Synthesis and Photonics Applications of Afzelechin Conjugated Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis and Characterization of Afzelechin Conjugated Silver Nanoparticles
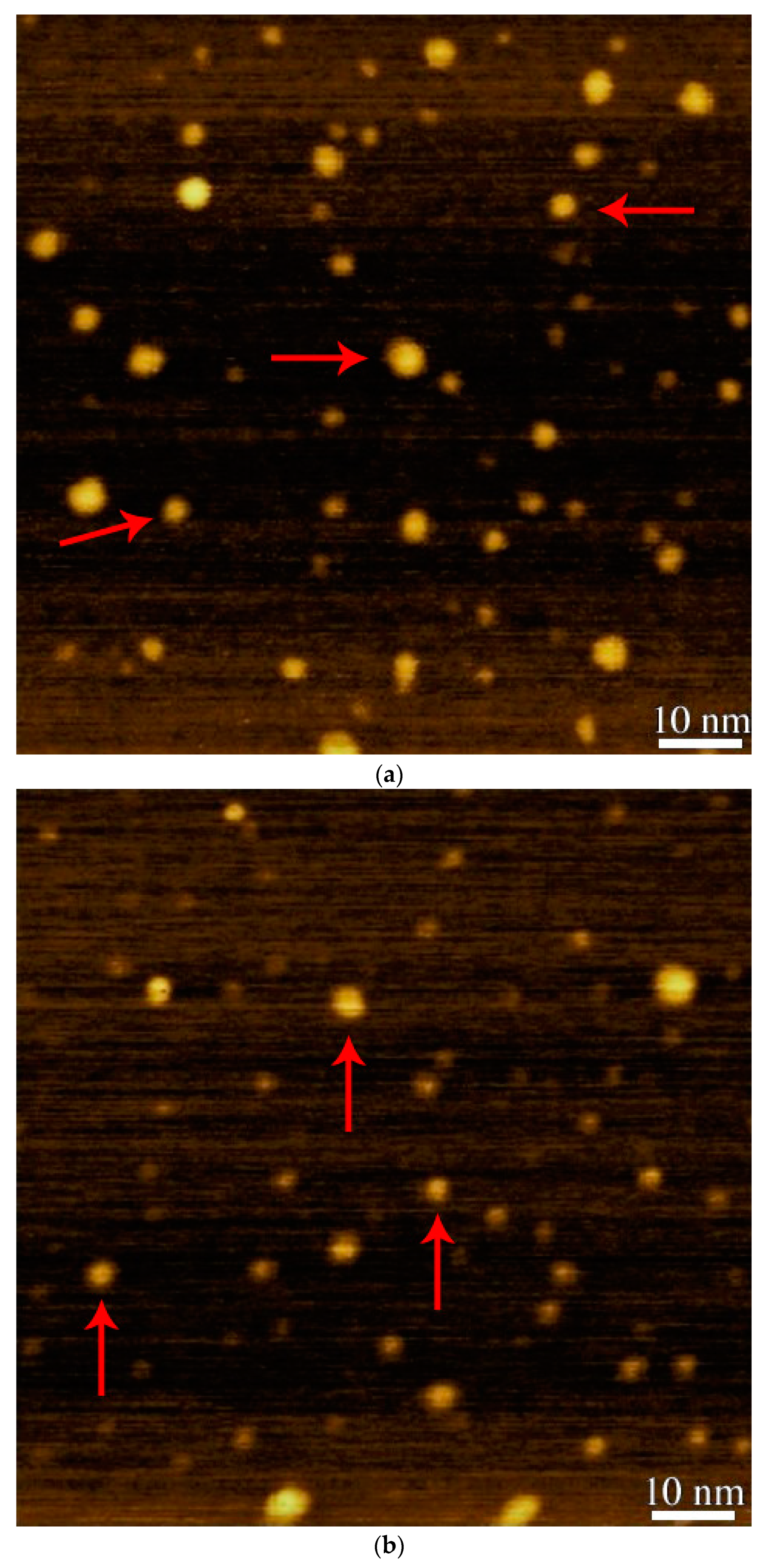
3.2. Atomic Force Microscopy
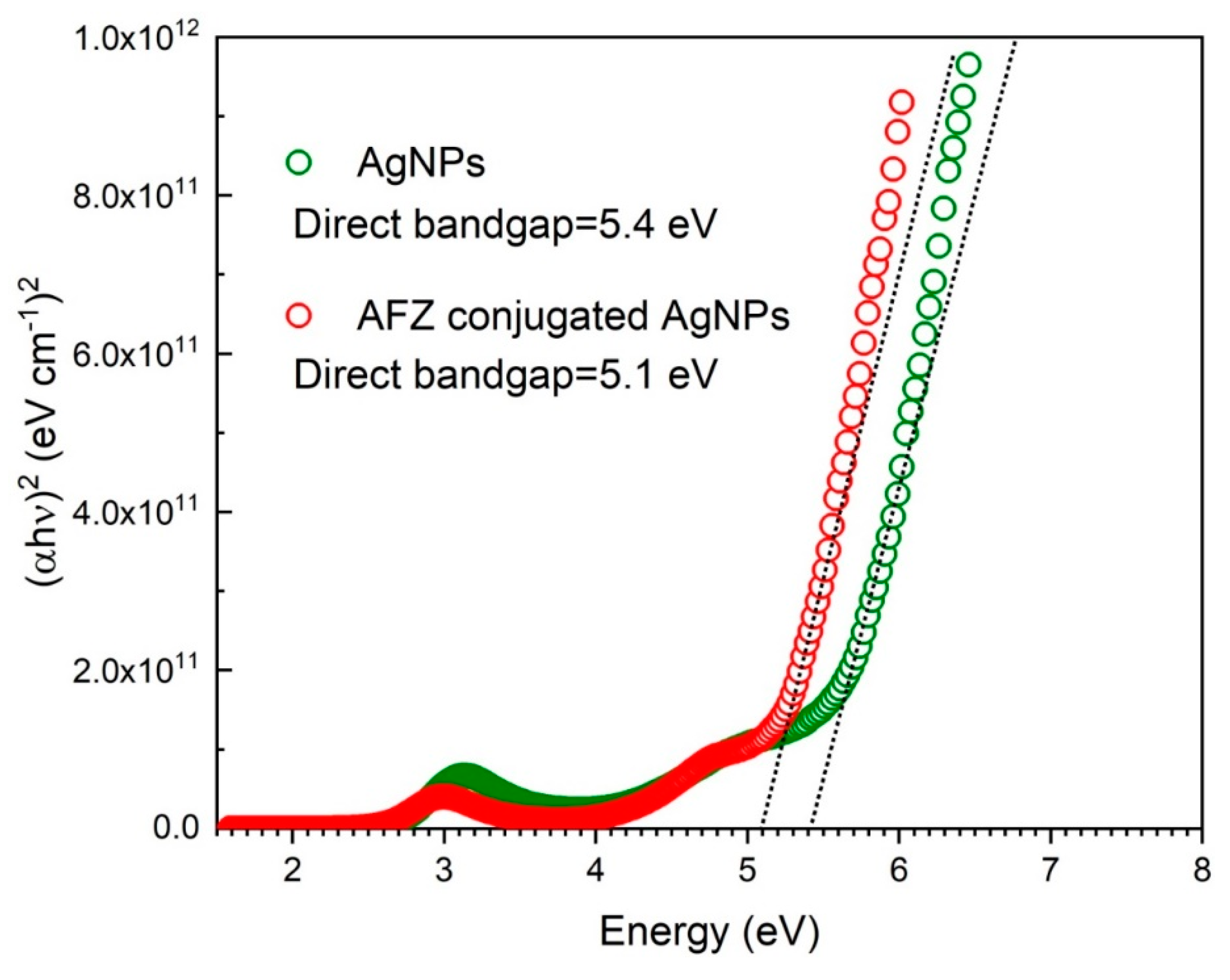
3.3. The Bandgap Energy
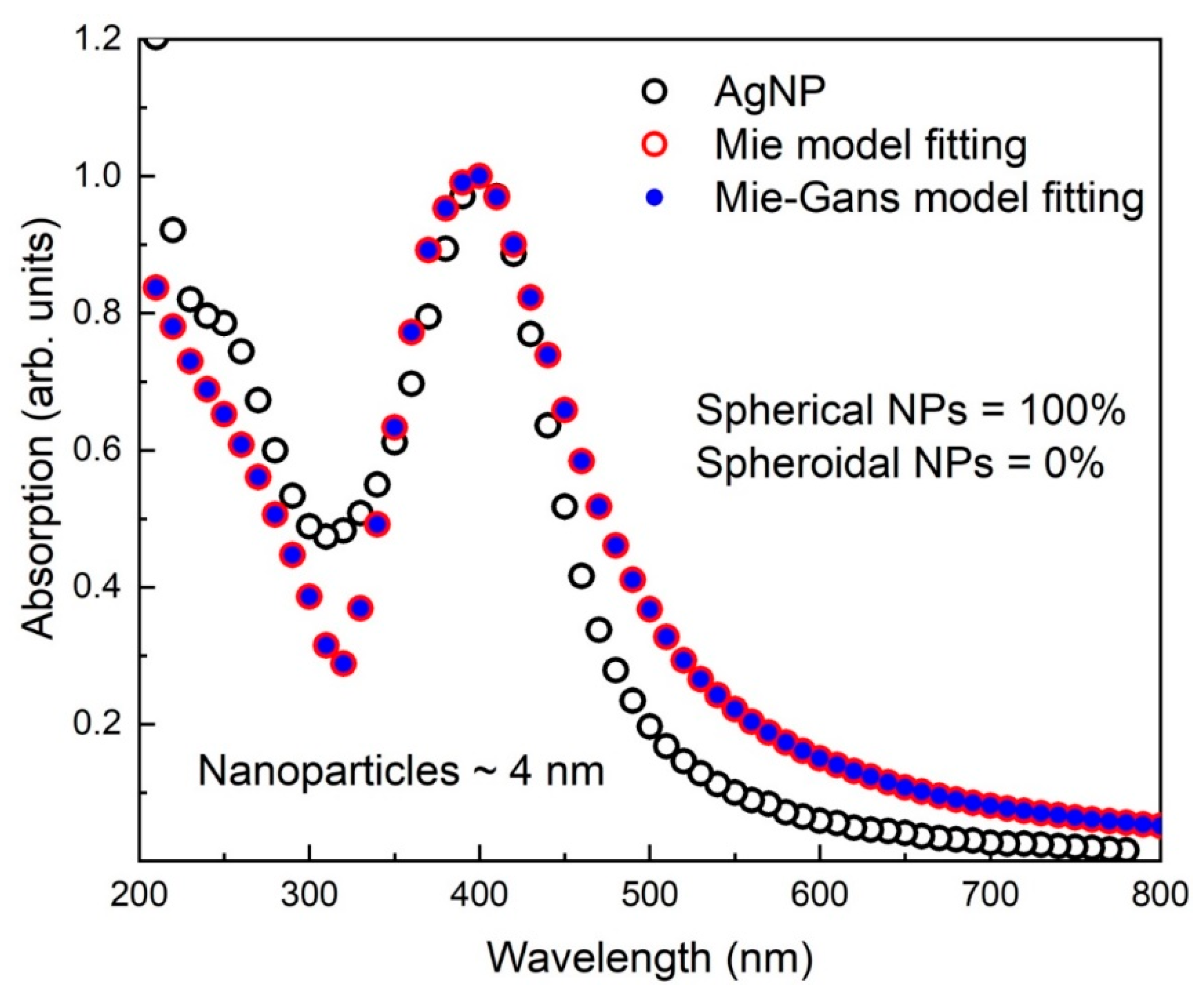
3.4. Mie and Mie-Gans Fitting Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forrest, S.R. ChemInform Abstract: Ultrathin Organic Films Grown by Organic Molecular Beam Deposition and Related Techniques. Chem. Rev. 2010, 97, 1793–1896. [Google Scholar] [CrossRef]
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Gerislioglu, B.; Dong, L.; Ahmadivand, A.; Hu, H.; Nordlander, P.; Halas, N.J. Monolithic Metal Dimer-on-Film Structure: New Plasmonic Properties Introduced by the Underlying Metal. Nano Lett. 2020, 20, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Ahmadivand, A.; Gerislioglu, B. Deep- and vacuum-ultraviolet metaphotonic light sources. Mater. Today 2021, in press. [Google Scholar] [CrossRef]
- Yan, F.; Mok, S.M.; Yu, J.; Chan, H.L.; Yang, M. Label-free DNA sensor based on organic thin film transistors. Biosens. Bioelectron. 2009, 24, 1241–1245. [Google Scholar] [CrossRef]
- Forrest, S.R.; Thompson, M.E. Introduction: Organic Electronics and Optoelectronics. Chem. Rev. 2007, 107, 923–925. [Google Scholar] [CrossRef]
- Rivnay, J.; Owens, R.M.; Malliaras, G.G. The Rise of Organic Bioelectronics. Chem. Mater. 2014, 26, 679–685. [Google Scholar] [CrossRef]
- Coropceanu, V.; Cornil, J.; da Silva Filho, D.A.; Olivier, Y.; Silbey, R.; Brédas, J.L. Charge Transport in Organic Semiconductors. Chem. Rev. 2007, 107, 926–952. [Google Scholar] [CrossRef]
- Klauk, H. Organic Electronics: Materials, Manufacturing, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Forrest, S.R. The road to high efficiency organic light emitting devices. Org. Electron. 2003, 4, 45–48. [Google Scholar] [CrossRef]
- Menard, E.; Meitl, M.A.; Sun, Y.; Park, J.-U.; Shir, D.J.-L.; Nam, Y.-S.; Jeon, A.S.; Rogers, J.A. Micro- and Nanopatterning Techniques for Organic Electronic and Optoelectronic Systems. Chem. Rev. 2007, 107, 1117–1160. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Integrated Nanoparticle–Biomolecule Hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed. 2004, 43, 6042–6108. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Baron, R.; Willner, B. Integrated nanoparticle–biomolecule systems for biosensing and bioelectronics. Biosens. Bioelectron. 2007, 22, 1841–1852. [Google Scholar] [CrossRef]
- Niemeyer, C.M. Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angew. Chem. Int. Ed. 2001, 40, 4128–4158. [Google Scholar] [CrossRef]
- Decher, G.; Lehr, B.; Lowack, K.; Lvov, Y.; Schmitt, J. New nanocomposite films for biosensors: Layer-by-layer adsorbed films of polyelectrolytes, proteins or DNA. Biosens. Bioelectron. 1994, 9, 677–684. [Google Scholar] [CrossRef]
- Willner, I.; Willner, B.; Katz, E. Biomolecule–nanoparticle hybrid systems for bioelectronic applications. Bioelectrochemistry 2007, 70, 2–11. [Google Scholar] [CrossRef]
- Ateeq, M.; Shah, M.R.; Ain, N.U.; Bano, S.; Anis, I.; Lubna; Faizi, S.; Bertino, M.F.; Naz, S.S. Green synthesis and molecular recognition ability of patuletin coated gold nanoparticles. Biosens. Bioelectron. 2015, 63, 499–505. [Google Scholar] [CrossRef]
- Shah, M.R.; Ali, S.; Ateeq, M.; Perveen, S.; Ahmed, S.; Bertino, M.F.; Ali, M. Morphological analysis of the antimicrobial action of silver and gold nanoparticles stabilized with ceftriaxone on Escherichia coli using atomic force microscopy. N. J. Chem. 2014, 38, 5633–5640. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Size Evaluation of Gold Nanoparticles by UV−vis Spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Rodríguez-Gattorno, G.; Díaz, D.; Rendón, L.; Hernández-Segura, G.O. Metallic Nanoparticles from Spontaneous Reduction of Silver(I) in DMSO. Interaction between Nitric Oxide and Silver Nanoparticles. J. Phys. Chem. B 2002, 106, 2482–2487. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Theoretical Considerations. In Optical Properties of Metal Clusters; Springer: Berlin/Heidelberg, Germany, 1995; pp. 13–201. [Google Scholar]
- Ali, S.; Ali, N.; Iqbal, Y.; Samreen, A.; Hayat, Q.; Hayat, K.; Ajmal, M.; Iqbal, M.J. Structural modifications induced in silicate glass by field-aided solid-state diffusion of gold and chromium ions. J. Non-Cryst. Solids 2015, 420, 38–42. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Ateeq, M.; Shah, M.R.; Ali, H.; Kabir, N.; Khan, A.; Nadeem, S. Hepatoprotective and urease inhibitory activities of garlic conjugated gold nanoparticles. N. J. Chem. 2015, 39, 5003–5007. [Google Scholar] [CrossRef]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Laser Ablation Synthesis of Gold Nanoparticles in Organic Solvents. J. Phys. Chem. B 2006, 110, 7232–7237. [Google Scholar] [CrossRef] [PubMed]
- Quinten, M. Optical Properties of Nanoparticle Systems: Mie and Beyond; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Rahim, M.; Fazil, P.; Ahmad, M.S.; Ullah, A.; Shah, M.R.; Rukh, G.; Ateeq, M.; Khattak, R.; Khan, M.S.; et al. Synthesis and Photonics Applications of Afzelechin Conjugated Silver Nanoparticles. Coatings 2021, 11, 1295. https://doi.org/10.3390/coatings11111295
Ali S, Rahim M, Fazil P, Ahmad MS, Ullah A, Shah MR, Rukh G, Ateeq M, Khattak R, Khan MS, et al. Synthesis and Photonics Applications of Afzelechin Conjugated Silver Nanoparticles. Coatings. 2021; 11(11):1295. https://doi.org/10.3390/coatings11111295
Chicago/Turabian StyleAli, Shahid, Muhammad Rahim, Perveen Fazil, Malik Shoaib Ahmad, Azeem Ullah, Muhammad Raza Shah, Gul Rukh, Muhammad Ateeq, Rozina Khattak, Muhammad Sufaid Khan, and et al. 2021. "Synthesis and Photonics Applications of Afzelechin Conjugated Silver Nanoparticles" Coatings 11, no. 11: 1295. https://doi.org/10.3390/coatings11111295
APA StyleAli, S., Rahim, M., Fazil, P., Ahmad, M. S., Ullah, A., Shah, M. R., Rukh, G., Ateeq, M., Khattak, R., Khan, M. S., Abu Ali, O. A., & Saleh, D. I. (2021). Synthesis and Photonics Applications of Afzelechin Conjugated Silver Nanoparticles. Coatings, 11(11), 1295. https://doi.org/10.3390/coatings11111295





