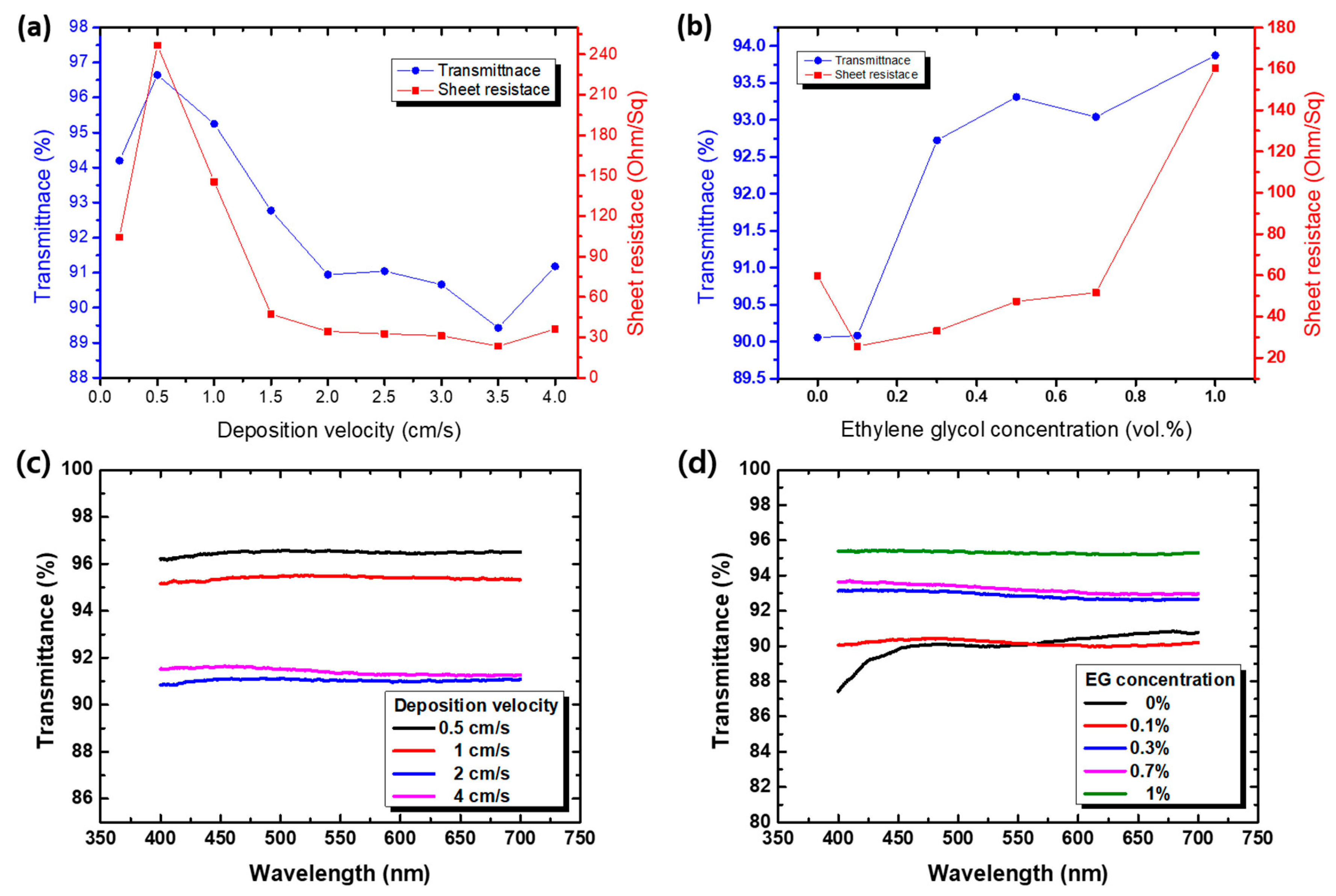
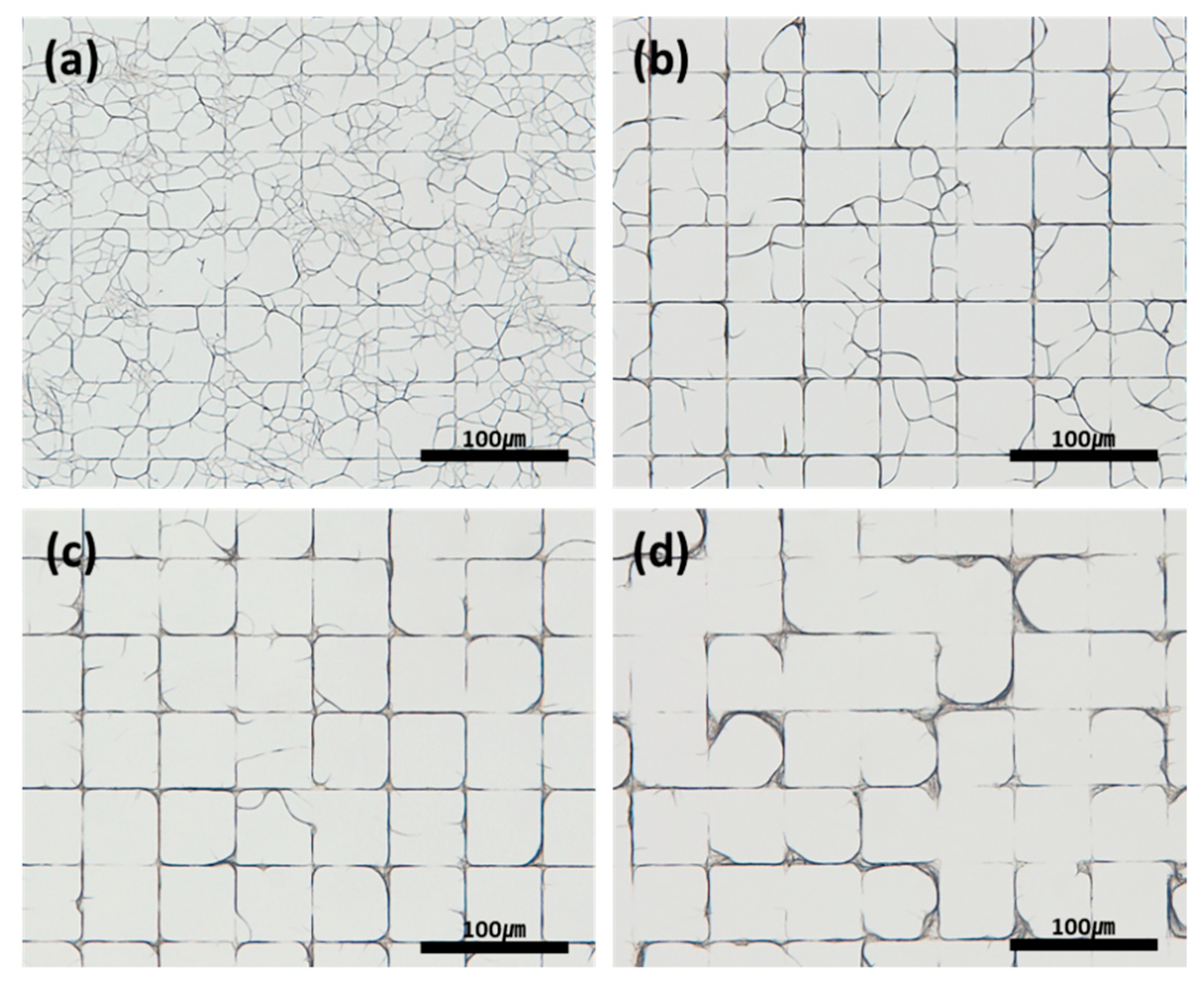
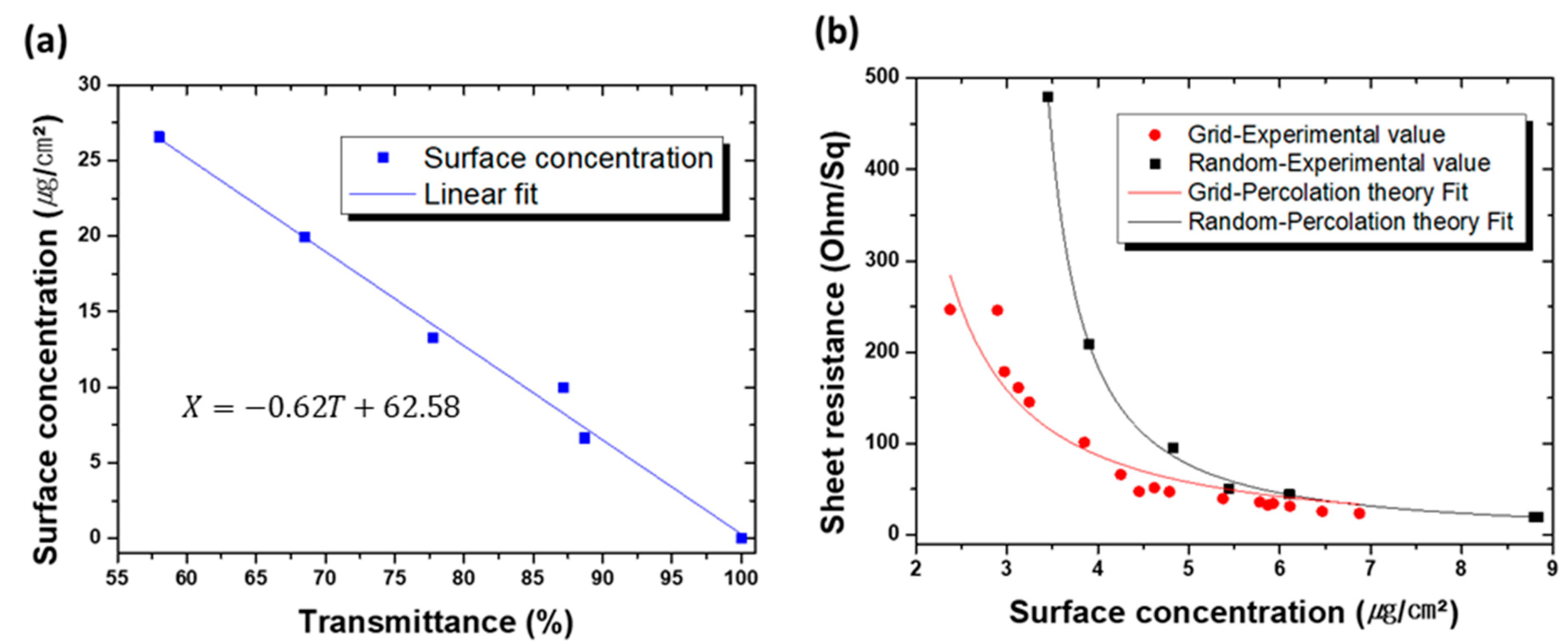
3.4. Effect of the Deposition Conditions
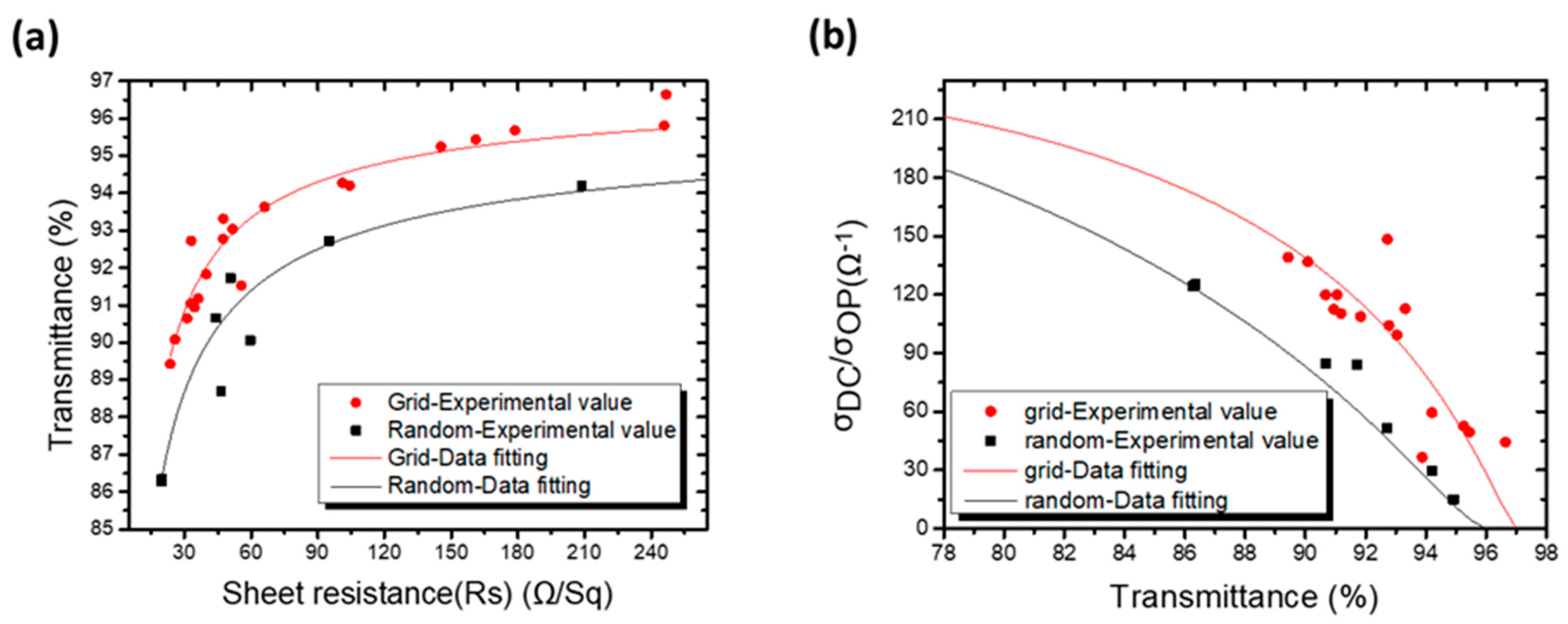
3.5. Performance of Grid-Structured AgNW Electrodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, L.; Tjong, S.C. Nanostructured transparent conductive films: Fabrication, characterization and applications. Mater. Sci. Eng. R Rep. 2016, 109, 1–101. [Google Scholar] [CrossRef]
- Pang, S.; Hernandez, Y.; Feng, X.; Müllen, K. Graphene as transparent electrode material for organic electronics. Adv. Mater. 2011, 23, 2779–2795. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-K.; Huang, J.-T.; Lee, T.-H.; Wang, J.-J.; Liu, D.-S. Flexibility of the indium tin oxide transparent conductive film deposited onto the plastic substrate. Smart Sci. 2014, 2, 7–12. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zeng, H. ZnO-based transparent conductive thin films: Doping, performance, and processing. J. Nanomater. 2013, 2013, 196521. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Liu, Y.-F.; Xiong, P.-P.; Chen, P.; Li, H.-Y.; Hou, J.-W.; Kang, B.-N.; Duan, Y. Aluminum-doped zinc oxide transparent electrode prepared by atomic layer deposition for organic light emitting devices. IEEE Trans. Nanotechnol. 2017, 16, 634–638. [Google Scholar] [CrossRef]
- Dan, B.; Irvin, G.C.; Pasquali, M. Continuous and scalable fabrication of transparent conducting carbon nanotube films. ACS Nano 2009, 3, 835–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Chen, Z.; Du, X.; Logan, J.M.; Sippel, J.; Nikolou, M.; Kamaras, K.; Reynolds, J.R.; Tanner, D.B.; Hebard, A.F. Transparent, conductive carbon nanotube films. Science 2004, 305, 1273–1276. [Google Scholar] [CrossRef]
- Ko, Y.U.; Cho, S.-R.; Choi, K.S.; Park, Y.; Kim, S.T.; Kim, N.H.; Kim, S.Y.; Chang, S.T. Microlitre scale solution processing for controlled, rapid fabrication of chemically derived graphene thin films. J. Mater. Chem. 2012, 22, 3606–3613. [Google Scholar] [CrossRef]
- Layani, M.; Darmawan, P.; Foo, W.L.; Liu, L.; Kamyshny, A.; Mandler, D.; Magdassi, S.; Lee, P.S. Nanostructured electrochromic films by inkjet printing on large area and flexible transparent silver electrodes. Nanoscale 2014, 6, 4572–4576. [Google Scholar] [CrossRef]
- Guo, H.; Lin, N.; Chen, Y.; Wang, Z.; Xie, Q.; Zheng, T.; Gao, N.; Li, S.; Kang, J.; Cai, D. Copper nanowires as fully transparent conductive electrodes. Sci. Rep. UK 2013, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.; Song, S.K.; Kim, N.H.; Chang, S.T. Highly transparent and stretchable conductors based on a directional arrangement of silver nanowires by a microliter-scale solution process. Langmuir 2016, 32, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.; Kwon, J.W. Silver nanowire-based transparent electrode as FTO replacement for dye-sensitized solar cell. Int. Nano Lett. 2019, 9, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Gebeyehu, M.B.; Chala, T.F.; Chang, S.-Y.; Wu, C.-M.; Lee, J.-Y. Synthesis and highly effective purification of silver nanowires to enhance transmittance at low sheet resistance with simple polyol and scalable selective precipitation method. RSC Adv. 2017, 7, 16139–16148. [Google Scholar] [CrossRef] [Green Version]
- Korte, K.E.; Skrabalak, S.E.; Xia, Y. Rapid synthesis of silver nanowires through a CuCl-or CuCl2-mediated polyol process. J. Mater. Chem. 2008, 18, 437–441. [Google Scholar] [CrossRef]
- Yun, H.D.; Seo, D.M.; Lee, M.Y.; Kwon, S.Y.; Park, L.S. Effective synthesis and recovery of silver nanowires prepared by tapered continuous flow reactor for flexible and transparent conducting electrode. Metals-Basel 2016, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Ouyang, Z.; Jia, B.; Gu, M. Large-size, high-uniformity, random silver nanowire networks as transparent electrodes for crystalline silicon wafer solar cells. Opt. Express 2013, 21, A355–A362. [Google Scholar] [CrossRef]
- Lee, C.; Oh, Y.; Yoon, I.S.; Kim, S.H.; Ju, B.-K.; Hong, J.-M. Flash-induced nanowelding of silver nanowire networks for transparent stretchable electrochromic devices. Sci. Rep. UK 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Xiong, W.; Liu, H.; Chen, Y.; Zheng, M.; Zhao, Y.; Kong, X.; Wang, Y.; Zhang, X.; Kong, X.; Wang, P. Highly conductive, air-stable silver nanowire@ iongel composite films toward flexible transparent electrodes. Adv. Mater. 2016, 28, 7167–7172. [Google Scholar] [CrossRef]
- Correa, D.; Medeiros, E.; Oliveira, J.; Paterno, L.; Mattoso, L.H. Nanostructured conjugated polymers in chemical sensors: Synthesis, properties and applications. J. Nanosci. Nanotechnol. 2014, 14, 6509–6527. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, X.; Yang, H.; Chao, Y.; Guo, S.; Wang, C. One-step synthesis of silver nanowires with ultra-long length and thin diameter to make flexible transparent conductive films. Materials 2019, 12, 401. [Google Scholar] [CrossRef] [Green Version]
- Seok, H.-J.; Kim, J.-K.; Kim, H.-K. Effective passivation of Ag nanowire network by transparent tetrahedral amorphous carbon film for flexible and transparent thin film heaters. Sci. Rep. UK 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-W.; Chang, W.S.; Cho, S.H. Laser direct patterning of AgNW/CNT hybrid thin films. Opt. Laser. Eng. 2015, 73, 40–45. [Google Scholar] [CrossRef]
- Lee, M.-S.; Kim, J.; Park, J.; Park, J.-U. Studies on the mechanical stretchability of transparent conductive film based on graphene-metal nanowire structures. Nanoscale. Res. Lett. 2015, 10, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Song, T.; Cui, W.; Liu, Y.; Xu, W.; Lee, S.-T.; Sun, B. Solution-processed highly conductive PEDOT: PSS/AgNW/GO transparent film for efficient organic-Si hybrid solar cells. ACS Appl. Mater. Interfaces 2015, 7, 3272–3279. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Min, S.Y.; Kim, T.S.; Jeong, S.H.; Won, J.Y.; Kim, H.; Xu, W.; Jeong, J.K.; Lee, T.W. Versatile metal nanowiring platform for large-scale nano-and opto-electronic devices. Adv. Mater. 2016, 28, 9109–9116. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-S.; Liu, C.; Chen, H.-J.; Cao, W.; Qiu, J.-S.; Shieh, H.-P.D.; Yang, B.-R. Electrically robust silver nanowire patterns transferrable onto various substrates. Nanoscale 2016, 8, 5507–5515. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, B.; Cho, S.M.; Lee, H.-J.; Hwang, B. Etchant-free patterning of silver nanowire transparent electrode using dry-film photoresists for organic light-emitting diodes. Mater. Lett. 2017, 209, 433–436. [Google Scholar] [CrossRef]
- Ko, D.; Gu, B.; Kang, S.J.; Jo, S.; Hyun, D.C.; Kim, C.S.; Kim, J. Critical work of adhesion for economical patterning of silver nanowire-based transparent electrodes. J. Mater. Chem. A 2019, 7, 14536–14544. [Google Scholar] [CrossRef]
- Liao, Q.; Wang, H.; Zhu, X.; Li, M. Liquid droplet movement on horizontal surface with gradient surface energy. Sci. China Ser. E 2006, 49, 733–741. [Google Scholar] [CrossRef]
- Calvert, J.; Georger, J.; Schnur, J.; Schoen, P.; Peckerar, M.; Pehrsson, P. Deep UV photochemistry and patterning of self-assembled monolayer films. Thin Solid Film. 1992, 210, 359–363. [Google Scholar] [CrossRef]
- Lee, S.M.; Oh, S.; Chang, S.T. Highly transparent, flexible conductors and heaters based on metal nanomesh structures manufactured using an all-water-based solution process. ACS Appl. Mater. Interfaces 2019, 11, 4541–4550. [Google Scholar] [CrossRef] [PubMed]
- Sett, S.; Yan, X.; Barac, G.; Bolton, L.W.; Miljkovic, N. Lubricant-infused surfaces for low-surface-tension fluids: Promise versus reality. ACS. Appl. Mater. Interfaces 2017, 9, 36400–36408. [Google Scholar]
- Li, Q.; Zhou, P.; Yan, H. Pinning–depinning mechanism of the contact line during evaporation on chemically patterned surfaces: A lattice Boltzmann study. Langmuir 2016, 32, 9389–9396. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, B.J.; Ko, Y.; Cho, J.H.; Chang, S.T. Surface energy engineered, high-resolution micropatterning of solution-processed reduced graphene oxide thin films. Adv. Mater. 2013, 25, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Budiman, A.G.; Florijanto, C.; Palen, J. Breakdown of evaporating falling films as a function of surface tension gradient. Heat Transf. Eng. 1996, 17, 72–81. [Google Scholar] [CrossRef]
- Sepulveda-Mora, S.B.; Cloutier, S.G. Figures of merit for high-performance transparent electrodes using dip-coated silver nanowire networks. J. Nanomater. 2012, 2012, 286104. [Google Scholar] [CrossRef]
- Bellet, D.; Lagrange, M.; Sannicolo, T.; Aghazadehchors, S.; Nguyen, V.H.; Langley, D.P.; Muñoz-Rojas, D.; Jiménez, C.; Bréchet, Y.; Nguyen, N.D. Transparent electrodes based on silver nanowire networks: From physical considerations towards device integration. Materials 2017, 10, 570. [Google Scholar] [CrossRef]
- Xiong, J.; Li, S.; Ye, Y.; Wang, J.; Qian, K.; Cui, P.; Gao, D.; Lin, M.F.; Chen, T.; Lee, P.S. A deformable and highly robust ethyl cellulose transparent conductor with a scalable silver nanowires bundle micromesh. Adv. Mater. 2018, 30, 1802803. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.Y.; Kim, J.; Kim, J.H. Highly reliable AgNW/PEDOT: PSS hybrid films: Efficient methods for enhancing transparency and lowering resistance and haziness. J. Mater. Chem. C 2014, 2, 5636–5643. [Google Scholar] [CrossRef]
- Ramadhan, Z.R.; Han, J.W.; Lee, D.J.; Entifar, S.A.N.; Hong, J.; Yun, C.; Kim, Y.H. Surface-functionalized silver nanowires on chitosan biopolymers for highly robust and stretchable transparent conducting films. Mater. Res. Lett. 2019, 7, 124–130. [Google Scholar] [CrossRef]
- Su, Q.; Xue, T.; Zhang, Y.; Lan, K.; Zou, Q. Fabrication of enhanced silver nanowire films via self-assembled gold nanoparticles without post-treatment. Mater. Lett. 2019, 236, 218–221. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, J.; Lee, S.M.; Eom, H.-S.; Chang, S.T. Highly Transparent Conducting Electrodes Based on a Grid Structure of Silver Nanowires. Coatings 2021, 11, 30. https://doi.org/10.3390/coatings11010030
You J, Lee SM, Eom H-S, Chang ST. Highly Transparent Conducting Electrodes Based on a Grid Structure of Silver Nanowires. Coatings. 2021; 11(1):30. https://doi.org/10.3390/coatings11010030
Chicago/Turabian StyleYou, Jinseon, Sung Min Lee, Hong-Sik Eom, and Suk Tai Chang. 2021. "Highly Transparent Conducting Electrodes Based on a Grid Structure of Silver Nanowires" Coatings 11, no. 1: 30. https://doi.org/10.3390/coatings11010030



