Effect of Annealing on Surface Morphology and Structure of Nickel Coatings Deposited from Deep Eutectic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Coatings Electrodeposition and Heat Treatment
2.2. Research Techniques
3. Results and Discussion
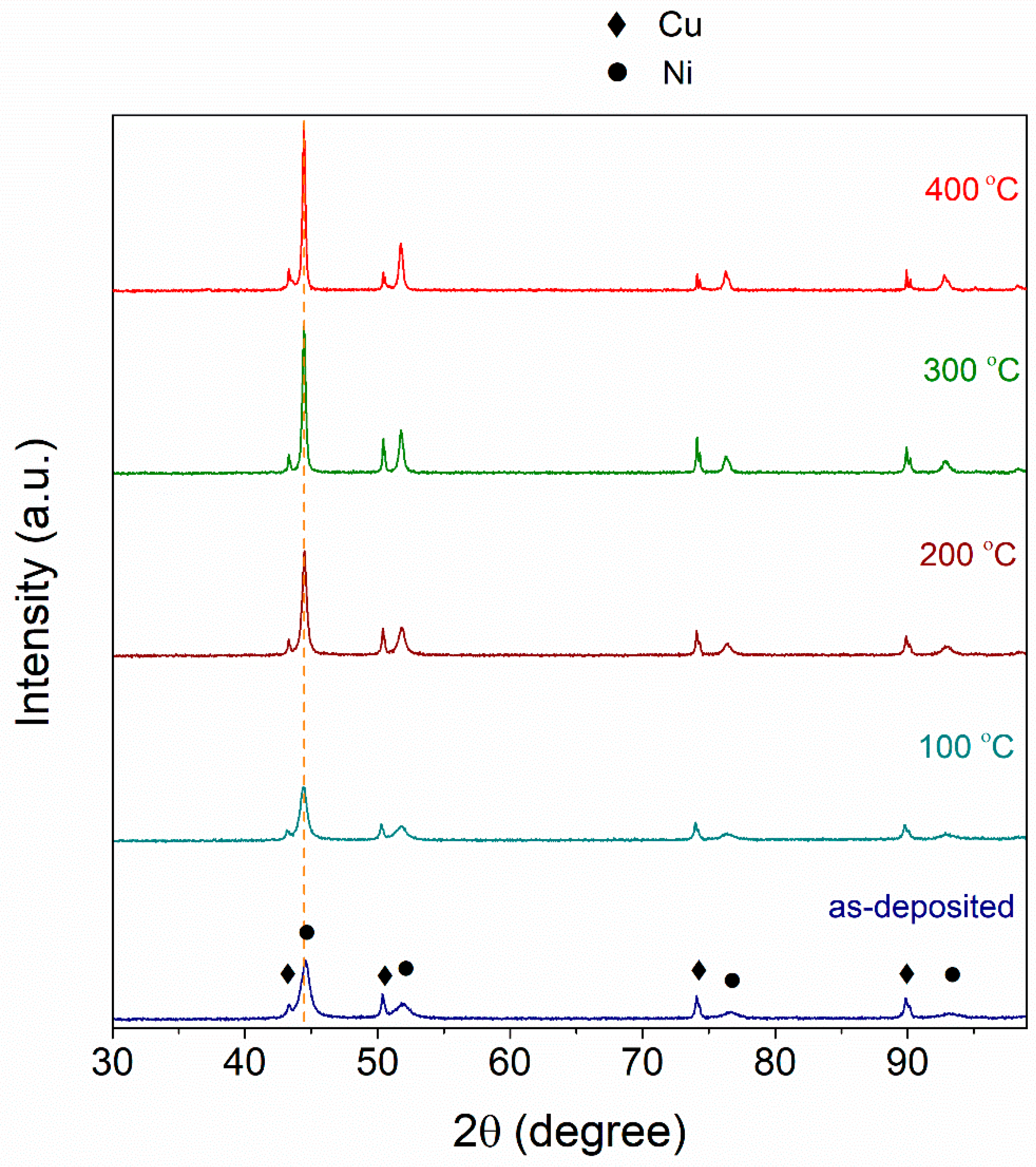
3.1. The Effect of Heat Treatment on the Structure and Microhardness of the Coatings
3.2. The Effect of Heat Treatment on the Morphology and Microstructure of the Coatings
3.3. The Effect of Heat Treatment on the Surface Chemistry: XPS Analysis
3.4. The Effect of Heat Treatment on Corrosion Resistance of the Coatings
3.4.1. Linear Polarization Resistance
3.4.2. Electrochemical Impedance Spectroscopy
4. Conclusions
- Nanocrystalline nickel coatings were deposited on a copper base material from deep eutectic solvent of choline chloride and ethylene glycol (1:2 molar ratio) containing 1 mol dm−3 NiCl2·6H2O under galvanostatic conditions at jc = 6 mA cm−2 and the bath temperature of 70 °C. The surface of 7–8 μm thick coatings were composed of spheroidal agglomerates with the size of several hundred nanometers interspersed with lamellar crystals.
- Modification of the properties of the coatings was achieved by subjecting the samples to heat treatment for two hours at the temperature of: 100, 200, 300 and 400 °C. It has been shown that the temperature of annealing, surface morphology, chemistry of oxidized surface, corrosion resistance and microhardness created quite a complex network of relationships.
- As a result of heat treatment, coatings were gradually covered by a layer of oxidized nickel species. XPS analyses showed that NiOOH and Ni(OH)2 dominated among them. However, with the increase in annealing temperature, the share of these compounds began to decline in the face of the increasing share of NiO. This phenomenon intensified after exceeding the annealing temperature of 200 °C, when metallic nickel was evidently oxidizing to NiO while the content of compounds containing –OH groups did not decrease.
- After annealing of the coatings at 300 °C and 400 °C the previously observed spheroidal morphology of nickel disappeared, although single nano-sized plates embedded in a clearly granular layer were visible. It is very likely that after annealing at 300 °C and 400 °C, the surface layer was of considerable thickness and was mainly composed of NiO, with a very low Ni(OH)2 content. On this basis, it can be assumed that the change of the main component of the oxidized nickel layer influenced its morphology and barrier properties. This, in turn, clearly translated into a deterioration of the corrosion resistance of Ni coatings annealed at 300 °C, and especially at 400 °C, during exposure in NaCl solution. For the coating annealed at 200 °C polarization resistance reached a value of ca. 2 MΩ cm2, while at 400 °C it was only about 100 kΩ cm2. The resistances determined from EIS measurements, 1.7 MΩ cm2 and 18 kΩ cm2, respectively, related to the oxidized surface layer also seemed to confirm this trend.
- The temperature of heat treatment of nickel coatings electrodeposited in DES bath largely influenced their microhardness. It has been shown that the observed behavior cannot be associated neither with changes in the surface composition nor the surface topography. Structural studies using the XRD technique showed that as the temperature of the heat treatment increased from 100 °C to 400 °C, the mean crystallite size increased from 13 to 35 nm (at 10 nm for the unannealed coating). Microhardness also changes—the maximum value of which was measured for the annealed coating at 200 °C.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winiarski, J.; Cieślikowska, B.; Tylus, W.; Kunicki, P.; Szczygieł, B. Corrosion of nanocrystalline nickel coatings electrodeposited from choline chloride:ethylene glycol deep eutectic solvent exposed in 0.05 M NaCl solution. Appl. Surf. Sci. 2018, 470, 331–339. [Google Scholar] [CrossRef]
- Abbott, A.; Ballantyne, A.; Harris, R.; Juma, J.A.; Ryder, K.; Forrest, G. A Comparative study of nickel electrodeposition using deep eutectic solvents and aqueous solutions. Electrochim. Acta 2015, 176, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Valladares, L.D.L.S.; Ionescu, A.; Holmes, S.; Barnes, C.H.W.; Domínguez, A.B.; Quispe, O.A.; Gonzalez, J.C.; Milana, S.; Barbone, M.; Ferrari, A.C.; et al. Characterization of Ni thin films following thermal oxidation in air. J. Vac. Sci. Technol. B 2014, 32, 051808. [Google Scholar] [CrossRef] [Green Version]
- Laszczyńska, A.; Tylus, W.; Szczygiel, B. Influence of post−deposition heat treatment on the properties of electrodeposited Ni−Mo alloy coatings. Appl. Surf. Sci. 2018, 462, 432–443. [Google Scholar] [CrossRef]
- Yavuz, A.; Kaplan, K.; Bedir, M. Annealing of electrodeposited nickel on low carbon steel for supercapacitor applications. Dig. J. Nanomater. Biostruct. 2019, 14, 1061–1068. [Google Scholar]
- Thuvander, M.; Abraham, M.; Cerezo, A.; Smith, G. Thermal stability of electrodeposited nanocrystalline nickel and iron–nickel alloys. Mater. Sci. Technol. 2001, 17, 961–970. [Google Scholar] [CrossRef]
- Lü, B.; Hu, Z.-F.; Wang, X.-H.; Xu, B.-S. Thermal stability of electrodeposited nanocrystalline nickel assisted by flexible friction. Trans. Nonferr. Met. Soc. China 2015, 25, 3297–3304. [Google Scholar] [CrossRef]
- Czerwinski, F.; Li, H.; Megret, M.; Szpunar, J.; Clark, D.; Erb, U. The evolution of texture and grain size during annealing of nanocrystalline Ni-45% Fe electrodeposits. Scr. Mater. 1997, 37, 1967–1972. [Google Scholar] [CrossRef]
- Subramanian, B.; Jayakumar, S.; Jayachandran, M.; Jayakrishnan, S. Studies on nickel electrodeposits on dc magnetron sputtered copper substrates. Surf. Eng. 2005, 21, 151–155. [Google Scholar] [CrossRef]
- Ul-Hamid, A.; Quddus, A.; Al-Yousef, F.; Mohammed, A.; Saricimen, H.; Al-Hadhrami, L. Microstructure and surface mechanical properties of electrodeposited Ni coating on Al 2014 alloy. Surf. Coat. Technol. 2010, 205, 2023–2030. [Google Scholar] [CrossRef]
- Jinlong, L.; Zhuqing, W.; Tongxiang, L.; Suzuki, K.; Hideo, M. Effect of tungsten on microstructures of annealed electrodeposited Ni-W alloy and its corrosion resistance. Surf. Coat. Technol. 2018, 337, 516–524. [Google Scholar] [CrossRef]
- Winiarski, J.; Cieślikowska, B.; Szczygieł, B. Electrodeposition of nickel coatings in choline chloride: Ethylene glycol deep eutectic solvent. Ochr. Przed Koroz. 2018, 61, 93–95. [Google Scholar] [CrossRef]
- Winiarski, J.; Niciejewska, A.; Ryl, J.; Darowicki, K.; Baśladyńska, S.; Winiarska, K.; Szczygieł, B. Ni/cerium molybdenum oxide hydrate microflakes composite coatings electrodeposited from choline chloride: Ethylene glycol deep eutectic solvent. Materials 2020, 13, 924. [Google Scholar] [CrossRef] [Green Version]
- Oliver, W.; Pharr, G. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ballantyne, A.; Harris, R.C.; Juma, J.A.; Ryder, K.S. Bright metal coatings from sustainable electrolytes: The effect of molecular additives on electrodeposition of nickel from a deep eutectic solvent. Phys. Chem. Chem. Phys. 2017, 19, 3219–3231. [Google Scholar] [CrossRef] [Green Version]
- Laszczyńska, A.; Tylus, W.; Winiarski, J.; Szczygieł, I. Evolution of corrosion resistance and passive film properties of Ni-Mo alloy coatings during exposure to 0.5 M NaCl solution. Surf. Coat. Technol. 2017, 317, 26–37. [Google Scholar] [CrossRef]
- Danilov, F.; Protsenko, V.S.; Kityk, A.A.; Shaiderov, D.; Vasil’Eva, E.A.; Kumar, U.P.; Kennady, C.J. Electrodeposition of nanocrystalline nickel coatings from a deep eutectic solvent with water addition. Prot. Met. Phys. Chem. Surf. 2017, 53, 1131–1138. [Google Scholar] [CrossRef]
- Abraham, M.; Holdway, P.; Thuvander, M.; Cerezo, A.; Smith, G.D.W. Thermal stability of electrodeposited nanocrystalline nickel. Surf. Eng. 2002, 18, 151–156. [Google Scholar] [CrossRef]
- Schuh, C.A.; Nieh, T.G. Hardness and abrasion resistance of nanocrystalline nickel alloys near the hall-petch breakdown regime. MRS Proc. 2002, 740, 18. [Google Scholar] [CrossRef] [Green Version]
- Naik, S.N.; Walley, S.M. The Hall–Petch and inverse Hall–Petch relations and the hardness of nanocrystalline metals. J. Mater. Sci. 2019, 55, 2661–2681. [Google Scholar] [CrossRef] [Green Version]
- Popczyk, M.; Kubisztal, J.; Swinarew, A.S.; Waśkiewicz, Z.; Stanula, A.; Knechtle, B. Corrosion resistance of heat-treated Ni-W alloy coatings. Materials 2020, 13, 1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oriňáková, R.; Oriňák, A.; Kupková, M.; Sabalová, M.; Straková Fedorková, A.; Kabátová, M.; Kal’avskỳ, F.; Sedlaříková, M. Effect of heat treatment on the corrosion and mechanical properties of electrolytical composite Ni–B coatings. Int. J. Electrochem. Sci. 2014, 9, 4268–4286. [Google Scholar]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]









| Parameter | As-Deposited | 100 °C | 200 °C | 300 °C | 400 °C |
|---|---|---|---|---|---|
| D (nm) | 9.8 | 12.7 | 23.5 | 31.2 | 35.2 |
| HVIT | 588.7 | 666.5 (45.2) | 803.4 (72.1) | 618.3 (31.9) | 475.4 (50.7) |
| (29.7) | |||||
| EIT (MPa) | 185.6 | 183.3 (20.3) | 211.0 (33.9) | 237.3 (34.9) | 213.1 (30.5) |
| (20.8) |
| Coating | Ni 2p | O 1s | C 1s | O:Ni | |
|---|---|---|---|---|---|
| fresh | “as received” immediately after deposition, rinsing and drying | 44.79 | 18.68 | 36.53 | 0.42 |
| +Ar+ 60 s | 69.03 | 15.30 | 15.67 | 0.22 | |
| 20 °C | “as received” after 24 h of exposure to air at 20 °C | 38.32 | 30.87 | 30.82 | 0.81 |
| +Ar+ 60 s | 62.93 | 19.49 | 17.58 | 0.31 | |
| 100 °C | “as received” after 2 h annealing at 100 °C | 29.19 | 26.46 | 44.35 | 0.91 |
| +Ar+ 60 s | 49.32 | 20.27 | 30.41 | 0.41 | |
| 200 °C | “as received” after 2 h annealing at 200 °C | 26.93 | 40.26 | 32.80 | 1.49 |
| +Ar+ 90 s | 40.63 | 39.61 | 19.76 | 0.97 | |
| 300 °C | “as received” after 2 h annealing at 300 °C | 32.66 | 50.67 | 16.67 | 1.55 |
| +Ar+ 60 s | 43.42 | 51.28 | 5.3 | 1.18 | |
| 400 °C | “as received” after 2 h annealing at 400 °C | 25.4 (+16.0 Cu) | 44.52 | 14.11 | 1.75 |
| Nimetal | Ni(OH)2 | NiO | NiOOH | Niox:Nimetal | |
|---|---|---|---|---|---|
| as-deposited | 86.10 | 0.64 | 6.97 | 6.29 | 0.16 |
| as-deposited (stored for 24 h, open to air) | 70.44 | 0.04 | 6.38 | 23.14 | 0.42 |
| 100 °C, 2 h | 66.99 | 0.76 | 2.38 | 29.86 | 0.49 |
| 200 °C, 2 h | 31.02 | 2.09 | 29.56 | 37.33 | 2.22 |
| 300 °C, 2 h | 1.44 | 1.91 | 87.64 | 9.00 | 68.44 |
| 400 °C, 2 h | 0.18 | 4.56 | 95.25 | 0.00 | >500 |
| Sample | Rs (Ω cm2) | CPE1-T (Ω−1 cm−2 s−P) | CPE1-P | Rlayer (kΩ cm2) | Cdl (μF cm−2) | Rct (kΩ cm2) |
|---|---|---|---|---|---|---|
| as-deposited | 135 | 1.4 × 10−4 | 0.78 | 6.0 | 62 | 40.5 |
| 100 °C | 145 | 6.5 × 10−5 | 0.91 | 1524 | 69 | 846 |
| 200 °C | 145 | 4.1 × 10−5 | 0.90 | 1740 | 90 | 895 |
| 300 °C | 144 | 3.0 × 10−5 | 0.88 | 278 | 32 | 650 |
| 400 °C | 180 | 3.2 × 10−5 | 0.90 | 18 | 6.5 * | 118 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winiarski, J.; Niciejewska, A.; Tylus, W.; Winiarska, K.; Pazgan, K.; Szczygieł, B. Effect of Annealing on Surface Morphology and Structure of Nickel Coatings Deposited from Deep Eutectic Solvents. Coatings 2021, 11, 1347. https://doi.org/10.3390/coatings11111347
Winiarski J, Niciejewska A, Tylus W, Winiarska K, Pazgan K, Szczygieł B. Effect of Annealing on Surface Morphology and Structure of Nickel Coatings Deposited from Deep Eutectic Solvents. Coatings. 2021; 11(11):1347. https://doi.org/10.3390/coatings11111347
Chicago/Turabian StyleWiniarski, Juliusz, Anna Niciejewska, Włodzimierz Tylus, Katarzyna Winiarska, Karolina Pazgan, and Bogdan Szczygieł. 2021. "Effect of Annealing on Surface Morphology and Structure of Nickel Coatings Deposited from Deep Eutectic Solvents" Coatings 11, no. 11: 1347. https://doi.org/10.3390/coatings11111347
APA StyleWiniarski, J., Niciejewska, A., Tylus, W., Winiarska, K., Pazgan, K., & Szczygieł, B. (2021). Effect of Annealing on Surface Morphology and Structure of Nickel Coatings Deposited from Deep Eutectic Solvents. Coatings, 11(11), 1347. https://doi.org/10.3390/coatings11111347







