Abstract
Thermal barrier coatings (TBCs) play a vitally important role in protecting the hot parts of a gas turbine from high temperature and corrosion effectively. More and more attention has been paid to the performance modification of ZrO2-based ceramics and seeking for new ceramic materials to meet requirements of gas turbine TBCs. The working principle, merits, and demerits of main technologies for coating preparation are elaborated in this paper, and the properties of new ceramic materials are reviewed. It is found that the thermal conductivity, thermal stability, mechanical properties, and other performances of traditional ZrO2-based ceramics could be improved effectively by doping modification. The emphases for new ceramic materials research were put on pyrochlores, magnetoplumbites, rare-earth tantalates, etc. Rare-earth tantalates with great potentials as new top ceramic materials were described in detail. In the end, the development directions of advanced top ceramic coatings, combining doping modification with preparation technology to regulate and control structure property of high-performance ceramic material, were put forward.
1. Introduction
High-performance gas turbines have been widely used as power sources for aircrafts, large ships, and electric power production []. During service, hot-end components of gas turbines need to face complex and harsh conditions, such as high-temperature oxidation, erosion, and corrosion, while thermal barrier coatings (TBCs) can provide effective protection for them [,]. TBCs are usually composed of metal bond coat and top ceramic coating to form an organically combined and synergistic system, as shown in Figure 1a []. The thermal conductivity, high temperature stability, fracture toughness, and thermal expansion coefficient are the key performance parameters of TBCs, which determine its service effect and life [].
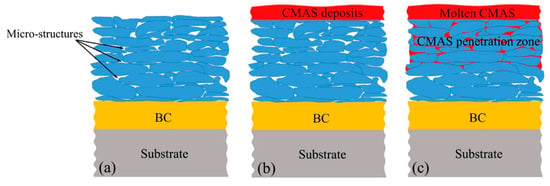
Figure 1.
Schematic diagram of the penetration process of CaO-MgO-Al2O3-SiO2 (CMAS) into atmospheric plasma spraying (APS) TBCs []: (a) Structure of TBCs, (b) CMAS depositing on the TBCs surface, (c) Liquefied CMAS penetrating the top ceramic coating.
In the study of TBCs, the double-layer structure TBCs system with 6–8 wt.% Y2O3 stabilized ZrO2 (6–8YSZ) as the top ceramic material and MCrAlY (M = Ni, Co, Ni + Co, etc.) alloy layer as the bond coat is most widely used [,]. However, with the continuous improvement of the thrust-weight ratio of aero-engines and the improvement of the operational effectiveness of ships, the inlet temperature of gas turbines is getting higher and higher. The most advanced gas turbine inlet temperature (TIT) has reached about 2273 K currently [,,], and the temperature at the surface of turbine blades is also over 1723 K. Although the blade temperature can be effectively reduced by 300–400 K through air cooling and the improvement of the manufacturing process [,], with long-term work under the high temperature of 1473 K, problems such as sintering, phase transformation (with changes of 3–5 vol.%), salt corrosion by CaO-MgO-Al2O3-SiO2 (CMAS) (as shown in Figure 1b,c), and Na2SO4 as well as V2O5, and formation of thermally grown oxide (TGO) layer at the interface between bond coat and ceramic layer, will result in an exponential decline in life expectancy [,,,]. Therefore, in recent years, new coating preparation methods, new coating materials, and coating structure design have become the hot spots of TBCs research. In the research and development of bond coat materials, high-temperature oxidation resistance, which can determine lifetime of TBCs to a large extent, is considered as a key performance of bond coat [,]. Adding some noble metals into MCrAlY to modify its performances is the main current. The addition of Hf, Pt revealed significant improvement of oxidation resistance and thermal cycling resistance [,,]. Cr2AlC with good oxidation resistance [,], CoCrAlY-TiB2 with significantly enhanced anti-oxidation performance [], CuAlNiCrFe with excellent oxidation and diffusion resistance [], are regarded as promising new bond coat materials. Especially in the research and development of new top ceramic materials, on the one hand, researchers doped ZrO2-based ceramic materials to improve their high-temperature resistance, corrosion resistance, and other properties, such as Gd2O3, Yb2O3, Nd2O3, Sc2O3, etc., on the other hand, they actively develop new ceramic materials to replace 6–8YSZ so as to meet the needs of development, such as ultra-high temperature rare-earth tantalate TBCs, which can work above 1873 K. The research and development of these new TBCs have greatly improved the performance and life of high-temperature end service materials. Mehboob et al. [] summarized the deterioration mechanism of TBCs and strategies to extend their service life, and introduced some new ceramic materials with lower thermal conductivity and greater stability at higher temperatures, such as rare-earth zirconates and stannates (RE2Zr2O7, where RE = Gd, Sm, Pr, Eu, Nd, and La, and RE2Sn2O7, where RE = Yb, Gd, Er, Sm, and La). Thakare et al. [] retrospected preparation techniques and current developments in ceramic materials, mechanical properties, high temperature resistance performance, deterioration mechanisms, and service life prediction models for TBCs. Zhang et al. [] reviewed the latest literature about the manufacturing technologies regarding La2Zr2O7 based TBCs; they evaluated the physical, thermal, and mechanical performances of the TBCs, presented theoretical studies on TBCs properties, and put forward future research orientations of La2Zr2O7 based TBCs. Motoc et al. [] summarized the applications of ZrO2 ceramics doped with mixed rare earth oxides and proposed a feasible recipe for ZrO2-based TBCs with lower costs and environmental influences. Lakiza et al. [] presented principles for choosing ceramic materials for TBCs, and thought that increasing its porosity and suppressing its sintering could reduce thermal conductivity of TBCs.
However, timely review specialized on ceramic materials of TBCs is still required for research references. In view of this, this research systematically gives an overview of preparation techniques and advancements in new ceramic materials. And the existing problems and future research directions were also discussed.
2. Preparation Methods for TBCs
At present, the main preparation methods of TBCs include plasma spraying, electron beam-physical vapor deposition, high velocity flame spraying, plasma spray-physical vapor deposition etc. [,,,,]. Among them, the plasma spraying method is widely used in TBCs preparation due to its advantages, such as being quick, convenient, economical and efficient, with stable performance, etc. Other methods are also explored for the preparation of TBCs due to their unique advantages.
As shown in Figure 2, the atmospheric plasma spraying method (APS) is used to convey spraying powders to the plasma flame flow at high temperature (>10,000 K) and high speed (600–2300 m/s) by powder feeder, and the powder particles are rapidly heated into a molten or half molten state in the flame flow, then they hit against the substrate surface at a speed of 30–500 m/s, spread and solidify to form lamellar coatings [,]. APS process is characterized by relatively simple operation, high heating temperature, loose requirements on coating materials, high deposition rate, and low preparation cost. The prepared coating structure is lamellar, with more voids, higher porosity, as well as good heat-insulating performance. However, there exist inherent defects such as slags and cracks in the coatings. And the strain tolerance of the layered structure coatings is low. Therefore, it is easy to fall off during the thermal cycling process. To control the spraying process and improve the reliability and repeatability of coating quality by adjusting spraying parameters (spraying power, spraying distance, gas flow, etc.) is the focus and development direction for the APS method. Sivakumar et al. [] prepared Sm2Zr2O7 and La2Zr2O7 with APS in a laminated stacking structure, and found that by adjusting the spraying parameters to reduce the porosity of the coating, the bonding force of the coating could be effectively improved, but the insulation performance of the coating could be reduced.
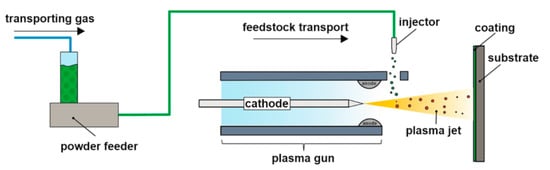
Figure 2.
Schematic representation of atmospheric plasma spraying [].
Electron Beam-Physical Vapor Deposition (EB-PVD) is a deposition method that utilizes high-energy electron beam to rapidly evaporate ceramics and other materials under high vacuum and deposit them on the substrate in the form of vapor []. The coating has a typical columnar structure with high strain tolerance and good thermal shock resistance []. However, the gap between adjacent columnar crystals also provides channels for the transfer of heat, oxygen, and corrosive molten salt, resulting in the decrease of thermal insulation performance and easy oxidation of the bond coat. By using EB-PVD method, Shen et al. [] prepared La2Zr2O7 series coatings respectively stabilized by rare-earth oxide (CeO2, La2Ce2O7, Ce2Zr2O7), which showed complex feathered nanostructures and intercolumn voids (as shown in Figure 3). The thermal cycling life and thermal shock resistance of the coatings were relatively ideal.
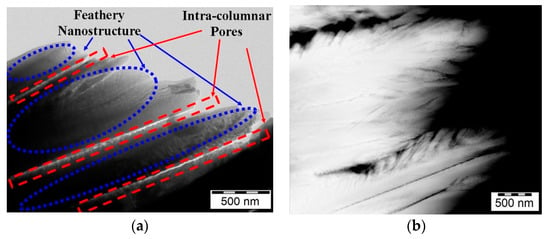
Figure 3.
Microstructure of Ce2Zr2O7 stabilized La2Zr2O7 coatings []: (a) Transmission electron microscopy image; (b) High angle annular dark fields image.
Through combining vacuum plasma spraying with the vapor deposition method, plasma spraying-physical vapor deposition method (PS-PVD) was made by Sulzer Metco Company. Its basic principle and the equipment were shown in Figure 4. Under the high vacuum degree, it adopts a high power single cathode vacuum plasma spray gun to form a plasma jet (up to 2000 mm in length, up to 400 mm in radius), so that the vapor deposition is mainly realized, with gas-liquid-solid multiphase mixed deposition. With the increase in spraying distance, coating will show a layered structure → layered-columnar mixed structure → columnar structure gradually, and the coating will be able to realize large areas of non-line-of-sight deposition and microstructure customization []. The PS-PVD method is the organic combination of EB-PVD method and PS method, and its strain tolerance and porosity of the coating are excellent. Zhao et al. [] prepared La2Ce2O7 coating by PS-PVD method and found that the microstructure of the coating was more sensitive to power and carrier gas flow than the spraying distance. Schmitt et al. [] also used the PS-PVD method to prepare YSZ coating co-doped with Gd2O3 and Yb2O3, in which the smaller the intercolumnar clearance and porosity of the coating was, the stronger the anti-erosion ability was.
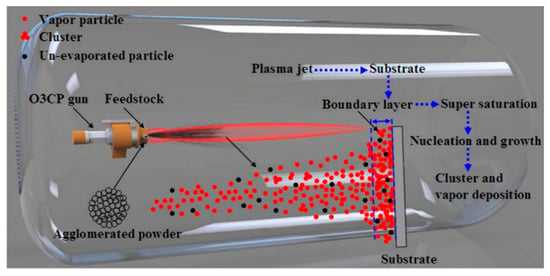
Figure 4.
Scheme of PS-PVD working principle and equipment [].
3. Doping Modification of ZrO2-Based TBCs Material
At present, ZrO2-based ceramic materials are still the most widely used TBC materials, of which 6–8 YSZ prepared TBC has properties of high melting point (2973 K), low thermal conductivity (2.5 W/(m·K)), higher coefficient of thermal expansion (11 × 10−6/K), lower elastic modulus (40 GPa), higher fracture toughness (3.4 MPa·m1/2), high hardness (14 GPa) and good erosion resistance, therefore, it can remain long-term service under the environment of 1473 K or even slightly higher [,,]. In view of the problems such as sintering, corrosion, phase transformation, and bond coat oxidation that occur when ZrO2-based ceramic materials are in service, many researchers have tried to use various materials to modify them by doping, hoping to further improve their service life.
3.1. ZrO2 Single-Phase Ceramic Doped by Oxide
In the process of cyclic heating and cooling of ZrO2 ceramics, allotropic crystalline transformation will occur along with volume change, resulting in internal stress and coating failure. ZrO2 doped with CeO2, Nd2O3, and other materials can reduce the thermal conductivity of the coating and improve the phase stability, but the improvement of anti-high-temperature sintering performance is not obvious, unable to replace YSZ. By using the co-doped ZrO2 of various oxides such as Al2O3, CeO2, Gd2O3, Sc2O3, TiO2 to replace the single doping of Y2O3, multipoint dislocation effects, lattice defects, and complementary effects can be formed, which can effectively inhibit phase transformation, improve thermal stability, slightly improve thermal expansion coefficient (as shown in Figure 5), reduce thermal conductivity, and improve bond strength, wear resistance and fracture toughness [,,,,].
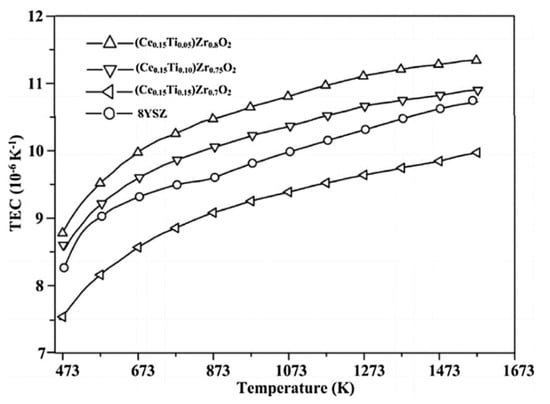
Figure 5.
The thermal expansion coefficient of ZrO2-CeO2-TiO2 and 8YSZ within a temperature range from 473 to 1573 K [].
3.2. YSZ Doped by Oxide
Studies on YSZ thermal conductivity have found that the difference between doped ions and Zr4+ radii will form more oxygen vacancies and high concentration of lattice defects, resulting in enhanced phonon scattering and reduced thermal conductivity of the coating [,]. The doping of CeO2, Yb2O3, Er2O3, Gd2O3, and other materials can effectively reduce the thermal conductivity of YSZ coating, and the greater the difference between the doped ions and Zr4+ ion radii is, the stronger the phonon scattering is; the slower the phonon propagation speed is, the lower the thermal conductivity will be [,]. Wenge Li et al. prepared Yb2O3-Gd2O3 co-doped YSZ coatings with different thicknesses by the APS method, and the stable thermal insulation temperature at 1473 K was above 240 K, as shown in Figure 6. The addition ofAl2O3, Dy2O3 can decrease lattice energy and increase the coefficient of thermal expansion [,]. Part of Zr4+ was replaced by doping rare-earth or alkaline earth oxide, substitutional type solid solution was formed, which can play the role of stabilizing ZrO2 tetragonal phase structure, and materials such as Gd2O3 and Yb2O3 can make YSZ maintain phase stability effectively [,]. Based on the theory of Lewis Acid and Alkali, adopting Gd2O3, Yb2O3, La2O3, Sc2O3, and other strong acidic oxides to partly replace Y2O3 can reduce driving force for the reaction and improve Na2SO4 + V2O5 molten salt thermal corrosion resistance of YSZ [,,,,,], adding rare-earth oxides with high basicity index such as Dy2O3 and Gd2O3 can form an impermeable reaction layer and improve CMAS hot corrosion resistance of YSZ, and multiple doping has better effects [,,,]. After doping ZrO2, one or more rare-earth oxides such as RE2O3(RE = La, Yb, Ce, Gd, Sc) and Y2O3—due to the differences in the mass and radius of doping ions with Zr4+ and Y3+ ions—can form at multiple points a dislocation compound effect, which can not only effectively improve the sintering resistance, stability, and mechanical properties, etc., of the materials, but also make up the deficiencies of stabilizing alone, improve the comprehensive performance, reduce the thermal conductivity, and improve the fracture toughness and thermal cycle life [,,].
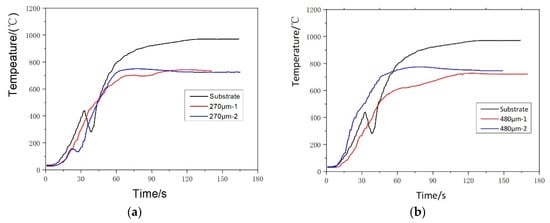
Figure 6.
Thermal barrier effect of Yb2O3-Gd2O3 co-doped YSZ coatings with different thickness: (a) 270 μm coatings, (b) 480 μm coatings.
3.3. YSZ Doped by Other Materials
In addition to modification by oxide doping, studies show that doping MoSi2 in YSZ can repair cracks to a certain extent [], doping SiC fibers can improve the thermal cycling life and fracture toughness, as well as repair and prevent cracks, and prevent ZrO2 martensitic phase transition by reducing the pressure in the lattice []. The addition of multiwall carbon nanotubes (MWCNT) will be detrimental in the hot corrosion environment, due to the production of cracks [,].
The above doped materials can modify ZrO2 ceramics to make up for the deficiencies of stabilizing alone, but the range is limited, and there are still some problems such as thermal expansion coefficient mismatch. The ZrO2 co-doped by La2O3, Gd2O3, Yb2O3, Y2O3, and other rare-earth oxides also made the composition and structure of the materials complicated. The addition amount, ratio, and synergistic mechanism of stabilizers still needs to be further studied.
4. New Type TBCs Ceramic Materials
In the development of high-performance TBCs, in addition to the modification research of ZrO2 ceramic coating, some new top ceramic materials have also been explored. These new materials should generally meet the following properties: thermal conductivity less than 2 W/(m·K), thermal expansion coefficient greater than 9 × 10−6/K, good high-temperature phase stability, better sintering resistance, higher toughness, stronger corrosion resistance, and good chemical stability and adhesion with TGO layer at high temperature.
4.1. A2B2O7 Type Compounds
A2B2O7 (A as rare-earth element, B as tetravalent element such as Zr and Ce) compounds are divided into Pyrochlore structure (seen as an ordered defective fluorite structure) and fluorite structure, according to the order of oxygen vacancy. A2B2O7 type compounds have the advantages of high melting point, good high-temperature phase stability, no phase transition below the melting point, and high CMAS impedance. Moreover, its crystal structure is more complex, with more vacancies inside the crystal, and the rare-earth atoms with larger mass in the crystal cell can significantly increase phonon scattering, which reduces the average phonon free path and reduces the thermal conductivity. As some parameters of materials such as thermal conductivity of A2Sn2O7 have too large deviation to be suitable for preparing TBCs, current studies on A2B2O7 ceramic compounds mainly focus on materials of A2Zr2O7 and A2Ce2O7 types. Meanwhile, some new materials such as Gd2Hf2O7 also draw some attention [].
4.1.1. Rare-Earth Zirconate (A2Zr2O7)
A2Zr2O7 has performance advantages such as high melting point, high temperature stability and resistance to sintering, and part of the rare-earth ions in rare-earth zirconate (such as Gd3+) will react with molten salt in CMAS to generate dense and stable reaction layer thus inhibit the infiltration of molten salt into the coating [,,]. It has high corrosion resistance, but low thermal expansion coefficient and fracture toughness make its service life much less than the YSZ coating. On the one hand, rare-earth oxides such as Sm2O3, Ce2O3, and Yb2O3 can be used for double rare-earth doping and multi-rare-earth doping, which will improve the thermal conductivity, elastic modulus and other thermal physical properties of rare-earth zirconate to some extent [,]. Due to the complex crystal structure of A2Zr2O7, each A2Zr2O7 unit has an oxygen vacancy. Meanwhile, the differences of ionic radius and atomic weight after doping also increase the scattering of phonon diffusion by point defects. These result in lower thermal conductivity and slightly increased thermal expansion coefficient. As potential TBCs material, Gd2Zr2O7 has lower thermal conductivity, better phase stability, sintering resistance, and corrosion resistance than YSZ at temperatures above 1473 K [,,]. SrZrO3, MgO etc. was used for doping Gd2Zr2O7, modifying its poor fracture toughness and thermal expansion coefficient, and enhancing the corrosion resistance and reducing the thermal conductivity further [,,]. The application of La2Zr2O7 with low thermal conductivity, high phase stability, and good corrosion resistance is limited by its low fracture toughness [,,]. Nd, Dy oxides were also employed to improve the fracture toughness and reduce the thermal conductivity []. Guo et al. [] found that MWCNTs could improve the fracture toughness and inhibit crack propagation. On the other hand, it can be tried to improve the thermal expansion coefficient of the compounds by partially replacing Zr4+. For example, Ce4+ is used to partially replace Zr4+, which can reduce the order degree of crystal structure, reduce the thermal conductivity, reduce the overall bond energy, significantly reduce Young’s modulus, and increase its thermal expansion coefficient [,].
4.1.2. Rare-Earth Cerium Oxides (A2Ce2O7)
With fluorite structure mainly, rare-earth cerate has the high temperature stability, higher thermal expansion coefficient, and lower thermal conductivity. Among them, the most concerned La2Ce2O7 can achieve very low thermal conductivity of 0.50–0.75 W/(m·K) [], and can still maintain fluorite structure after heat treatment for a long time at the temperature of 1673 K []. The thermal expansion coefficient under the 1473 K can be up to 14 × 10−6/K (block), very close to the thermal expansion coefficient of bond coat alloy. La2Ce2O7 doped by Gd2O3 shows significantly good resistance against volcanic ash []. However, the rapid rundown of thermal expansion coefficient at low temperature (may be caused by the transverse vibration of oxygen ion in chemical bonds) limits its application [,]. Therefore, by means of replacing Ce4+ with Ta5+, the oxygen vacancy concentration is reduced and the sudden drop of coefficient of thermal expansion coefficient at 623 K is restrained (Figure 7). Relatively low thermal conductivity (0.54–0.71 W/(m·K), 298–1273 K) is maintained, thermal shock resistance, and thermal cycle life are improved, but the too-low fracture toughness is still the leading cause of the failure [,]. In view of this, YSZ is added to enhance its fracture toughness [,], and it is found that (Ca, Fe) or (Sr, Mn) substituting La2Ce2O7 could increase its infrared emittances and thermal conductivity, and decrease the fracture toughness [].
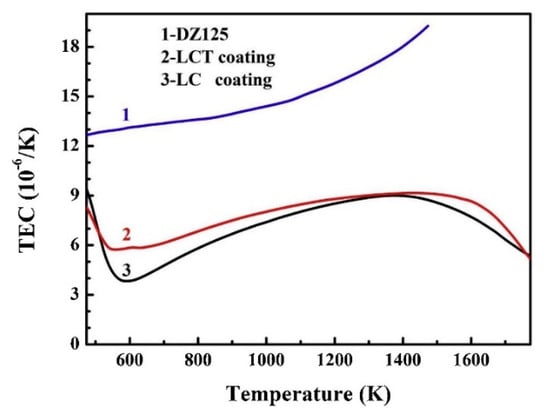
Figure 7.
Thermal expansion coefficient trend chart of La2Ce2O7 (LC) coatings, Ta2O5 doped La2Ce2O7 (LCT) coatings and DZ125 substrate [].
The above studies showed that the coating prepared by A2B2O7 type compounds exhibited relatively comprehensive excellent properties, with lower thermal conductivity, higher thermal stability, and higher thermal expansion coefficient compared with traditional ceramic materials. With regard to doping of different elements, whether single element doping or multi-element doping, the thermodynamic properties of materials have been improved to varying degrees. However, there are also some shortcomings, such as mismatching of thermal expansion coefficient (larger than 8YSZ, but still smaller than the bond coat) and low fracture toughness.
4.2. Perovskite Structure Oxide
The perovskite structure oxide (ABO3, A is rare-earth or alkaline earth element, and B is generally transition metal element) is a multi-purpose oxide material with a cubic symmetrical structure. It is compatible with larger radius change of A and B ions, and the doping of ions with large radius does not change the basic structure. It has many advantages, such as high melting point, low thermal conductivity (generally lower than 2.2 W/(m·K)), relatively high thermal expansion coefficient (generally higher than 8.5 × 10−6/K), and so on. The SrZrO3 has high melting point (2923 K), thermal conductivity close to YSZ, good chemical compatibility, and a relatively high thermal expansion coefficient, which has received more attention. Although the phase stability is good, there is a second phase t-ZrO2 in the prepared coatings; phase change is easy to take place at temperatures above 1723 K, and performance disadvantages exist such as low fracture toughness, easy sintering, and poor corrosion resistance. Many researches have optimized the material properties of SrZrO3 further [,]. Matiullah et al. [,] doped SrZrO3 with Yb2O3, La2Ce2O7 to increase the concentration of point defects and phonon scattering, and reduce the thermal conductivity. Meanwhile, it is found that Yb2O3 could increase the fracture toughness of SrZrO3 []. In addition to SrZrO3, other ABO3 type compounds have also been explored. La0.8Ba0.2TiO3 shows excellent hot corrosion resistance against Na2SO4 + V2O5 []. Materials, such as SrCeO3 [], LaAlO3 [], Ba(Sr1/3Ta2/3)O3 [], Ba(Mg1/3Ta2/3)O3 [] show common problems including low thermal expansion coefficient and low fracture toughness leading to premature coating failure. It is indicated that the complex perovskite compounds will decompose during deposition with second phase formation.
Therefore, the perovskite structural material coating has the advantages of low thermal conductivity and high thermal expansion coefficient, which can reduce the mismatch of thermal expansion coefficient, but its application is greatly limited by its disadvantages such as low fracture toughness.
4.3. Rare-Earth Tantalate
Rare-earth tantalate (RETaO4, RE3TaO7, RE = Nd, Dy, Gd, etc.) has high melting point and good thermal stability. The complex lattice structure and the lattice non-simple harmonic vibration caused by the difference in the size and mass of ions enhance phonon scattering and reduce the thermal conductivity. The high temperature ferroelasticity can buffer the stress variation and greatly improve the high temperature fracture toughness of the material. Phase transition is hard to occur in service, and the volume change caused by phase transition is not large enough to cause coating failure. It is almost an insulator for oxygen ion transport, and the growth rate of TGO is negligible compared with ZrO2 material. The low Young’s modulus enables it to have low thermal stress, and the coating thickness under the same external conditions can increase by 30% compared with YSZ [,,]. Current researches on rare-earth tantalates mainly focus on the key thermophysical properties such as thermal conductivity and thermal expansion coefficient, as shown in Table 1. Chen et al. [] found that the addition of La3+ with higher ionic-radius could result in reduced thermal conductivity, resulting from large atomic mass, high oxygen vacancies, and complex microstructure. Ye et al. [] indicated that ScTaO4 could resist the corrosion of CMAS effectively, due to low solubility of Sc3+ in the CMAS.

Table 1.
Thermal physical properties of some rare-earth tantalates.
It can be seen that the thermal conductivity of rare-earth tantalate is lower than YSZ and the thermal expansion coefficient is relatively high. The thermal expansion coefficient of some materials such as Ca3Ln3Ce7Ta2O26.5 (Ln = Dy, Nd) even exceed YSZ. Above studies have also proved that it has good comprehensive thermal and mechanical properties. Therefore, rare-earth tantalates are considered to be the most promising new generation thermal barrier coating materials.
4.4. LnMAl11O19 Type Magnetoplumbite Compounds
Rare-earth aluminate compounds LnMAl11O19 (Ln is La, Pr, Nd, Ce, Sm, Gd, Eu, etc., M is Mg, Ni, Co, Mn, Fe, etc.) with high melting point, high stress tolerance, high thermal expansion coefficient, low sintering rate, and low thermal conductivity, can be used for a long time below 1673 K without phase transition. Compounds such as LnMgAl11O19 (LnMA, Ln = Nd, Sm, Gd) [] have excellent corrosion resistance. Double-ceramic-layer SrAl12O19/YSZ TBCs prepared by APS showed a much longer cycling life than the YSZ coating []. GdMgAl11O19 TBCs showed excellent CMAS corrosion resistance compared to the YSZ coating, due to the formations of Ca2Gd8(SiO4)6O2, MgAl2O4, and CaAl2Si2O8, which could effectively arrest the molten CMAS, and heal the vertical microcracks induced by crystallization of amorphous phase []. LaMgAl11O19 with defective magnetoplumbite structure has attracted the most attention. Its thermal conductivity at 1273 K is 1.7 W/(m·K). Young’s modulus is 295 Gpa, and thermal expansion coefficient is 9.6 × 10−6/K (1773 K). At 1673 K, LnMAl11O19 still has high resistance to sintering []. However, in the process of preparing plasma spraying, because of the rapid solidification, amorphous phase can be produced and its content is increased with the increase in spraying power. A larger volume shrinkage is also produced in the process of thermal cycling, seriously affecting the service life, and easy to react with V2O5 + Na2SO4 and other molten salts [,]. Study results of Sun et al. [,,] indicated that LaMgAl11O19 with lower content of amorphous phase and moderate grain size has the longest thermal cycle life expectancy. Some researchers used Nd2O3, Y3Al5O12, etc. for doping modification to improve its thermal expansion coefficient and thermal cycle life [].
As a result, the rare-earth aluminate compound with magnetoplumbite structure has good comprehensive performance. On the one hand, the presence of too many amorphous phases will reduce the coefficient of thermal expansion, leading to excessive thermal stress and reduced thermal cycle life, on the other hand, the corrosion resistance of amorphous phase of different kinds of magnetoplumbite compounds varies greatly, which can seriously affect their high temperature corrosion resistance.
4.5. Other New Type Ceramic Materials
In addition to the well-studied ceramic materials, some new types of thermal barrier coating ceramic materials, such as Yttrium Aluminium Garnet (YAG), Forsterite, and Rare-earth Silicate, are also paid attention to.
Yttrium aluminum garnet (Y3Al5O12, YAG) has excellent stability under 2243 K, with thermal expansion coefficient being 7.5–9.1 × 10−6/K, Vickers hardness being 16.5–17 GPa, fracture toughness being 1.8 MPa·m1/2 (298 K). With a strong creep resistance, its low oxygen permeability makes bond coat hard to oxidating, and it hardly reacts with CMAS [,]. Although its thermal conductivity is slightly higher (2.4–3.1 W/(m·K)) at high temperature, it can be greatly reduced through the structure design (0.91 W/(m·K), 1273 K) []. Gd3+ etc. can partially replace Y3+ to reduce the thermal conductivity (1.51 W/(m·K), 1473 K) and increase thermal expansion coefficient to a small extent [,], Pd doping can enhance the high-temperature oxidation resistance and thermal shock resistance effectively []. but low thermal expansion coefficient and extremely low fracture toughness are still the main reasons limiting its application.
As a new thermal barrier coating material, Mg2SiO4 can still maintain good phase stability at 1573 K, with low sintering rate and thermal conductivity about 20% lower than 8YSZ at 1273 K, with the hardness of 10 GPa and fracture toughness of 2.8 MPa·m1/2. However, its corrosion resistance is poor, and the recrystallization and thermal expansion coefficient mismatching (8.6–11.3 × 10−6/K, 473–1623 K) are easy to lead to coating failure [].
Some rare-earth silicate ceramics have low thermal conductivity and good phase stability and corrosion resistance. Among them, the thermal expansion coefficient of X1-RE2SiO5 (RE = La, Nd, Sm, Eu, Gd), with a large rare-earth ion radius, is 8.3–9.2 × 10−6/K. The resistance to CMAS corrosion increases with the increase of the ion radius of rare-earth elements, and the elastic modulus increases with the decrease of the ion radius [,]. The comparison of the properties of X1-RE2SiO5 is shown in Figure 8. The intrinsic properties of X2-RE2SiO5 (RE = Tb, Dy, Ho, Er, Tm, Yb, Lu, Y) with a small radius of rare-earth ions are shown in Figure 9, indicating that the thermal conductivity and other properties are greatly different from the elements []. It is revealed that influences from distortion of RE polyhedrons and stretching of RE-O bonds can determine the thermal expansion behaviors of X2-RE2SiO5 [].
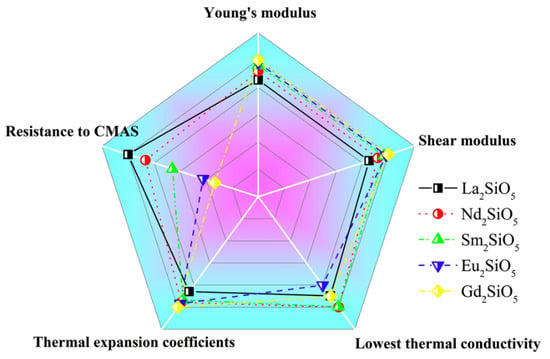
Figure 8.
Spider chart for a comparison of the properties of X1-RE2SiO5 [].
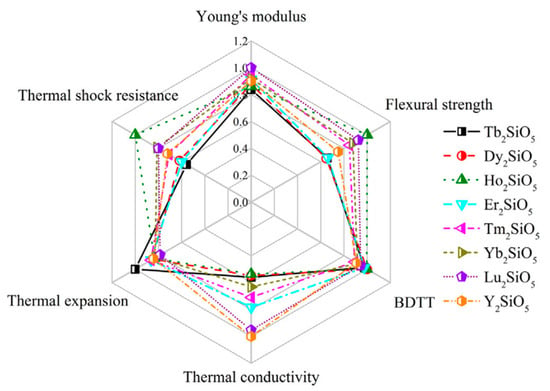
Figure 9.
Radar chart for comparison of the properties of X2-RE2SiO5 [].
In addition, materials such as Ba2REAlO5 (RE = Dy, Er, Yb) can effectively inhibit CMAS or V2O5 + Na2SO4 molten salt corrosion [,,]; Sm2SrAl2O7 has strong corrosion resistance to MgO and NiO []; Yb2O3 has excellent comprehensive mechanical properties, such as fracture toughness and elastic strength []; cuspidine structural Ln4Al2O9 (Ln = Y, Sm, Eu, Gd, Tb) [] has a relatively high thermal expansion coefficient; LaPO4 has low thermal conductivity and a larger thermal expansion coefficient; a series of Y2O3 fully stabilized HfO2 ceramics (Hf1−xYxO2−0.5x, x = 0.20, 0.24, 0.28, 0.32, 0.36 and 0.40) indicated good resistance to sintering []; RENbO4 has low thermal conductivities, higher coefficients of thermal expansion (12.8 × 10−6 K−1), and high fracture toughness (1.93–2.77 MPa m1/2) []; and Yttria-stabilized hafnia (Hf0.84Y0.16O1.92, YSH16) [] has superior sintering-resistance, excellent thermal stability, and mechanical properties; the above are also potential thermal barrier coating materials [].
Based on the above researches, thermal conductivity and thermal expansion coefficient have become important performance index to choose top ceramic coating materials. At present, most of the new materials studied have relatively low thermal conductivity and can meet performance requirements. However, their common defects are that some parameters such as thermal expansion coefficient and fracture toughness are relatively low, which has become the most important factors restricting their application. Preparation method has a great influence on the structure, corrosion resistance and thermal conductive performance of the coating, which will affect the service life of coating. The thermal conductivity of rare-earth tantalate is lower than the YSZ, while thermal expansion coefficient is relatively high, even higher than YSZ. With strong fracture toughness, and superior comprehensive performance, it is considered to be the most potential new generation of thermal barrier coating material.
5. Conclusions
Through this paper, the principles, advantages, and disadvantages of the preparation methods of TBCs in recent years are described, and the research and development direction of the doping modification of traditional ZrO2-based materials and new ceramic materials are summarized. According to the research results, it is found that the preparation technology and methods of TBCs have important effects on the structure and properties of coatings, and the newly developed plasma spraying-physical vapor deposition method has obvious advantages in the properties of coatings. The doping modification of traditional ZrO2-based ceramic coating can improve the thermal insulation performance, thermal corrosion resistance and other properties of the coatings, but there are still some problems, such as material composition being too complex and thermal expansion coefficient mismatching caused by doping. Compared with YSZ coating, rare-earth zirconate and other new ceramic materials have better thermal insulation resistance, high-temperature oxidation resistance, high-temperature phase stability and other properties. However, the problems of thermal expansion coefficient mismatching and insufficient fracture toughness are still the keys to limit the application of these new materials. Rare-earth tantalate becomes the most potential new generation of TBC material, with its superior thermal and mechanical performance. Therefore, to study the structure-function relationship between preparation technology, structure and properties comprehensively, and improve the thermal expansion coefficient mismatch and insufficient fracture toughness, are the key to improve development and application of the ZrO2-based coating and new top ceramic materials.
Author Contributions
Conceptualization, W.L. (Wenge Li) and S.W.; methodology, S.W. and Y.Z.; software, S.W.; validation, W.L. (Wenge Li), Y.Z., and S.W.; investigation, S.W.; data curation, S.W., W.L. (Weilai Liu), Y.W., and F.L.; Writing—Original draft preparation, S.W. and W.L. (Weilai Liu); Writing—Review and editing, S.W., Y.Z., and W.L. (Wenge Li); supervision, Y.Z.; project administration, W.L. (Wenge Li); funding acquisition, W.L. (Wenge Li). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by special fund for green manufacturing system integration projects in 2018, the Ministry of Industry and Information Technology of China, key green process system integration of advanced turbine engine hot end components.
Data Availability Statement
Data sharing not applicable. No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Krishna Anand, V.G.; Parammasivam, K.M. Thermal barrier coated surface modifications for gas turbine film cooling: A review. J. Therm. Anal. Calorim. 2020, 1–36. [Google Scholar] [CrossRef]
- Essa, S.K.; Chen, K.; Liu, R.; Wu, X.; Yao, M.X. Failure mechanisms of APS-YSZ-CoNiCrAlY thermal barrier coating under isothermal oxidation and solid particle erosion. J. Therm. Spray Technol. 2020, 1–18. [Google Scholar] [CrossRef]
- Zhou, D.; Mack, D.E.; Bakan, E.; Mauer, G.; Sebold, D.; Guillon, O.; Vaßen, R. Thermal cycling performances of multilayered yttria-stabilized zirconia/gadolinium zirconate thermal barrier coatings. J. Am. Ceram. Soc. 2020, 103, 2048–2061. [Google Scholar] [CrossRef]
- Cai, Z.; Jiang, J.; Wang, W.; Liu, Y.; Cao, Z. CMAS penetration-induced cracking behavior in the ceramic top coat of APS TBCs. Ceram. Int. 2019, 45, 14366–14375. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, J.; Song, W.; Zhou, X.; Dong, S.; Duo, S.; Wang, J.; Yang, X.; Jiang, J.; Deng, L.; et al. Composition, mechanical properties and thermal cycling performance of YSZ toughened La2Ce2O7 composite thermal barrier coatings. Ceram. Int. 2020, 46, 6641–6651. [Google Scholar] [CrossRef]
- Jiang, C.; Li, S.; Liu, H.; Bao, Z.; Zhang, J.; Zhu, S.; Wang, F. Effect of Hf addition in (Ni,Pt)Al bond coat on thermal cycling behavior of a thermal barrier coating system at 1100 °C. Corros. Sci. 2020, 166, 108424. [Google Scholar] [CrossRef]
- Qu, L.; Choy, K.L.; Wheatley, R. Enhanced doping effects of multielement on anisotropic thermal expansion in ZrO2 with new compositions. J. Am. Ceram. Soc. 2020, 103, 5881–5890. [Google Scholar] [CrossRef]
- Ma, X.; Rivellini, K.; Ruggiero, P.; Wildridge, G. Toward durable thermal barrier coating with composite phases and low thermal conductivity. J. Therm. Spray Technol. 2020, 29, 423–432. [Google Scholar] [CrossRef]
- Negami, M.; Hibino, S.; Kawano, A.; Nomura, Y.; Tanaka, R.; Igashira, K. Development of highly durable thermal barrier coating by suppression of thermally grown oxide. In Proceedings of the ASME Turbo Expo, Charlotte, NC, USA, 26–30 June 2017. [Google Scholar]
- Zhang, X.; Deng, Z.; Li, H.; Mao, J.; Deng, C.; Deng, C.; Niu, S.; Chen, W.; Song, J.; Fan, J.; et al. Al2O3-modified PS-PVD 7YSZ thermal barrier coatings for advanced gas-turbine engines. NPJ Mater. Degrad. 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Shi, L.; Sun, Z.; Lu, Y. The combined influences of film cooling and thermal barrier coatings on the cooling performances of a film and internal cooled vane. Coatings 2020, 10, 861. [Google Scholar] [CrossRef]
- Han, J.C. Advanced cooling in gas turbines 2016 Max Jakob memorial award paper. J. Heat Transf. 2018, 140, 11. [Google Scholar] [CrossRef]
- Bakan, E.; Vaßen, R. Ceramic top coats of plasma-sprayed thermal barrier coatings: Materials, processes, and properties. J. Therm. Spray Technol. 2017, 26, 992–1010. [Google Scholar] [CrossRef]
- Chang, H.; Cai, C.; Wang, Y.; Zhou, Y.; Yang, L.; Zhou, G. Calcium-rich CMAS corrosion induced microstructure development of thermal barrier coatings. Surf. Coat. Technol. 2017, 324, 577–584. [Google Scholar] [CrossRef]
- De la Roche, J.; Alvarado-Orozco, J.M.; Toro, A. Hot corrosion mechanism of yttria-stabilized zirconia powder in the presence of molten Na2SO4 + V2O5 salts. Rare Met. 2020. [Google Scholar] [CrossRef]
- Tao, S.; Yang, J.; Li, W.; Shao, F.; Zhong, X.; Zhao, H.; Zhuang, Y.; Ni, J.; Tao, S.; Yang, K. Thermal stability of plasma-sprayed thick thermal barrier coatings using triplex ProTM-200 torch. Coatings 2020, 10, 894. [Google Scholar] [CrossRef]
- Karaoglanli, A.C.; Ozgurluk, Y.; Doleker, K.M. Comparison of microstructure and oxidation behavior of CoNiCrAlY coatings produced by APS, SSAPS, D-gun, HVOF and CGDS techniques. Vacuum 2020, 180, 109609. [Google Scholar] [CrossRef]
- Weng, W.X.; Wang, Y.M.; Liao, Y.M.; Li, C.C.; Li, Q. Comparison of microstructural evolution and oxidation behaviour of NiCoCrAlY and CoNiCrAlY as bond coats used for thermal barrier coatings. Surf. Coat. Technol. 2018, 352, 285–294. [Google Scholar] [CrossRef]
- Zhao, C.; Luo, L.; Xiao, C.; Zhao, X.; Wang, X.; Guo, F.; Xiao, P. The oxidation performance of plasma-sprayed NiAl bond coat: Effect of Hf addition in bond coat and substrate. Surf. Coat. Technol. 2018, 352, 49–58. [Google Scholar] [CrossRef]
- Audigié, P.; Rouaix-Vande Put, A.; Malié, A.; Thouron, C.; Monceau, D. High-temperature cyclic oxidation of Pt-rich γ-γ’ bond-coatings. Part II: Effect of Pt and Al on TBC system lifetime. Corros. Sci. 2019, 150, 1–8. [Google Scholar] [CrossRef]
- Sokol, M.; Yang, J.; Keshavan, H.; Barsoum, M.W. Bonding and oxidation protection of Ti2AlC and Cr2AlC for a Ni-based superalloy. J. Eur. Ceram. Soc. 2019, 39, 878–882. [Google Scholar] [CrossRef]
- Go, T.; Sohn, Y.J.; Mauer, G.; Vaßen, R.; Gonzalez-Julian, J. Cold spray deposition of Cr2AlC MAX phase for coatings and bond-coat layers. J. Eur. Ceram. Soc. 2019, 39, 860–867. [Google Scholar] [CrossRef]
- An, Q.; Huang, L.; Wei, S.; Zhang, R.; Rong, X.; Wang, Y.; Geng, L. Enhanced interfacial bonding and superior oxidation resistance of CoCrAlY-TiB2 composite coating fabricated by air plasma spraying. Corros. Sci. 2019. [Google Scholar] [CrossRef]
- Xu, Q.L.; Zhang, Y.; Liu, S.H.; Li, C.J.; Li, C.X. High-temperature oxidation behavior of CuAlNiCrFe high-entropy alloy bond coats deposited using high-speed laser cladding process. Surf. Coat. Technol. 2020, 398, 126093. [Google Scholar] [CrossRef]
- Mehboob, G.; Liu, M.J.; Xu, T.; Hussain, S.; Mehboob, G.; Tahir, A. A review on failure mechanism of thermal barrier coatings and strategies to extend their lifetime. Ceram. Int. 2020, 46, 8497–8521. [Google Scholar] [CrossRef]
- Thakare, J.G.; Pandey, C.; Mahapatra, M.M.; Mulik, R.S. Thermal barrier coatings—A state of the art review. Met. Mater. Int. 2020, 1–22. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.; Jung, Y.G.; Li, L.; Knapp, J. Lanthanum zirconate based thermal barrier coatings: A review. Surf. Coat. Technol. 2017, 323, 18–29. [Google Scholar] [CrossRef]
- Motoc, A.M.; Valsan, S.; Slobozeanu, A.E.; Corban, M.; Valerini, D.; Prakasam, M.; Botan, M.; Dragut, V.; Vasile, B.S.; Surdu, A.V.; et al. Design, fabrication, and characterization of new materials based on zirconia doped with mixed rare earth oxides: Review and first experimental results. Metals 2020, 10, 746. [Google Scholar] [CrossRef]
- Lakiza, S.M.; Grechanyuk, M.I.; Ruban, O.K.; Redko, V.P.; Glabay, M.S.; Myloserdov, O.B.; Dudnik, O.V.; Prokhorenko, S.V. Thermal barrier coatings: Current status, search, and analysis. Powder Met. Met. Ceram. 2018, 57, 82–113. [Google Scholar] [CrossRef]
- Li, D.C.; Zhao, H.Y.; Zhong, X.H.; Tao, S.Y. Research progresses of atmospheric plasma sprayed splat. J. Inorg. Mater. 2017, 32, 571–580. [Google Scholar] [CrossRef][Green Version]
- Sivakumar, S.; Praveen, K.; Shanmugavelayutham, G.; Yugeswaran, S.; Mostaghimi, J. Thermo-physical behavior of atmospheric plasma sprayed high porosity lanthanum zirconate coatings. Surf. Coat. Technol. 2017, 326, 173–182. [Google Scholar] [CrossRef]
- Ozgurluk, Y.; Doleker, K.M.; Ozkan, D.; Ahlatci, H.; Karaoglanli, A.C. Cyclic hot corrosion failure behaviors of EB-PVD TBC systems in the presence of sulfate and vanadate molten salts. Coatings 2019, 9, 166. [Google Scholar] [CrossRef]
- Vijay, S.; Wang, L.; Lyphout, C.; Nylen, P.; Markocsan, N. Surface characteristics investigation of HVAF sprayed cermet coatings. Appl. Surf. Sci. 2019, 493, 956–962. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, J.; Shao, F.; Zhong, X.; Zhao, H.; Zhuang, Y.; Sheng, J.; Ni, J.; Tao, S. Thermal stability of PS-PVD YSZ coatings with typical dense layered and columnar structures. Crystals 2020, 10, 826. [Google Scholar] [CrossRef]
- Wang, T.; Wang, N.; Li, Y.; Wang, H.; Tang, J.; Wang, Y. Study on preparation technologies of thermal barrier coatings. Surf. Rev. Lett. 2017, 24, 1730004. [Google Scholar] [CrossRef]
- Łatka, L.; Pawłowski, L.; Winnicki, M.; Sokołowski, P.; Małachowska, A.; Kozerski, S. Review of functionally graded thermal sprayed coatings. Appl. Sci. 2020, 10, 5153. [Google Scholar] [CrossRef]
- Wee, S.; Do, J.; Kim, K.; Lee, C.; Seok, C.; Choi, B.G.; Choi, Y.; Kim, W. Review on mechanical thermal properties of superalloys and thermal barrier coating used in gas turbines. Appl. Sci. 2020, 10, 5476. [Google Scholar] [CrossRef]
- Mauer, G.; Vaßen, R. Coatings with columnar microstructures for thermal barrier applications. Adv. Eng. Mater. 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Shen, Z.; He, L.; Xu, Z.; Mu, R.; Huang, G. Rare earth oxides stabilized La2Zr2O7 TBCs: EB-PVD, thermal conductivity and thermal cycling life. Surf. Coat. Technol. 2019, 357, 427–432. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, J.; Shao, F.; Zhong, X.; Zhao, H.; Zhuang, Y.; Ni, J.; Tao, S. Thermal stability of YSZ coatings deposited by plasma spray-physical vapor deposition. Coatings 2019, 9, 464. [Google Scholar] [CrossRef]
- Zhao, C.; He, W.; Wei, L.; Guo, H. Microstructures of La2Ce2O7 coatings produced by plasma spray-physical vapor deposition. J. Eur. Ceram. Soc. 2020, 40, 1462–1470. [Google Scholar] [CrossRef]
- Schmitt, M.P.; Harder, B.J.; Wolfe, D.E. Process-structure-property relations for the erosion durability of plasma spray-physical vapor deposition (PS-PVD) thermal barrier coatings. Surf. Coat. Technol. 2016, 297, 11–18. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhou, K.S.; Liu, M.; Deng, C.M.; Deng, C.G.; Mao, J.; Deng, Z.Q. Mechanisms governing the thermal shock and tensile fracture of PS-PVD 7YSZ TBC. Ceram. Int. 2018, 44, 3973–3980. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, N.; Zeng, Y.; Song, X.; Lin, C.; Liu, Z.; Zhang, J. Effect of different types of pores on thermal conductivity of YSZ thermal barrier coatings. Coatings 2019, 9, 138. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Q.; Liu, Y.; Ning, X. Microstructure, thermal characteristics, and thermal cycling behavior of the ternary rare earth oxides (La2O3, Gd2O3, and Yb2O3) co-doped YSZ coatings. Surf. Coat. Technol. 2020, 403, 126387. [Google Scholar] [CrossRef]
- Doleker, K.M.; Karaoglanli, A.C. Comparison of oxidation behavior of YSZ and Gd2Zr2O7 thermal barrier coatings (TBCs). Surf. Coat. Technol. 2017, 318, 198–207. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wang, X.L.; Wang, X.Y.; Yang, Y.; Zhang, C.; Sun, W.W.; Ma, Y.D.; Cui, Y.H.; Wang, L.; Dong, Y.C. Effect of CeO2 on the microstructure and properties of plasma-sprayed Al2O3-ZrO2 ceramic coatings. J. Mater. Eng. Perform. 2020, 29, 6390–6401. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, S.; Wang, X. Low–thermal–conductivity and high–toughness CeO2–Gd2O3 co–stabilized zirconia ceramic for potential thermal barrier coating applications. J. Eur. Ceram. Soc. 2018, 38, 3986–3993. [Google Scholar] [CrossRef]
- Tabatabaeian, M.R.; Rahmanifard, R.; Seyed Jalili, Y. The study of phase stability and thermal shock resistance of a Scandia–Ceria stabilized zirconia as a new TBC material. Surf. Coat. Technol. 2019, 374, 752–762. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Jing, Q.; Liu, B.; Zhang, H.; Yongsheng, Y.; Yuan, J.; Dong, S.; Zhou, X.; Cao, X. Phase stability and thermo-physical properties of ZrO2-CeO2-TiO2 ceramics for thermal barrier coatings. J. Eur. Ceram. Soc. 2018, 38, 2841–2850. [Google Scholar] [CrossRef]
- Sun, W.W.; Wang, X.L.; Sun, X.W.; Yang, Y.; Zhang, C.; Wang, Y.W.; Cui, Y.H.; Ma, Y.D. Microstructure and properties of Al2O3-ZrO2-Y2O3 coatings during high temperature and thermal shock resistance. Appl. Phys. A 2020, 126. [Google Scholar] [CrossRef]
- Khan, M.; Zeng, Y.; Lan, Z.; Wang, Y. Reduced thermal conductivity of solid solution of 20% CeO2 + ZrO2 and 8% Y2O3 + ZrO2 prepared by atmospheric plasma spray technique. Ceram. Int. 2019, 45, 839–842. [Google Scholar] [CrossRef]
- Bobzin, K.; Zhao, L.; Öte, M.; Königstein, T. A highly porous thermal barrier coating based on Gd2O3–Yb2O3 co-doped YSZ. Surf. Coat. Technol. 2019, 366, 349–354. [Google Scholar] [CrossRef]
- Boissonnet, G.; Chalk, C.; Nicholls, J.R.; Bonnet, G.; Pedraza, F. Phase stability and thermal insulation of YSZ and erbia-yttria co-doped zirconia EB-PVD thermal barrier coating systems. Surf. Coat. Technol. 2020, 389, 125566. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, Y.; Wang, X.; Wang, Q.; Ai, L.; Zhao, L.; Chu, Y.; Guo, S.; Hu, J.; Zhang, Q. Preparation and thermophysical properties of Ti4+ doped zirconia matrix thermal barrier coatings. J. Alloys Compd. 2019, 777, 646–654. [Google Scholar] [CrossRef]
- Dong, K.; Lu, F.; Huang, W.; Zhu, L. Residual stress and fracture toughness of thick 8YSZ-Al2O3 composite coatings via a modified Vickers indentation method. Vacuum 2020, 177, 109437. [Google Scholar] [CrossRef]
- Bobzin, K.; Zhao, L.; Öte, M.; Königstein, T. Effect of long-term heat-treatment at 1150 °C on the microstructure and properties of thermal barrier coatings based on ZrO2–4 mol.% Y2O3–1 mol.% Gd2O3–1 mol.% Yb2O3. Surf. Coat. Technol. 2017, 318, 142–146. [Google Scholar] [CrossRef]
- Bahamirian, M.; Hadavi, S.M.M.; Farvizi, M.; Rahimipour, M.R.; Keyvani, A. Phase stability of ZrO29.5Y2O35.6Yb2O35.2Gd2O3 compound at 1100 °C and 1300 °C for advanced TBC applications. Ceram. Int. 2019, 45, 7344–7350. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C. Hot corrosion behavior of nanostructured Gd2O3 doped YSZ thermal barrier coating in presence of Na2SO4 + V2O5 molten salts. Prog. Nat. Sci. Mater. Int. 2017, 27, 507–513. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, C.; Li, M.; Sun, W.; Zhang, Z.; Ye, F. Hot corrosion evaluation of Gd2O3-Yb2O3 co-doped Y2O3 stabilized ZrO2 thermal barrier oxides exposed to Na2SO4 + V2O5 molten salt. Ceram. Int. 2017, 43, 2780–2785. [Google Scholar] [CrossRef]
- Khajezadeh, M.H.; Mohammadi, M.; Ghatee, M. Hot corrosion performance and electrochemical study of CoNiCrAlY/YSZ/YSZ-La2O3 multilayer thermal barrier coatings in the presence of molten salt. Mater. Chem. Phys. 2018, 220, 23–34. [Google Scholar] [CrossRef]
- Chen, C.; Liang, T.; Guo, Y.; Chen, X.; Man, Q.; Zhang, X.; Zeng, J.; Ji, V. Effect of scandia content on the hot corrosion behavior of Sc2O3 and Y2O3 co-doped ZrO2 in Na2SO4 + V2O5 molten salts at 1000 °C. Corros. Sci. 2019. [Google Scholar] [CrossRef]
- Bahamirian, M.; Hadavi, S.M.M.; Farvizi, M.; Keyvani, A.; Rahimipour, M.R. ZrO2 9.5Y2O3 5.6Yb2O3 5.2Gd2O3; a promising TBC material with high resistance to hot corrosion. J. Asian Ceram. Soc. 2020. [Google Scholar] [CrossRef]
- Avci, A.; Eker, A.A.; Karabas, M. An investigation of oxidation, hot corrosion, and thermal shock behavior of atmospheric plasma-sprayed YSZ–Al2O3 composite thermal barrier coatings. Int. J. Mater. Res. 2020, 111, 567–580. [Google Scholar] [CrossRef]
- Fan, W.; Bai, Y.; Liu, Y.F.; Kang, Y.X.; Wang, Y.; Wang, Z.Z.; Tao, W.Z. Corrosion behavior of Sc2O3–Y2O3 co-stabilized ZrO2 thermal barrier coatings with CMAS attack. Ceram. Int. 2019, 45, 15763–15767. [Google Scholar] [CrossRef]
- Bahamirian, M.; Hadavi, S.M.M.; Farvizi, M.; Keyvani, A.; Rahimipour, M.R. Hot corrosion behavior of ZrO2 9.5Y2O3 5.6Yb2O3 5.2Gd2O3 TBCs in CMAS: CaO-MgO-Al2O3-SiO2. J. Aust. Ceram. Soc. 2020. [Google Scholar] [CrossRef]
- Wu, D.; Yao, Y.; Shan, X.; Yang, F.; Zhao, X.; Xiao, P. Equimolar YO1.5 and TaO2.5 co-doped ZrO2 as a potential CMAS-resistant material for thermal barrier coatings. J. Am. Ceram. Soc. 2020. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Q.; Liu, Y.; Ning, X. Investigation of ternary rare earth oxide-doped YSZ and its high temperature stability. J. Alloys Compd. 2019, 806, 580–586. [Google Scholar] [CrossRef]
- Shi, Q.; Yuan, W.; Chao, X.; Zhu, Z. Phase stability, thermal conductivity and crystal growth behavior of RE2O3 (RE = La, Yb, Ce, Gd) co-doped Y2O3 stabilized ZrO2 powder. J. Sol-Gel Sci. Technol. 2017, 84, 341–348. [Google Scholar] [CrossRef]
- Fan, W.; Wang, Z.Z.; Bai, Y.; Che, J.W.; Wang, R.J.; Ma, F.; Tao, W.Z.; Liang, G.Y. Improved properties of scandia and yttria co-doped zirconia as a potential thermal barrier material for high temperature applications. J. Eur. Ceram. Soc. 2018, 38, 4502–4511. [Google Scholar] [CrossRef]
- Kulczyk-Malecka, J.; Zhang, X.; Carr, J.; Nozahic, F.; Estournès, C.; Monceau, D.; Carabat, A.L.; Sloof, W.G.; van der Zwaag, S.; Withers, P.J.; et al. Thermo—mechanical properties of SPS produced self-healing thermal barrier coatings containing pure and alloyed MoSi2 particles. J. Eur. Ceram. Soc. 2018, 38, 4268–4275. [Google Scholar] [CrossRef]
- Fang, Y.; Cui, X.; Jin, G.; Lu, B.; Wang, F.; Liu, M.; Wen, X. Influence of SiC fiber on thermal cycling lifetime of SiC fibers/YSZ thermal barrier coatings by atmospheric plasma spraying. Ceram. Int. 2018, 20, 1–15. [Google Scholar] [CrossRef]
- Thakare, J.G.; Mulik, R.S.; Mahapatra, M.M. Estimation of residual stress in air plasma sprayed MWCNT-reinforced 8YSZ–alumina composite coating. Arch. Civ. Mech. Eng. 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Thakare, J.G.; Mulik, R.S.; Mahapatra, M.M. Evaluation of cyclic hot corrosion resistance of plasma-sprayed composite coating in Na2SO4-60%V2O5 molten salt environment. J. Therm. Spray Technol. 2020, 29, 811–824. [Google Scholar] [CrossRef]
- Yang, P.; An, Y.; Yang, D.; Li, Y.; Chen, J. Structure, thermal properties and hot corrosion behaviors of Gd2Hf2O7 as a potential thermal barrier coating material. Ceram. Int. 2020, 46, 21367–21377. [Google Scholar] [CrossRef]
- Deng, W.; Fergus, J.W. Effect of CMAS Composition on hot corrosion behavior of gadolinium zirconate thermal barrier coating materials. J. Electrochem. Soc. 2017, 164, C526–C531. [Google Scholar] [CrossRef]
- Zhou, X.; He, L.; Cao, X.; Xu, Z.; Mu, R.; Sun, J.; Yuan, J.; Zou, B. La2(Zr0.7Ce0.3)2O7 thermal barrier coatings prepared by electron beam-physical vapor deposition that are resistant to high temperature attack by molten silicate. Corros. Sci. 2017, 115, 143–151. [Google Scholar] [CrossRef]
- Li, M.; Cheng, Y.; Guo, L.; Zhang, Y.; Zhang, C.; He, S.; Sun, W.; Ye, F. Preparation of nanostructured Gd2Zr2O7-LaPO4 thermal barrier coatings and their calcium-magnesium-alumina-silicate (CMAS) resistance. J. Eur. Ceram. Soc. 2017, 37, 3425–3434. [Google Scholar] [CrossRef]
- Xu, Z.H.; Shen, Z.Y.; Mu, R.D.; He, L.M. Phase structure, thermophysical properties and thermal cycling behavior of novel (Sm0.2La0.8)2(Zr0.7Ce0.3)2O7 thermal barrier coatings. Vacuum 2018, 157, 105–110. [Google Scholar] [CrossRef]
- Zhao, F.A.; Xiao, H.Y.; Bai, X.M.; Liu, Z.J.; Zu, X.T. Effects of doping Yb3+, La3+, Ti4+, Hf4+, Ce4+ cations on the mechanical properties, thermal conductivity, and electronic structures of Gd2Zr2O7. J. Alloys Compd. 2019, 776, 306–318. [Google Scholar] [CrossRef]
- Jiang, T.; Xie, M.; Wang, X.; Song, X. Effects of Nb5+ doping on thermal properties of Gd2(Zr1−xNbx)2O7+x ceramics. Adv. Appl. Ceram. 2020, 119, 212–217. [Google Scholar] [CrossRef]
- Zhu, R.B.; Zou, J.P.; Mao, J.; Deng, Z.Q.; Zhang, X.F.; Deng, C.M.; Liu, M. A comparison between novel Gd2Zr2O7 and Gd2Zr2O7/YSZ thermal barrier coatings fabricated by plasma spray-physical vapor deposition. Rare Met. 2020. [Google Scholar] [CrossRef]
- Xue, Z.; Wu, S.; Qian, L.; Byon, E.; Zhang, S. Influence of Y2O3 and Ta2O5 Co-doping on Microstructure and Thermal Conductivity of Gd2Zr2O7 Ceramics. J. Mater. Eng. Perform. 2020, 29, 1206–1213. [Google Scholar] [CrossRef]
- Guo, Y.; He, W.; Guo, H. Thermo-physical and mechanical properties of Yb2O3 and Sc2O3 co-doped Gd2Zr2O7 ceramics. Ceram. Int. 2020, 46, 18888–18894. [Google Scholar] [CrossRef]
- Guo, L.; Xin, H.; Zhang, Z.; Ye, F.; Yan, Z. Preparation of (Gd0.9Sc0.1)2Zr2O7/YSZ thermal barrier coatings and their corrosion resistance to V2O5 molten salt. Surf. Coat. Technol. 2020, 389, 125677. [Google Scholar] [CrossRef]
- Wang, D.; Dong, S.; Zeng, J.; Liang, P.; Liao, H.; Wang, Y.; Jiang, J.; Deng, L.; Zhou, X.; Cao, X. Influence of doping Mg2+ or Ti4+ captions on the microstructures, thermal radiation and thermal cycling behavior of plasma-sprayed Gd2Zr2O7 coatings. Ceram. Int. 2020, 46, 13054–13065. [Google Scholar] [CrossRef]
- Satpathy, R.; Rani, S.; Alam, Z.; Besra, L. Effectiveness of lanthanum zirconate and Yttria stabilised zirconia freestanding APS thermal barrier coatings against natural CMAS attack at high temperatures. Mater. High Temp. 2020, 37, 416–424. [Google Scholar] [CrossRef]
- Lyu, G.; Song, D.; Choi, B.G.; Jung, Y.G. Infiltration behavior of CMAS in LZ-YSZ composite thermal barrier coatings. JOM 2020, 1–10. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, J.; Yang, L.; Zhou, Y.; Wang, Q.; Chen, H.; Yang, G.; Gao, Y.; Liu, B. Preparation and corrosion resistance of nonstoichiometric lanthanum zirconate coatings. J. Eur. Ceram. Soc. 2020, 40, 3122–3128. [Google Scholar] [CrossRef]
- Karabaş, M. Production and characterization of Nd and Dy doped lanthanum zirconate-based thermal barrier coatings. Surf. Coat. Technol. 2020, 394, 125864. [Google Scholar] [CrossRef]
- Jin, G.; Fang, Y.; Cui, X.; Wang, C.; Zhang, D.; Wen, X.; Mi, Q. Effect of YSZ fibers and carbon nanotubes on bonding strength and thermal cycling lifetime of YSZ-La2Zr2O7 thermal barrier coatings. Surf. Coat. Technol. 2020, 397, 125986. [Google Scholar] [CrossRef]
- Feng, B.B.; Wang, Y.; Jia, Q.; Huang, W.; Suo, H.L.; Ma, W. Thermophysical properties of solution precursor plasma-sprayed La2Ce2O7 thermal barrier coatings. Rare Met. 2019, 38, 689–694. [Google Scholar] [CrossRef]
- Xiaoge, C.; Hongsong, Z.; Kun, S.; Xudan, D.; Haoming, Z.; Bo, R.; An, T. Thermal conductivity and expansion coefficient of (Sm1−xYbx)2Ce2O7 ceramics for thermal barrier coatings. J. Mater. Eng. Perform. 2017, 26, 6193–6197. [Google Scholar] [CrossRef]
- Kandasamy, P.; Govindarajan, S.; Gurusamy, S. Volcanic ash infiltration resistance of new-generation thermal barrier coatings at 1150 °C. Surf. Coat. Technol. 2020, 401, 126226. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yi, H.; Che, J.W.; Liang, G.Y. Phase, compositional, structural, and chemical stability of La2Ce2O7 after high temperature heat treatment. Ceram. Int. 2019, 45, 5030–5035. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Dong, S.; Yuan, J.; Zhou, X.; Duo, S.; Chen, S.; Huo, P.; Jiang, J.; Deng, L.; et al. Mechanical properties and thermal cycling behavior of Ta2O5 doped La2Ce2O7 thermal barrier coatings prepared by atmospheric plasma spraying. J. Alloys Compd. 2019, 785, 1068–1076. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Duo, S.; Zhou, X.; Yuan, J.; Dong, S.; Yang, X.; Zeng, J.; Jiang, J.; Deng, L.; et al. Thermal and mechanical properties of Ta2O5 doped La2Ce2O7 thermal barrier coatings prepared by atmospheric plasma spraying. J. Eur. Ceram. Soc. 2019, 39, 2379–2388. [Google Scholar] [CrossRef]
- Dehkharghani, A.M.F.; Rahimipour, M.R.; Zakeri, M. Improving the thermal shock resistance and fracture toughness of synthesized La2Ce2O7 thermal barrier coatings through formation of La2Ce2O7/YSZ composite coating via air plasma spraying. Surf. Coat. Technol. 2020, 399, 126174. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, W.; Wang, Y.; Liu, K.; Wang, Z.Z.; Bai, Y. Oxidation resistance of plasma-sprayed double-layered LC/YSZ coatings with different thickness ratios at high temperatures. Oxid. Met. 2020, 94, 397–408. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, F.; Li, N.; Zeng, J.; Liang, P.; Zhang, H.; Liao, H.; Jiang, J.; Deng, L.; Cao, X. Thermal radiation and cycling properties of (Ca, Fe) or (Sr, Mn) co-doped La2Ce2O7 coatings. J. Eur. Ceram. Soc. 2020, 40, 2020–2029. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Wen, J.; Bai, Y.; Sun, L.; Chen, B.; Dong, H.; Shuang, Y. Preparation of SrZrO3 thermal barrier coating by solution precursor plasma spray. J. Therm. Spray Technol. 2017, 26, 371–377. [Google Scholar] [CrossRef]
- Ma, W.; Li, X.; Meng, X.; Xue, Y.; Bai, Y.; Chen, W.; Dong, H. Microstructure and thermophysical properties of SrZrO3 thermal barrier coating prepared by solution precursor plasma spray. J. Therm. Spray Technol. 2018, 27, 1056–1063. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, Y.; Li, E.; Qi, Y.; Liu, C.; Dong, H.; Jia, R.; Ma, W. Preparation and characterization of SrZrO3–La2Ce2O7 composite ceramics as a thermal barrier coating material. Mater. Chem. Phys. 2020, 247, 122904. [Google Scholar] [CrossRef]
- Khan, M.; Zeng, Y. Achieving low thermal conductivity in Sr(Zr0.9Yb0.05Gd0.05)O2.95: A suitable material for high temperature applications. Ceram. Int. 2020, 46, 28778–28784. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, Y.; Li, E.; Dong, H.; Ma, W. Yb2O3-Gd2O3 codoped strontium zirconate composite ceramics for potential thermal barrier coating applications. Int. J. Appl. Ceram. Technol. 2020, 17, 1608–1618. [Google Scholar] [CrossRef]
- Fang, Y.; Cui, X.; Jin, G.; Liu, E.; Zhang, D.; Wen, X.; Mi, Q. Microstructural evolution and hot corrosion behavior of La0.8Ba0.2TiO3–δ-YSZ double-layer thermal barrier coatings in Na2SO4 + V2O5 molten salt at 900 °C. Surf. Coat. Technol. 2020, 399, 126175. [Google Scholar] [CrossRef]
- Yuan, J.; Sun, J.; Wang, J.; Zhang, H.; Dong, S.; Jiang, J.; Deng, L.; Zhou, X.; Cao, X. SrCeO3 as a novel thermal barrier coating candidate for high–temperature applications. J. Alloys Compd. 2018, 740, 519–528. [Google Scholar] [CrossRef]
- Vourdas, N.; Marathoniti, E.; Pandis, P.K.; Argirusis, C.; Sourkouni, G.; Legros, C.; Mirza, S.; Stathopoulos, V.N. Evaluation of LaAlO3 as top coat material for thermal barrier coatings. Trans. Nonferrous Met. Soc. China 2018, 28, 1582–1592. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Q.; Ning, X.; Liu, Y. Evaluation of the phase stability, and mechanical and thermal properties of Ba(Sr1/3Ta2/3)O3 as a potential ceramic material for thermal barrier coatings. Ceram. Int. 2019, 45, 12989–12993. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Q.; Liu, Y.; Ning, X.; Wang, H. Characteristics and thermal cycling behavior of plasma-sprayed Ba(Mg1/3Ta2/3)O3 thermal barrier coatings. Ceram. Int. 2017, 43, 10955–10959. [Google Scholar] [CrossRef]
- Wu, P.; Chong, X.; Wu, F.; Hu, M.; Guo, H.; Feng, J. Investigation of the thermophysical properties of (Y1−xYbx)TaO4 ceramics. J. Eur. Ceram. Soc. 2020, 40, 3111–3121. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.; Guo, J.; Chong, X.; Feng, J. Mechanical and thermal properties of RETaO4 (RE = Yb, Lu, Sc) ceramics with monoclinic-prime phase. J. Mater. Sci. Technol. 2020, 52, 20–28. [Google Scholar] [CrossRef]
- Yang, K.; Chen, L.; Wu, F.; Zheng, Q.; Li, J.; Song, P.; Wang, Y.; Liu, R.; Feng, J. Thermophysical properties of Yb(TaxNb1−x)O4 ceramics with different crystal structures. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Chen, X.G.; Zhang, H.S.; Tong, Y.P.; Yang, X.F.; Sang, W.W.; Zhang, H.M.; Zhao, Y. Thermophysical properties of Ln(3)Ce(7)Ta(2)O(23)(.5) (Ln = Nd and La) composite oxides. Ceram. Int. 2020, 46, 8903–8909. [Google Scholar]
- Ye, F.; Yuan, Y.; Yan, S.; Guo, L.; Yu, J. High-temperature corrosion mechanism of a promising scandium tantalate ceramic for next generation thermal barrier coating under molten calcium–magnesium-aluminosilicate (CMAS). Mater. Chem. Phys. 2020, 256, 123679. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, Y.; Tong, Y.P.; Sang, W.W.; Zhao, Y.T.; Yan, X.F.; Tang, A. Thermal-physical performances of novel pyrochlore-type Ca3Ln3Ce7Ta2O26.5(Ln = Nd and Dy) oxides. Ceram. Int. 2020, 46, 11416–11420. [Google Scholar]
- Zheng, Q.; Wu, F.; Chen, L.; Qian, F.; Yang, K.; Ge, Z.; Song, P.; Feng, J. Thermophysical and mechanical properties of YTaO4 ceramic by niobium substitution tantalum. Mater. Lett. 2020, 268, 127586. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhou, Y.; Wu, P.; Song, P.; Chong, X.Y.; Feng, J. Thermal properties of Y1−xMgxTaO4−x/2 ceramics via anion sublattice adjustment. RARE Met. 2020, 39, 545–554. [Google Scholar] [CrossRef]
- Chen, X.G.; Yang, S.S.; Song, Y.; Zhang, H.S.; Yang, X.F.; Sang, W.W. Phase-structures, thermophysical properties of Sm3Ce7Ta2O23.5 and Gd3Ce7Ta2O23.5 oxides for thermal barrier coating applications. Ceram. Int. 2020, 46, 8238–8243. [Google Scholar] [CrossRef]
- Chen, L.; Feng, J. Thermal and mechanical properties optimization of ABO4 type EuNbO4 by the B-site substitution of Ta. Engineering 2020, 6, 178–185. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, K.; Wang, S.-H.; Zhang, H.-S.; Yan, X.F.; Sang, W.W.; Zhao, Y.T.; Tang, A. Synthesis and thermophysical performances of (Nd1−xYbx)2AlTaO7 oxides for heat-insulation coating applications. Ceram. Int. 2020, 46, 26754–26759. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Y.; Chen, D.; Li, J.; Li, W.; Zeng, D.; Wu, D.; Zou, B.; Cao, X. A comparative investigation on the corrosion degradation of plasma sprayed YSZ and LnMgAl11O19 (Ln = Nd, Sm, Gd) coatings exposed to the molten V2O5 + Na2SO4 salt mixture at 1100 °C. J. Eur. Ceram. Soc. 2019, 39, 3778–3787. [Google Scholar] [CrossRef]
- Zhou, X.; Song, W.; Yuan, J.; Gong, Q.; Zhang, H.; Cao, X.; Dingwell, D.B. Thermophysical properties and cyclic lifetime of plasma sprayed SrAl12O19 for thermal barrier coating applications. J. Am. Ceram. Soc. 2020, 103, 5599–5611. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, H.; Chen, X.; Deng, C.; Wu, D.; Cao, X.; Li, W. Degradation of the plasma sprayed GdMgAl11O19 thermal barrier coating resistant to calcium-magnesium-aluminum-silicate attack at 1350 °C. Corros. Sci. 2020, 169, 108593. [Google Scholar] [CrossRef]
- Khorramirad, M.M.; Rahimipour, M.R.; Hadavi, S.M.M.; Shirvani, K. High temperature oxidation behavior of Inc-738/NiCrAlY/LaMA thermal barrier coating system. Surf. Coat. Technol. 2019, 364, 70–80. [Google Scholar] [CrossRef]
- Tsukada, S.; Kuroda, S.; Nishijima, M.; Araki, H.; Yumoto, A.; Watanabe, M. Effects of amorphous phase on hot corrosion behavior of plasma-sprayed LaMgAl11O19 coating. Surf. Coat. Technol. 2019, 363, 95–105. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Zhou, X.; Dong, S.; Deng, L.; Jiang, J.; Cao, X. Microstructure and thermal cycling behavior of plasma-sprayed LaMgAl11O19 coatings. Ceram. Int. 2018, 44, 5572–5580. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Zhou, X.; Hui, Y.; Dong, S.; Li, L.; Deng, L.; Jiang, J.; Cao, X. Thermal cycling behavior of the plasma-sprayed coating of lanthanum hexaaluminate. J. Eur. Ceram. Soc. 2018, 38, 1919–1929. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Zhang, H.; Yuan, J.; Dong, S.; Jiang, J.; Deng, L.; Zhou, X.; Cao, X. Preparation, structure, mechanical properties and thermal cycling behavior of porous LaMgAl11O19 coating. J. Alloys Compd. 2018, 750, 1007–1016. [Google Scholar] [CrossRef]
- Jana, P.; Jayan, P.S.; Mandal, S.; Biswas, K. Thermal cycling life and failure analysis of rare earth magnesium hexaaluminate based advanced thermal barrier coatings at 1400 °C. Surf. Coat. Technol. 2017, 328, 398–409. [Google Scholar] [CrossRef]
- Kumar, R.; Jordan, E.; Gell, M.; Roth, J.; Jiang, C.; Wang, J.; Rommel, S. CMAS behavior of yttrium aluminum garnet (YAG) and yttria-stabilized zirconia (YSZ) thermal barrier coatings. Surf. Coat. Technol. 2017, 327, 126–138. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Yu, Y.; Yuan, Q.; Tan, Y.; Feng, Z. Hot corrosion behavior of Y4Al2O9 ceramics for thermal barrier coatings exposed to calcium-magnesium-alumina-silicate at 1250 °C. J. Eur. Ceram. Soc. 2019, 39, 1487–1495. [Google Scholar] [CrossRef]
- Gell, M.; Wang, J.; Kumar, R.; Roth, J.; Jiang, C.; Jordan, E.H. Higher temperature thermal barrier coatings with the combined use of yttrium aluminum garnet and the solution precursor plasma spray process. J. Therm. Spray Technol. 2018, 27, 543–555. [Google Scholar] [CrossRef]
- Xue, Z.; Ma, Y.; Guo, H. The influence of Gd doping on thermophysical properties, elasticity modulus and phase stability of garnet-type (Y1−xGdx)3Al5O12 ceramics. J. Eur. Ceram. Soc. 2017, 37, 4171–4177. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Z.; Xiang, H.; Dai, F.Z.; Xu, W.; Sun, K.; Liu, J.; Zhou, Y. High entropy (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12: A novel high temperature stable thermal barrier material. J. Mater. Sci. Technol. 2020, 48, 57–62. [Google Scholar] [CrossRef]
- Li, M.; Wang, D.; Xue, J.; Jia, R. Preparation of Pd-doped Y3Al5O12 thermal barrier coatings using cathode plasma electrolytic deposition. Ceram. Int. 2020, 46, 7019–7024. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, X.; Song, W.; Sun, J.; Zhang, H.; Jiang, J.; Deng, L.; Dong, S.; Cao, X. Mg2SiO4 as a novel thermal barrier coating material for gas turbine applications. J. Eur. Ceram. Soc. 2019, 39, 2397–2408. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, J.; Zhang, T.; Ren, X.; Hu, W.; Zheng, L.; Wang, J. Towards thermal barrier coating application for rare earth silicates RE2SiO5 (RE = La, Nd, Sm, Eu, and Gd). J. Eur. Ceram. Soc. 2019, 39, 1463–1476. [Google Scholar] [CrossRef]
- Tian, Z.; Zheng, L.; Wang, J.; Wan, P.; Li, J.; Wang, J. Theoretical and experimental determination of the major thermo-mechanical properties of RE2SiO5 (RE = Tb, Dy, Ho, Er, Tm, Yb, Lu, and Y) for environmental and thermal barrier coating applications. J. Eur. Ceram. Soc. 2016, 36, 189–202. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wang, J. Theoretical investigation of phonon contributions to thermal expansion coefficients for rare earth monosilicates RE2SiO5 (RE = Dy, Ho, Er, Tm, Yb and Lu). J. Eur. Ceram. Soc. 2020. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, C.; He, Q.; Yu, J.; Yan, Z.; Ye, F.; Dan, C.; Ji, V. Microstructure evolution and hot corrosion mechanisms of Ba2REAlO5 (RE = Yb, Er, Dy) exposed to V2O5 + Na2SO4 molten salt. J. Eur. Ceram. Soc. 2018, 40, 2658–2666. [Google Scholar] [CrossRef]
- Wei, L.; Guo, L.; Li, M.; Guo, H. Calcium-magnesium-alumina-silicate (CMAS) resistant Ba2REAlO5 (RE = Yb, Er, Dy) ceramics for thermal barrier coatings. J. Eur. Ceram. Soc. 2017, 37, 4991–5000. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Guo, L.; Yu, Y.; Zhao, Y.; Yuan, Q.; Wang, H.; Feng, Z. Hot corrosion behavior of Ba2DyAlO5 exposed to calcium-magnesium-alumina-silicate at 1300 °C and 1350 °C. Vacuum 2018, 155, 307–317. [Google Scholar] [CrossRef]
- Baskaran, T.; Arya, S.B. Hot corrosion resistance of air plasma sprayed ceramic Sm2SrAl2O7 (SSA) thermal barrier coatings in simulated gas turbine environments. Ceram. Int. 2018, 44, 17695–17708. [Google Scholar] [CrossRef]
- Zhong, X.; Niu, Y.; Li, H.; Zheng, X.; Ding, C.; Sun, J. Microstructure and thermomechanical properties of atmospheric plasma-sprayed Yb2O3 coating. J. Therm. Spray Technol. 2018, 27, 959–967. [Google Scholar] [CrossRef]
- Morán-Ruiz, A.; Vidal, K.; Larrañaga, A.; Arriortua, M.I. Characterization of Ln4Al2O9 (Ln = Y, Sm, Eu, Gd, Tb) rare-earth aluminates as novel high-temperature barrier materials. Ceram. Int. 2018, 44, 8761–8767. [Google Scholar] [CrossRef]
- Li, C.; He, J.; Ma, Y. Sintering behavior and thermal conductivity of Y2O3 fully stabilized HfO2 ceramics. Rare Met. 2020. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, Y.; Li, Y.; Pan, W.; Zong, P.; Huang, M.; Han, Y.; Yang, Z.; Chen, H.; Gong, Q.; et al. Thermal and mechanical properties of ferroelastic RENbO4 (RE = Nd, Sm, Gd, Dy, Er, Yb) for thermal barrier coatings. Scr. Mater. 2020, 180, 51–56. [Google Scholar] [CrossRef]
- Li, C.; He, J.; Ma, Y.; Guo, H. Evolution mechanism of the microstructure and mechanical properties of plasma-sprayed yttria-stabilized hafnia thermal barrier coating at 1400 °C. Ceram. Int. 2020, 46, 23417–23426. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, W.; Zhang, H.; Wang, F.; Sun, D.; Yang, X.; Su, Z.; Duan, Q.; Mai, W. Y3Ce7Ta2O23.5 and Yb3Ce7Ta2O23.5—Two kinds of novel ceramics for thermal barrier coatings. Ceram. Int. 2019, 45, 10414–10419. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).