RETRACTED: Curing of Functionalized Superhydrophobic Inorganic/Epoxy Nanocomposite and Application as Coatings for Steel
Abstract
1. Introduction
2. Experimental
2.1. Materials
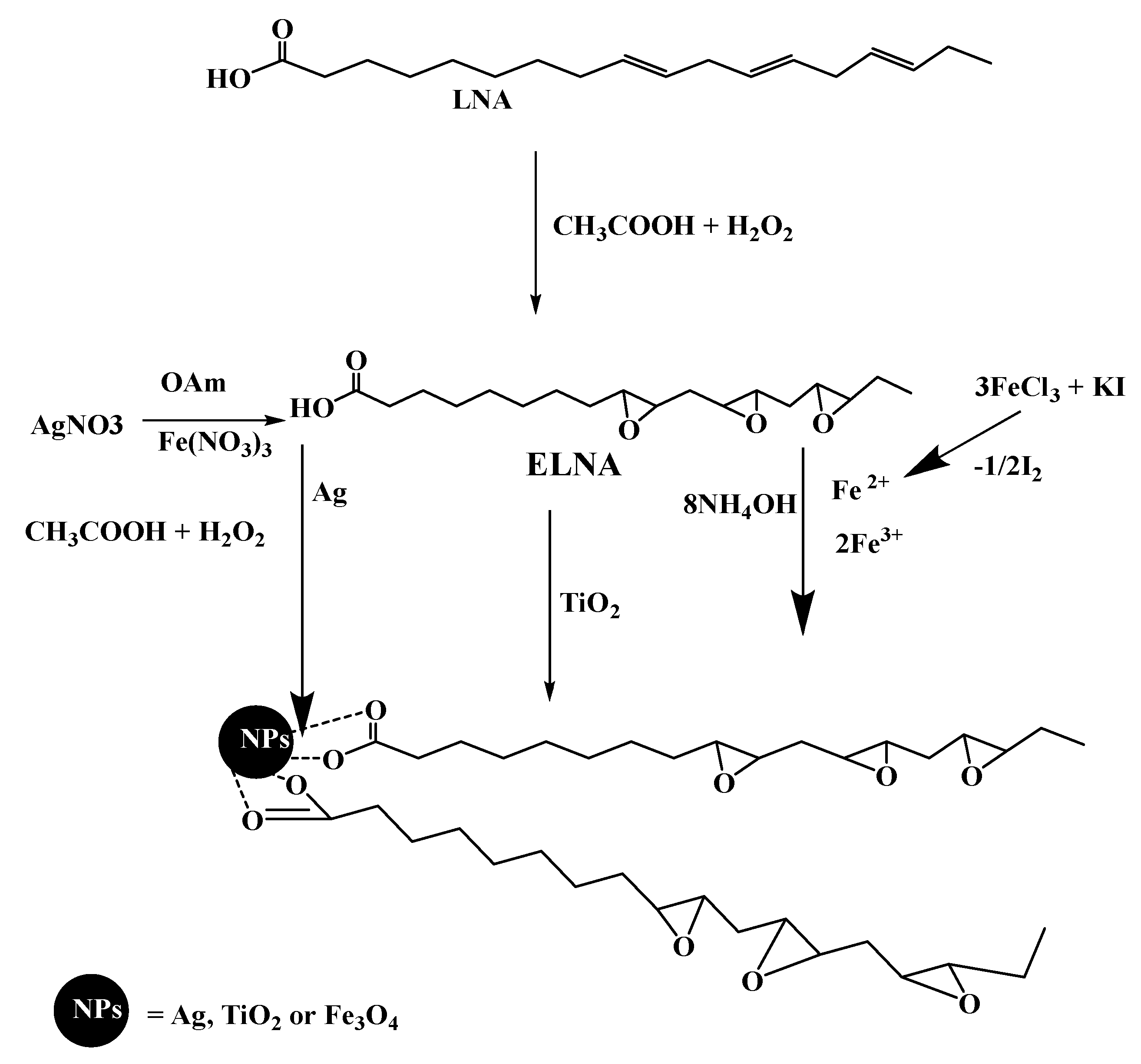
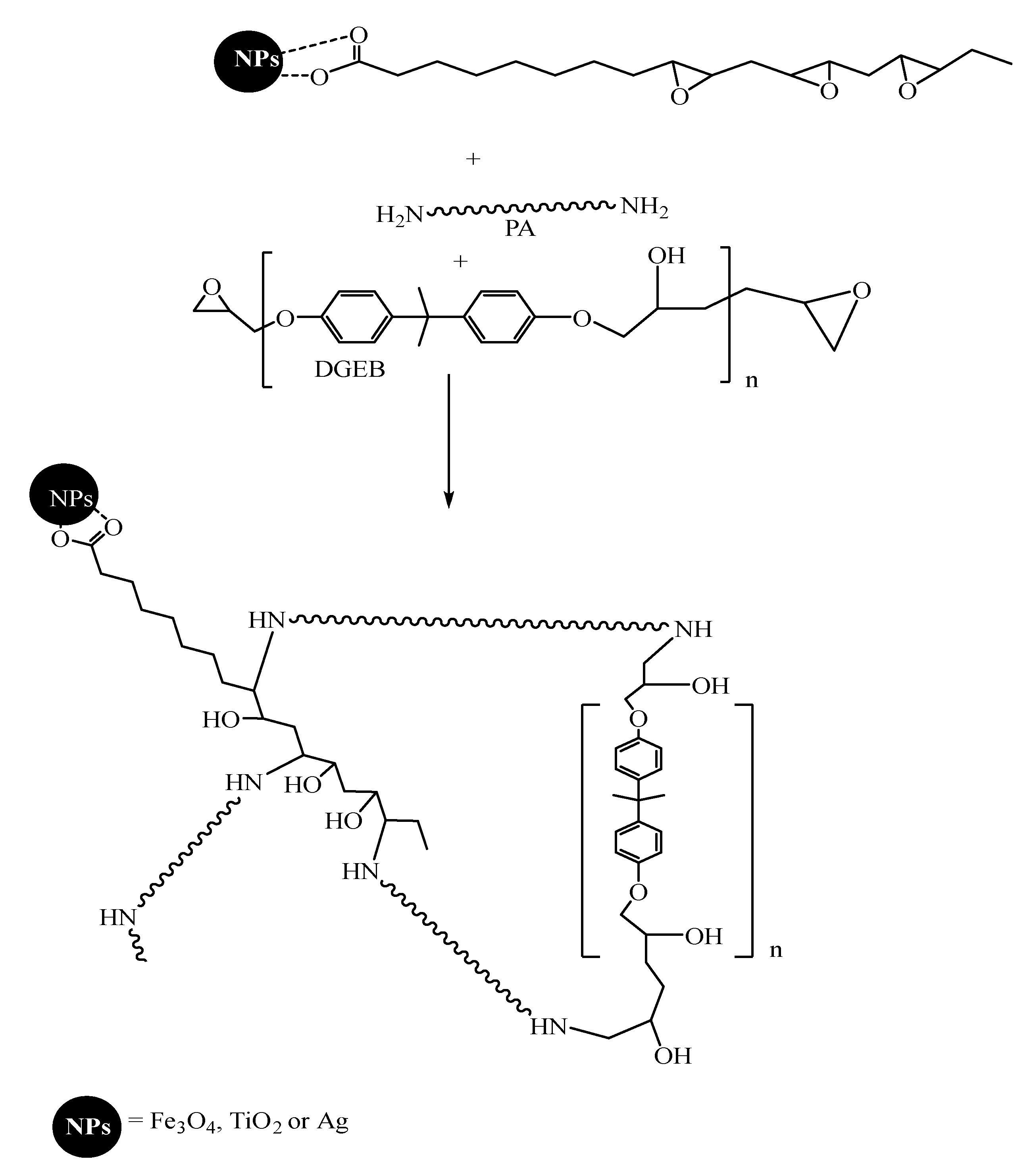
2.2. Preparation of Hydrophobic Modified NPs
2.2.1. Synthesis of Hydrophobically Modified Magnetite NPs
2.2.2. Synthesis of Hydrophobically Modified TiO2 NPs
2.2.3. Synthesis of Hydrophobically Modified Ag NPs
2.3. Characterization of Hydrophobic Modified NPs
2.4. Curing and Thermomechanical Properties of DGEB/PA Epoxy Nanocomposites
2.5. Preparation of DGEB/PA Epoxy Nanocomposites Coatings
2.6. Properties of DGEB/PA Epoxy Nanocomposites Coatings
3. Results and Discussion
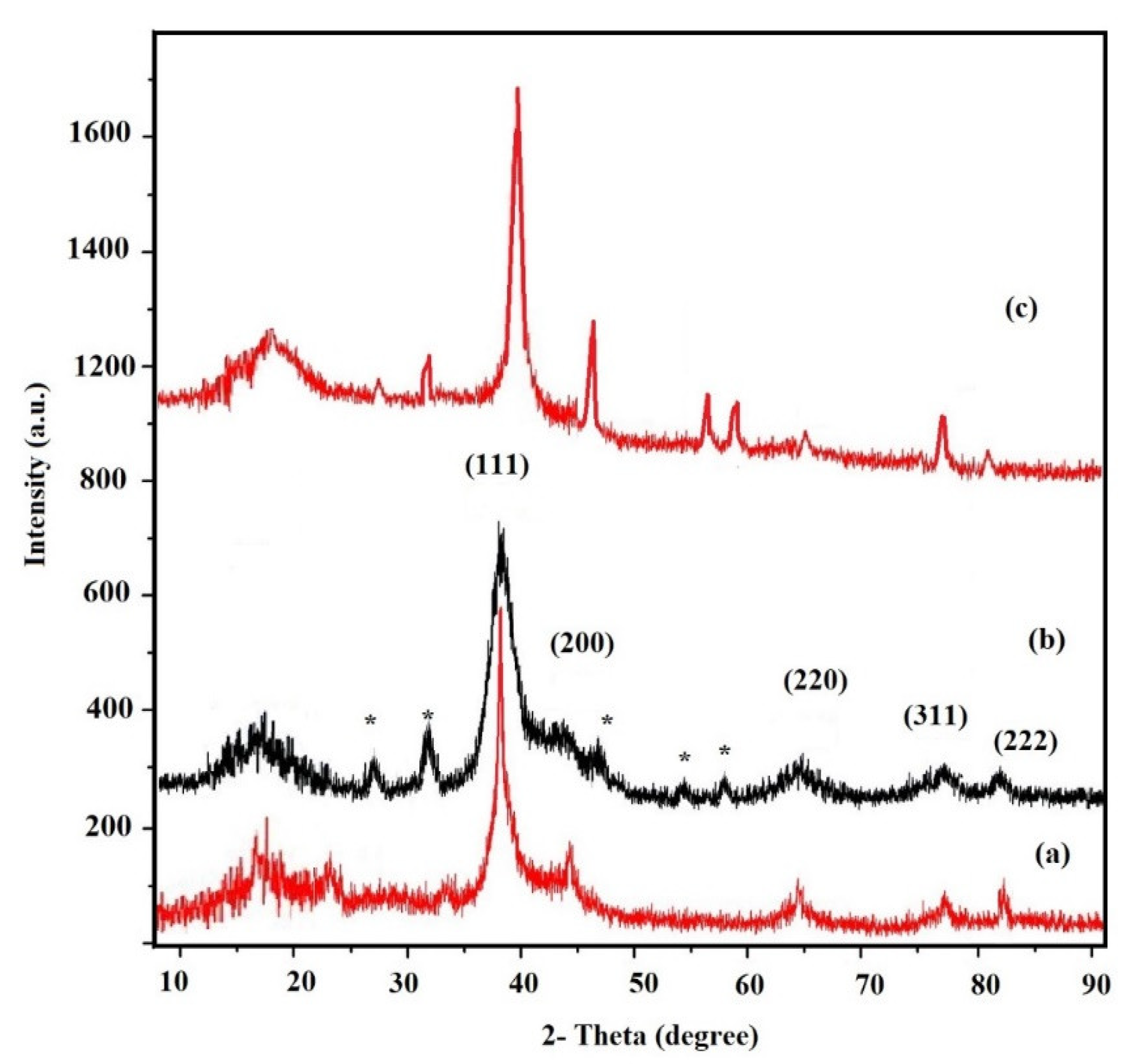
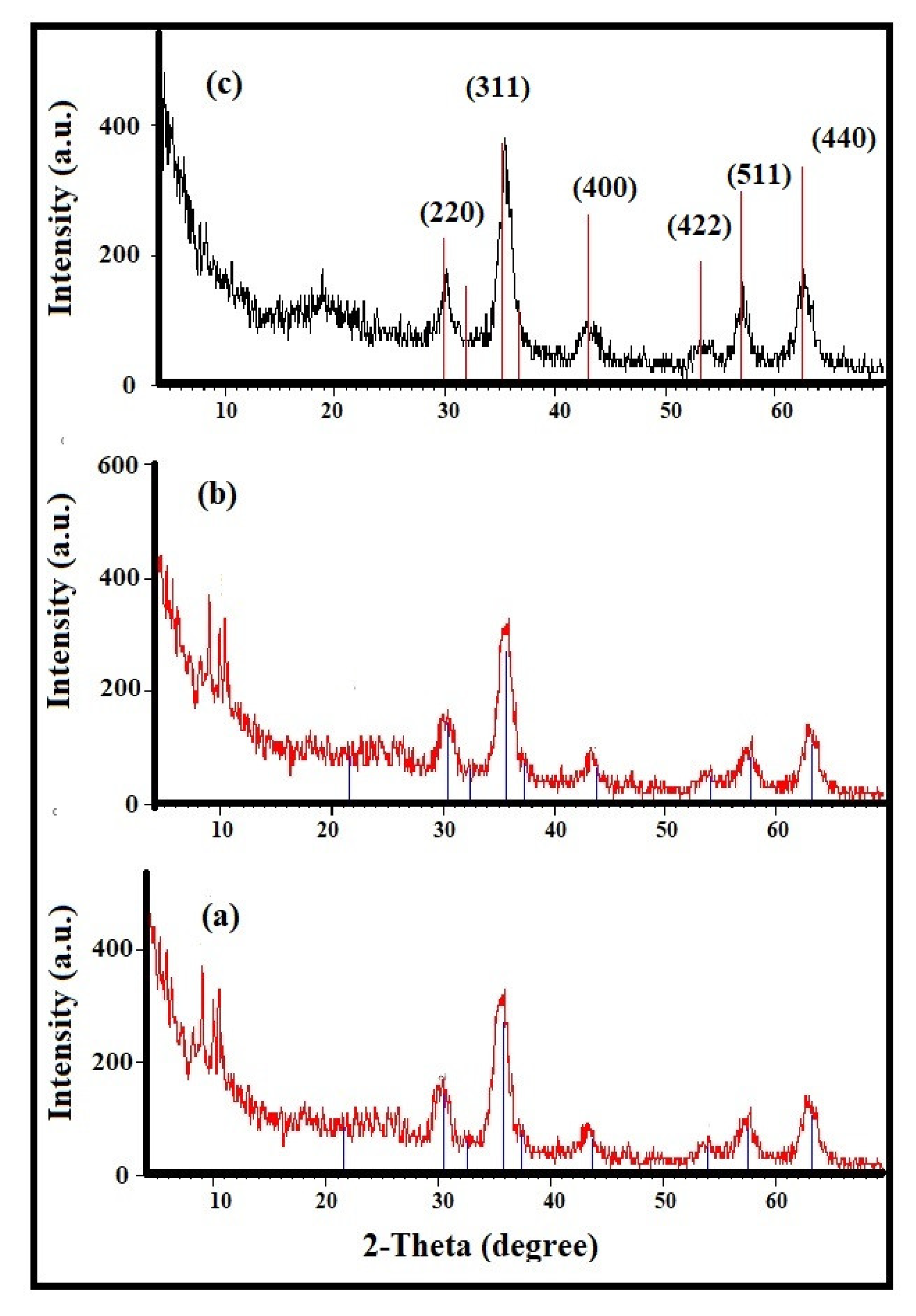
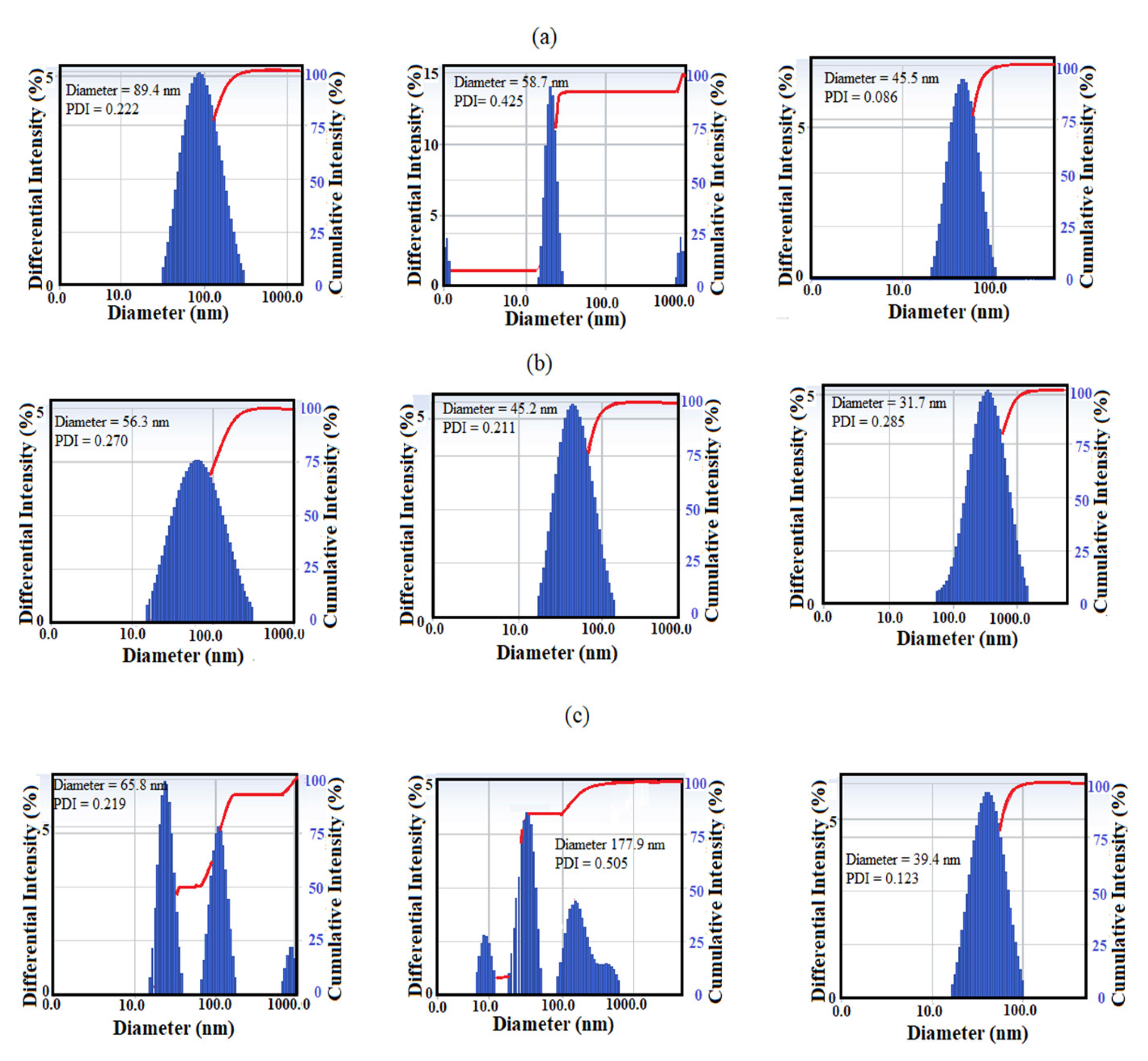
3.1. Characterization of the Modified NPs
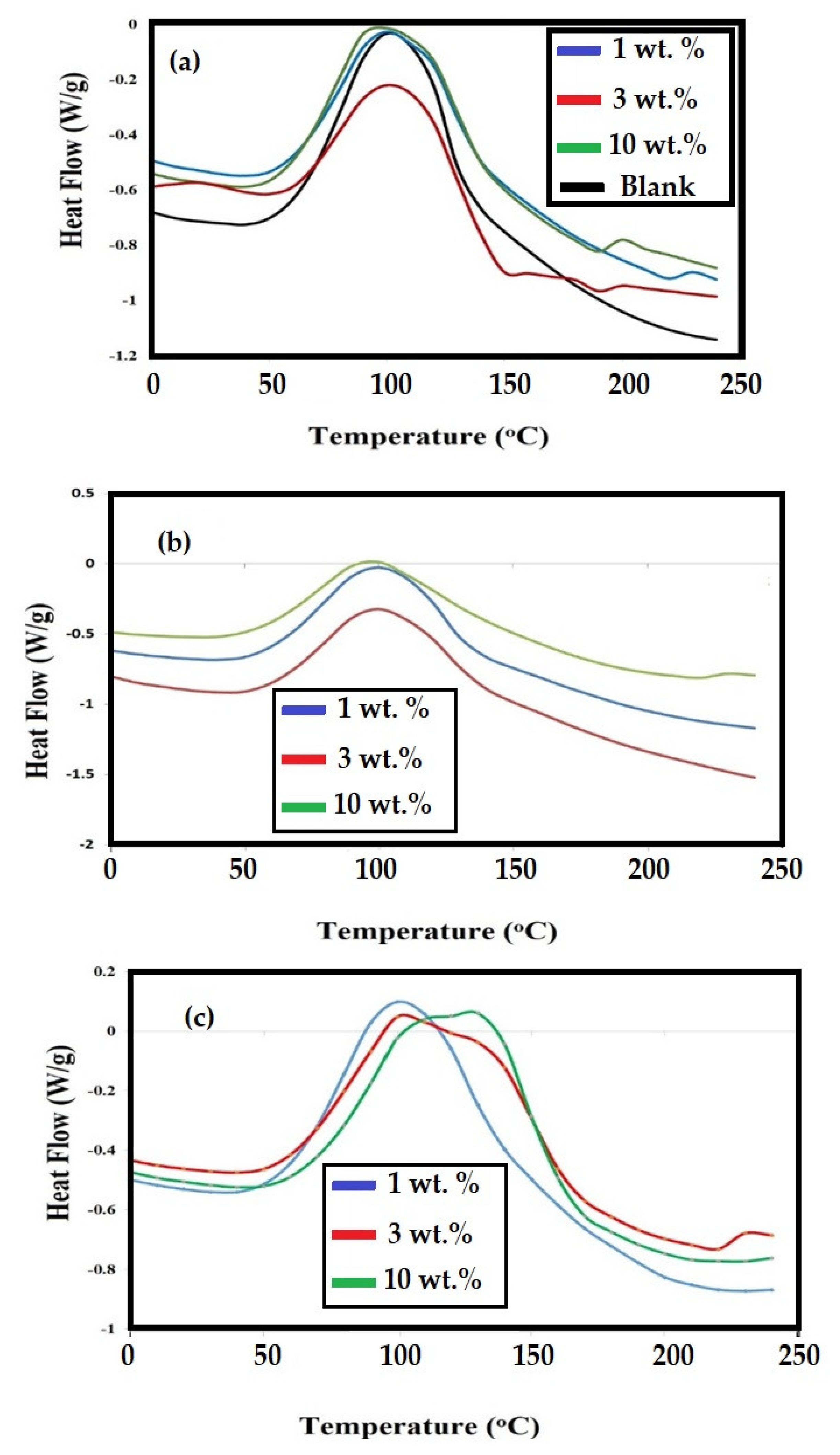
3.2. Dispersion of Modified NPs and Their Curing with DGEB/PA
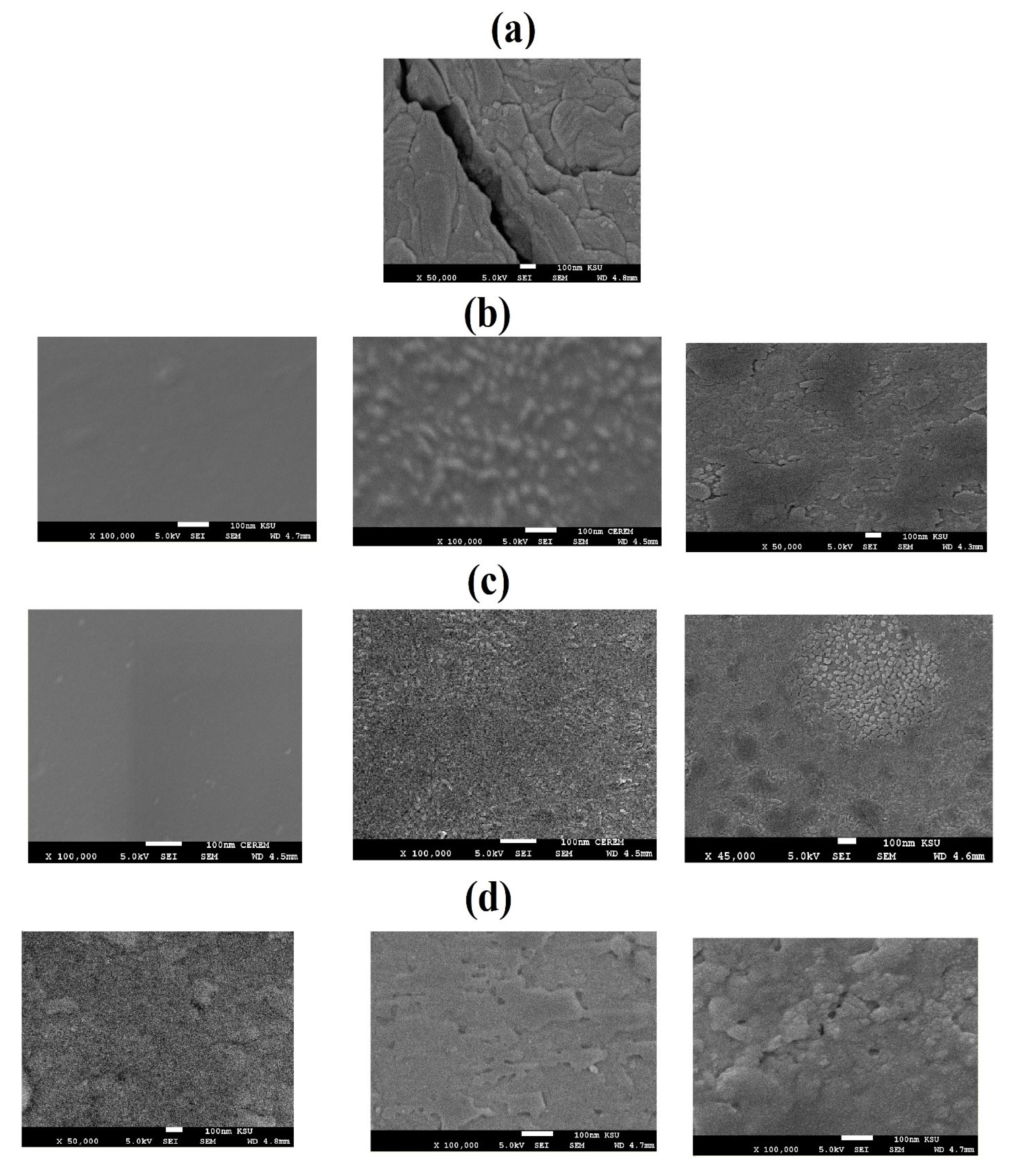
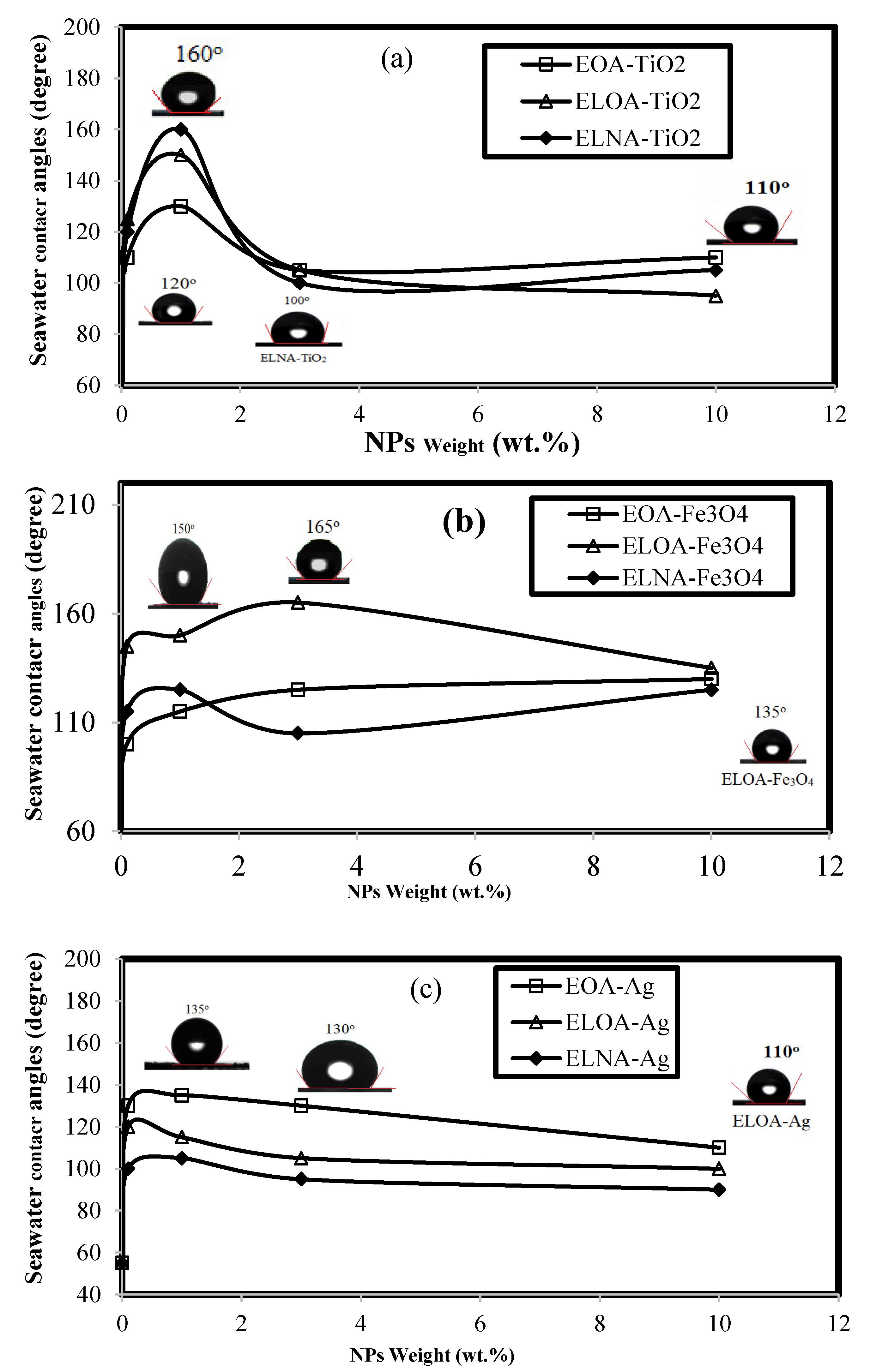
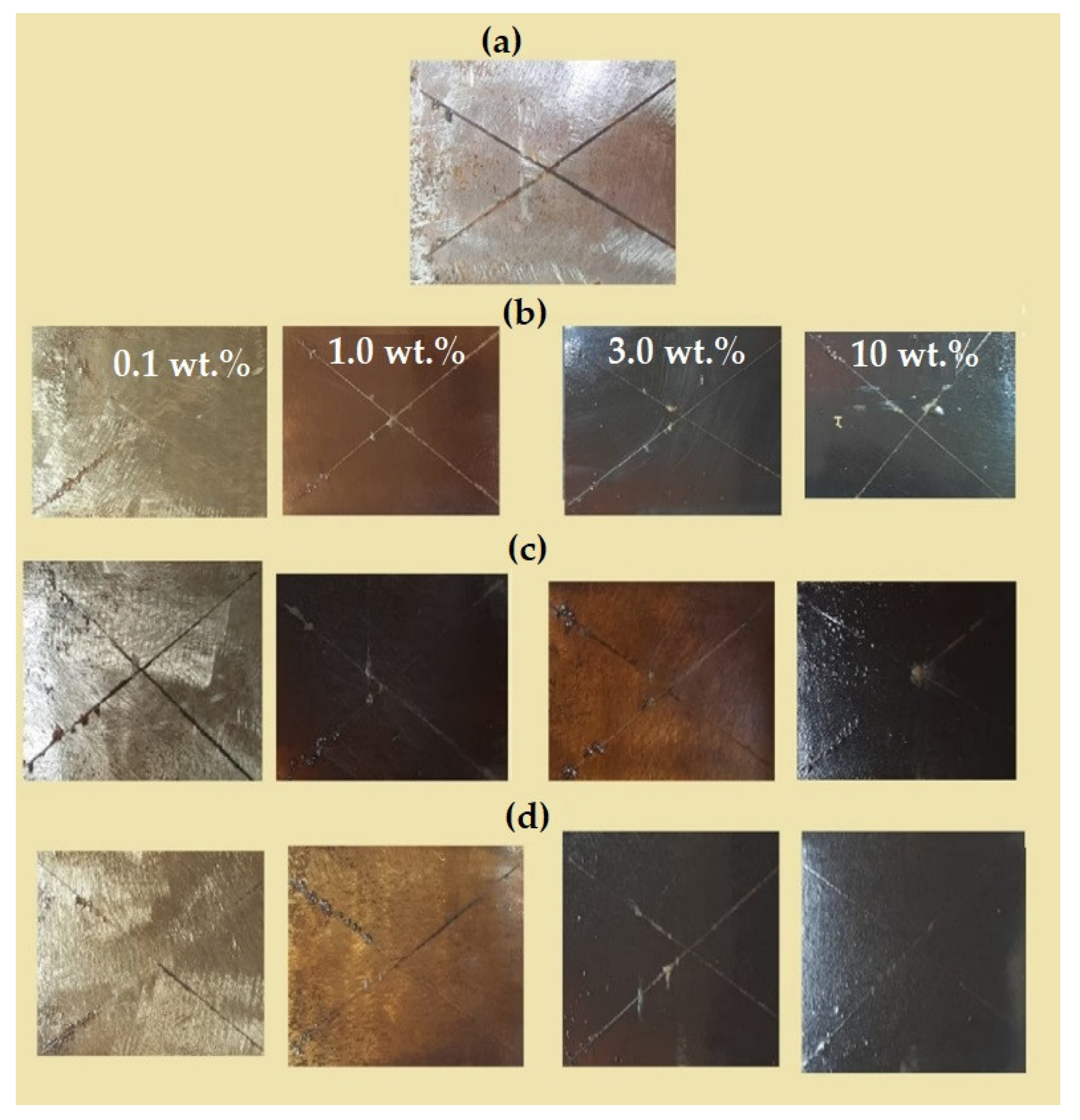
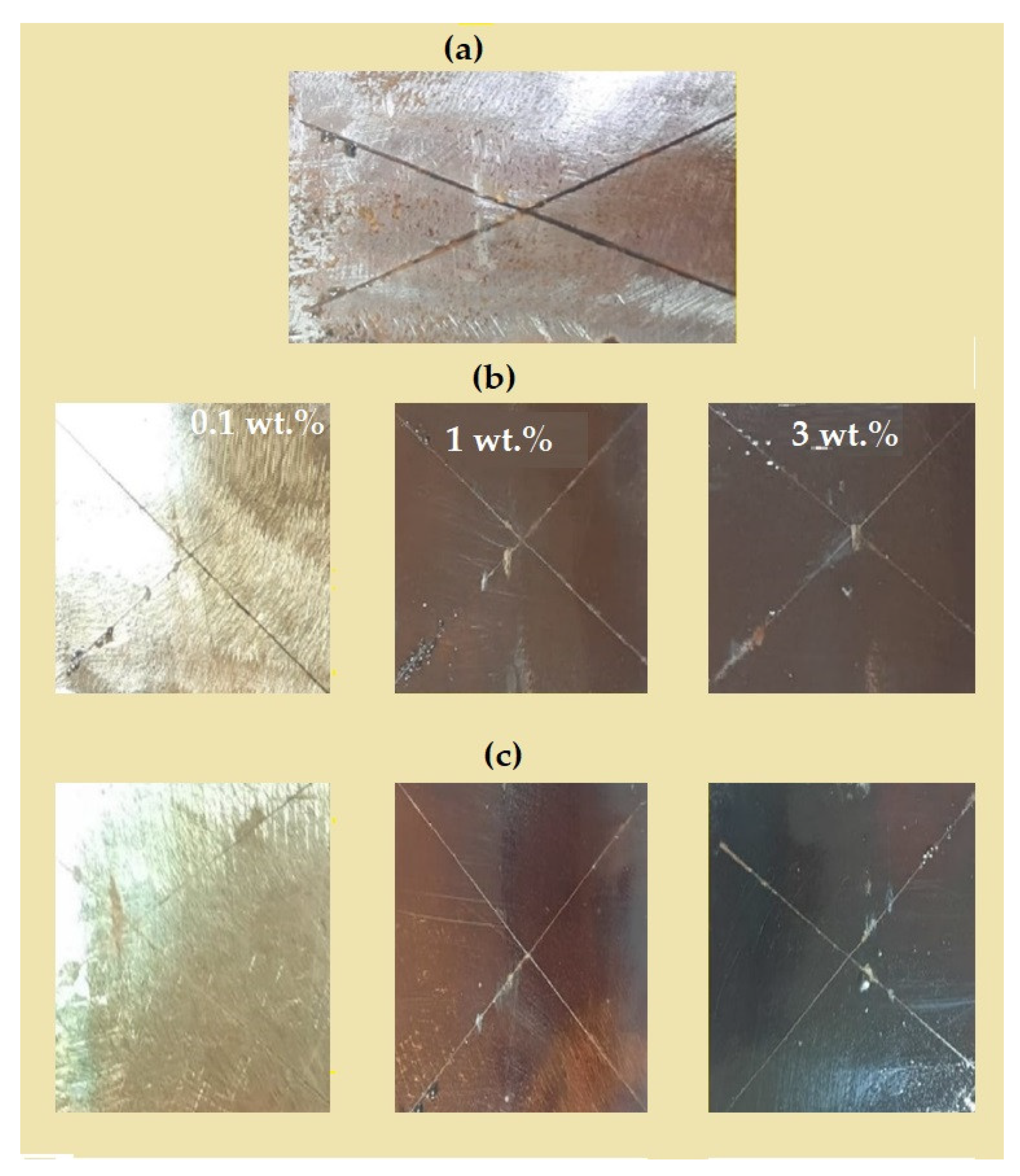
3.3. DGEB/PA Epoxy Nanocomposites Coatings Performances
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jyotishkumar, P.; Koetz, J.; Tiersch, B.; Strehmel, V.; Özdilek, C.; Moldenaers, P.; Hässler, R.; Thomas, S. Complex phase separation in poly (acrylonitrile–butadiene—Styren2e)-modified epoxy/4, 4′-diaminodiphenyl sulfone blends: Generation of new micro-and nanosubstructures. J. Phys. Chem. B 2009, 113, 5418–5430. [Google Scholar] [CrossRef]
- Tuncer, E.; Sauers, I.; James, D.R.; Ellis, A.R.; Paranthaman, M.P.; Aytuğ, T.; Sathyamurthy, S.; More, K.L.; Li, J.; Goyal, A. Electrical properties of epoxy resin based nano-composites. Nanotechnology 2006, 18, 025703. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.; Wu, S.; Guo, Z. Kinetic Study of Epoxy Resin Decomposition in Near-Critical Water. Chem. Eng. Technol. 2012, 35, 713–719. [Google Scholar] [CrossRef]
- Campo, A.G.; Orchard, K.L.; Sato, N.; Shaffer, M.S.; Williams, C.K. One-pot, in situ synthesis of ZnO-carbon nanotube–epoxy resin hybrid nanocomposites. Chem. Commun. 2009, 27, 4034–4036. [Google Scholar] [CrossRef] [PubMed]
- Olad, A.; Barati, M.; Behboudi, S. Preparation of PANI/epoxy/Zn nanocomposite using Zn nanoparticles and epoxy resin as additives and investigation of its corrosion protection behavior on iron. Prog. Org. Coat. 2012, 74, 221–227. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Song, L.; Kan, Y.; Qian, X.; Hu, Y. In situ preparation of functionalized graphene oxide/epoxy nanocomposites with effective reinforcements. J. Mater. Chem. 2011, 21, 13290–13298. [Google Scholar] [CrossRef]
- Chan, C.-M.; Wu, J.; Li, J.-X.; Cheung, Y.-K. Polypropylene/calcium carbonate nanocomposites. Polymer 2002, 43, 2981–2992. [Google Scholar] [CrossRef]
- Guo, Z.; Pereira, T.; Choi, O.; Wang, Y.; Hahn, H.T. Surface functionalized alumina nanoparticle filled polymeric nanocomposites with enhanced mechanical properties. J. Mater. Chem. 2006, 16, 2800–2808. [Google Scholar] [CrossRef]
- Kang, Y.; Taton, T.A. Micelle-encapsulated carbon nanotubes: A route to nanotube composites. J. Am. Chem. Soc. 2003, 125, 5650–5651. [Google Scholar] [CrossRef]
- Pron, A.; Rannou, P. Processible conjugated polymers: From organic semiconductors to organic metals and superconductors. Prog. Polym. Sci. 2002, 27, 135–190. [Google Scholar] [CrossRef]
- Atta, A.M.; Ezzat, A.O.; Saeed, A.M.E.; Wahby, M.H.; Abdallah, M.M. Superhydrophobic organic and inorganic clay nanocomposites for epoxy steel coatings. Prog. Org. Coat. 2020, 140, 105502. [Google Scholar] [CrossRef]
- Gu, H.; Tadakamalla, S.; Huang, Y.; Colorado, H.A.; Luo, Z.; Haldolaarachchige, N.; Young, D.P.; Wei, S.; Guo, Z. Polyaniline stabilized magnetite nanoparticle reinforced epoxy nanocomposites. ACS Appl. Mater. Interfaces 2012, 4, 5613–5624. [Google Scholar] [CrossRef] [PubMed]
- Alhabill, F.N.; Ayoob, R.; Andritsch, T.; Vaughan, A.S. Influence of filler/matrix interactions on resin/hardener stoichiometry, molecular dynamics, and particle dispersion of silicon nitride/epoxy nanocomposites. J. Mater. Sci. 2018, 53, 4144–4158. [Google Scholar] [CrossRef]
- Vertuccio, L.; Russo, S.; Raimondo, M.; Lafdi, K.; Guadagno, L. Influence of carbon nanofillers on the curing kinetics of epoxy-amine resin. RSC Adv. 2015, 5, 90437–90450. [Google Scholar] [CrossRef]
- Singh, S.K.; Kumar, A.; Jain, A. Improving tensile and flexural properties of SiO2-epoxy polymer nanocomposite. Mater. Today Proc. 2018, 5, 6339–6344. [Google Scholar] [CrossRef]
- Tao, P.; Viswanath, A.; Li, Y.; Siegel, R.W.; Benicewicz, B.C.; Schadler, L.S. Bulk transparent epoxy nanocomposites filled with poly (glycidyl methacrylate) brush-grafted TiO2 nanoparticles. Polymer 2013, 54, 1639–1646. [Google Scholar] [CrossRef]
- Azeez, A.A.; Rhee, K.Y.; Park, S.J.; Hui, D. Epoxy clay nanocomposites—Processing, properties and applications: A review. Compos. Part B Eng. 2013, 45, 308–320. [Google Scholar] [CrossRef]
- Wernik, J.; Meguid, S. On the mechanical characterization of carbon nanotube reinforced epoxy adhesives. Mater. Des. 2014, 59, 19–32. [Google Scholar] [CrossRef]
- Singh, A.K.; Panda, B.P.; Mohanty, S.; Nayak, S.K.; Gupta, M.K. Synergistic effect of hybrid graphene and boron nitride on the cure kinetics and thermal conductivity of epoxy adhesives. Polym. Adv. Technol. 2017, 28, 1851–1864. [Google Scholar] [CrossRef]
- Alamri, H.; Shahrani, A.A.; Bovero, E.; Khaldi, T.; Alabedi, G.; Obaid, W.; Taie, I.A.; Fihri, A. Self-cleaning superhydrophobic epoxy coating based on fibrous silica-coated iron oxide magnetic nanoparticles. J. Colloid Interface Sci. 2018, 513, 349–356. [Google Scholar] [CrossRef]
- Faham, A.E.; Atta, A.M.; Osman, S.M.; Ezzat, A.O.; Saeed, A.M.E.; Othman, Z.A.A.; Lohedan, H.A.A. Silver-embedded epoxy nanocomposites as organic coatings for steel. Prog. Org. Coat. 2018, 123, 209–222. [Google Scholar] [CrossRef]
- He, S.; Shia, J.; Huang, J.; Hu, J.; Laic, Y.; Chen, Z. Rational designed structured superhydrophobic iron oxide surface towards sustainable anti-corrosion and self-cleaning. Chem. Eng. J. 2020, 127768. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Paran, S.M.R.; Naderi, G.; Saeb, M.R.; Thomas, S. Curing epoxy with polyvinylpyrrolidone (PVP) surface-functionalized ZnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105227. [Google Scholar] [CrossRef]
- Xiong, H.; Qi, F.; Zhao, N.; Yuan, H.; Wan, P.; Liao, B.; Ouyang, X. Effect of organically modified sepiolite as inorganic nanofiller on the anti-corrosion resistance of epoxy coating. Mater. Lett. 2020, 260, 126941. [Google Scholar] [CrossRef]
- Khan, M.; Khurram, A.A.; Li, T.; Zhao, T.; Subhani, T.; Gul, I.; Ali, Z.; Patel, V. Synergistic effect of organic and inorganic nano fillers on the dielectric and mechanical properties of epoxy composites. J. Mater. Sci. Technol. 2018, 34, 2424–2430. [Google Scholar] [CrossRef]
- Malekshahinezhad, K.; Khaneghah, A.A.; Behniafar, H. Amine-functionalized TiO2 nanoparticles covalently loaded into epoxy networks via thermal and microwave curing processes. Macromol. Res. 2020, 28, 567–572. [Google Scholar] [CrossRef]
- Mishra, K.; Pandey, G.; Singh, R.P. Enhancing the mechanical properties of an epoxy resin using polyhedral oligomeric silsesquioxane (POSS) as nano-reinforcement. Polym. Test. 2017, 62, 210–218. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Jazani, O.M.; Navarchian, A.H.; Shabanian, M.; Vahabi, H.; Saeb, M.R. Surface engineering of nanoparticles with macromolecules for epoxy curing: Development of super-reactive nitrogen-rich nanosilica through surface chemistry manipulation. Appl. Surf. Sci. 2018, 447, 152–164. [Google Scholar] [CrossRef]
- ASTM International. ASTM D4541-02, Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- ASTM International. ASTM D4060-19, Standard Test Method for Abrasion Resistance of Organic Coatings by the Taber Abraser; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM International. ASTM B117-19, Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Bundjali, B.; Masykuri, M.; Hartanti, F.W.; Arcana, I.M. Poly (urethane) synthesized from 9-ethoxy-1, 10-octadecanediol obtained by modification of palm oil oleic acid. J. Math. Fundam. Sci. 2018, 50, 13–27. [Google Scholar] [CrossRef]
- Atta, A.M.; Mahdy, G.A.E.; Lohedan, H.A.A.; Ezzat, A.O. Preparation of crosslinked amphiphilic silver nanogel as thin film corrosion protective layer for steel. Molecules 2014, 19, 10410–10426. [Google Scholar] [CrossRef]
- Atta, A.M.; Lohedan, H.A.A.; Ezzat, A.O.; Al-Hussain, S.A. Characterization of superhydrophobic epoxy coatings embedded by modified calcium carbonate nanoparticles. Prog. Org. Coat. 2016, 101, 577–586. [Google Scholar] [CrossRef]
- Shi, Y.-Y.; Bin, S.; Zhe, Z.; Wu, Y.-T.; Zhu, M.-F. Size-controlled and large-scale synthesis of organic-soluble Ag nanocrystals in water and their formation mechanism. Prog. Nat. Sci. Mater. Int. 2011, 21, 447–454. [Google Scholar] [CrossRef]
- Manikandan, V.; Velmurugan, P.; Park, J.-H.; Chang, W.-S.; Park, Y.-J.; Jayanthi, P.; Cho, M.; Oh, B.-T. Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. 3 Biotech 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley: Boston, MA, USA, 1978. [Google Scholar]
- Arnold, C.L.; Eyckens, D.J.; Servinis, L.; Nave, M.D.; Yin, H.; Marceau, R.K.; Pinson, J.; Demir, B.; Walsh, T.R.; Henderson, L.C. Simultaneously increasing the hydrophobicity and interfacial adhesion of carbon fibres: A simple pathway to install passive functionality into composites. J. Mater. Chem. A 2019, 7, 13483–13494. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Lu, Y.; Mei, Y.; Schrinner, M.; Ballauff, M.; Möller, M.W.; Breu, J. In situ formation of Ag nanoparticles in spherical polyacrylic acid brushes by UV irradiation. J. Phys. Chem. C 2007, 111, 7676–7681. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Muthukumaran, T.; Philip, J. Effect of phosphate and oleic acid capping on structure, magnetic properties and thermal stability of iron oxide nanoparticles. J. Alloy Compd. 2016, 689, 959–968. [Google Scholar] [CrossRef]
- Aqida, S.; Maurel, M.; Brabazon, D.; Naher, S.; Rosso, M. Thermal stability of laser treated die material for semi-solid metal forming. Int. J. Mater. Form. 2009, 2, 761. [Google Scholar] [CrossRef]
- Seyhan, M.; Kucharczyk, W.; Yarar, U.E.; Rickard, K.; Rende, D.; Baysal, N.; Bucak, S.; Ozisik, R. Interfacial surfactant competition and its impact on poly (ethylene oxide)/Au and poly (ethylene oxide)/Ag nanocomposite properties. Nanotechnol. Sci. Appl. 2017, 10, 69. [Google Scholar] [CrossRef]
- Basu, S.K.; Scriven, L.; Francis, L.; McCormick, A. Mechanism of wrinkle formation in curing coatings. Prog. Org. Coat. 2005, 53, 1–16. [Google Scholar] [CrossRef]
- Atta, A.M.; Mohamed, N.H.; Rostom, M.; Al-Lohedan, H.A.; Abdullah, M.M. New hydrophobic silica nanoparticles capped with petroleum paraffin wax embedded in epoxy networks as multifunctional steel epoxy coatings. Prog. Org. Coat. 2019, 128, 99–111. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, N.; Qi, F.; Zhang, B.; Liao, B.; Ouyang, X. Reinforced Superhydrophobic Anti-Corrosion Epoxy Resin Coating by Fluorine–Silicon–Carbide Composites. Coatings 2020, 10, 1244. [Google Scholar] [CrossRef]
- Li, S.; Page, K.; Sathasivam, S.; Heale, F.; He, G.; Lu, Y.; Lai, Y.; Chen, G.; Carmalt, C.J.; Parkin, I.P. Efficiently texturing hierarchical superhydrophobic fluoride-free translucent films by AACVD with excellent durability and self-cleaning ability. J. Mater. Chem. A 2018, 6, 17633–17641. [Google Scholar] [CrossRef]
- Ahn, B.K.; Wang, H.; Robinson, S.; Shrestha, T.B.; Troyer, D.L.; Bossmann, S.H.; Sun, X.S. Ring opening of epoxidized methyl oleate using a novel acid-functionalized iron nanoparticle catalyst. Green Chem. 2012, 14, 136–142. [Google Scholar]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Saeb, M.R.; Ray, S.S. Curing epoxy with polyvinylpyrrolidone (PVP) surface-functionalized NixFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105259. [Google Scholar] [CrossRef]
- Huang, Y.; Tian, Y.; Li, Y.; Tan, X.; Li, Q.; Cheng, J.; Zhang, J. High mechanical properties of epoxy networks with dangling chains and tunable microphase separation structure. RSC Adv. 2017, 7, 49074–49082. [Google Scholar] [CrossRef]
- Binks, F.C.; Cavalli, G.; Henningsen, M.; Howlin, B.J.; Hamerton, I. Investigating the mechanism through which ionic liquids initiate the polymerisation of epoxy resins. Polymer 2018, 139, 163–176. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Ogale, S.B.; Vijayamohanan, K.P. Tuning the hydrophobic properties of silica particles by surface silanization using mixed self-assembled monolayers. J. Colloid Interface Sci. 2008, 318, 372–379. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Liang, F.; Liu, R.; Zhang, Y.; Zhang, W.; Zhu, T.; Yi, B.; Tang, Y.; Lai, Y. A PDMS-in-water emulsion enables mechanochemically robust superhydrophobic surfaces with self-healing nature. Nanoscale Horiz. 2020, 5, 65–73. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic Surfaces: Insights from Theory and Experiment. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef]
- Atta, A.M.; Ahmed, M.A.; Saeed, A.M.E.; Elenien, O.M.A.; Sockary, M.A.E. Hybrid ZrO2/Cr2O3 Epoxy Nanocomposites as Organic Coatings for Steel. Coatings 2020, 10, 997. [Google Scholar] [CrossRef]
- Davis, G.D.; Krebs, L.A.; Dacres, C.M. Coating evaluation and validation of accelerated test conditions using an in-situ corrosion sensor. J. Coat. Technol. 2002, 74, 69–74. [Google Scholar] [CrossRef]
- ASTM International. ASTM D1654-08(2016)e1, Standard Test Method for Evaluation of Painted or Coated Specimens Subjected to Corrosive Environments; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Elzaabalawy, A.; Meguid, S.A. Development of novel superhydrophobic coatings using siloxane modified epoxy nanocomposites. Chem. Eng. J. 2020, 398, 125403. [Google Scholar] [CrossRef]
- Zhang, F.; Qian, H.C.; Wang, L.; Wang, Z.; Du, C.; Li, X.; Zhang, D. Superhydrophobic carbon nanotubes/epoxy nanocomposite coating by facile one-step spraying. Surf. Coat. Technol. 2018, 341, 15–23. [Google Scholar] [CrossRef]

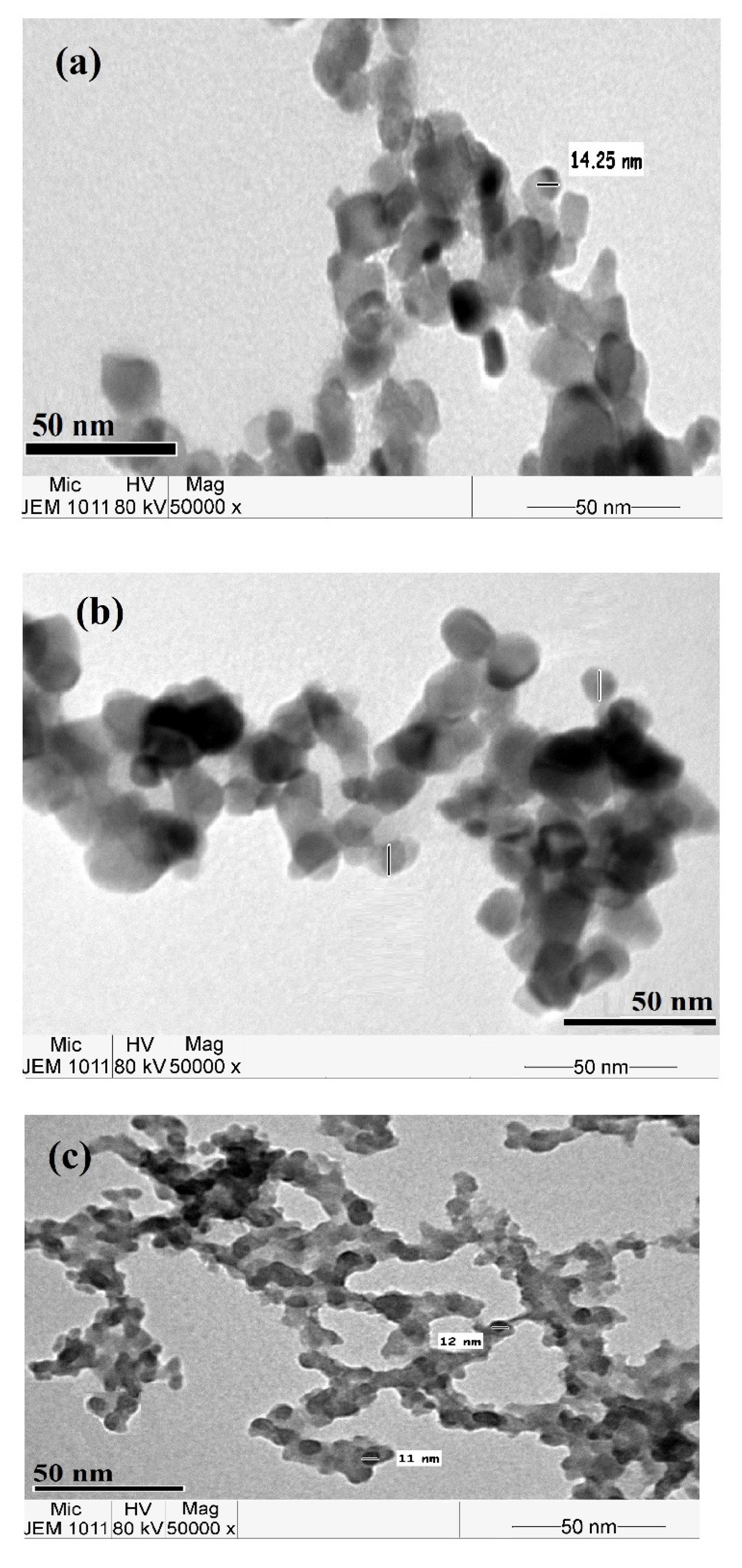


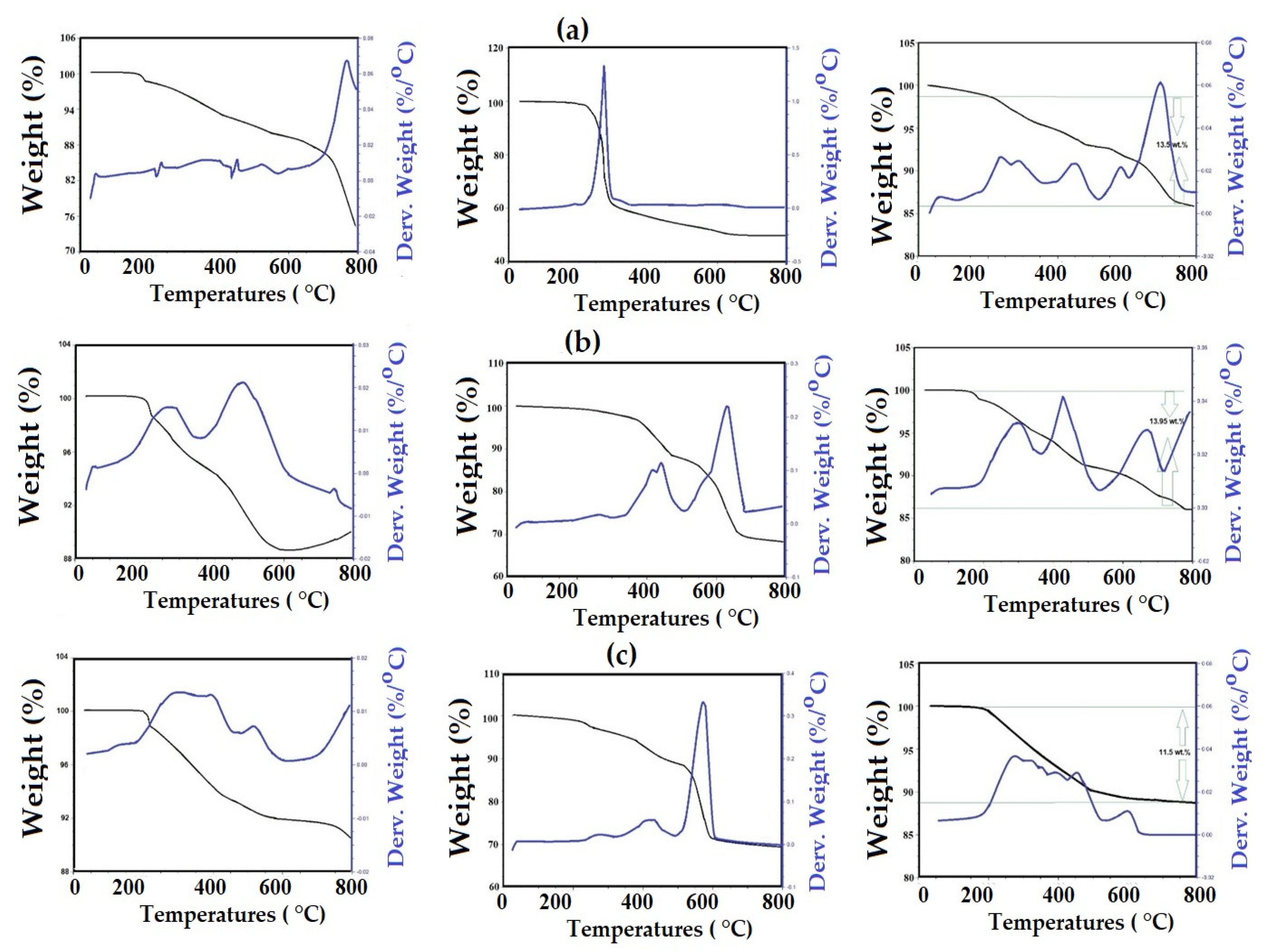
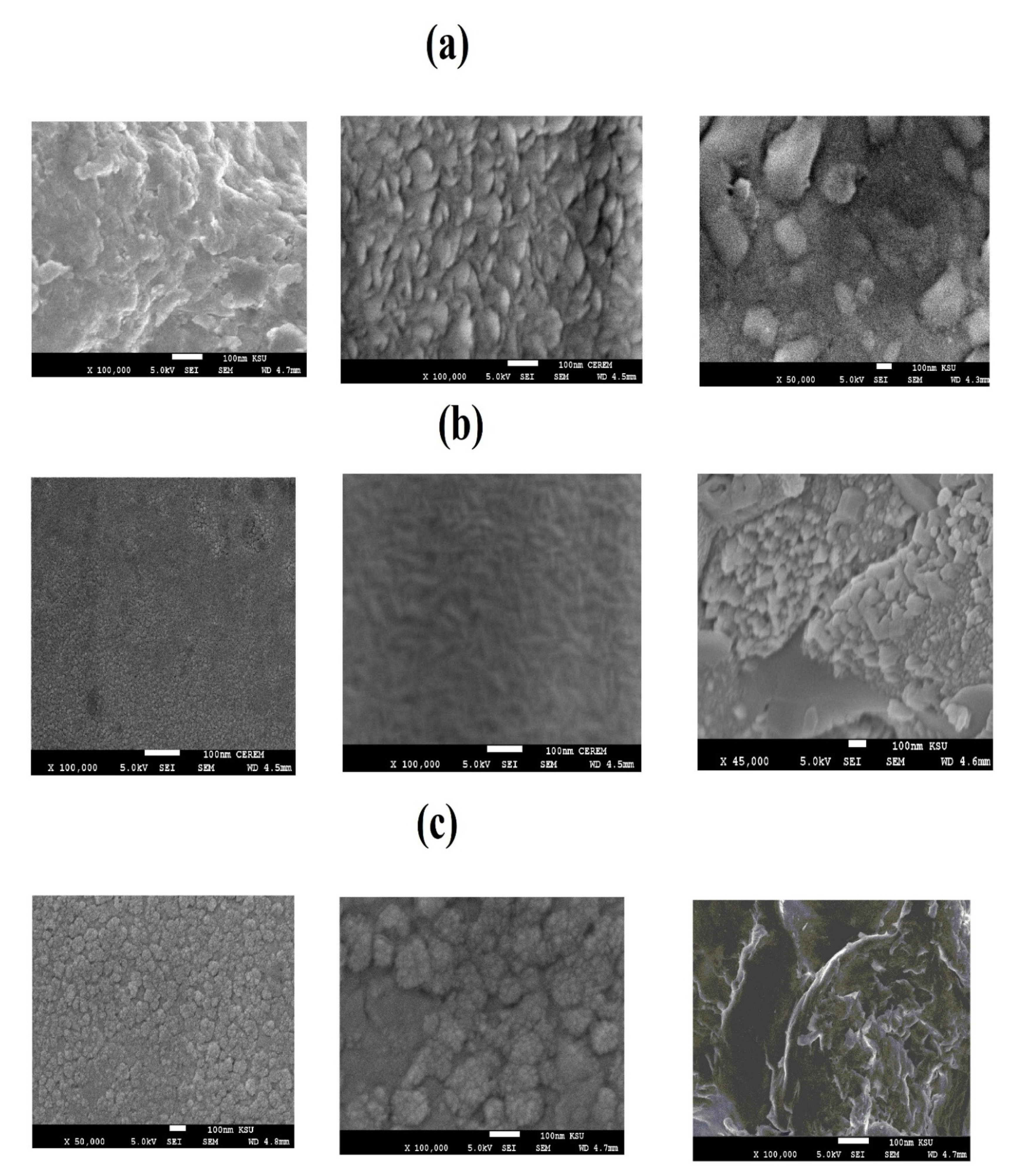
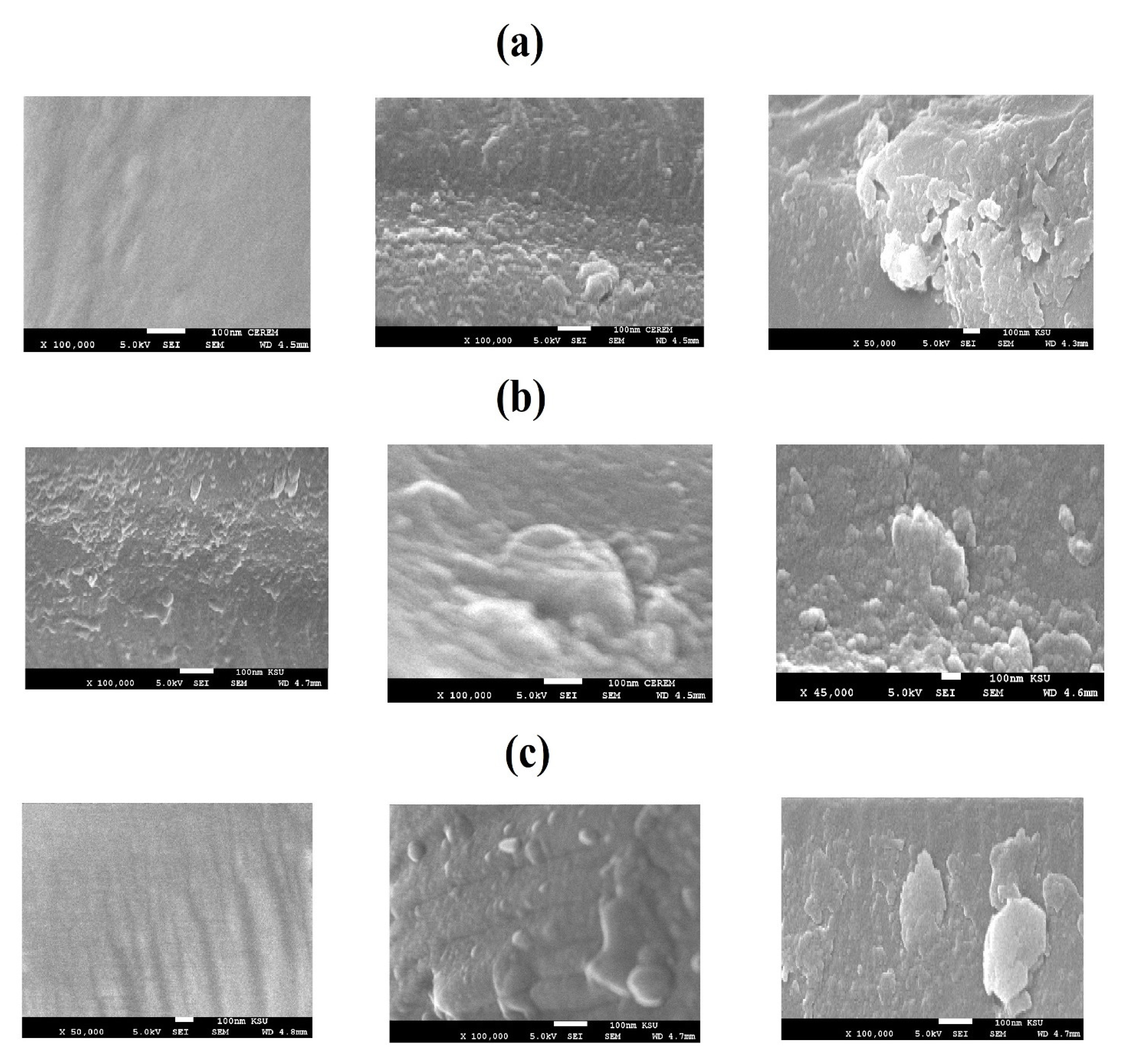


| NPs | IDT (°C) | Tmax (°C) | Rs % (wt %) | Capping % (wt %) |
|---|---|---|---|---|
| EOA-Fe3O4 | 240 | 750 | 85.50 | 13.50 |
| ELOA-Fe3O4 | 250 | 750 | 86.05 | 13.95 |
| ELNA-Fe3O4 | 275 | 700 | 88.50 | 11.50 |
| EOA-TiO2 | 235 | 700 | 86.50 | 13.50 |
| ELOA-TiO2 | 225 | 600 | 89.00 | 11.00 |
| ELNA-TiO2 | 215 | 600 | 91.60 | 8.40 |
| EOA-Ag | 220 | 650 | 50.00 | 50.00 |
| ELOA-Ag | 350 | 670 | 67.00 | 33.00 |
| ELNA-Ag | 230 | 600 | 70.00 | 30.00 |
| DGEB/PA/NPs | NPs (wt %) | Types of Epoxide Fatty Acids | Tg (°C) | DMA Data | ||
|---|---|---|---|---|---|---|
| DSC | DMA | E’ (MPa) | ρ × 103 (mol·dm−3) | |||
| 0 | 0 | Blank | 108.6 | 120.3 | 2800 | 0.762 |
| Fe3O4 | 1 | EOA | 85.4 | 90.3 | 3000 | 0.875 |
| ELOA | 90.6 | 95.8 | 3400 | 0.976 | ||
| ELNA | 99.8 | 106.3 | 3800 | 1.070 | ||
| 3 | EOA | 82.6 | 83.4 | 3400 | 1.006 | |
| ELOA | 87.4 | 90.3 | 3650 | 1.065 | ||
| ELNA | 93.6 | 98.6 | 3780 | 1.079 | ||
| 10 | EOA | 100.4 | 103.2 | 3700 | 1.046 | |
| ELOA | 98.5 | 105.7 | 3850 | 1.081 | ||
| ELNA | 101.5 | 110.3 | 4000 | 1.113 | ||
| TiO2 | 1 | EOA | 98.6 | 100.5 | 3850 | 1.097 |
| ELOA | 100.5 | 103.5 | 3920 | 1.106 | ||
| ELNA | 102.3 | 105.6 | 4080 | 1.146 | ||
| 3 | EOA | 90.3 | 102.3 | 3950 | 1.120 | |
| ELOA | 94.3 | 105.3 | 4150 | 1.168 | ||
| ELNA | 98.6 | 106.6 | 4250 | 1.191 | ||
| 10 | EOA | 92.6 | 104.5 | 4200 | 1.182 | |
| ELOA | 94.7 | 105.6 | 4450 | 1.250 | ||
| ELNA | 96.4 | 108.3 | 4650 | 1.300 | ||
| Ag | 1 | EOA | 110.3 | 125.4 | 4900 | 1.312 |
| 3 | EOA | 120.4 | 132.3 | 5200 | 1.377 | |
| 10 | EOA | 130.6 | 143.2 | 5420 | 1.401 | |
| DGEB/ PA- NPs | Types of Fatty Acid | NPs (wt %) | Adhesion Strength (MPa) | Abrasion Resistance Weight Lost (2000 cycles) (mg) | Exposure Time (h) | Rust Area % | Rating Number (ASTM D-1654) [58] | Adhesion Strength After Salt Spray Exposure Time (MPa) |
|---|---|---|---|---|---|---|---|---|
| blank | 0 | 5.00 ± 0.08 | 56 ± 4.85 | 500 | 10 ± 0.05 | 5 | 4 | |
| TiO2 | EOA | 0.1 | 5.00 ± 0.04 | 45 ± 1.95 | 1000 | 2 ± 0.08 | 8 | 3.2 |
| 1 | 5.80 ± 0.01 | 11 ± 1.75 | 1000 | 2 ± 0.04 | 8 | 3.4 | ||
| 3 | 6.25 ± 0.05 | 12 ± 1.85 | 750 | 30 ± 0.04 | 4 | F | ||
| 10 | 7.06 ± 0.04 | 27 ± 3.05 | 1000 | 12 ± 0.04 | 6 | 3.2 | ||
| ELOA | 0.1 | 6.81 ± 0.05 | 21 ± 1.85 | 1500 | 12 ± 0.04 | 6 | 7.2 | |
| 1 | 7.42 ± 0.03 | 30 ± 3.05 | 1500 | 2 ± 0.04 | 8 | 6.9 | ||
| 3 | 8.34 ± 0.04 | 37 ± 1.95 | 1500 | 2 ± 0.04 | 8 | 4.8 | ||
| 10 | 7.37 ± 0.02 | 17 ± 2.05 | 1500 | 12± 0.04 | 6 | 4.8 | ||
| ELNA | 0.1 | 6.53 ± 0.01 | 7 ± 2.35 | 1500 | 10 ± 0.05 | 5 | 6.4 | |
| 1 | 7.18 ± 0.02 | 18 ± 2.15 | 2000 | 2 ± 0.04 | 8 | 7.2 | ||
| 3 | 9.17 ± 0.04 | 13 ± 1.75 | 2000 | 1 ± 0.08 | 9 | 8.8 | ||
| 10 | 6.68 ± 0.03 | 16 ± 1.95 | 2000 | 8± 0.04 | 7 | 6.4 | ||
| Fe3O4 | EOA | 0.1 | 9.00 ± 0.05 | 22 ± 2.85 | 1500 | 10 ± 0.08 | 5 | 9.0 |
| 1 | 9.30 ± 0.07 | 18 ± 1.35 | 2000 | 12± 0.04 | 6 | 9.3 | ||
| 3 | 11.19 ± 0.09 | 20 ± 2.05 | 2000 | 2 ± 0.04 | 8 | 7.2 | ||
| 10 | 10.83 ± 0.08 | 25 ± 1.95 | 2000 | 12± 0.04 | 6 | 5.8 | ||
| ELOA | 0.1 | 7.30 ± 0.05 | 9 ± 1.55 | 1000 | 8± 0.04 | 7 | 3.2 | |
| 1 | 8.21 ± 0.06 | 11 ± 1.25 | 1500 | 8± 0.04 | 7 | 7.2 | ||
| 3 | 9.75 ± 0.04 | 7 ± 1.85 | 2000 | 1 ± 0.08 | 9 | 8.9 | ||
| 10 | 7.63 ± 0.01 | 13 ± 1.05 | 1500 | 10 ± 0.05 | 5 | 3.2 | ||
| ELNA | 0.1 | 10.20 ± 0.03 | 14 ± 1.45 | 1500 | 10 ± 0.05 | 5 | 9.6 | |
| 1 | 12.50 ± 0.09 | 18 ± 1.35 | 1500 | 5 ± 0.05 | 5 | 5.9 | ||
| 3 | 15.60 ± 0.08 | 16 ± 1.15 | 2000 | 2 ± 0.04 | 8 | 8.0 | ||
| 10 | 7.70 ± 0.01 | 16 ± 1.65 | 2000 | 2 ± 0.04 | 8 | 7.2 | ||
| Ag | EOA | 0.1 | 12.50 ± 0.06 | 10 ± 1.25 | 1000 | 2 ± 0.04 | 8 | 5.6 |
| 1 | 13.50 ± 0.05 | 13 ± 1.05 | 1500 | 12± 0.04 | 6 | 6.4 | ||
| 3 | 12.00 ± 0.02 | 16 ± 1.45 | 1500 | 8± 0.04 | 7 | 5.6 | ||
| 10 | 8.50 ± 0.03 | 22 ± 1.85 | 500 | 2 ± 0.04 | 8 | F | ||
| OA | 0.1 | 7.50 ± 0.05 | 17 ± 2.15 | 1000 | 10 ± 0.05 | 5 | 6.4 | |
| 1 | 7.00 ± 0.04 | 24 ± 1.05 | 1000 | 12± 0.04 | 6 | 7.0 | ||
| 3 | 6.50 ± 0.01 | 28 ± 1.8 | 1000 | 8± 0.04 | 7 | 6.5 | ||
| 10 | 5.50 ± 0.02 | 35 ± 2.1 | 1000 | 12± 0.04 | 6 | 6.3 |
| Epoxy Nanocomposite | Adhesion Test | Abrasion Weight Loss (mg) |
|---|---|---|
| Graphene-polydopamine (GP) and SiO2 | Pass cross-cut tester | 10.7–17.1 (2000 cycles) |
| siloxane-modified epoxy nanocomposites | Pass adhesion with 3M-3939 adhesive tape, with an adhesion peel strength of 6.3 N/cm | 190 cycles did not reduce the WCA |
| Carbon nanotubes/epoxy nanocomposite [55] | Pass adhesion with 3M-3939 adhesive tape | Pass 40 cycles of abrasion using 240-grit sandpaper under the load of 100 g |
| Carbon nanotubes/epoxy nanocomposite [56] | Pass adhesion with 3M-3939 adhesive tape | Pass 40 cycles of abrasion using 240-grit sandpaper under the load of 100 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahby, M.H.; Atta, A.M.; Moustafa, Y.M.; Ezzat, A.O.; Hashem, A.I. RETRACTED: Curing of Functionalized Superhydrophobic Inorganic/Epoxy Nanocomposite and Application as Coatings for Steel. Coatings 2021, 11, 83. https://doi.org/10.3390/coatings11010083
Wahby MH, Atta AM, Moustafa YM, Ezzat AO, Hashem AI. RETRACTED: Curing of Functionalized Superhydrophobic Inorganic/Epoxy Nanocomposite and Application as Coatings for Steel. Coatings. 2021; 11(1):83. https://doi.org/10.3390/coatings11010083
Chicago/Turabian StyleWahby, Mohamed H., Ayman M. Atta, Yasser M. Moustafa, Abdelrahman O. Ezzat, and Ahmed I. Hashem. 2021. "RETRACTED: Curing of Functionalized Superhydrophobic Inorganic/Epoxy Nanocomposite and Application as Coatings for Steel" Coatings 11, no. 1: 83. https://doi.org/10.3390/coatings11010083
APA StyleWahby, M. H., Atta, A. M., Moustafa, Y. M., Ezzat, A. O., & Hashem, A. I. (2021). RETRACTED: Curing of Functionalized Superhydrophobic Inorganic/Epoxy Nanocomposite and Application as Coatings for Steel. Coatings, 11(1), 83. https://doi.org/10.3390/coatings11010083




