Hybrid ZnO Flowers-Rods Nanostructure for Improved Photodetection Compared to Standalone Flowers and Rods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Cleaning
2.2. Sensors Fabrication with Different Morphologies
2.2.1. ZnO Nanorods (NRs) Deposition
2.2.2. ZnO Nanoflowers (NFs) Deposition
2.2.3. Hybrid ZnO Flower-Rod (HZFR) Deposition
2.3. Final Device Fabrication
2.4. Characterization Tools
3. Results and Discussion
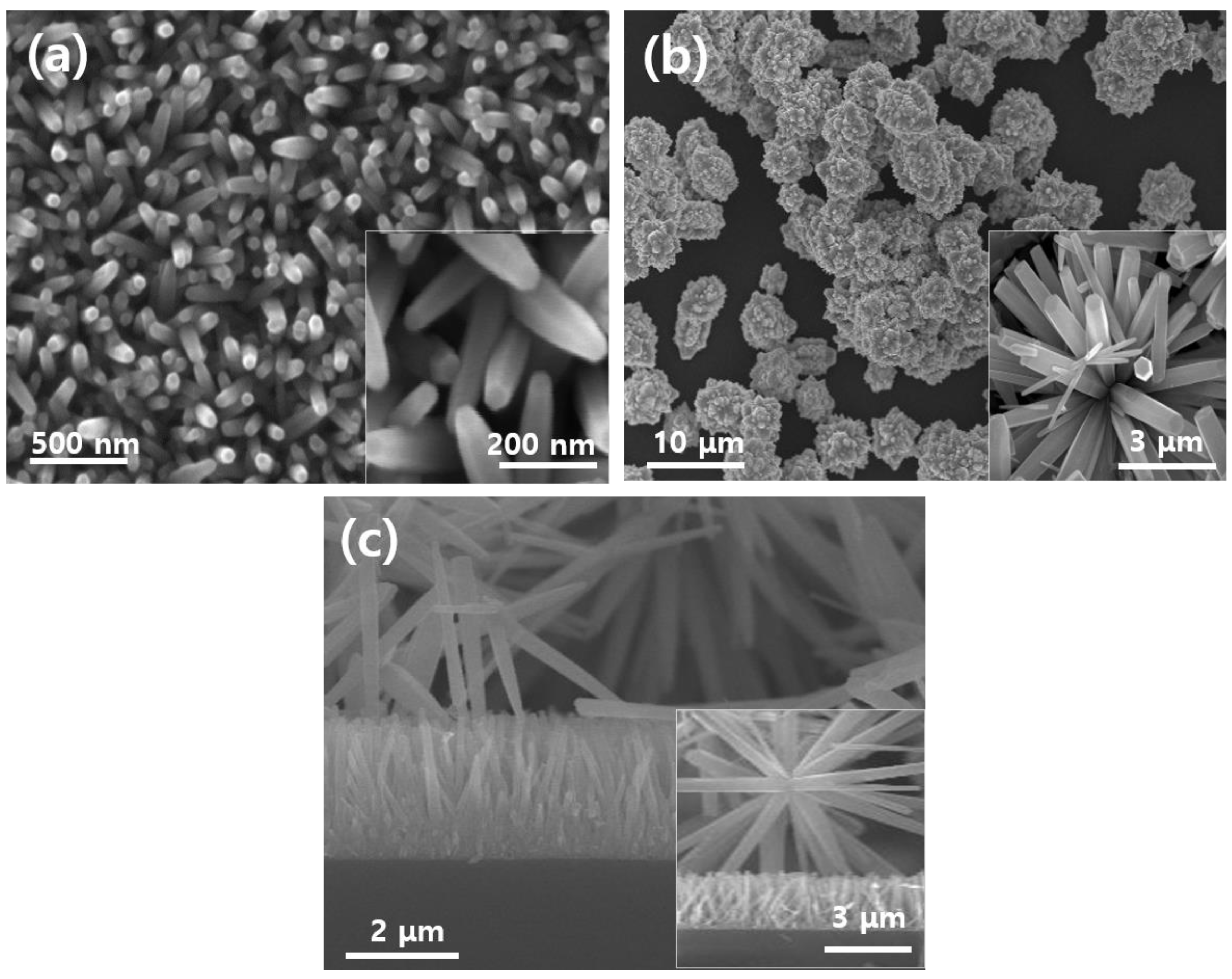
3.1. Novel Nanostructured Morphologies
3.2. Nanostructured XRD Structural and PL Optical Characteristics
3.3. Electrical (UV On-Off) Characteristics and Impulse Response
3.4. Responsivity and Photoconductive Gain
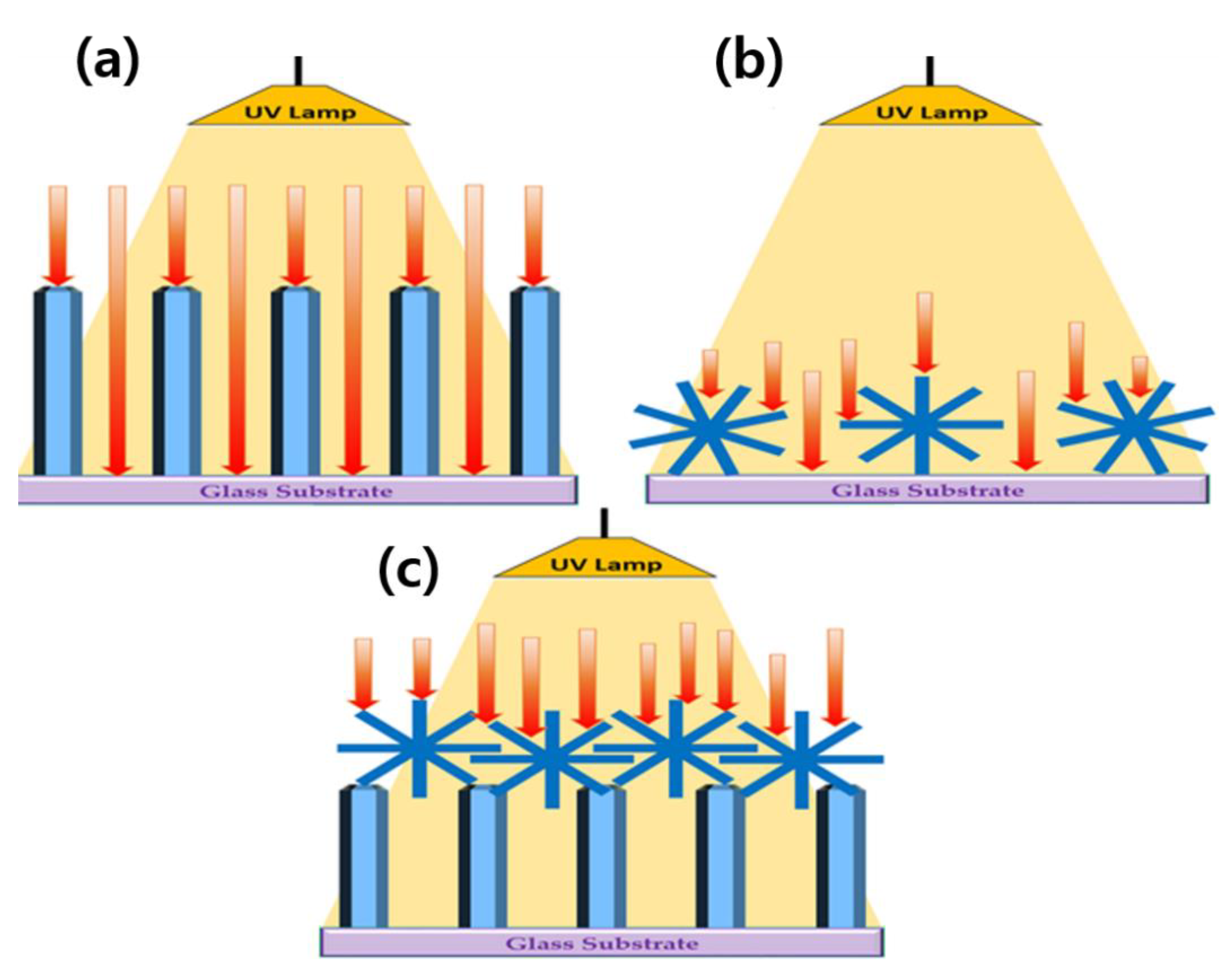
3.5. Proposed UV Sensor Mechanism for HZFR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amin, M.; Shah, N.A.; Bhatti, A.S. Development of highly sensitive UV sensor using morphology tuned ZnO nanostructures. Appl. Phys. A 2015, 118, 595–603. [Google Scholar] [CrossRef]
- Zhang, X.; Han, X.; Su, J.; Zhang, Q.; Gao, Y. Well vertically aligned ZnO nanowire arrays with an ultra-fast recovery time for UV photodetector. Appl. Phys A 2012, 107, 255–260. [Google Scholar] [CrossRef]
- Caruge, J.M.; Halpert, J.E.; Wood, V.; Bulovic, V.; Bawendi, M.G. Colloidal quantum-dot light-emitting diodes with metal-oxide charge transport layers. Nat. Photonics 2008, 2, 247–250. [Google Scholar] [CrossRef]
- Anikeeva, P.O.; Halpert, J.E.; Bawendi, M.G.; Bulovic, V. Electroluminescence from a mixed red–green–blue colloidal quantum dot monolayer. Nano Lett. 2007, 7, 2196–2200. [Google Scholar] [CrossRef]
- Rana, A.S.; Kim, H.S. NH4OH Treatment for an optimum morphological trade-off to hydrothermal Ga-doped n-ZnO/p-Si heterostructure characteristics. Materials 2018, 11, 37. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, X.; Liu, N.; Ding, L.; Tao, J.; Wang, S.; Su, J.; Li, L.; Gao, Y. Enhanced photo-response properties of a single ZnO microwire photodetector by coupling effect between localized Schottky barriers and piezoelectric potential. Opt. Express 2015, 23, 21204–21212. [Google Scholar] [CrossRef]
- Rana, A.S.; Kang, M.; Jeong, E.S.; Kim, H.S. Transition between ZnO Nanorods and ZnO Nanotubes with Their Antithetical Properties. J. Nanosci. Nanotechnol. 2016, 16, 10772–10776. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Su, J.; Li, H.; Zhang, Q.; Gao, Y. Ag nanoparticles@ZnO nanowire composite arrays: An absorption enhanced UV photodetector. Opt. Express 2014, 22, 30148–30155. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.; Park, J.; Lim, M.; Lee, R.; Cho, S.J.; Kumaresan, Y.; Oh, M.K.; Jun, G.Y. Surface conversion of ZnO nanorods to ZIF-8 to suppress surface defects for a visible-blind UV photodetector. Nanoscale 2018, 10, 21168–21177. [Google Scholar] [CrossRef]
- Grottrup, J.; Postica, V.; Smazna, D.; Hoppe, M.; Kaidas, V.; Mishra, Y.K.; Lupan, O.; Adelunga, R. UV detection properties of hybrid ZnO tetrapod 3-D networks. Vacuum 2017, 146, 492–500. [Google Scholar] [CrossRef]
- Weber, M.; Kim, J.Y.; Lee, J.H.; Kim, J.H.; Iatsunskyi, I.; Coy, E.; Miele, P.; Bechelany, M.; Kim, S.S. Highly efficient hydrogen sensors based on Pd nanoparticles supported on boron nitride coated ZnO nanowires. J. Mater. Chem. A 2019, 7, 8107–8116. [Google Scholar] [CrossRef]
- Rana, A.S.; Lee, J.Y.; Hong, Y.P.; Kim, H.S. Transient Current Response for ZnO Nanorod-Based Doubly Transparent UV Sensor Fabricated on Flexible Substrate. Phys. Status Solidi RRL 2018, 12, 1800001. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, M.; Zhao, Q.; Qiu, H.; Li, J.; Li, X.; Shao, J. Dielectrophoretic-Assembled Single and Parallel-Aligned Ag Nanowire–ZnO-Branched Nanorod Heteronanowire Ultraviolet Photodetectors. ACS Appl. Mater. Interfaces 2017, 9, 22837–22845. [Google Scholar] [CrossRef]
- Boscarino, S.; Filice, S.; Sciuto, A.; Libertino, S.; Scuderi, M.; Galati, C.; Scalese, S. Investigation of ZnO-decorated CNTs for UV light detection applications. Nanomaterials 2019, 9, 1099. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Kumar, Y.; Kumar, H.; Vyas, S.; Periasamy, C.; Chakrabarti, P.; Park, S.H. A study of hydrothermally grown ZnO nanorod-based metal-semiconductor-metal UV detectors on glass substrates. Nanomater. Nanotechnol. 2017, 7, 1847980417702144. [Google Scholar] [CrossRef] [Green Version]
- Tahira, A.; Mazzaro, R.; Rigoni, F.; Nafady, A.; Shaikh, S.F.; Al-Othman, A.A.; Alshgari, R.A.; Ibupoto, Z.H. A simple and efficient visible light photodetector based on Co3O4/Zno composite. Opt. Quantum Electron. 2021, 53, 1–9. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.; Mao, S.S.; Lin, L. Fabrication and characterization of ZnO nanowires based UV photodiodes. Sens. Actuators A-Phys. 2006, 127, 201–206. [Google Scholar] [CrossRef]
- Zhou, J.; Gu, Y.; Hu, Y.; Mai, W.; Yeh, P.H.; Bao, G.; Sood, A.K.; Polla, D.L.; Wang, Z.L. Gigantic enhancement in response and reset time of ZnO UV nanosensor by utilizing Schottky contact and surface functionalization. Appl. Phys. Lett. 2009, 94, 191103. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, S.F.; Al-Enizi, A.M.; Agyeman, D.A.; Ghani, F.; Nah, I.W.; Shahid, A. Intrinsic control in defects density for improved ZnO nanorod-based UV sensor performance. Nanomaterials 2020, 10, 142. [Google Scholar]
- Yang, P.; Yan, H.; Mao, S.; Russo, R.; Johnson, J.; Saykally, R.; Morris, N.; Pham, J.; He, R.; Choi, H.J. Controlled growth of ZnO nanowires and their optical properties. Adv. Funct. Mater. 2002, 12, 323–331. [Google Scholar] [CrossRef]
- Park, W.I.; Yi, G.C.; Kim, M.; Pennycook, S.J. ZnO nanoneedles grown vertically on Si substrates by non-catalytic vapor-phase epitaxy. Adv. Mater. 2002, 14, 1841–1843. [Google Scholar] [CrossRef]
- Sun, Y.; Fuge, G.M.; Ashfold, M.N. Growth of aligned ZnO nanorod arrays by catalyst-free pulsed laser deposition methods. Chem. Phys. Lett. 2004, 396, 21–26. [Google Scholar] [CrossRef]
- Yao, B.D.; Chan, Y.F.; Wang, N. Formation of ZnO nanostructures by a simple way of thermal evaporation. Appl. Phys. Lett. 2002, 81, 757–759. [Google Scholar] [CrossRef]
- Rana, A.S.; Ko, K.; Hong, S.; Kang, M.; Kim, H.S. Fabrication and characterization of ZnO nanorods on multiple substrates. J. Nanosci. Nanotechnol. 2015, 15, 8375–8380. [Google Scholar] [CrossRef]
- Rana, A.S.; Kang, M.; Kim, H.S. Microwave-assisted facile and ultrafast growth of ZnO nanostructures and proposition of alternative microwave-assisted methods to address growth stoppage. Sci. Rep. 2016, 6, 24870. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A review of microwave synthesis of zinc oxide nanomaterials: Reactants, process parameters and morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Chang, S.B.; Chae, H.U.; Kim, H.S. Structural, optical, electrical and morphological properties of different concentration sol-gel ZnO seeds and consanguineous ZnO nanostructured growth dependence on seeds. J. Alloys Comp. 2017, 729, 571–582. [Google Scholar]
- Luo, J.; Ma, S.Y.; Sun, A.M.; Cheng, L.; Yang, G.J.; Wang, T.; Li, W.Q.; Li, X.B.; Mao, Y.Z.; Gz, D.J. Ethanol sensing enhancement by optimizing ZnO nanostructure: From 1D nanorods to 3D nanoflower. Mater. Lett. 2014, 137, 17–20. [Google Scholar] [CrossRef]
- Sahdan, M.Z.; Mamat, M.H.; Salina, M.; Khusaimi, Z.; Noor, U.M.; Rusop, M. Heat treatment effects on the surface morphology and optical properties of ZnO nanostructures. Phys. Status Solidi C 2010, 7, 2286–2289. [Google Scholar] [CrossRef]
- Keskenler, E.F.; Tomakin, M.; Dogan, S.; Turgut, G.; Aydın, S.; Duman, S.; Gurbulak, B. Growth and characterization of Ag/n-ZnO/p-Si/Al heterojunction diode by solegel spin technique. J. Alloys Compd. 2013, 550, 129–132. [Google Scholar] [CrossRef]
- Rana, A.S.; Lee, J.Y.; Shahid, A.; Kim, H.S. Growth method-dependent and defect density-oriented structural, optical, conductive, and physical properties of solution-grown ZnO nanostructures. Nanomaterials 2017, 7, 266. [Google Scholar] [CrossRef] [Green Version]
- Janotti, A.; Van de Walle, C.G. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Look, D.C.; Jogai, B. Combined effects of screening and band gap renormalization on the energy of optical transitions in ZnO and GaN. J. Appl. Phys. 2000, 88, 5760–5763. [Google Scholar] [CrossRef]
- Fair, R.B. The effect of strain-induced band-gap narrowing on high concentration phosphorus diffusion in silicon. J. Appl. Phys. 1979, 50, 860–868. [Google Scholar] [CrossRef]
- Rodwihok, C.; Choopun, S.; Ruankham, P.; Gardchareon, A.; Phadungdhitidhada, S.; Wongratanaphisan, D. UV sensing properties of ZnO nanowires/nanorods. Appl. Surf. Sci. 2019, 477, 159–165. [Google Scholar] [CrossRef]
- Eom, T.H.; Han, J.I. Single fiber UV detector based on hydrothermally synthesized ZnO nanorods for wearable computing devices. Appl. Surf. Sci. 2018, 428, 233–241. [Google Scholar] [CrossRef]
- Makhlouf, H.; Karam, C.; Lamouchi, A.; Tingry, S.; Miele, P.; Habchi, R.; Chtourou, R.; Bechelany, M. Analysis of ultraviolet photo-response of ZnO nanostructures prepared by electrodeposition and atomic layer deposition. Appl. Surf. Sci. 2018, 444, 253–259. [Google Scholar] [CrossRef]
- Yu, J.; Shan, C.X.; Qiao, Q.; Xie, X.H.; Wang, S.P.; Zhang, Z.Z.; Shen, D.Z. Enhanced responsivity of photodetectors realized via impact ionization. Sensors 2012, 12, 1280–1287. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hu, Y.; Wang, Z.; Fang, Z.; Peng, L.M. Performance boosting of flexible ZnO UV sensors with rational designed absorbing antireflection layer and humectant encapsulation. ACS Appl. Mater. Interf. 2016, 8, 381–389. [Google Scholar] [CrossRef]
- Wang, X.; Liu, K.; Chen, X.; Li, B.; Jiang, M.; Zhang, Z.; Zhao, H.; Shen, D. Highly wavelength-selective enhancement of responsivity in Ag nanoparticle-modified ZnO UV photodetector. ACS Appl. Mater. Interf. 2017, 9, 5574–5579. [Google Scholar] [CrossRef]
- Li, Y.; Gong, J.; He, G.; Deng, Y. Enhancement of photoresponse and UV-assisted gas sensing with Au decorated ZnO nanofibers. Mater. Chem. Phys. 2012, 134, 1172–1178. [Google Scholar] [CrossRef]
- Alenezi, M.R.; Henley, S.J.; Silva, S.R.P. On-chip fabrication of high performance nanostructured ZnO UV detectors. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]





| Sample | Two Theta (Degree) | Full Width at Half Maximum (Radian) | Crystallite Size (nm) |
|---|---|---|---|
| NRs | 34.43 | 0.0026 | 67 |
| NFs | 34.60 | 0.0031 | 51 |
| HZFRs | 34.78 | 0.0023 | 66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Enizi, A.M.; Shaikh, S.F.; Tamboli, A.M.; Marium, A.; Ijaz, M.F.; Ubaidullah, M.; Moydeen Abdulhameed, M.; Ekar, S.U. Hybrid ZnO Flowers-Rods Nanostructure for Improved Photodetection Compared to Standalone Flowers and Rods. Coatings 2021, 11, 1464. https://doi.org/10.3390/coatings11121464
Al-Enizi AM, Shaikh SF, Tamboli AM, Marium A, Ijaz MF, Ubaidullah M, Moydeen Abdulhameed M, Ekar SU. Hybrid ZnO Flowers-Rods Nanostructure for Improved Photodetection Compared to Standalone Flowers and Rods. Coatings. 2021; 11(12):1464. https://doi.org/10.3390/coatings11121464
Chicago/Turabian StyleAl-Enizi, Abdullah M., Shoyebmohamad F. Shaikh, Asiya M. Tamboli, Afifa Marium, Muhammad Fazal Ijaz, Mohd Ubaidullah, Meera Moydeen Abdulhameed, and Satish U. Ekar. 2021. "Hybrid ZnO Flowers-Rods Nanostructure for Improved Photodetection Compared to Standalone Flowers and Rods" Coatings 11, no. 12: 1464. https://doi.org/10.3390/coatings11121464
APA StyleAl-Enizi, A. M., Shaikh, S. F., Tamboli, A. M., Marium, A., Ijaz, M. F., Ubaidullah, M., Moydeen Abdulhameed, M., & Ekar, S. U. (2021). Hybrid ZnO Flowers-Rods Nanostructure for Improved Photodetection Compared to Standalone Flowers and Rods. Coatings, 11(12), 1464. https://doi.org/10.3390/coatings11121464








