Corrosion and Biocompatibility of Pure Zn with a Micro-Arc-Oxidized Layer Coated with Calcium Phosphate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Microstructure and Composition Characterization
2.3. Electrochemical Tests
2.4. Immersion Tests
2.5. In-Vitro Tests
2.6. Wettability Measurement
3. Results and Discussion
3.1. Microstructure and Composition Identification of MAO Coating before and after HT
3.2. Electrochemical Characterization
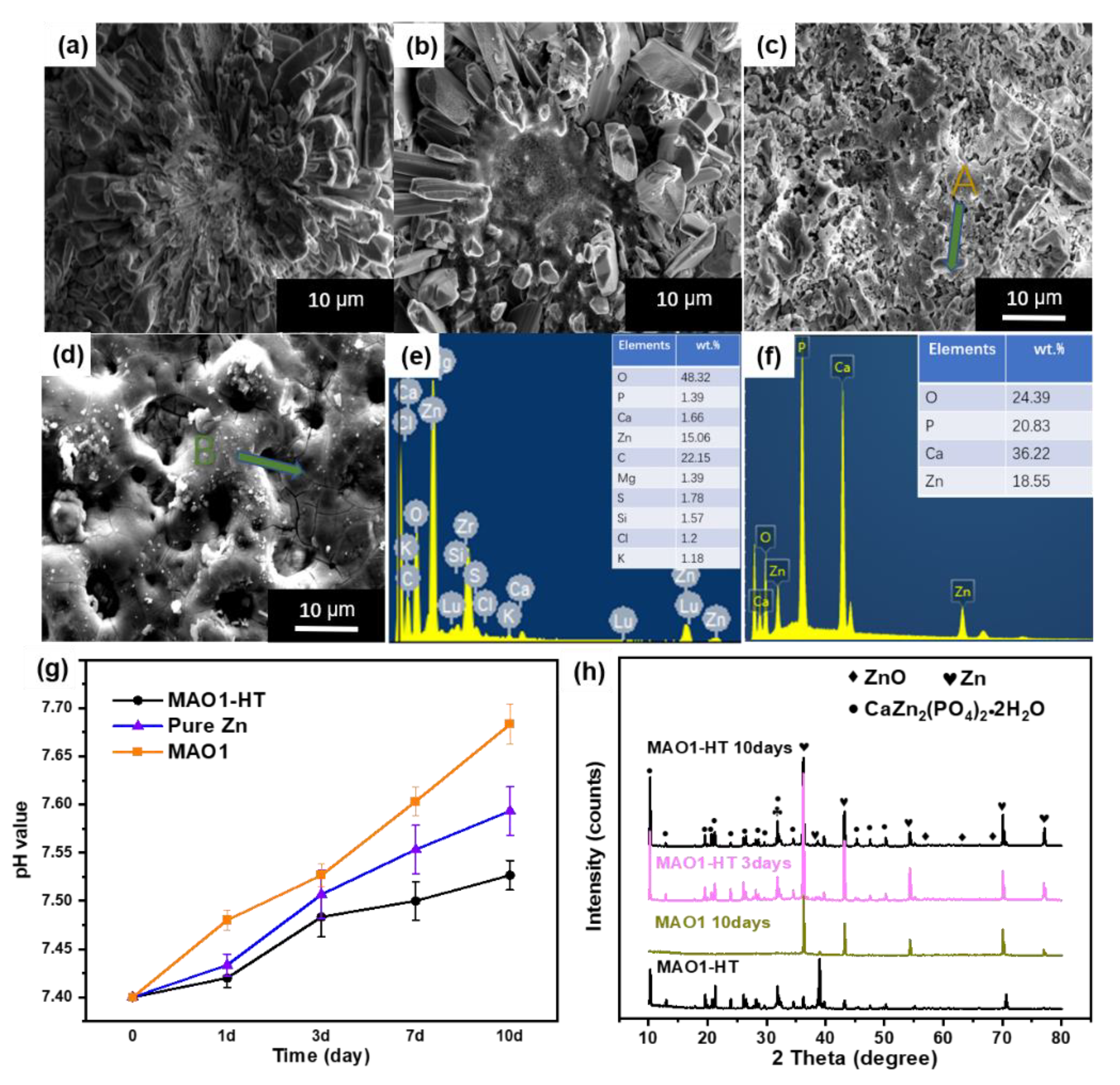
3.3. Immersion Tests
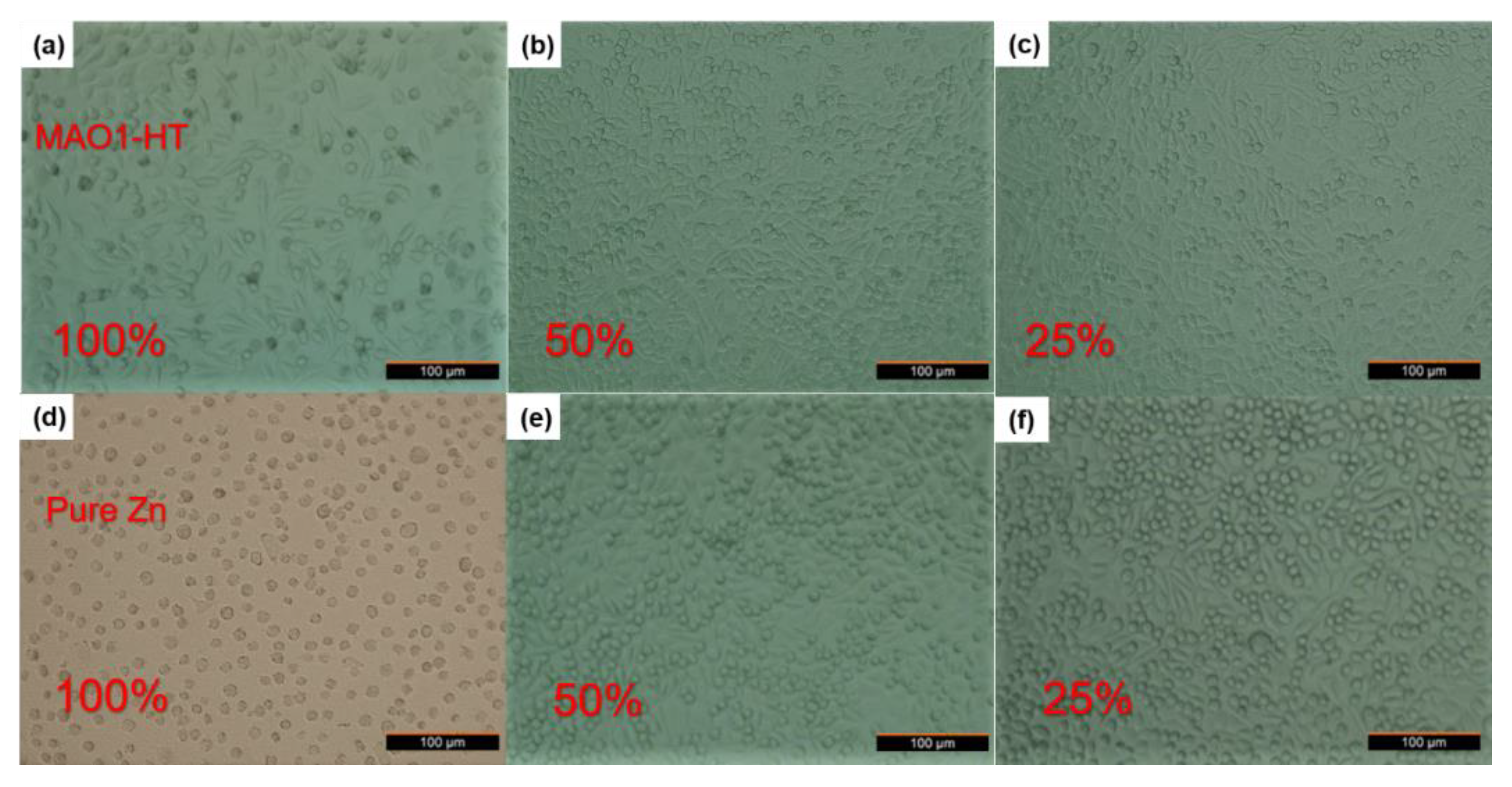
3.4. Cytotoxicity and Cell Morphology
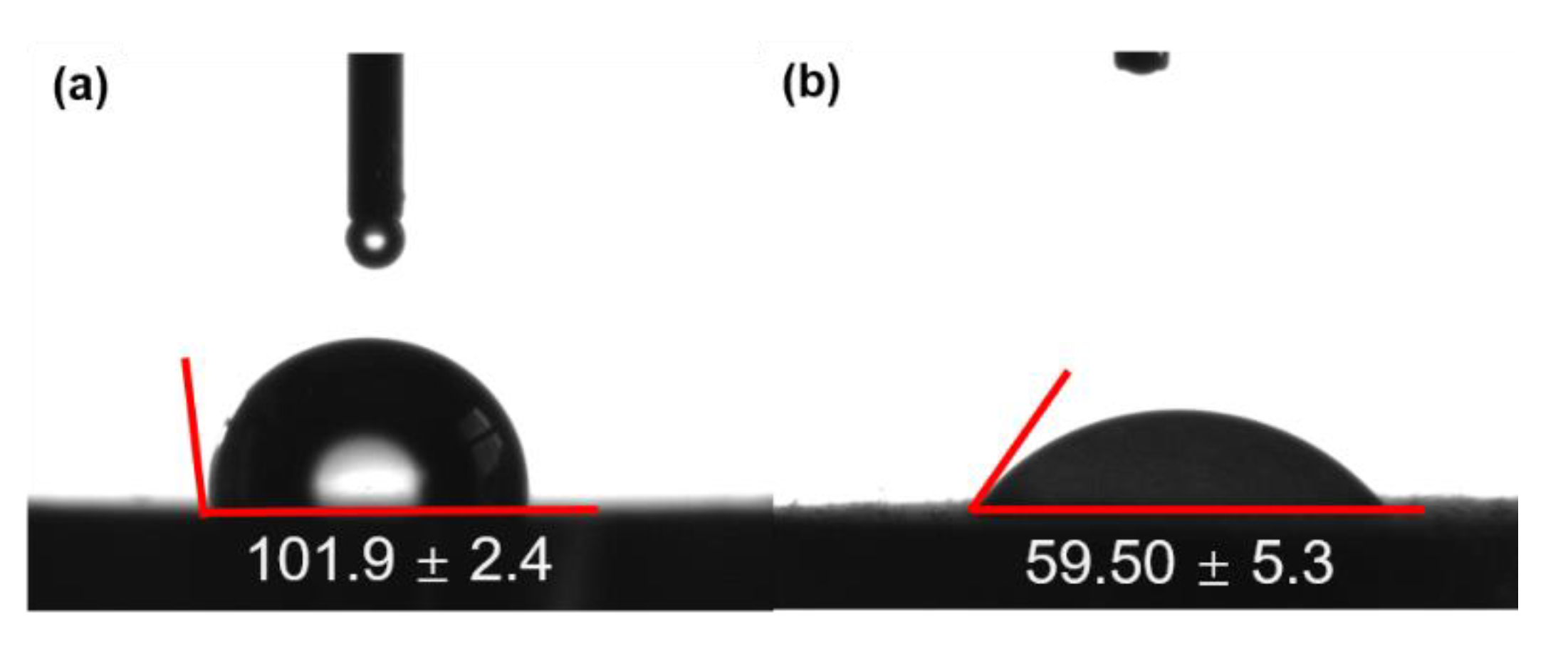
3.5. Wettability
4. Conclusions
- A dual-layer structure MAO1−HT coating consisting of ZnO and CaZnP processed under MAO and HT could be an available surface modification technique. Sufficient dense and rich CaZnP formed on the dual-layer MAO1−HT coating, which is a practical approach to adjust the degradation rate and improve biocompatibility.
- The MAO1 coating could accelerate the corrosion of Zn because of the crevice corrosion between the substrate and the coating. The corrosion performance confirmed by electrochemical measurements could be ranked as: MAO1−HT > pure Zn > MAO1.
- Cytotoxicity was detected in different extract groups, and each group revealed excellent cytocompatibility by MAO-HT treated Zn, whereas pure Zn in low concentration of extract. In addition, the wettability of MAO1−HT treated Zn with a contact angle of 59° also promoted cell adhesion compared with pure Zn with a contact angle of 101°.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys a review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Fischerauer, S.; Treichler, S.; Martinelli, E.; Eichler, J.; Myrissa, A.; Weinberg, A.M. The influence of biodegradable magnesium implants on the growth plate. Acta Biomater. 2018, 66, 109–117. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, H.; Sun, J. Long-term in vivo evolution of high-purity Mg screw degradation—Local and systemic effects of Mg degradation products. Acta Biomater. 2018, 71, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Tew, K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef]
- Zhu, D.; Su, Y.; Young, M.L.; Ma, J.; Zheng, Y.; Tang, L. Biological responses and mechanisms of human bone marrow mesenchymal stem cells to zn and mg biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 27453–27461. [Google Scholar] [CrossRef]
- Lin, J.; Tong, X.; Sun, Q.; Luan, Y.; Zhang, D.; Shi, Z.; Wen, C. Biodegradable ternary Zn-3Ge-0.5X (X=Cu, Mg, and Fe) alloys for orthopedic applications. Acta Biomater. 2020, 115, 432–446. [Google Scholar] [CrossRef]
- Li, H.F.; Xie, X.H.; Zheng, Y.F.; Cong, Y.; Zhou, F.Y.; Qiu, K.J.; Qin, L. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015, 5, 10719. [Google Scholar] [CrossRef]
- Cashman, K.D. Milk minerals (including trace elements) and bone health. Int. Dairy J. 2006, 16, 1389–1398. [Google Scholar] [CrossRef]
- Kaur, K.; Gupta, R.; Saraf, S.A.; Saraf, S.K. Zinc: The metal of life. Compr. Rev. Food Sci. F 2014, 13, 358–376. [Google Scholar] [CrossRef]
- Jaiswal, S.; McHale, P.; Duffy, B. Preparation and rapid analysis of antibacterial silver, copper and zinc doped sol–gel surfaces. Colloids Surf. B Biointerfaces 2012, 94, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Wang, X.; Shao, X.; Dai, T.; Xu, F.; Zhou, J.G.; Qu, G.; Liu, Y. In vivo study of the efficacy, biosafety, and degradation of a zinc alloy osteosynthesis system. Acta Biomater. 2019, 92, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, K.; Gao, J.; Yang, Y.; Qin, Y.X.; Zheng, Y.; Zhu, D. Enhanced cytocompatibility and antibacterial property of zinc phosphate coating on biodegradable zinc materials. Acta Biomater. 2019, 98, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Kazuyuki, Y.; Yamamoto, O.; Fukuda, M.; Koyota, S.; Koizumi, Y.; Sugiyama, T. In vitro prominent bone regeneration by release zinc ion from Zn-modified implant. Biochem. Biophys. Res. Commun. 2011, 412, 273–278. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Shi, Z.Z.; Gao, X.X.; Liu, X.F.; Wang, J.Q.; Wang, L.N. Adjusting comprehensive properties of biodegradable Zn-Mn alloy through solution heat-treatment. Mater. Today Commun. 2020, 23, 101150. [Google Scholar] [CrossRef]
- Mo, X.; Qian, J.; Chen, Y.; Zhang, W.; Xian, P.; Tang, S.; Wan, G. Corrosion and degradation decelerating alendronate embedded zinc phosphate hybrid coating on biodegradable Zn biomaterials. Corros. Sci. 2021, 184, 109398. [Google Scholar] [CrossRef]
- Yang, H.; Qu, X.; Lin, W.; Wang, C.; Zhu, D.; Dai, K.; Zheng, Y. In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 2018, 71, 200–214. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Ebrahimi, S.; Yarmand, B. In-vitro corrosion and bioactivity behavior of tailored calcium phosphate-containing zinc oxide coating prepared by plasma electrolytic oxidation. Corros. Sci. 2020, 173, 108781. [Google Scholar] [CrossRef]
- Su, Y.; Yang, H.; Gao, J.; Qin, Y.X.; Zheng, Y.; Zhu, D. Interfacial zinc phosphate is the key to controlling biocompatibility of metallic zinc implants. Adv. Sci. 2019, 6, 1900112. [Google Scholar] [CrossRef]
- Shi, Y.; Qi, M.; Chen, Y.; Shi, P. MAO-DCPD composite coating on Mg alloy for degradable implant applications. Mater. Lett. 2011, 65, 2201–2204. [Google Scholar] [CrossRef]
- Sun, J.; Wang, G. Preparation and corrosion resistance of cerium conversion coatings on AZ91D magnesium alloy by a cathodic electrochemical treatment. Surf. Coat. Technol. 2014, 254, 42–48. [Google Scholar] [CrossRef]
- Sealy, M.P.; Guo, Y.B. Surface integrity and process mechanics of laser shock peening of novel biodegradable magnesium–calcium (Mg–Ca) alloy. J. Mech. Behav. Biomed. Mater. 2010, 3, 488–496. [Google Scholar] [CrossRef]
- Gu, X.N.; Zheng, W.; Cheng, Y.; Zheng, Y.F. A study on alkaline heat treated Mg–Ca alloy for the control of the biocorrosion rate. Acta Biomater. 2009, 5, 2790–2799. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.O.; Northwood, D.O.; Nie, X. The effect of processing parameters and substrate composition on the corrosion resistance of plasma electrolytic oxidation (PEO) coated magnesium alloys. Surf. Coat. Technol. 2013, 237, 357–368. [Google Scholar] [CrossRef]
- Rojaee, R.; Fathi, M.; Raeissi, K. Controlling the degradation rate of AZ91 magnesium alloy via sol–gel derived nanostructured hydroxyapatite coating. Mater. Sci. Eng. C 2013, 33, 3817–3825. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Vashaee, D.; Tayebi, L. In vitro study of nanostructured diopside coating on Mg alloy orthopedic implants. Mater. Sci. Eng. C 2014, 41, 168–177. [Google Scholar] [CrossRef]
- Hou, Y. Study of ZA43 Alloy Micro-Arc Oxidation; Jiangsu University: Zhenjiang, China, 2005. [Google Scholar]
- Zhou, R.; Wei, D.; Cao, J.; Feng, W.; Cheng, S.; Du, Q.; Li, B.; Wang, Y.; Jia, D.; Zhou, Y. Conformal coating containing Ca, P, Si and Na with double-level porous surface structure on titanium formed by a three-step microarc oxidation. RSC Adv. 2015, 5, 28908–28920. [Google Scholar] [CrossRef]
- Kaseem, M.; Choe, H.C. Triggering the hydroxyapatite deposition on the surface of PEO-coated Ti–6Al–4V alloy via the dual incorporation of Zn and Mg ions. J. Alloy. Compd. 2020, 819, 153038. [Google Scholar] [CrossRef]
- Yuan, W.; Li, B.; Chen, D.; Zhu, D.; Han, Y.; Zheng, Y. Formation mechanism, corrosion behavior, and cytocompatibility of microarc oxidation coating on absorbable high-purity zinc. ACS Biomater. Sci. Eng. 2019, 5, 487–489. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef]
- Du, Q.; Wei, D.; Wang, S.; Cheng, S.; Wang, Y.; Li, B.; Jia, D.; Zhou, Y. Rapidly formation of the highly bioactive surface with hydroxyapatite crystals on the titania micro arc oxidation coating by microwave hydrothermal treatment. Appl. Surf. Sci. 2019, 487, 708–718. [Google Scholar] [CrossRef]
- Onoki, T.; Yamamoto, S.Y.; Onodera, H.; Nakahira, A. New technique for bonding hydroxyapatite ceramics and magnesium alloy by hydrothermal hot-pressing method. Mater. Sci. Eng. C 2011, 31, 499–502. [Google Scholar] [CrossRef]
- Sun, J.; Cai, S.; Wei, J.; Ling, R.; Liu, J.; Xu, G. Long-term corrosion resistance and fast mineralization behavior of micro-nano hydroxyapatite coated magnesium alloy in vitro. Ceram. Int. 2020, 46, 824–832. [Google Scholar] [CrossRef]
- Yang, Q.; Troczynski, T.; Liu, D.-M. Influence of apatite seeds on the synthesis of calcium phosphate cement. Biomaterials 2020, 23, 2751–2760. [Google Scholar] [CrossRef]
- Kroese-Deutman, H.C.; Wolke, J.G.C.; Spauwen, P.H.M.; Jansen, J.A. Closing capacity of cranial bone defects using porous calcium phosphate cement implants in a rabbit animal model. J. Biomed. Mater. Res. Part A 2006, 79, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Kizalaite, A.; Grigoraviciute-Puroniene, I.; Asuigui, D.R.C.; Stoll, S.L.; Cho, S.H.; Sekino, T.; Kareiva, A.; Zarkov, A. Dissolution–Precipitation Synthesis and Characterization of Zinc Whitlockite with Variable Metal Content. ACS Biomater. Sci. Eng. 2021, 7, 3586–3593. [Google Scholar] [CrossRef] [PubMed]
- Raja, F.N.; Worthington, T.; Isaacs, M.A.; Rana, K.S.; Martin, R.A. The antimicrobial efficacy of zinc doped phosphate-based glass for treating catheter associated urinary tract infections. Mater. Sci. Eng. C 2019, 103, 109868. [Google Scholar] [CrossRef]
- Cama, G.; Nkhwa, S.; Gharibi, B.; Lagazzo, A.; Cabella, R.; Carbone, C.; Dubruel, P.; Haugen, H.; di Silvio, L.; Deb, S. The role of new zinc incorporated monetite cements on osteogenic differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C 2017, 78, 485–494. [Google Scholar] [CrossRef] [Green Version]
- Boanini, E.; Silingardi, F.; Gazzano, M.; Bigi, A. Synthesis and Hydrolysis of Brushite (DCPD): The Role of Ionic Substitution. Cryst. Growth Des. 2021, 21, 1689–1697. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- FAN, X.; Jian, C.; Zou, J.P.; Qian, W.; Zhou, Z.C.; Ruan, J.M. Bone-like apatite formation on HA/316L stainless steel composite surface in simulated body fluid. Trans. Nonferrous Met. Soc. China 2009, 19, 347–352. [Google Scholar] [CrossRef]
- Narayanan, T.S.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Mardziah, C.M.; Ramesh, S.; Wahid, M.A.; Chandran, H.; Sidhu, A.; Krishnasamy, S.; Purbolaksono, J. Effect of zinc ions on the structural characteristics of hydroxyapatite bioceramics. Ceram. Int. 2020, 46, 13945–13952. [Google Scholar] [CrossRef]
- Canham, L.T.; Reeves, C.L.; Loni, A.; Houlton, M.R.; Newey, J.P.; Simons, A.J.; Cox, T.I. Calcium phosphate nucleation on porous silicon: Factors influencing kinetics in acellular simulated body fluids. Thin Solid Film. 1997, 297, 304–307. [Google Scholar] [CrossRef]
- Bigi, A.; Foresti, E.; Gandolfi, M.; Gazzano, M.; Roveri, N. Isomorphous substitutions in β-tricalcium phosphate: The different effects of zinc and strontium. J. Inorg. Biochem. 1997, 66, 259–265. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Dulski, M.; Dudek, K.; Grelowski, M.; Kubacki, J.; Hertlein, J.; Wojtyniak, M.; Goryczka, T. Impact of annealing on features of BCP coating on NiTi shape memory alloy: Preparation and physicochemical characterization. Appl. Surf. Sci. 2018, 437, 28–40. [Google Scholar] [CrossRef]
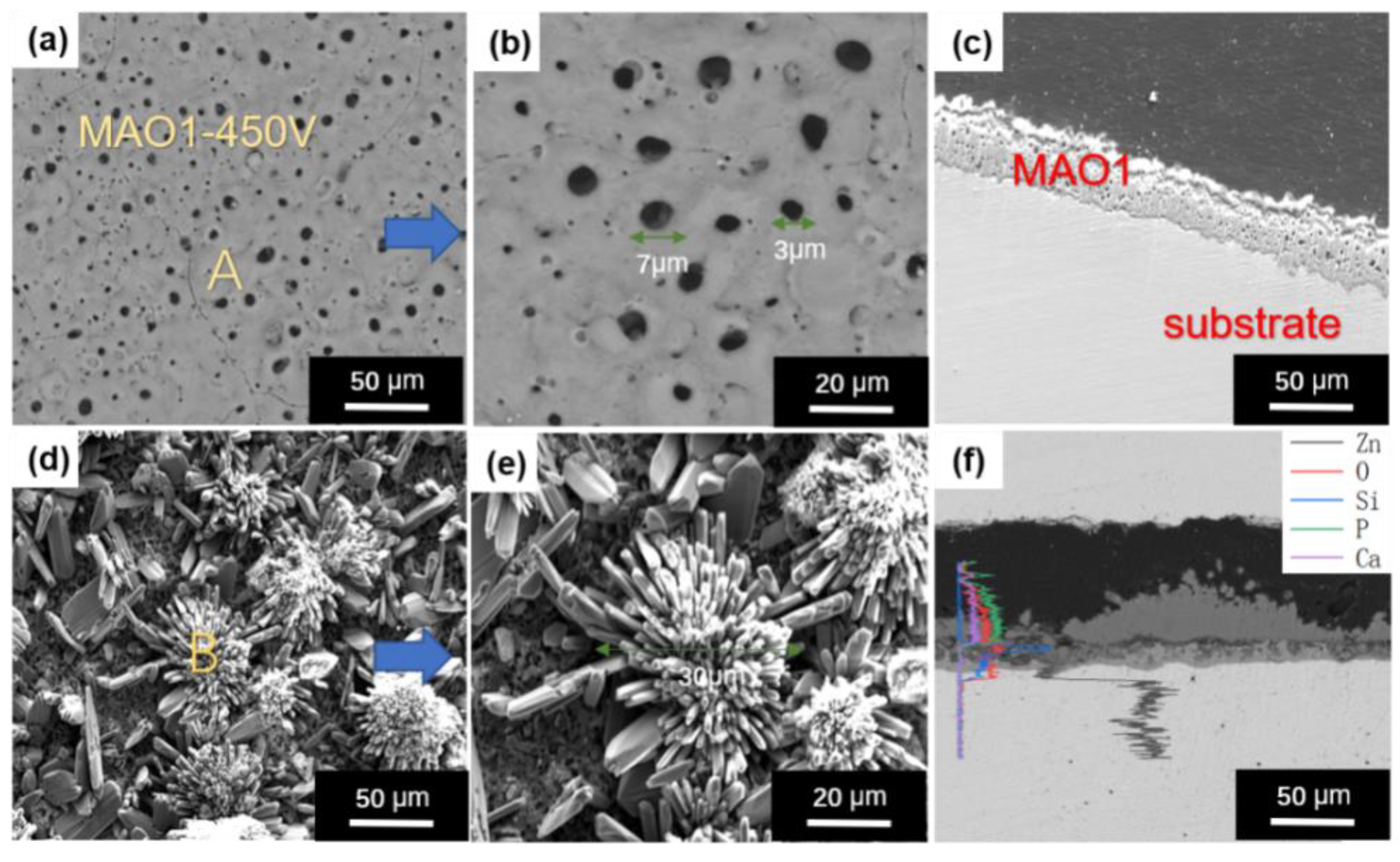
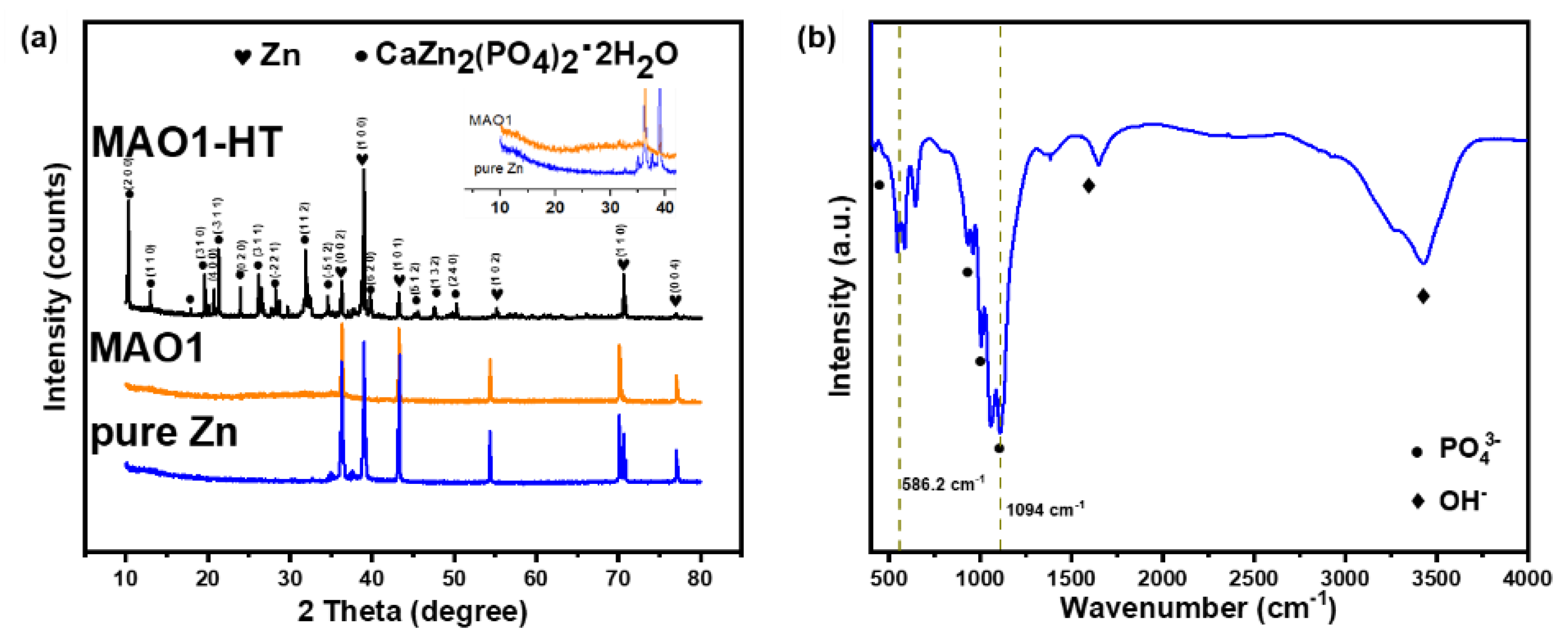
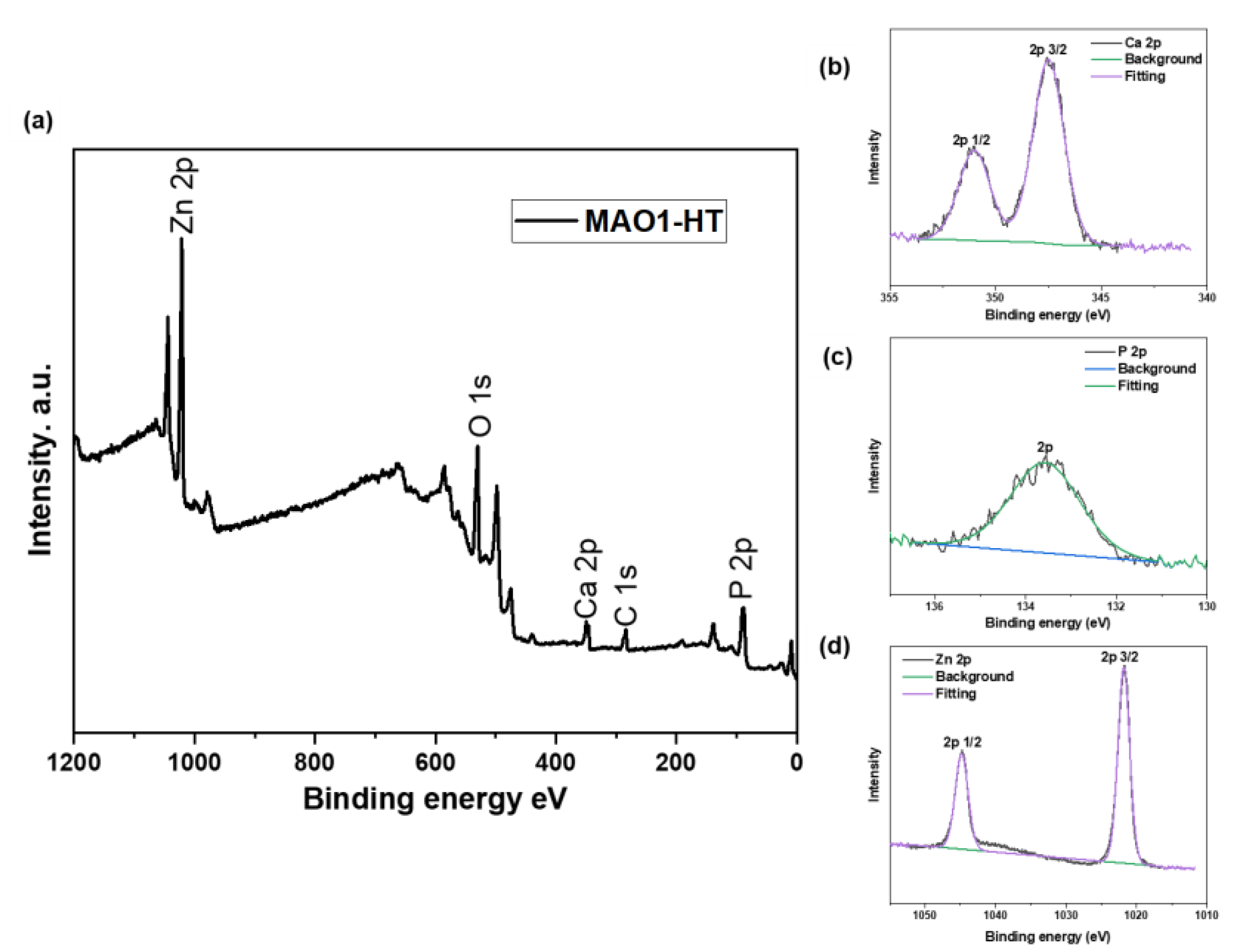


| Element | wt.% | |
|---|---|---|
| MAO1 | MAO1−HT | |
| O | 23.19 | 34.84 |
| Zn | 55.54 | 6.8 |
| P | / | 23.99 |
| Ca | / | 34.13 |
| Si | 21.27 | 0.24 |
| Sample | icorr (µA·cm−2) | Tafel Slopes (V/dec) | Ecorr (V) | Rp (kΩ) | Corrosion Rate (mm·Year−1) |
|---|---|---|---|---|---|
| Pure Zn | 17.0 | 0.11 (bc), 0.13 (ba) | −1.17 | 1.52 | 0.518 |
| MAO1 | 30.1 | 0.10 (bc), 0.24 (ba) | −1.34 | 1.02 | 0.897 |
| MAO1−HT | 16.3 | 0.08 (bc), 0.23 (ba) | −1.31 | 1.61 | 0.491 |
| Sample | Q1 (10−6·Ω−1·cm−2·s) | n | R1 (Ω·cm−2) | Qdl (10−4·Ω−1·cm−2·s) | Rct (Ω·cm−2) |
|---|---|---|---|---|---|
| Pure Zn | 5.115 | 0.85 | 2710 | 5.5 | 4.7 × 103 |
| MAO1 | 7.96 | 0.61 | 232 | 9.025 | 1.4 × 103 |
| MAO1−HT | 4.72 | 0.59 | 346 | 2.68 | 8.3 × 103 |
| Element | Concentration (mg/L) | |
|---|---|---|
| Pure Zn | MAO1−HT | |
| Zn | 4.947 | 5.342 |
| Ca | 63.12 | 107.24 |
| P | 28.97 | 58.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Yang, L.; Wang, L.; Zhang, Q.; Zhu, X.; Sun, W.; Shen, J.; Lu, T.; Song, Z.; Liu, H. Corrosion and Biocompatibility of Pure Zn with a Micro-Arc-Oxidized Layer Coated with Calcium Phosphate. Coatings 2021, 11, 1425. https://doi.org/10.3390/coatings11111425
Shi Y, Yang L, Wang L, Zhang Q, Zhu X, Sun W, Shen J, Lu T, Song Z, Liu H. Corrosion and Biocompatibility of Pure Zn with a Micro-Arc-Oxidized Layer Coated with Calcium Phosphate. Coatings. 2021; 11(11):1425. https://doi.org/10.3390/coatings11111425
Chicago/Turabian StyleShi, Yixuan, Lijing Yang, Lucai Wang, Qingke Zhang, Xinglong Zhu, Wensheng Sun, Jianwei Shen, Ting Lu, Zhenlun Song, and Huinan Liu. 2021. "Corrosion and Biocompatibility of Pure Zn with a Micro-Arc-Oxidized Layer Coated with Calcium Phosphate" Coatings 11, no. 11: 1425. https://doi.org/10.3390/coatings11111425







