Abstract
A dye-sensitized solar cell (DSC) is the third generation of solar technology, utilizing TiO2 nanoparticles with sizes of 20–30 nm as the photoelectrode material. The integration of smaller nanoparticles has the advantage of providing a larger surface area, yet the presence of grain boundaries is inevitable, resulting in a higher probability of electron trapping. This study reports on the improvement of charge transport through the integration of quantum dot (QD) TiO2 with a size of less than 10 nm as the dye absorption photoelectrode layer. The QD TiO2 samples were synthesized through sol–gel and reflux methods in a controlled pH solution without surfactants. The synthesized samples were analyzed using microscopic, diffraction, absorption, as well as spectroscopic analyses. A current–voltage and impedance analysis was used to evaluate the performance of a DSC integrated with synthesized TiO2 as the photoelectrode material. The sample with smaller crystallite structures led to a large surface area and exhibited a higher dye absorption capability. Interestingly, a DSC integrated with QD TiO2 showed a higher steady-state electron density and a lower electron recombination rate. The shallow distribution of the trap state led to an improvement of the electron trapping/de-trapping process between the Fermi level and the conduction band of oxide photoelectrode material, hence improving the lifetime of generated electrons and the overall performance of the DSC.
1. Introduction
A dye-sensitized solar cell (DSC) is the third generation of photovoltaic cells that has a similar mechanism to photosynthesis in nature, with efficient performance under low-light conditions [1,2]. Unlike Si PV, the effective charge separation of a DSC allows for better performance in diffused sunlight, allowing for easy integration in the facade of buildings and indoor applications such as glass walls, sunblinds, awnings, roofing, self-powered Internet of Things (IoT) devices, and wireless sensor networks [3,4]. The structure of a DSC consists of three parts, namely, a dye-sensitized photoanode, a redox couple electrolyte, and a catalytic conductive substrate [5]. In the presence of sunlight, photons strike the dyes with enough energy, producing dye-excited states, resulting in the injection of electrons into the conduction band of the photoelectrode TiO2. The original dye state was restored subsequently by electron donation of the redox electrolyte [6,7,8,9,10]. In actual conditions, the generated electrons could travel in the reverse direction and recombine with the oxidized dyes before the regeneration process occurs (Figure 1). The dynamic competition between the generation and recombination of the electrons is the bottleneck restricting the development of a DSC with a higher performance efficiency [11,12,13].
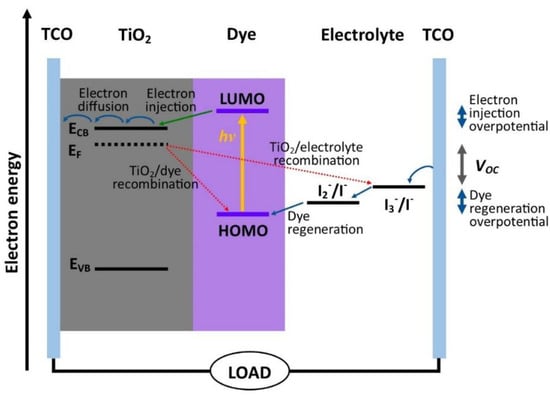
Figure 1.
Schematic energy diagram and working mechanism of a DSC.
The porous nanostructure photoelectrode material, typically TiO2, is the heart of a DSC. The photoelectrode is responsible for providing a large surface area for dye sensitizers, acting as an electron acceptor and an electronic conductor [14]. An extensive simulation study by Usami (1997) concluded that the optimal configuration of a photoelectrode consists of three parts, namely, a thin rutile blocking and passivation layer, a dye absorption layer, and a top scattering layer [15]. The rutile layer deposited on the conductive substrate acts as a blocking layer by avoiding direct contact of the conductive substrate with the redox electrolyte and, at the same time, improving the adhesion of the photoelectrode film to the conductive substrate. The dye absorption layer is the middle layer in between the thin rutile layer and the scattering layer. It provides sufficient surface area to anchor the dye sensitizer molecules for the absorption of light [16]. Meanwhile, the top scattering layer helps to improve the optical path length of incident light within the photoelectrode film. The layer is made up of either micron-sized particles [17], a dual function micro–nano structure such as aggregates [18,19], multiscattering structures [20,21], one-dimensional (1D) nanostructures such as nanowires [22] or nanotubes [16], and so forth. The proposed structure allows for light confinement, thus improving light absorption through multiple scattering and the total reflection of the incident light [23].
The nanostructure TiO2 was synthesized through various routes, namely, sol–gel [24,25,26,27], hydrothermal [28,29,30,31], solvothermal [32,33,34,35], co-precipitation [36,37], polyol synthesis [38,39], and so forth. Amongst the reported methods, sol–gel has been the most explored and preferred method due to low-temperature processing procedures, ease of controlling the reaction parameters, and high homogeneity [40]. The sol–gel method produced polydispersed amorphous nanoparticles, which require further calcination to improve crystallinity. Moreover, it is very challenging to control the shape and size of the nanoparticles due to the high rate of the hydrolysis reaction, hence resulting in agglomeration [41]. Usually, a surfactant is added to control the hydrolysis rate, the shape, and the size of the nanoparticle [41,42,43]. The surfactant prevents agglomeration by reducing the solid–liquid interface energy, thus reducing collision and contact between nucleates [43]. Nevertheless, the presence of a surfactant leads to tedious washing procedures that reduce the active site of the nanomaterials [44,45].
In most conventional DSCs, the dye absorption layer consists of TiO2 nanoparticles with sizes of about 20 nm [8,16,17,18,19,22]. Theoretically, further reducing the size of nanoparticles gives an added advantage of a large surface area and contact points, allowing for a higher dye absorption [46]. However, a study conducted by Chou et al. (2007) has shown that the amount of absorbed dye reduces as the size of nanoparticles reduces from about 23 nm to 10 nm [47]. It is anticipated that the capability of nanoparticles to absorb dye molecules is influenced by the facets and the surface energy of the particles [47]. Moreover, smaller nanoparticles result in higher grain boundaries, increasing the probability of electron trapping, hence affecting the performance of a DSC [5,46,47,48]. On the other hand, a study reported by Tang et al. (2009) has shown that, despite having low dye-absorption, a low-crystalline film exhibits a comparable performance with a high-crystalline film, provided the presence of a well mesoporous inner structure [49].
Here, we report on the improvement of charge transport through the integration of QD TiO2 with a size of less than 10 nm as the dye absorption photoelectrode layer. The QD TiO2 was successfully synthesized in a controlled pH solution at high water to the precursor molar ratio without surfactants. Most of the previously reported studies related to QD TiO2 were related to the implementation of it as a thin passivation layer [50] or the composite of TiO2 nanoparticles with QD materials such as graphene [51,52,53] and cadmium selenide [54,55]. However, in this study, the QD TiO2 was utilized as the photoelectrode dye absorbing layer. To the best of the authors’ knowledge, there is a limited number of reports focusing on the utilization of QD TiO2 as the photoelectrode dye absorbing layer. The higher grain boundaries of QD TiO2 increase the transport resistance of generated electrons [5,46,47,48]. Interestingly, the synthesized QD TiO2 reported in this study exhibits a higher dye absorption and a slower rate of electron recombination, thus improving the overall performance efficiency of the DSC despite the presence of smaller nanoparticles of less than 10 nm compared with the commercially available TiO2 nanoparticles.
2. Materials and Methods
2.1. Synthesis of Crystalline QD TiO2
A TiO2 nanoparticles sample was synthesized through the sol–gel reflux method using titanium isopropoxide (CAS No. 546-68-9, MERCK, Selangor, Malaysia) as a precursor. In a typical procedure, a pH 2 water solution was first prepared using deionized water and glacial acetic acid (CH3COOH, CAS No. 64-19-7, MERCK, Selangor, Malaysia). Then, 0.2 M titanium isopropoxide in an absolute ethanol solution was added dropwise under vigorous stirring. The molar ratio of the acidic water to the titanium isopropoxide was fixed at 110:1. The solution refluxed at 90 °C for 4 h. The precipitate was collected by evaporating the solution using a rotary evaporator at 80 °C with a speed of 50 rpm. Then, the sample was dried in a convection oven at 90 °C overnight.
2.2. Characterization of Crystalline QD TiO2
A high-resolution transmission electron microscope (HRTEM, TECNAI F20 X-Twin, Hillsboro, Oregon, United States) was used to evaluate the particle size and morphology of the synthesized TiO2. Surface area and porosity analyzers (TriStar II 3020, Micromeritics, Norcross, Georgia, USA) were used to evaluate the surface area, pore size, and pore volume degassed at 300 °C for 3 h. The phase of the prepared photoelectrode film was evaluated using an X-ray diffraction analysis (XRD, Empyrean, PANalytical, Malvern, UK) operated at 45 kV using a Ni-filtered Cu Kα radiation (λ = 1.54 Å) with a step scan size of 0.01° and an omega of 2 in the range of 2θ of 10° to 80°. An ultraviolet-visible spectroscopy (UV-Vis, Cary 100, Agilent, Santa Clara, CA, USA) was used to evaluate the dye absorption capability of the printed photoelectrode films in the wavelength range of 200 nm to 800 nm.
2.3. Fabrication and Performance Analysis of DSC
The pastes were prepared based on a previously reported study [56,57]. An amount of 6 g of the synthesized QD TiO2 sample was converted into a paste by mixing it with 20 mL of ethanol as the solvent, 1 mL of acetic acid and 5 mL of deionized water as the dispersion agent, 3 g of ethyl cellulose in 30 g of ethanol as the binder, and 20 g of terpineol as the rheology agent. A control sample was prepared using commercially available TiO2 nanoparticles (CAS No. 1317-70-0, MERCK, Selangor, Malaysia). The paste was then printed on a fluorine tin oxide (FTO)-coated glass substrate and heat-treated in a six-zone belt furnace with the temperatures of 300 °C, 510 °C, 505 °C, 350 °C, 270 °C, and 180 °C for the consecutive zones with a conveyor speed of 140 mm/min. The photoelectrode films were soaked overnight in 0.254 mM of di-tetrabutylammonium cis-bis(isothiocyanate)bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium (II) (N719) dye at room temperature. The dye coated photoelectrode films were assembled in a sandwich layer with a platinum (Pt) counter electrode and sealed with a hot-melt thermoplastic gasket. An iodide/triiodide (high-stability electrolyte, EL-HSE) as redox electrolyte was then injected through a pre-drilled hole on the counter electrode side. The redox electrolyte was composed of a mixture of 1-propyl-3-methyl-imidazolium iodide, 4-tert-butyl pyridine, lithium iodide, guanidinium thiocyanate, and iodine in acetonitrile as a solvent. The hole was sealed using aluminium backed bynel. The N719 dye, platinum paste, electrolyte and aluminium backed bynel were sourced from Greatcell Solar, Queanbeyan, Australia.
The performance of the fabricated DSCs was verified using a universal photovoltaic test system (UPTS, Greatcell Solar, Queanbeyan, Australia) at 100 mW/cm2 of simulated light of air mass at a 1.5 (AM-1.5) radiation angle under variable load. The system was integrated with a solar simulator (Greatcell Solar, Queanbeyan, Australia), a Keithley 2420 source meter (Tektronix, Beaverton, OR, USA), and a current–voltage (I–V) testing software (I-V Software, Version 9, 2015, Michael Kelzenberg, Pasadena, CA, USA). The results were taken based on the average value of at least five samples per batch and repeated for three batches. The external quantum efficiencies (EQEs) of the DSCs were obtained using an incident-photon-to-electron conversion efficiency measurement system (IPCE, Greatcell Solar, Queanbeya, Australia) with a 300 W xenon lamp. The analyzed active areas were about 0.25 cm2. An electrochemical impedance spectroscopy (EIS) analysis was carried out in the dark at various forwards bias voltages in the 300 kHz to 0.1 Hz of frequency range and an alternating current (AC) modulation signal of 10 mV using PCI4-300 Potentiostat/Galvanostat/ZRA (Gamry Instruments, Warminster, PA, USA). Echem Analyst (Version 7.8.2, Gamry Instruments, Warminster, Pennsylvania, USA) software was used to fit the frequency-dependent impedance and the performance compared with commercial TiO2-based DSCs.
3. Results
The morphology of the synthesized QD TiO2 and commercial TiO2 nanoparticles was analyzed using HRTEM, and the images are shown in Figure 2. The HRTEM analysis shows that the synthesized QD TiO2 (Figure 2a,b) is in the form of irregular shapes with a size of about 5 nm. Meanwhile, the commercial sample (Figure 2c,d) was in the form of nanoparticles with a size of about 30 nm. At higher magnifications, the samples clearly show the interlayer spacing of approximately 0.358 nm and 0.383 nm for the synthesized QD TiO2 and commercial TiO2, respectively, corresponding to a lattice plane of the TiO2 anatase phase. Figure 2a,b prove that the synthesized QD TiO2 sample is free of submicron-size aggregates. The presence of a large molar ratio of water to the precursor in a controlled pH solution controlled the hydrolysis rate, leading to the separation of the nucleation and the growth stage, thus ensuring a complete hydrolysis process and preventing the formation of submicron-size aggregates.

Figure 2.
HRTEM images of the synthesized QD TiO2 at (a) 88 KX and (b) 530 KX, and commercial TiO2 nanoparticles at (c) 88 KX and (d) 360 KX.
An analysis using a surface area and pore size analyser shows that both the synthesized QD TiO2 and the commercial TiO2 nanoparticles exhibit type IV adsorption–desorption isotherms (Figure 3a). The inflection of the volume of nitrogen adsorbed at high relative pressure, P/P°, corresponds to the presence of the mesoporous structure. Based on the Brunauer–Emmett–Teller and Barrett–Joyner–Halenda (BET–BJH) analysis, the synthesized QD TiO2 exhibits a surface area of 152.41 m2/g, a pore size of about 6 nm, and a pore volume of 0.23 cm3/g. Meanwhile, the commercial TiO2 nanoparticles sample exhibits a surface area of 53.36 m2/g, a pore size of 20.24 nm, and a pore volume of 0.27 cm3/g. Based on the plot of pore diameter (Figure 3b), the synthesized sample exhibits a narrow pore volume distribution that indicates a well-dispersed QD TiO2. The successful synthesis of dispersed QD TiO2 without utilizing surfactant was contributed by the presence of a large molar ratio of water to the precursor in a controlled pH environment, leading to the separation of the nucleation and growth stages, thus ensuring a complete hydrolysis process. On the other hand, the commercial TiO2 nanoparticle sample exhibits a region with larger pores of more than 50 nm due to the submicron size agglomerations. The large size of the pore diameters corresponds to the agglomerated particles emphasized in the red circle of Figure 2c while the pore distribution below 25 nm represents the porous structure of the nanoparticle.
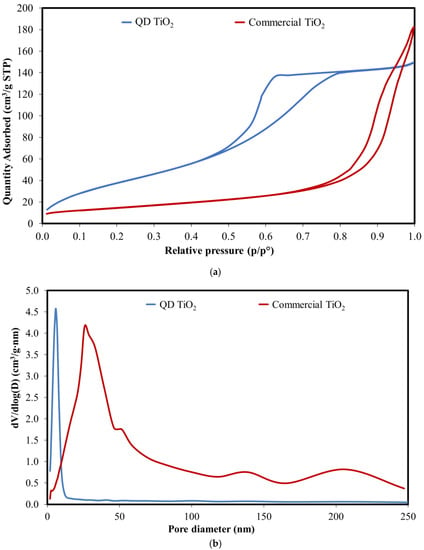
Figure 3.
The (a) absorption–desorption isotherm and (b) pore size distribution of the synthesized QD TiO2 and commercial TiO2 nanoparticles.
The prepared photoelectrode films were tested using an XRD analysis to evaluate the crystallinity and the phase presence. Figure 4 shows the XRD of the samples deposited on the FTO substrate. A bare FTO was also tested as a comparison. An XRD analysis confirms that both TiO2 samples were in the form of an anatase phase with peaks at 25.3°, 37.8°, 48.0°, 53.9°, 55.0°, 61.7°, 62.7°, 68.8°, 70.3°, and 75.1° corresponding to Miller indices of (101), (004), (200), (105), (211), (213), (204), (116), (220), and (215), respectively. The peaks at about 26.5°, 33. 7°, 37.8°, 51.5°, 61.6°, and 65.6° correspond to the tin oxide phase (PDF 00-046-1088); peaks at 31.2°, 51.5°, 61.6°, 65.6°, 70.9°, and 78.5° correspond to tin fluoride (PDF 01-085-2126); while peaks at about 26.5°, 42.4°, and 54.6° correspond to the silicon dioxide phase (PDF 01-089-8937). The presence of these phases is well matched with the compositions of the glass substrate coated with a thin layer of FTO. The crystallite size calculated using Scherer’s formula gave average crystal sizes of about 8.6 nm and 25 nm for the synthesized QD TiO2 and the commercial TiO2 nanoparticles, respectively. The results obtained were in good agreement with the size estimated from HRTEM analysis.
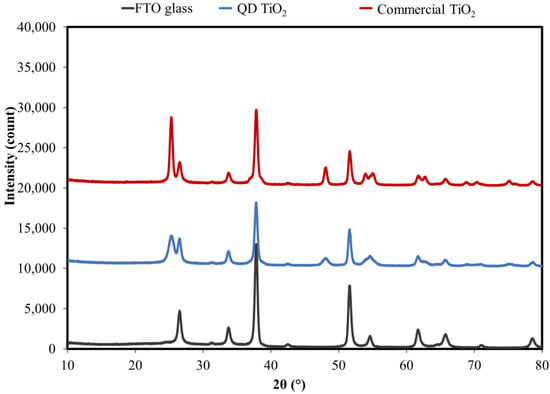
Figure 4.
The XRD of the QD TiO2 and commercial TiO2 samples printed on FTO substrates.
A UV-Vis analysis of the desorbed dye solution of the dyed photoelectrode film was tested to evaluate the capability of photoelectrode film to anchor dye molecules. Figure 5 shows the absorbance spectrum of the N719 solution desorbed using diluted ammonium hydroxide for the synthesized and commercial TiO2 nanoparticles. Based on a UV-Vis analysis, both samples exhibit typical absorbance spectra of N719 dye solutions with three absorbance peaks at about 310 nm, 370 nm, and 510 nm, attributed to the ligand-centered charge transfer (LCCT) transitions and the metal-to-ligand charge transfer (MTLC) transitions of the N719 dye molecules [58,59]. The analysis confirmed that the synthesized TiO2 nanoparticle sample shows higher absorbance for the entire wavelength region of 200–600 nm corresponding to the higher dye concentration in the diluted ammonium hydroxide. The higher amount of dye molecules increased the number of injected electrons into the conduction band of the photoelectrode network, thus increasing the value of the short-circuit current.
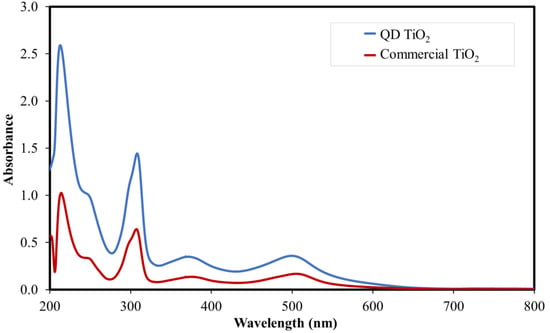
Figure 5.
Absorbance spectra of the desorbed dye solution of the QD TiO2 and commercial TiO2 nanoparticles.
The prepared photoelectrode film was then integrated into the DSC with a Pt counter electrode and iodide electrolyte. The fabricated DSC based on the synthesized nanoparticle sample was tested for I–V characteristic and the result was compared with the DSC based on the commercial TiO2 nanoparticle sample as the photoelectrode film. Figure 6 shows the I–V curve of the synthesized and commercial TiO2 nanoparticles tested under a 100 mW/cm2 intensity of an illuminated xenon lamp at an AM-1.5 radiation angle. Table 1 shows the photovoltaic properties, namely, short-circuit current density (Jsc), open-circuit voltage (Voc), fill factor (FF), and performance efficiency (η) of the fabricated DSC using the synthesized and commercial TiO2 nanoparticles.
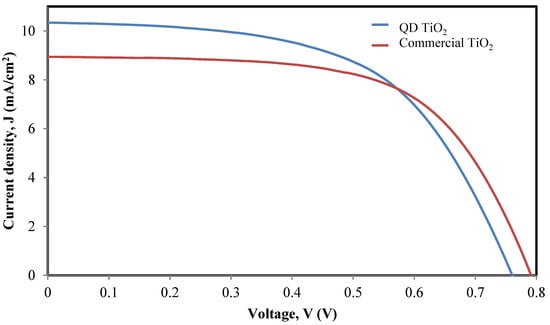
Figure 6.
J–V curve of the QD TiO2 and commercial TiO2 nanoparticle-based DSCs.

Table 1.
Photovoltaic properties of the QD TiO2 and commercial TiO2 nanoparticle-based DSCs.
The I–V analysis shows that the QD TiO2-based DSC exhibits higher Jsc, hence leading to a better performance efficiency of 4.44 ± 0.09%. The improvement of Jsc was related to the smaller size of nanoparticles, resulting in the availability of a large surface area for anchoring dye molecules. Increasing the number of anchored dye molecules increased the chances of harvesting the photon radiance, increasing the number of generated electrons and thus increasing the current density. The higher amount of dye molecules has increased the number of the injected electrons into the conduction band of the photoelectrode network, thus increasing the value of Jsc.
Table 1 compares the reported photovoltaic properties of typical DSCs composed of a TiO2 photoelectrode film, a N719 dye, and an iodide and tri-iodide redox electrolyte. Based on the tabulated data, the previous studies reported performance efficiencies in the range of 4.1% to 5.48%. However, the photovoltaic properties except for Voc of the fabricated DSC based on the synthesized QD TiO2 as the photoelectrode film are lower compared with those reported by Subalakshmi and Senthilselvan in 2018. This is due to the larger active area used to fabricate the DSC in this study. Increasing the active area prevents a smooth transport of electrons within the photoelectrode film due to the deepened trap state distribution, thus increasing the rate of electron recombination in the higher voltage region and reducing the charge collection efficiency [62].
Figure 7 shows the EQE of DSCs integrated with QD TiO2 and commercial TiO2 photoelectrode films. The photocurrent action spectra exhibit two peaks at wavelengths of about 335 nm and 530 nm. The small distribution of photocurrent peaks at the lower wavelength (~335 nm) corresponds to direct light harvesting by the TiO2 semiconductor with an energy of approximately 3.2 eV. A broader spectrum in the visible region with a maximum peak at about 530 nm was attributed to the absorption of N719 dye molecules. The result is in good agreement with the J–V analysis, where the QD TiO2 exhibits higher photocurrent efficiency, suggesting the enhancement of quantum efficiency compared with the commercial TiO2 based DSC. Moreover, the commercial TiO2 nanoparticles exhibit a broad spectrum in the wavelength range of 380 nm to 450 nm. The broad spectrum corresponds to the large, agglomerated particles, as evident in the FESEM analysis [12,63].
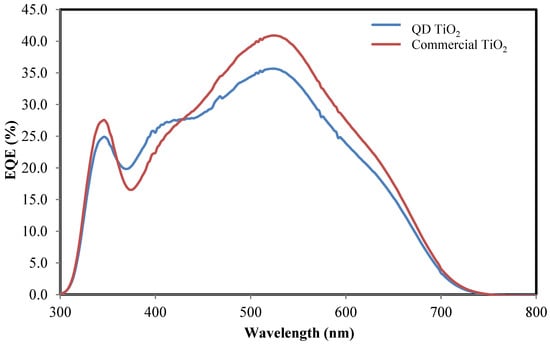
Figure 7.
IPCE analyses of the QD TiO2 and commercial TiO2 nanoparticle-based DSCs.
The QD TiO2 exhibits a lower value of Vocand FF compared with the commercial TiO2 nanoparticles. The variation in the Voc and FF values was related to the electron transport properties and the internal resistance of the cells. An EIS analysis was used to evaluate the electrochemical properties of the DSCs. Figure 8 shows the Nyquist, Bode, and phase plots of fabricated DSCs based on synthesized and commercial TiO2 nanoparticles. The data were fitted based on the equivalent circuit, as shown in the inset of Figure 8. The Rs of the equivalent circuit signifies the sheet resistance of the substrate, and the contact resistance between the substrate and photoelectrode film. The value was measured from the gap between the y-axis and the small semicircle of the Nyquist plot. The R1 and CPE1 signify the resistance and constant phase element of the charge transfer resistance at the counter electrode, represented by the small semicircle of the Nyquist plot at the high-frequency range. The large semicircle of the Nyquist plot represents the electron transport properties of the photoelectrode and electrolyte interface. A Bisquert open model was used to fit the data [64]. Their measured and calculated electrochemical properties, namely, transport resistance (Rt), recombination resistance (Rbr), chemical capacitance (Cμ), electron lifetime (τn), reaction rate constant for recombination (k), electrons diffusion coefficient (Dn), effective diffusion length (Ln), and steady-state electron density in the conduction band (ns) are tabulated in Table 2.
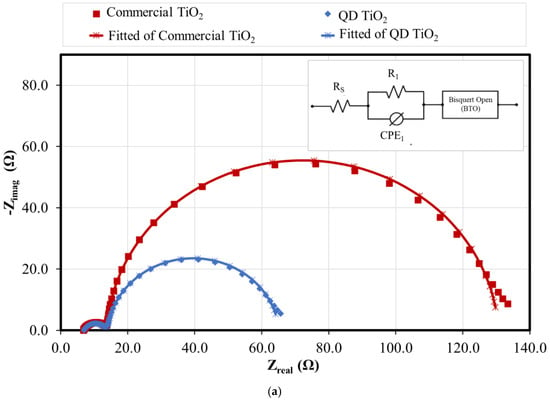
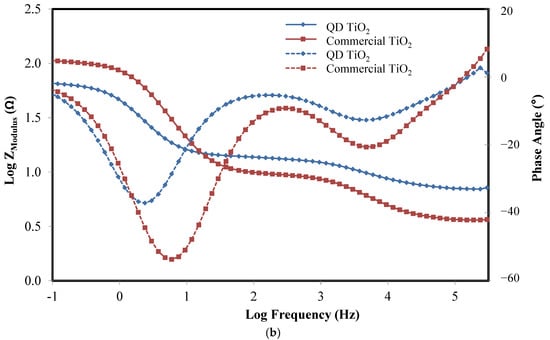
Figure 8.
(a) Nyquist plot, and (b) Bode and phase plot of the QD TiO2 and commercial TiO2 nanoparticle-based DSCs.

Table 2.
Electrochemical properties of the QD TiO2 and commercial TiO2 nanoparticle-based DSCs.
The EIS analysis reveals that the DSC fabricated with the synthesized QD TiO2 as the photoelectrode film exhibited higher transport resistance and lower electron recombination resistance compared with the commercial TiO2 nanoparticle sample. This was due to the presence of a smaller size of nanoparticles resulting in the presence of larger grain boundaries and structural defects, preventing the smooth transportation of electrons, and reducing the value of electron diffusion and electron diffusion length as tabulated in Table 2. This condition promotes the recombination of the injected electrons in the conduction band of TiO2 with the oxidized species of electrolyte and dyes instead of the injected electrons travelling to the external circuit. Even though the Rbr value of the QD TiO2 is smaller, the cell exhibits a slower k compared with the commercial TiO2-based DSC, thus leading to higher τn. Moreover, the fabricated cell based on the synthesized QD TiO2 sample also shows a higher value of steady-state electron density in the conduction band of TiO2 compared with the commercial TiO2-based DSC. The outcome can be explained by the larger surface area of the QD TiO2, which allows for a larger amount of dye to be anchored, thus increasing the number of generated electrons, which improves the number of injected electrons from the LUMO to the conduction band of TiO2.
The high ns value reflects the high Cµ value of the synthesized QD TiO2 compared with the commercial TiO2 nanoparticle sample. The Cµ resembles the change in the electron density over a small variation of chemical potential and expressed as in Equation (1):
where is the occupation factor of the Fermi–Dirac distribution function while gn(E) is the density of state, which is expressed as in Equation (2):
where kB is the Boltzmann constant; NL is the density of localized states; and To is a parameter with a temperature unit that determines the depth of the distribution below the lower edge of the conduction band, Ec. Based on these two equations (Equations (1) and (2)), an exponential distribution of chemical capacitance was derived and expressed as Equation (3) [65,66,67,68]:
where α is the measure of the depth of trap energy distribution, which equals T/To. The trap state distribution of the fabricated DSC was analyzed by conducting EIS analyses at different bias potentials (from 0.8–0.35 V). Figure 9 shows the measured chemical capacitance based on the EIS analyses of the cells integrated with synthesized QD TiO2 and commercial TiO2 nanoparticle samples.
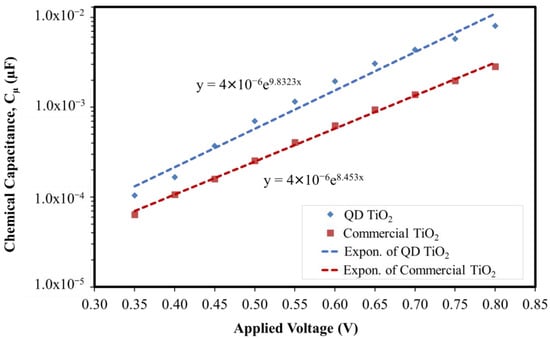
Figure 9.
The chemical capacitance of the QD TiO2 and commercial TiO2 nanoparticle-based DSCs obtained from the EIS analyses in the dark.
The plotted graph shows that the QD TiO2 exhibits an exponential trend with a steeper slope compared with the commercial TiO2 nanoparticle-based DSC. Based on the analysis, the α values of the QD TiO2 and the commercial TiO2 nanoparticle-based DSC were calculated to be about 0.253 and 0.217, respectively. A bigger α indicates a narrower distribution of the electronic state’s density, which helps with the electron trapping and de-trapping phenomena [65,67,69]. A shallow distribution of the trap state helps the trapped electrons at the Fermi level to be de-trapped back to the conduction band of the oxide photoelectrode material. This finding supports the results of a lower rate of electron recombination and higher steady-state electron density, thus leading to a longer lifetime of electrons and a higher value of produced current density.
4. Conclusions
The QD TiO2 was successfully synthesized through the sol–gel reflux method in a controlled pH of the water–acetic acid solution without surfactants. The large surface area brought about by the small QD structure results in an increase in the capability of the photoelectrode material to anchor large amounts of dye, leading to a higher steady-state electron density and improving the generation of electrons. Moreover, the fabricated cell also exhibits a slower electron recombination rate due to the shallow distribution of the trap state of the synthesized QD TiO2-based DSC. This characteristic helps the trapped electrons at the Fermi level be released back to the conduction band of oxide photoelectrode materials, thus improving the lifetime of generated electrons. However, the successful development of QD TiO2 potentially applied as the thin blocking layer of the photoelectrode configuration in which the titanium chloride treatment is replaced still faces the challenge of substrate corrosion.
Author Contributions
Conceptualization, S.N.A.Z. and N.M.M.; methodology, S.N.A.Z.; formal analysis, S.N.A.Z., M.K. and M.U.S.; data curation, S.N.A.Z., M.K. and M.U.S.; writing—original draft preparation, S.N.A.Z.; writing—review and editing, S.N.A.Z. and N.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universiti Teknologi PETRONAS, under the University Research Internal Fund (URIF) for the project on the Development of Solar-Powered Applications (Cost Center: 015LB0-029).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
The authors acknowledge Universiti Teknologi PETRONAS for funding this project and the Centralized Analytical Laboratory of Universiti Teknologi PETRONAS for the sample characterizations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raïssi, M.; Pellegrin, Y.; Lefevre, F.X.; Boujtita, M.; Rousseau, D.; Berthelot, T.; Odobel, F. Digital printing of efficient dye-sensitized solar cells (DSSCs). Sol. Energy 2020, 199, 92–99. [Google Scholar] [CrossRef]
- Ikpesu, J.E.; Iyuke, S.E.; Daramola, M.; Oyetunde, O.A. Synthesis of improved dye-sensitized solar cell for renewable energy power generation. Sol. Energy 2020, 206, 918–934. [Google Scholar] [CrossRef]
- Saeed, M.A.; Kang, H.C.; Yoo, K.; Asiam, F.K.; Lee, J.-J.; Shim, J.W. Cosensitization of metal-based dyes for high-performance dye-sensitized photovoltaics under ambient lighting conditions. Dye. Pigment. 2021, 194, 109624. [Google Scholar] [CrossRef]
- Saeed, M.A.; Yoo, K.; Kang, H.C.; Shim, J.W.; Lee, J.-J. Recent developments in dye-sensitized photovoltaic cells under ambient illumination. Dye. Pigment. 2021, 194, 109626. [Google Scholar] [CrossRef]
- Karim, N.A.; Mehmood, U.; Zahid, H.F.; Asif, T. Nanostructured photoanode and counter electrode materials for efficient Dye-Sensitized Solar Cells (DSSCs). Sol. Energy 2019, 185, 165–188. [Google Scholar] [CrossRef]
- Grätzel, M. Mesoporous oxide junctions and nanostructured solar cells. Curr. Opin. Colloid Interface Sci. 1999, 4, 314–321. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Grätzel, M. Conversion of sunlight to electric power by nanocrystalline dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2004, 164, 3–14. [Google Scholar] [CrossRef]
- Hara, K.; Arakawa, H. Dye-Sensitized Solar Cells. In Handbook of Photovoltaic Science and Engineering; Luque, A., Hegedus, S., Eds.; John Wiley & Sons: London, UK, 2003; pp. 663–699. [Google Scholar]
- Liu, Q.; Wang, J. Dye-sensitized solar cells based on surficial TiO2 modification. Sol. Energy 2019, 184, 454–465. [Google Scholar] [CrossRef]
- Chou, T.P.; Zhang, Q.; Fryxell, G.E.; Cao, G.Z. Hierarchically Structured ZnO Film for Dye-Sensitized Solar Cells with Enhanced Energy Conversion Efficiency. Adv. Mater. 2007, 19, 2588–2592. [Google Scholar] [CrossRef]
- Zhang, Q.; Chou, T.P.; Russo, B.; Jenekhe, S.A.; Cao, G. Aggregation of ZnO Nanocrystallites for High Conversion Efficiency in Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2008, 47, 2402–2406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.F.; Dandeneau, C.S.; Zhou, X.Y.; Cao, G. ZnO nanostructures for dye-sensitized solar cells. Adv. Mater. 2009, 19, 4087–4108. [Google Scholar] [CrossRef]
- Wei, D. Dye sensitized solar cells. Int. J. Mol. Sci. 2010, 11, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Usami, A. Theoretical study of application of multiple scattering of light to a dye-sensitized nanocrystalline photoelectrichemical cell. Chem. Phys. Lett. 1997, 277, 105–108. [Google Scholar] [CrossRef]
- Mathew, A.; Rao, G.M.; Munichandraiah, N. Enhanced efficiency of tri-layered dye solar cells with hydrothermally synthesized titania nanotubes as light scattering outer layer. Thin Solid Films 2012, 520, 3581–3586. [Google Scholar] [CrossRef]
- Hwang, K.-J.; Park, D.-W.; Jin, S.; Kang, S.O.; Cho, D.W. Influence of dye-concentration on the light-scattering effect in dye-sensitized solar cell. Mater. Chem. Phys. 2015, 149, 594–600. [Google Scholar] [CrossRef]
- Liu, Z.H.; Su, X.J.; Hou, G.L.; Bi, S.; Xiao, Z.; Jia, H.-P. Enhanced performance for dye-sensitized solar cells based on spherical TiO2 nanorod-aggregate light-scattering layer. J. Power Sources 2012, 218, 280–285. [Google Scholar] [CrossRef]
- Bakhshayesh, A. Light scattering management of dye-sensitized solar cells based on double-layered photoanodes aided by uniform TiO2 aggregates. Mater. Res. Bull. 2016, 73, 268–275. [Google Scholar] [CrossRef]
- Zhao, L.; Li, J.; Shi, Y.; Wang, S.; Hu, J.; Dong, B.; Lu, H.; Wang, P. Double light-scattering layer film based on TiO2 hollow spheres and TiO2 nanosheets: Improved efficiency in dye-sensitized solar cells. J. Alloy. Compd. 2013, 575, 168–173. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, Y.; Lin, X.; Gu, X.; Qiang, Y. The effect of light-scattering layer on the performance of dye-sensitized solar cell assembled using TiO2 double-layered films as photoanodes. Superlattices Microstruct. 2013, 65, 152–160. [Google Scholar] [CrossRef]
- Bakhshayesh, A.; Mohammadi, M.; Dadar, H.; Fray, D. Improved efficiency of dye-sensitized solar cells aided by corn-like TiO2 nanowires as the light scattering layer. Electrochim. Acta 2013, 90, 302–308. [Google Scholar] [CrossRef]
- Zhang, Q.; Myers, D.; Lan, J.; Jenekhe, S.A.; Cao, G. Applications of light scattering in dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2012, 14, 14982–14998. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.D.; Méndez Medrano, M.G.; Remita, H.; Escobar-Barrios, V. Photocatalytic properties of BiOCl-TiO2 composites for phenol photodegradation. J. Environ. Chem. Eng. 2018, 6, 1601–1612. [Google Scholar] [CrossRef]
- Rathore, N.; Kulshreshtha, A.; Shukla, R.K.; Sharma, D. Study on morphological, structural and dielectric properties of sol-gel derived TiO2 nanocrystals annealed at different temperatures. Phys. B Condens. Matter 2019, 582, 411969. [Google Scholar] [CrossRef]
- Abbad, S.; Guergouri, K.; Gazaout, S.; Djebabra, S.; Zertal, A.; Barille, R.; Zaabat, M. Effect of silver doping on the photocatalytic activity of TiO2 nanopowders synthesized by the sol-gel route. J. Environ. Chem. Eng. 2020, 8, 103718. [Google Scholar] [CrossRef]
- Purcar, V.; Rădiţoiu, V.; Dumitru, A.; Nicolae, C.-A.; Frone, A.N.; Anastasescu, M.; Rădiţoiu, A.; Raduly, M.F.; Gabor, R.A.; Căprărescu, S. Antireflective coating based on TiO2 nanoparticles modified with coupling agents via acid-catalyzed sol-gel method. Appl. Surf. Sci. 2019, 487, 819–824. [Google Scholar] [CrossRef]
- Liu, B.-T.; Liou, J.-Y. High efficiency of dye-sensitized solar cells with two-layer mesoporous photoanodes fabricated in a low temperature process. Electrochimica Acta 2018, 261, 421–427. [Google Scholar] [CrossRef]
- Lin, A.; Qi, D.; Ding, H.; Wang, L.; Xing, M.; Shen, B.; Zhang, J. Carbon-doped titanum dioxide nanocrystals for highly efficient dye-sensitized solar cells. Catal. Today 2017, 281, 636–641. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, R.; Jiang, Y. Strong efficiency improvement in dye-sensitized solar cells by novel multi-dimensional TiO2 photoelectrode. Appl. Surf. Sci. 2017, 434, 11–15. [Google Scholar] [CrossRef]
- Guo, H.; Hu, Z.; Zhao, L.; Wu, Y.; Wang, S.; Dong, B.; Wan, L.; Li, J. Facile synthesis of chrysanthemum flowers-like TiO2 hierarchical microstructures assembled by nanotube for high performance dye-sensitized solar cells. Org. Electron. 2018, 55, 97–105. [Google Scholar] [CrossRef]
- Ali, S.; Khan, S.A.; Khan, I.; Yamani, Z.H.; Sohail, M.; Morsy, M.A. Surfactant-free synthesis of ellipsoidal and spherical shaped TiO2 nanoparticles and their comparative photocatalytic studies. J. Environ. Chem. Eng. 2017, 5, 3956–3962. [Google Scholar] [CrossRef]
- Kathirvel, S.; Pedaballi, S.; Su, C.; Chen, B.-R.; Li, W.-R. Morphological control of TiO2 nanocrystals by solvothermal synthesis for dye-sensitized solar cell applications. Appl. Surf. Sci. 2020, 519, 146082. [Google Scholar] [CrossRef]
- Lu, X.; Li, M.; Hoang, S.; Suib, S.L.; Gao, P.-X. Solvent effects on the heterogeneous growth of TiO2 nanostructure arrays by solvothermal synthesis. Catal. Today 2020, 360, 275–283. [Google Scholar] [CrossRef]
- Huang, J.; Liu, H.; Li, Z.; Zhong, J.; Wang, T.; Li, J.; Li, M. Photocatalytic activity of TiO2 prepared by different solvents through a solvothermal approach. Solid State Sci. 2019, 98, 106024. [Google Scholar] [CrossRef]
- Chandrika, M.; Ravindra, A.; Rajesh, C.; Ramarao, S.D.; Ju, S. Studies on structural and optical properties of nano ZnFe2O4 and ZnFe2O4-TiO2 composite synthesized by co-precipitation route. Mater. Chem. Phys. 2019, 230, 107–113. [Google Scholar] [CrossRef]
- Wattanawikkam, C.; Pecharapa, W.; Ishihara, K.N. X-ray absorption spectroscopy analysis and magnetic properties of M-doped TiO2 nanoparticles (M=Co, Mn, Ni and Zn) prepared by co-precipitation method. Ceram. Int. 2017, 43, S397–S402. [Google Scholar] [CrossRef]
- Su, P.-G.; Chen, F.-Y.; Wei, C.-H. Simple one-pot polyol synthesis of Pd nanoparticles, TiO2 microrods and reduced graphene oxide ternary composite for sensing NH3 gas at room temperature. Sensors Actuators B Chem. 2018, 254, 1125–1132. [Google Scholar] [CrossRef]
- Bargougui, R.; Bouazizi, N.; Hochepied, J.-F.; Le Derf, F.; Vieillard, J.; Ammar, S. Microwave-assisted polyol synthesis of mesoporous Ta doped mixed TiO2/SnO2: Application for CO2 capture. J. Alloy. Compd. 2017, 728, 391–399. [Google Scholar] [CrossRef]
- Parangi, T.; Mishra, M.K. Titania Nanoparticles as Modified Photocatalysts: A Review on Design and Development. Comments Inorg. Chem. 2019, 39, 90–126. [Google Scholar] [CrossRef]
- Maurya, I.C.; Senapati, S.; Singh, S.; Srivastava, P.; Maiti, P.; Bahadur, L. Effect of Particle Size on the Performance of TiO2 Based Dye-Sensitized Solar Cells. ChemistrySelect 2018, 3, 9872–9880. [Google Scholar] [CrossRef]
- Yuenyongsuwan, J.; Nithiyakorn, N.; Sabkird, P.; O’Rear, E.A.; Pongprayoon, T. Surfactant effect on phase-controlled synthesis and photocatalyst property of TiO2 nanoparticles. Mater. Chem. Phys. 2018, 214, 330–336. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, L.; Zhai, X.; Liang, C.; Yu, K. Preparation and photocatalytic activity analysis of nanometer TiO2 modified by surfactant. Nanomater. Nanotechnol. 2018, 8, 1847980418781973. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zang, B.; Wang, S. Surfactant-free synthesis of porous Au by a urea complex. RSC Adv. 2019, 9, 23081–23085. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Yuan, K.; Yu, J.; Delaunay, J.-J.; Che, R. Ultrafast self-assembly of silver nanostructures on carbon-coated copper grids for surface-enhanced Raman scattering detection of trace melamine. J. Colloid Interface Sci. 2017, 490, 23–28. [Google Scholar] [CrossRef]
- Nakade, S.; Saito, Y.; Kubo, W.; Kitamura, T.; Wada, A.Y.; Yanagida, S. Influence of TiO2 Nanoparticle Size on Electron Diffusion and Recombination in Dye-Sensitized TiO2 Solar Cells. J. Phys. Chem. B 2003, 107, 8607–8611. [Google Scholar] [CrossRef]
- Chou, T.P.; Zhang, Q.; Russo, B.; Fryxell, G.E.; Cao, G. Titania Particle Size Effect on the Overall Performance of Dye-Sensitized Solar Cells. J. Phys. Chem. C 2007, 111, 6296–6302. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.J.; Kang, J.S.; Yoon, J.; Kim, J.; Jeong, J.; Kang, J.; Lee, M.J.; Park, H.S.; Sung, Y.-E. Influence of TiO2 Particle Size on Dye-Sensitized Solar Cells Employing an Organic Sensitizer and a Cobalt(III/II) Redox Electrolyte. J. Phys. Chem. C 2018, 122, 7051–7060. [Google Scholar] [CrossRef]
- Tang, X.; Qian, J.; Wang, Z.; Wang, H.; Feng, Q.; Liu, G. Comparison of low crystallinity TiO2 film with nanocrystalline anatase film for dye-sensitized solar cells. J. Colloid Interface Sci. 2009, 330, 386–391. [Google Scholar] [CrossRef]
- Lee, H.; Zhai, P.; Cheng, R.; Huang, Y.-T.; Feng, S.-P. Study on transparency and hierarchical structure with TiO2 quantum dots for efficient back-side illuminated dye-sensitized solar cells. Electrochimica Acta 2016, 213, 155–162. [Google Scholar] [CrossRef]
- Kumar, D.K.; Suazo, D.D.; García, T.D.; Cook, N.P.; Ivaturi, A.; Hsu, M.; Marti, A.A.; Cabrera Martinez, C.R.; Chen, B.; Bennett, N.; et al. Low-temperature titania-graphene quantum dots paste for flexible dye-sensitised solar cell applications. Electrochim. Acta 2019, 305, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Peter, I.J.; Rajamanickam, N.; Vijaya, S.; Anandan, S.; Ramanchandran, K.; Nithiananthi, P. TiO2/Graphene Quantum Dots core-shell based photo anodes with TTIP treatment- A perspective way of enhancing the short circuit current. Solar Energy Mater. Solar Cells 2020, 205, 110239. [Google Scholar] [CrossRef]
- Shen, D.; Zhang, W.; Xie, F.; Li, Y.; Abate, A.; Wei, M. Graphene quantum dots decorated TiO2 mesoporous film as an efficient electron transport layer for high-performance perovskite solar cells. J. Power Sources 2018, 402, 320–326. [Google Scholar] [CrossRef]
- Du, X.; Li, W.; Zhao, L.; He, X.; Chen, H.; Fang, W. Electron transport improvement in CdSe-quantum dot solar cells using ZnO nanowires in nanoporous TiO2 formed by foam template. J. Photochem. Photobiol. A Chem. 2019, 371, 144–150. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Ge, Z.; Wang, T.; Xia, L.; Wu, Y.; Du, N.; Xiao, H.; Liu, J. Investigation of the CdS quantum dot sensitized solar cells based on a series of zinc titanium mixed metal oxides. Opt. Mater. 2020, 107, 110059. [Google Scholar] [CrossRef]
- Zaine, S.N.A.; Mohamed, N.M.; Khatani, M.; Samsudin, A.E.; Shahid, M.U. Trap state and charge recombination in nanocrystalline passivized Conductive and photoelectrode interface of dye-sensitized solar cell. Coatings 2020, 10, 284. [Google Scholar] [CrossRef] [Green Version]
- Zaine, S.N.A.; Mohamed, N.M.; Khatani, M.; Shahid, M.U. Effect of paste viscosity on direct-current resistance in improving the efficiency of dye-sensitized solar cell. J. Telecommun. Electron. Comput. Eng. 2018, 10, 73–78. [Google Scholar]
- Nazeeruddin, K.; Baranoff, E.; Grätzel, M. Dye-sensitized solar cells: A brief overview. Sol. Energy 2011, 85, 1172–1178. [Google Scholar] [CrossRef]
- Agarwala, S.; Kevin, M.; Wong, A.S.W.; Peh, C.K.N.; Thavasi, V.; Ho, G.W. Mesophase ordering of TiO2 film with high surface area and strong light harvesting for dye-sensitized solar cell. ACS Appl. Mater. Interfaces 2010, 2, 1844–1850. [Google Scholar] [CrossRef]
- Das, T.K.; Ilaiyaraja, P.; Sudakar, C. Template assisted nanoporous TiO2 nanoparticles: The effect of oxygen vacancy defects on photovoltaic performance of DSSC and QDSSC. Sol. Energy 2018, 159, 920–929. [Google Scholar] [CrossRef]
- Subalakshmi, K.; Senthilselvan, J. Effect of fluorine-doped TiO2 photoanode on electron transport, recombination dynamics and improved DSSC efficiency. Sol. Energy 2018, 171, 914–928. [Google Scholar] [CrossRef]
- Yan, W.; Huo, M.-M.; Hu, R.; Wang, Y. Working area effects on the energetic distribution of trap states and charge dynamics of dye-sensitized solar cells. RSC Adv. 2019, 9, 1734–1740. [Google Scholar] [CrossRef] [Green Version]
- Zaine, S.N.A.; Mohamed, N.M.; Khatani, M.; Samsudin, A.E.; Shahid, M.U. Improving the light scattering efficiency of photoelectrode dye-sensitized solar cell through optimization of core-shell structure. Mater. Today Commun. 2019, 19, 220–229. [Google Scholar] [CrossRef]
- Adachi, M.; Sakamoto, M.; Jiu, J.; Ogata, Y.; Isoda, S. Determination of Parameters of Electron Transport in Dye-Sensitized Solar Cells Using Electrochemical Impedance Spectroscopy. J. Phys. Chem. B 2006, 110, 13872–13880. [Google Scholar] [CrossRef] [PubMed]
- Bisquert, J. Chemical capacitance of nanostructured semiconductors: Its origin and significance for nanocomposite solar cells. Phys. Chem. Chem. Phys. 2003, 5, 5360–5364. [Google Scholar] [CrossRef]
- Bisquert, J. Theory of the impedance of charge transfer via surface states in dye-sensitized solar cells. J. Electroanal. Chem. 2010, 646, 43–51. [Google Scholar] [CrossRef]
- Wang, Q.; Ito, S.; Grätzel, M.; Fabregat-Santiago, F.; Mora-Seró, I.; Bisquert, J.; Bessho, T.; Imai, H. Characteristics of High Efficiency Dye-Sensitized Solar Cells. J. Phys. Chem. B 2006, 110, 25210–25221. [Google Scholar] [CrossRef]
- Bisquert, J.; Garcia, B.G.; Mora, S.I. Characterization of Capacitance, Transport and Recombination Parameters in Hybrid Perovskite and Organic Solar Cells. In Unconventional Thin Film Photovoltaics; Da Como, E., De Angelis, F., Snaith, H., Walker, A., Eds.; The Royal Society of Chemistry: London, UK, 2016; pp. 57–106. [Google Scholar]
- Fabregat-Santiago, F.; Randriamahazaka, H.; Zaban, A.; Garcia-Cañadas, J.; Garcia-Belmonte, G.; Bisquert, J. Chemical capacitance of nanoporous-nanocrystalline TiO2 in a room temperature ionic liquid. Phys. Chem. Chem. Phys. 2006, 8, 1827–1833. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).