Er:YAG Laser Cleaning of Painted Surfaces: Functional Considerations to Improve Efficacy and Reduce Side Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Painting Mock-Ups
2.2. Hyperspectral Sensor
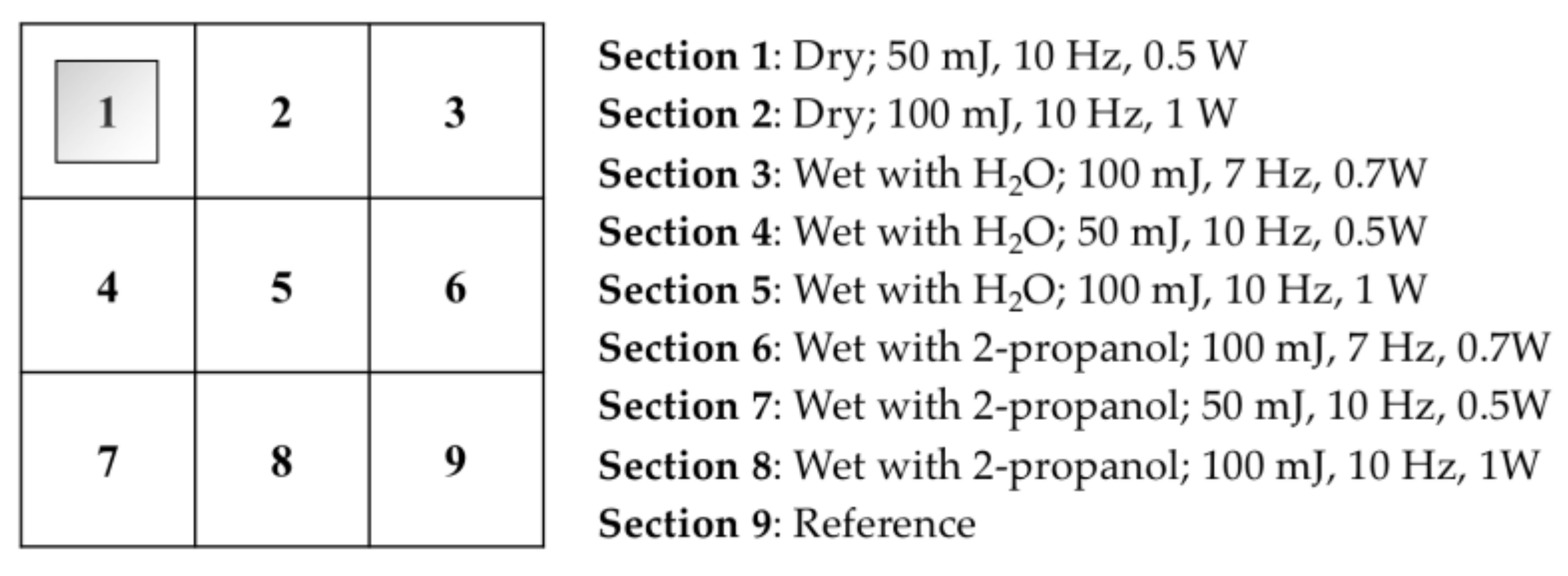
2.3. Varnish Removal by an Er:YAG Laser
2.4. Optical Microscopy
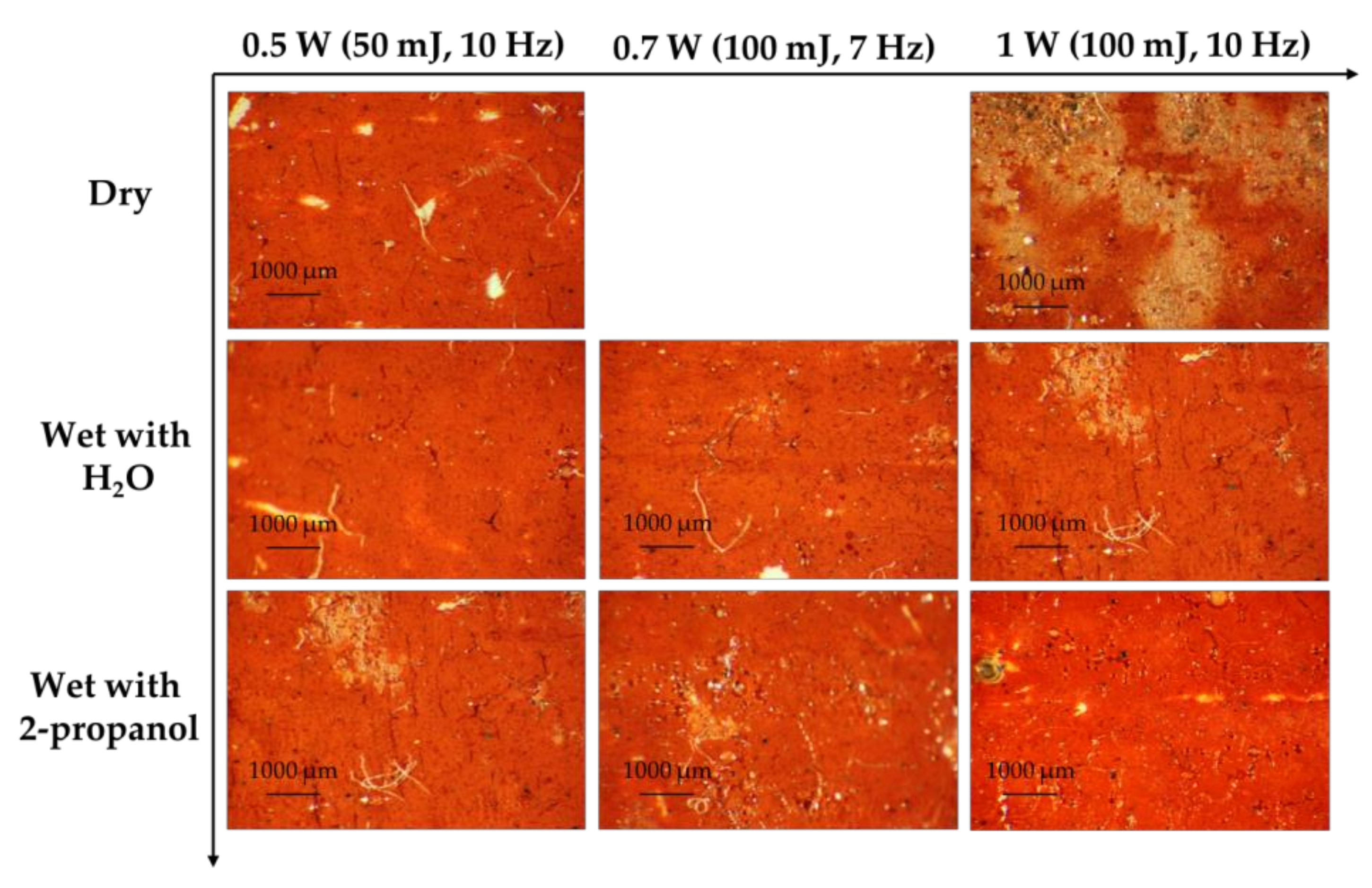
3. Results and Discussion
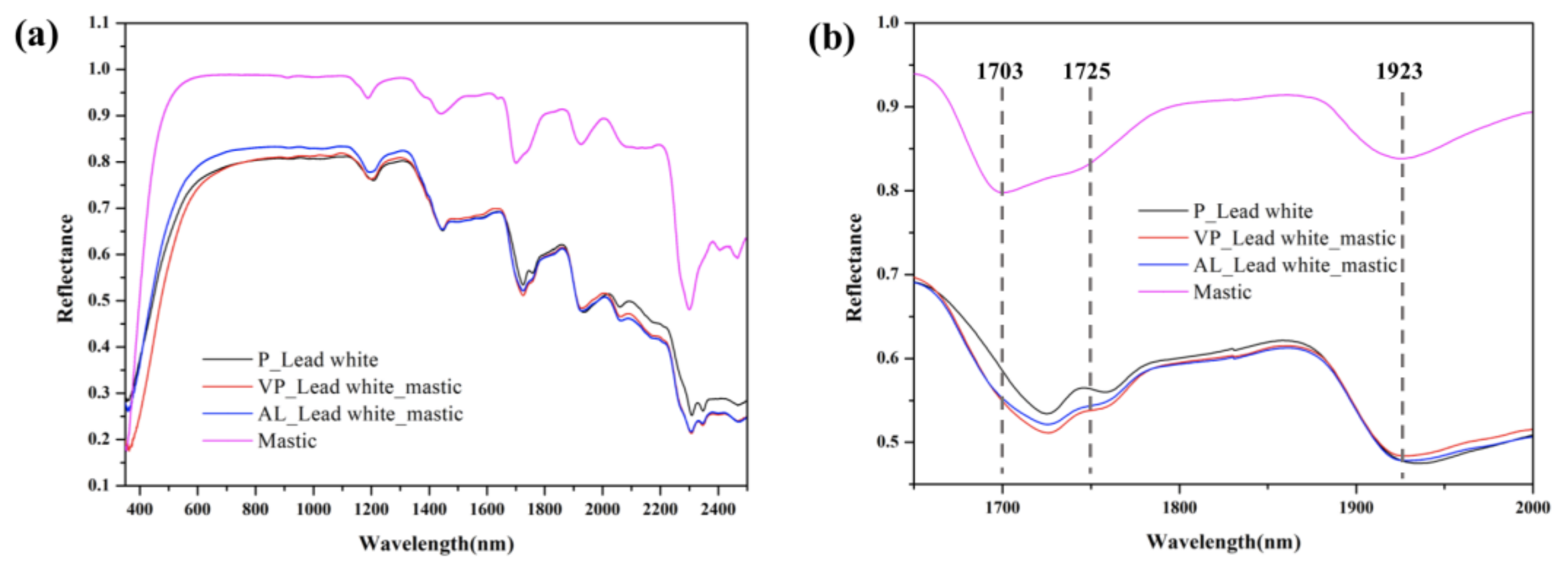
3.1. Assessment of the Cleaning of Mastic
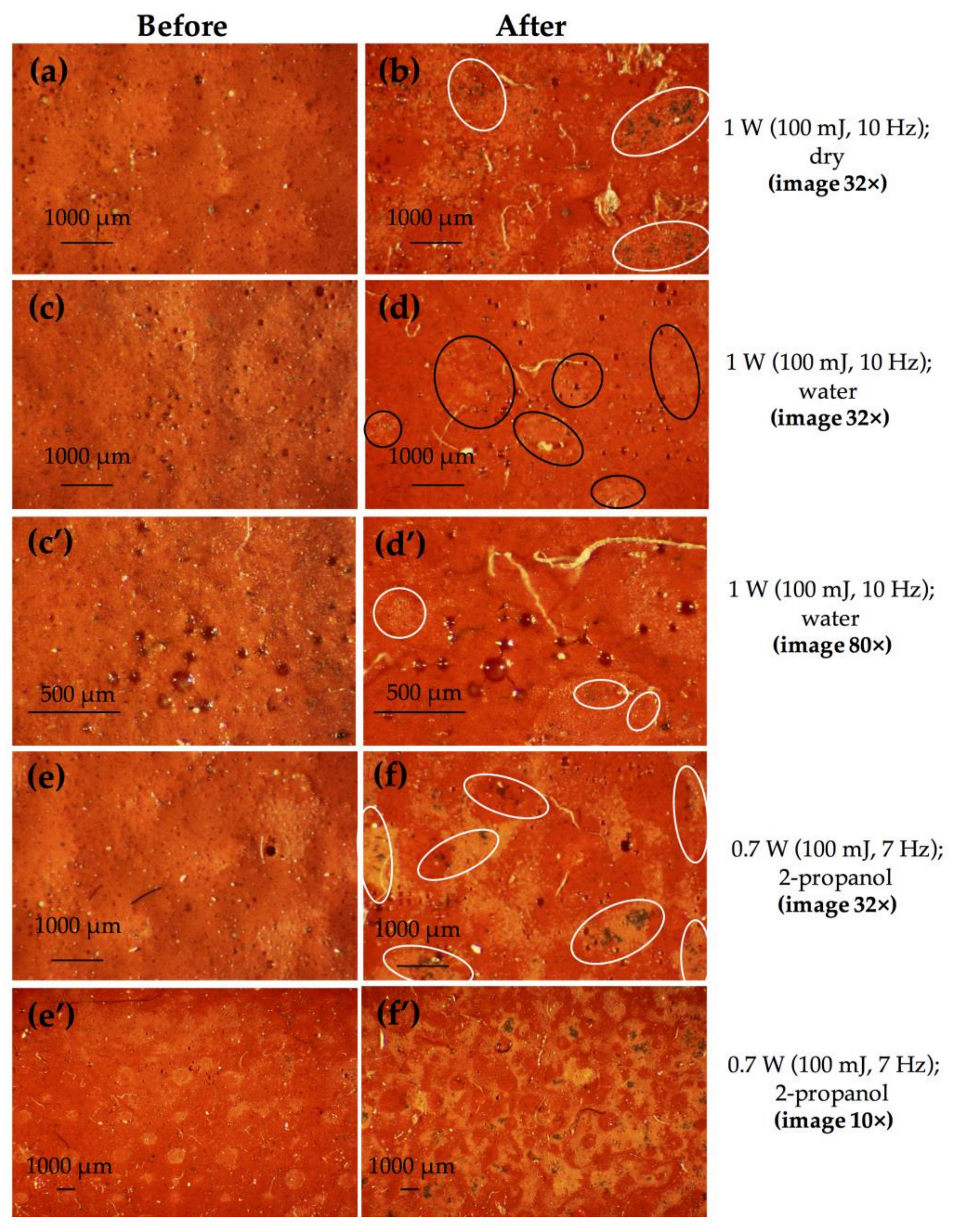
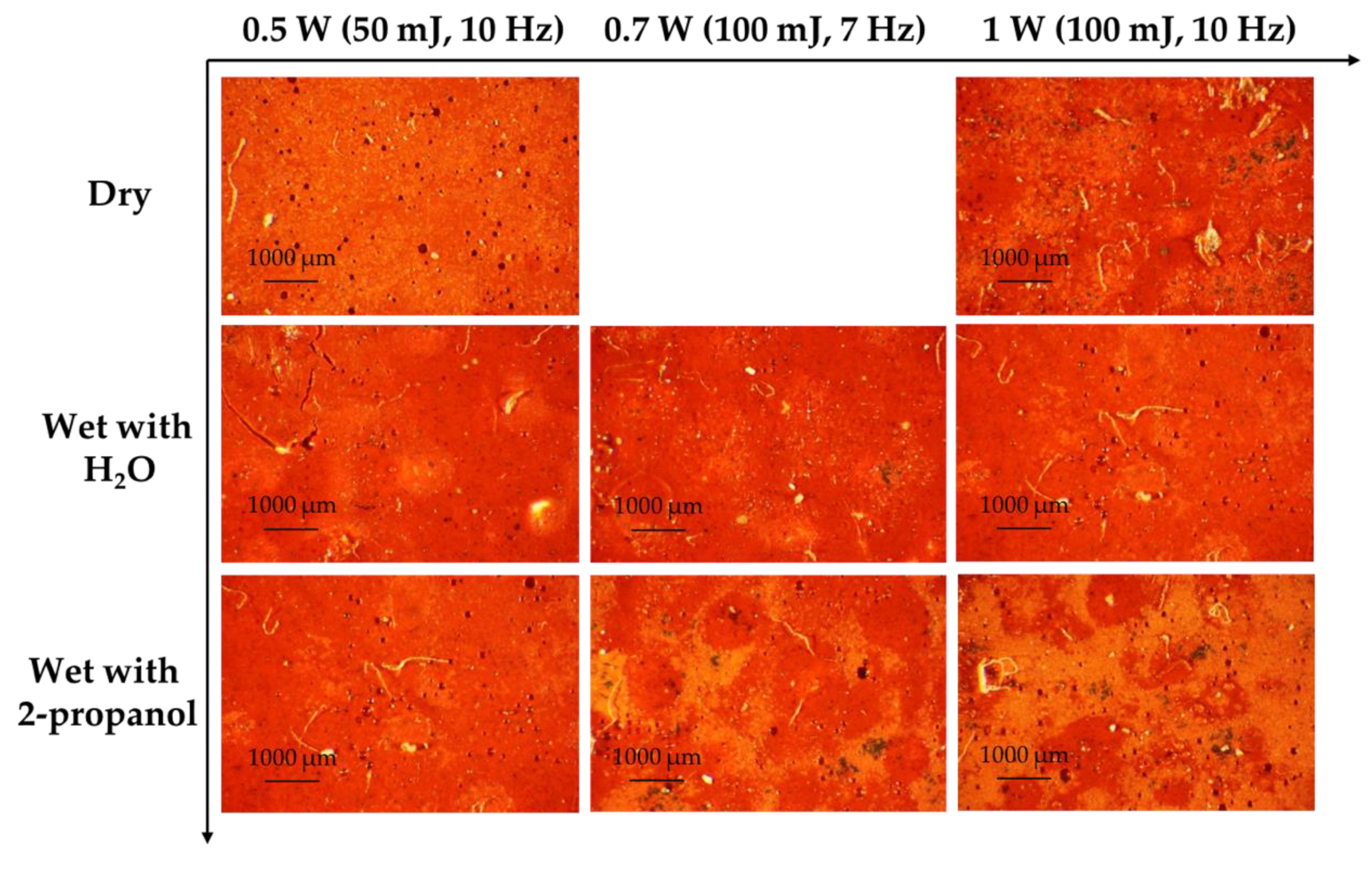
3.1.1. Cinnabar
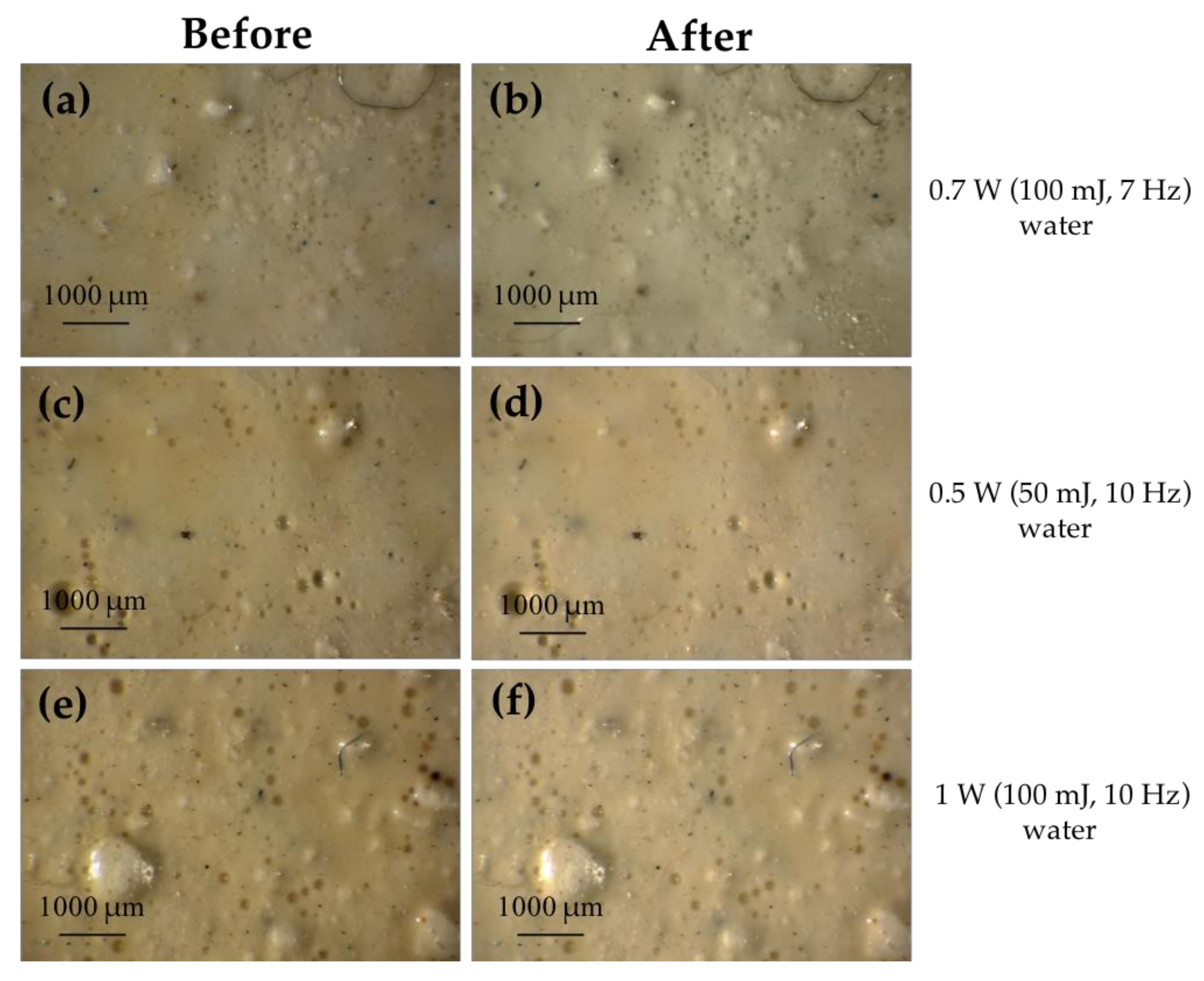
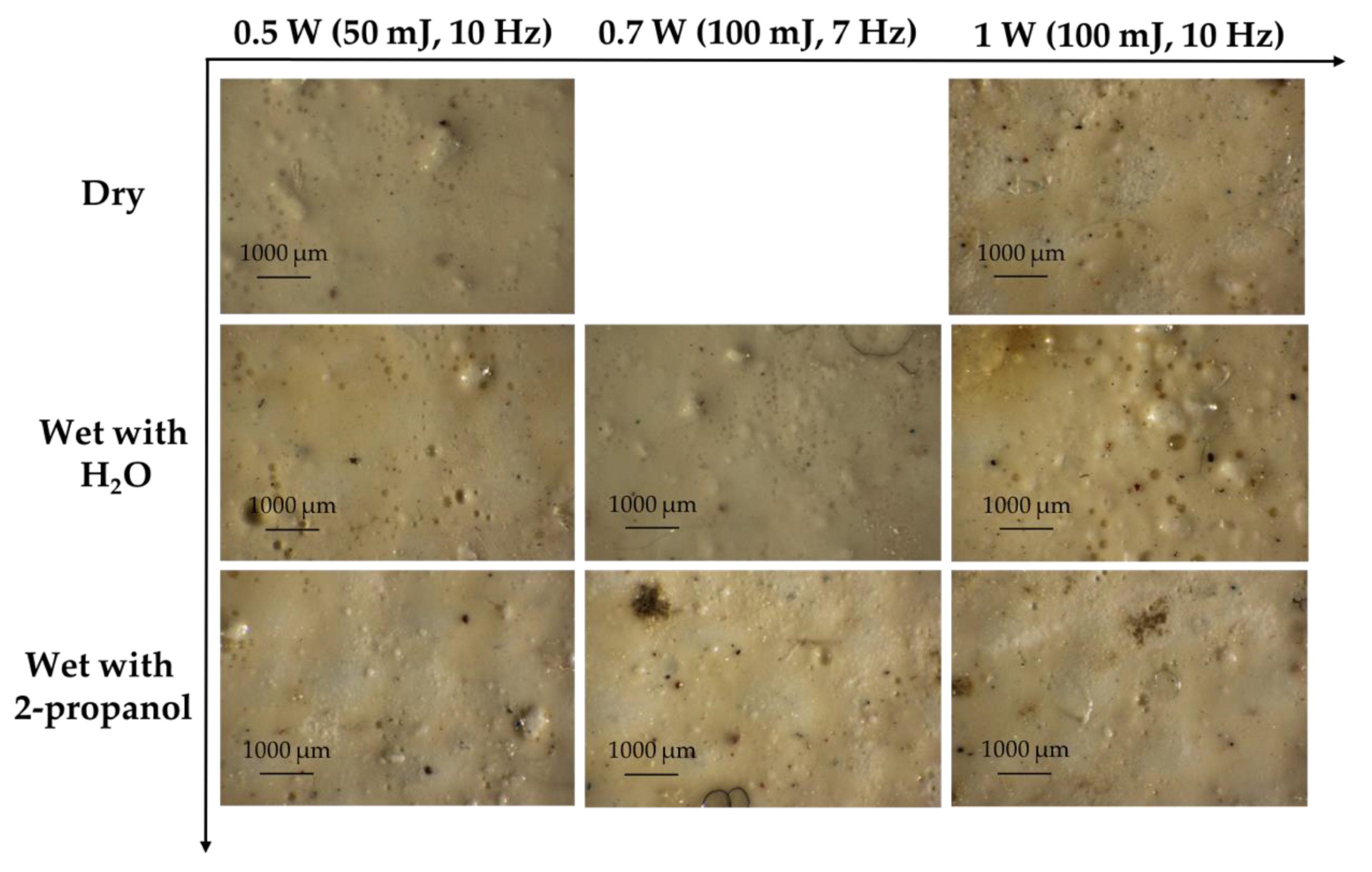
3.1.2. Lead White
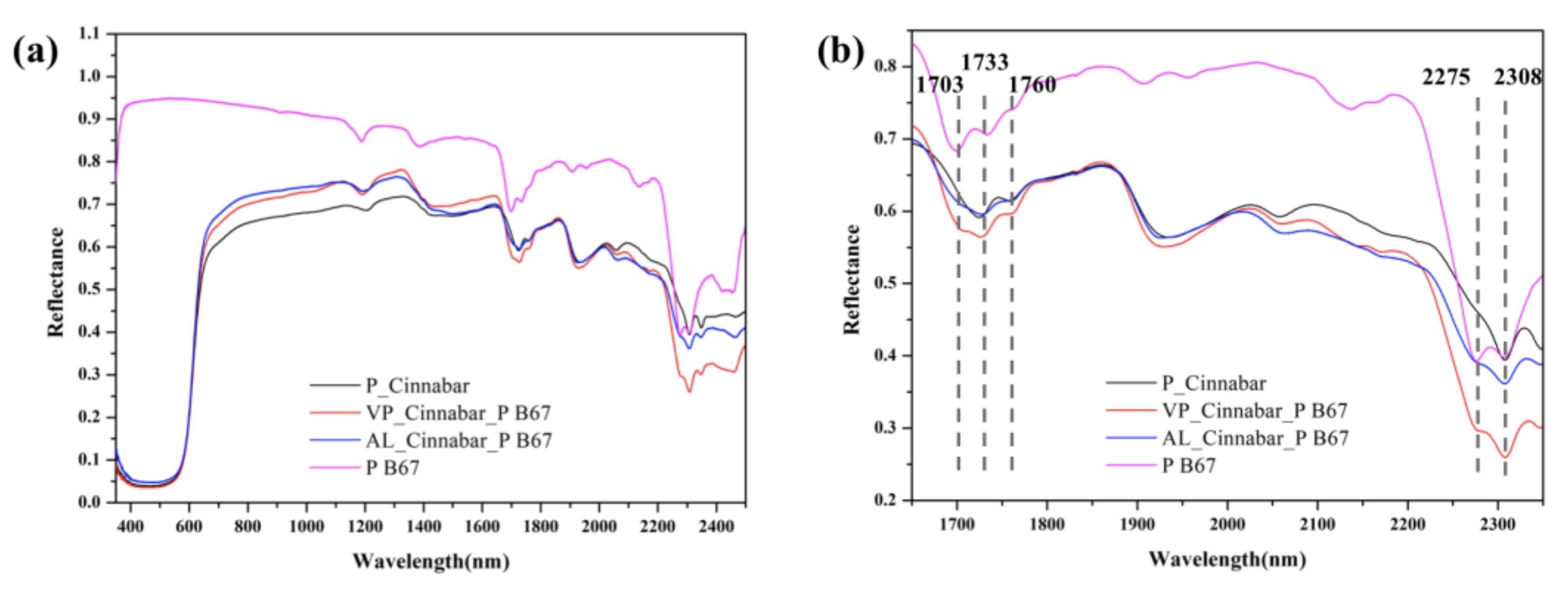
3.2. Assessment of the Cleaning of P B67
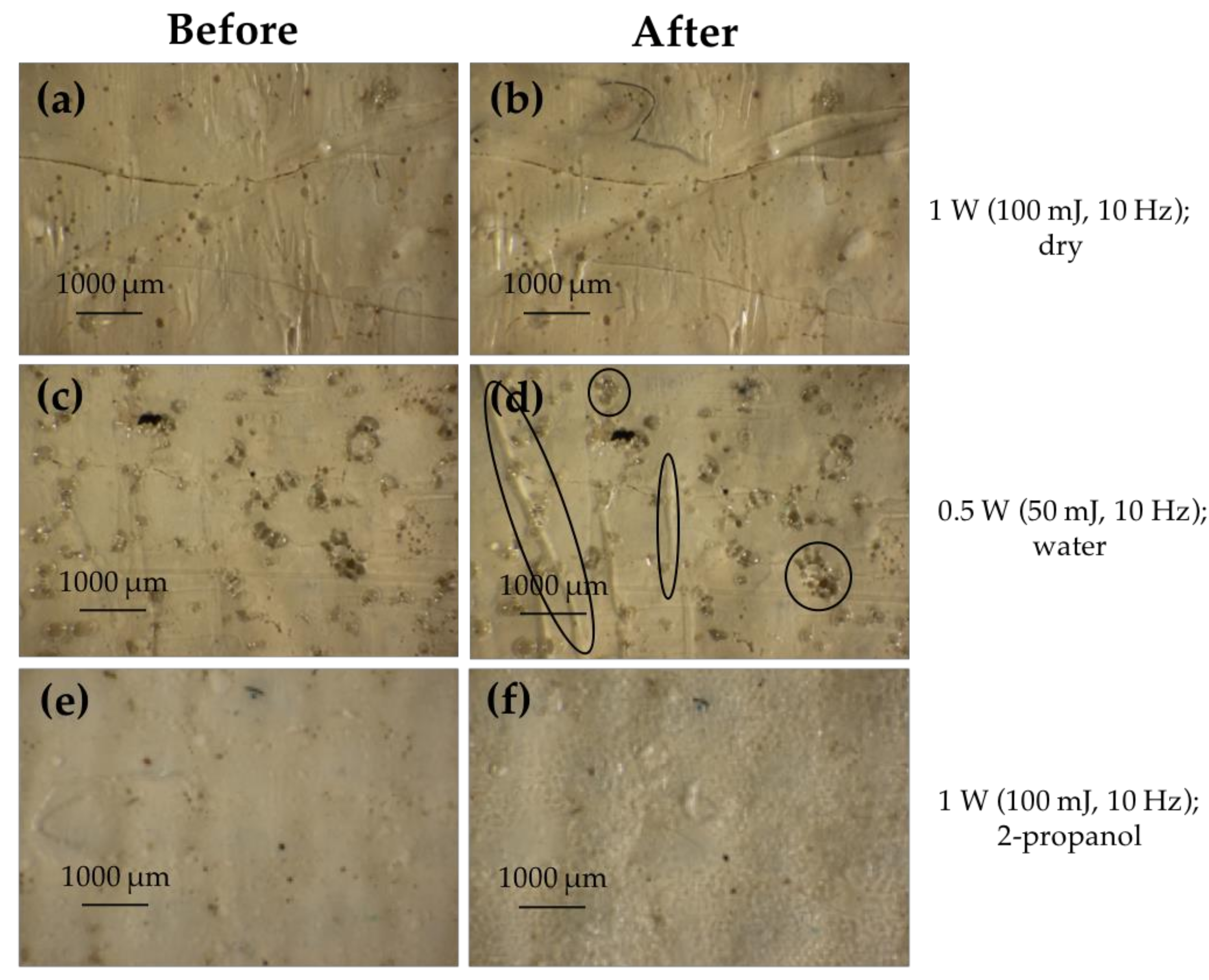
3.2.1. Cinnabar
3.2.2. Lead White
3.3. In Situ Monitoring of Cleaning by a Portable Hyperspectral Sensor
3.4. Best Condition for Laser Sensitive Pigments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De la Rie, E.R. The inpower of varnish on the appearance of paintings. Stud. Conserv. 1987, 32, 1–13. [Google Scholar]
- Reifsnyder, J.M. A note on a traditional technique of varnish application for paintings on panel. Stud. Conserv. 1996, 41, 120–122. [Google Scholar]
- Phenix, A.; Sutherland, K. The cleaning of paintings: Effects of organic solvents on oil paint films. Stud. Conserv. 2001, 46, 47–60. [Google Scholar] [CrossRef]
- Baij, L.; Hermans, J.; Ormsby, B.; Noble, P.; Iedema, P.; Keune, K. A review of solvent action on oil paint. Herit. Sci. 2020, 8, 43. [Google Scholar] [CrossRef]
- Khandekar, N. A survey of the conservation literature relating to the development of aqueous gel cleaning on painted and varnished surfaces. Stud. Conserv. 2000, 45, 10–20. [Google Scholar] [CrossRef]
- Erhardt, D.; Tsang, J.S. The extraction components of oil paint films. Stud. Conserv. 1990, 45, 93–97. [Google Scholar] [CrossRef]
- Carretti, E.; Dei, L.; Weiss, R.G.; Baglioni, P. A new class of gels for the conservation of painted surfaces. J. Cult. Herit. 2008, 9, 386–393. [Google Scholar] [CrossRef]
- Mastrangelo, R.; Chelazzi, D.; Poggi, G.; Fratini, E.; Buemi, L.P.; Petruzzellis, M.L.; Baglioni, P. Twin-chain polymer hydrogels based on poly (vinyl alcohol) as new advanced tool for the cleaning of modern and contemporary art. Proc. Natl. Acad. Sci. USA 2020, 117, 7011–7020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, B.; Morais, P.J.; Helena, G.; Young, C. Laser Cleaning of easel paintings: An overview. Laser Chem. 2006, 2006, 90279. [Google Scholar]
- Ganeev, R. Laser Cleaning of Art. In Laser—Surface Interactions, 1st ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 87–103. [Google Scholar]
- Asmus, J.F.; Guattari, G.; Lazzarini, L.; Musumeci, G.; Wuerker, R.F. Holography in the conservation of statuary. Stud. Conserv. 1973, 18, 49–63. [Google Scholar]
- Siano, S.; Agresti, J.; Cacciari, I.; Ciofini, D.; Mascalachi, M.; Osticioli, I.; Mencaglia, A. Laser cleaning in conservation of stone, metal, and painted artifacts: State of the art and new insights on the use of the Nd:YAG lasers. Appl. Phys. 2012, 106, 419–446. [Google Scholar] [CrossRef]
- Siano, S.; Giamello, M.; Bartoli, L.; Mencaglia, A.; Parfenov, V.; Salimbeni, R. Laser cleaning of stone by different laser pulse duration and wavelength. Laser Phys. 2008, 18, 27–36. [Google Scholar] [CrossRef]
- Osticioli, I.; Mascalchi, M.; Pinna, D.; Siano, S. Removal of verrucaria nigrescens from carrara marble artefacts using Nd:YAG lasers: Comparison among different pulse durations and wavelengths. Appl. Phys. A 2015, 118, 1517–1526. [Google Scholar] [CrossRef]
- Koh, Y.S.; Sárady, I. Cleaning of corroded iron artefacts using pulsed TEA CO2- and Nd:YAG-lasers. J. Cult. Herit. 2003, 4, 129–133. [Google Scholar] [CrossRef]
- Sansonetti, A.; Colella, M.; Letardi, P.; Salvadori, B.; Striova, J. Laser cleaning of a nineteenth-century bronze sculpture: In situ multi-analytical evaluation. Stud. Conserv. 2015, 60, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Bilmes, G.M.; Vallejo, J.; Costa Vera, C.; Garcia, M.E. High efficiencies for laser cleaning of glassware irradiated from the back: Application to glassware historical objects. Appl. Phys. A 2018, 124, 1–11. [Google Scholar] [CrossRef]
- Gaetani, C.; Santamaria, U. The laser cleaning of wall paintings. J. Cult. Herit. 2000, 1, S199–S207. [Google Scholar] [CrossRef]
- Andreotti, A.; Colombini, M.P.; Nevin, A.; Melessanaki, K.; Pouli, P.; Fotakis, C. Multianalytical study of laser pulse duration effects in the IR laser cleaning of wall paintings from the monumental cemetery of Pisa. Laser Chem. 2006, 2006, 39046. [Google Scholar] [CrossRef] [Green Version]
- De Cruz, A.; Wolbarsht, M.L.; Hauger, S.A. Laser removal of contaminants from painted surfaces. J. Cult. Herit. 2000, 1, 173–180. [Google Scholar] [CrossRef]
- Teppo, E. Introduction: Er:YAG lasers in the conservation of artworks. J. Inst. Conserv. 2020, 43, 2–11. [Google Scholar] [CrossRef]
- De Cruz, A.; Andreotti, A.; Ceccarini, A.; Colombini, M.P. Laser cleaning of works of art: Evaluation of the thermal stress induced by Er:YAG laser. Appl. Phys. B 2014, 117, 533–541. [Google Scholar] [CrossRef]
- Bracco, P.; Lanterna, G.; Matteini, M.; Nakahara, K.; Colombini, M.P. Er:YAG laser: An innovative tool for controlled cleaning of old paintings: Testing and evaluation. J. Cult. Herit. 2003, 4, 202–208. [Google Scholar] [CrossRef]
- Striova, J.; Salvadori, B.; Fontana, R.; Sansonetti, A.; Barucci, M.; Pampaloni, E.; Marconi, E.; Pezzati, L.; Colombini, M.P. Optical and spectroscopic tools for evaluating Er:YAG laser removal of shellac varnish. Stud. Conserv. 2015, 60, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Chillè, C.; Papadakis, V.M.; Theodorakopoulos, C. An analytical evaluation of Er:YAG laser cleaning tests on a nineteenth century varnished painting. Microchem. J. 2020, 158, 105086. [Google Scholar] [CrossRef]
- Brunetto, A.; Bono, G.; Frezzato, F. Er:YAG laser cleaning of ‘San Marziale in Gloria’ by Jacopo Tintoretto in the Church of San Marziale, Venice. J. Inst. Conserv. 2020, 43, 44–58. [Google Scholar] [CrossRef]
- Chillè, C.; Sala, F.; Wu, Q.; Theodorakopoulos, C. A study on the heat distribution and oxidative modification of aged dammar films upon Er:YAG laser irradiation. J. Inst. Conserv. 2020, 43, 59–78. [Google Scholar] [CrossRef]
- Pereira-Pardo, L.; Melita, L.N.; Korenberg, C. Tackling conservation challenges using erbium lasers: Case studies at the British Museum. J. Inst. Conserv. 2020, 43, 25–43. [Google Scholar] [CrossRef]
- Striova, J.; Fontana, R.; Barbetti, I.; Pezzati, L.; Fedele, A.; Riminesi, C. Multisensorial assessment of laser effects on shellac applied on wall paintings. Sensors 2021, 21, 3354. [Google Scholar] [CrossRef]
- Camaiti, M.; Vettori, S.; Benvenuti, M.; Chiarantini, L.; Costagliola, P.; Di Benedetto, F.; Moretti, S.; Paba, F.; Pecchioni, E. Hyperspectral sensor for gypsum detection on monumental buildings. J. Geophys. Eng. 2011, 8, S126–S131. [Google Scholar] [CrossRef]
- Wang, C.; Salvatici, T.; Camaiti, M.; Chiara, D.; Moretti, S. A new application of hyperspectral radiometry: The characterization of painted surfaces. In Proceedings of the EGU General Assembly 2016, Vienna, Austria, 23–29 April 2016. [Google Scholar]
- Wang, C. Hyperspectral Sensor: A New Approach for Evaluating the Efficacy of Laser Cleaning in the Removal of Varnishes and Overpaintings. Master’s Thesis, University of Bologna, Bologna, Italy, 2015. [Google Scholar]
- Camaiti, M.; Benvenuti, M.; Costagliola, P.; Benedetto, F.D.; Moretti, S. Hyperspectral sensors for the characterization of cultural heritage surfaces. In Sensing the Past; Masini, N., Soldovieri, F., Eds.; Springer International Publishing: Gewerbestrasse, Switzerland, 2017; Volume 16, pp. 289–311. [Google Scholar]
- Pouli, P.; Emmony, D.C.; Madden, C.E.; Sutherland, I. Studies towards a thorough understanding of the laser-induced discoloration mechanisms of medieval pigments. J. Cult. Herit. 2003, 4, 271s–275s. [Google Scholar] [CrossRef]
- Striova, J.; Camaiti, M.; Castellucci, E.M.; Sansonetti, A. Chemical, morphological and chromatic behavior of mural paintings under er:yag laser irradiation. Appl. Phys. A 2011, 104, 649–660. [Google Scholar] [CrossRef]
- Camaiti, M.; Matteini, M.; Sansonetti, A.; Striová, J.; Castelluci, E.; Andreotti, A.; Colombini, M.P.; De Cruze, A.; Palmer, R. The Interaction of Laser Radiation at 2.94 μm with Azurite and Malachite Pigments. Lasers in the Conservation of Artworks. In Proceedings of the International Conference Lacona VII, Madrid, Spain, 17–21 September 2008; pp. 253–258. [Google Scholar]
- Invernizzi, C.; Rovetta, T.; Licchelli, M.; Malagodi, M. Mid and near-infrared reflection spectral database of natural organic materials in the cultural heritage field. Int. J. Anal. Chem. 2018, 2018, 7823248. [Google Scholar] [CrossRef] [PubMed]
- Schwanninger, M.; Rodrigues, J.C.; Fackler, K. A review of band assignments in near infrared spectra of wood and wood components. J. Near Infrared Spectrosc. 2011, 19, 287–308. [Google Scholar] [CrossRef]





| Pigment | Mass of Pigment (g) | Mass of Egg Yolk (g) | Mass of Water (g) |
|---|---|---|---|
| Cinnabar | 3.529 | 3.528 | 0.700 |
| Lead white | 3.589 | 3.960 | 0.800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Cao, Y.; Tie, F.; Camaiti, M. Er:YAG Laser Cleaning of Painted Surfaces: Functional Considerations to Improve Efficacy and Reduce Side Effects. Coatings 2021, 11, 1315. https://doi.org/10.3390/coatings11111315
Wang C, Cao Y, Tie F, Camaiti M. Er:YAG Laser Cleaning of Painted Surfaces: Functional Considerations to Improve Efficacy and Reduce Side Effects. Coatings. 2021; 11(11):1315. https://doi.org/10.3390/coatings11111315
Chicago/Turabian StyleWang, Cong, Yijian Cao, Fude Tie, and Mara Camaiti. 2021. "Er:YAG Laser Cleaning of Painted Surfaces: Functional Considerations to Improve Efficacy and Reduce Side Effects" Coatings 11, no. 11: 1315. https://doi.org/10.3390/coatings11111315





