Laboratory Experimental Study on Influencing Factors of Drainage Pipe Crystallization in Highway Tunnel in Karst Area
Abstract
:1. Introduction
2. Theoretical Tests
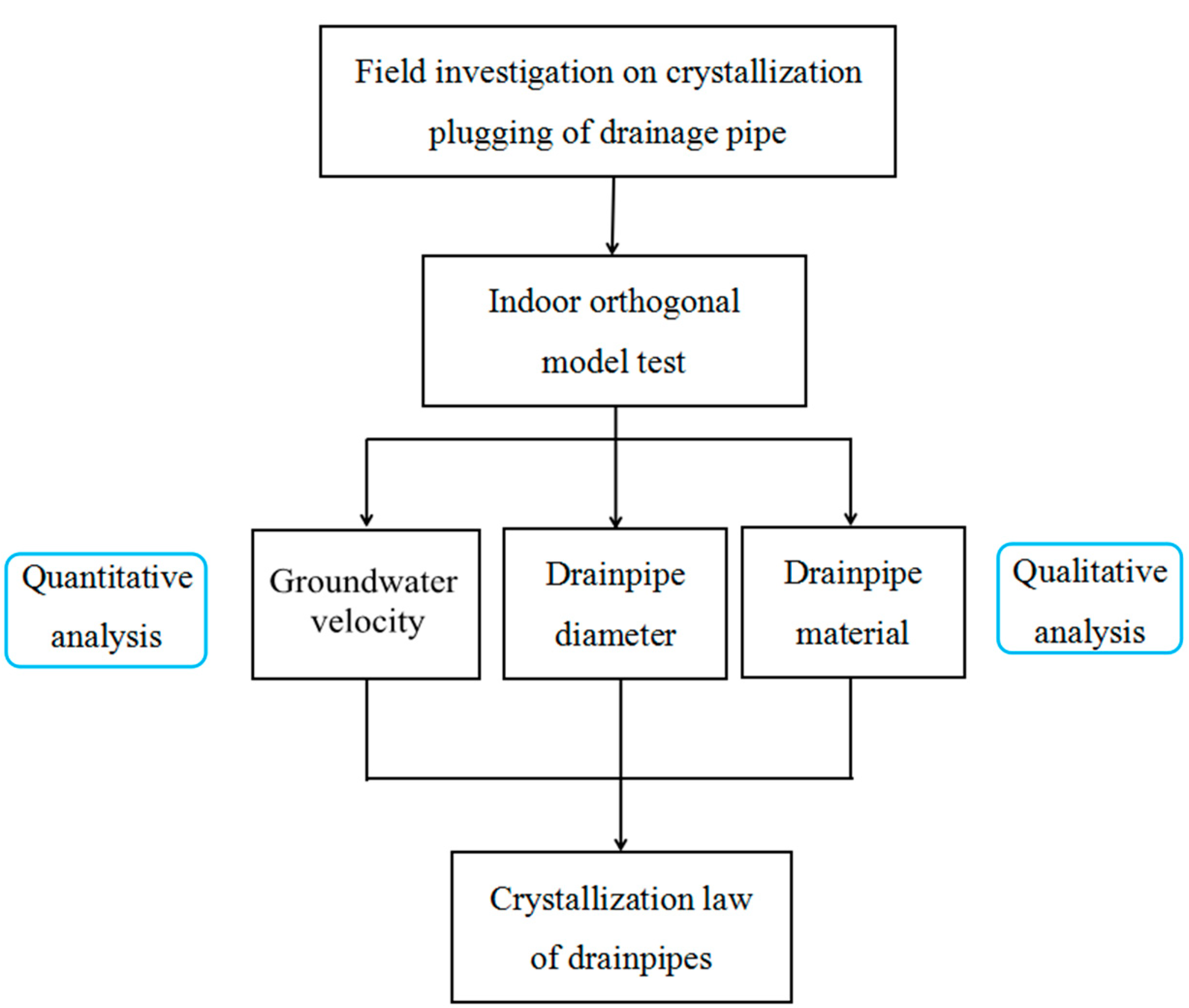
2.1. Research Route
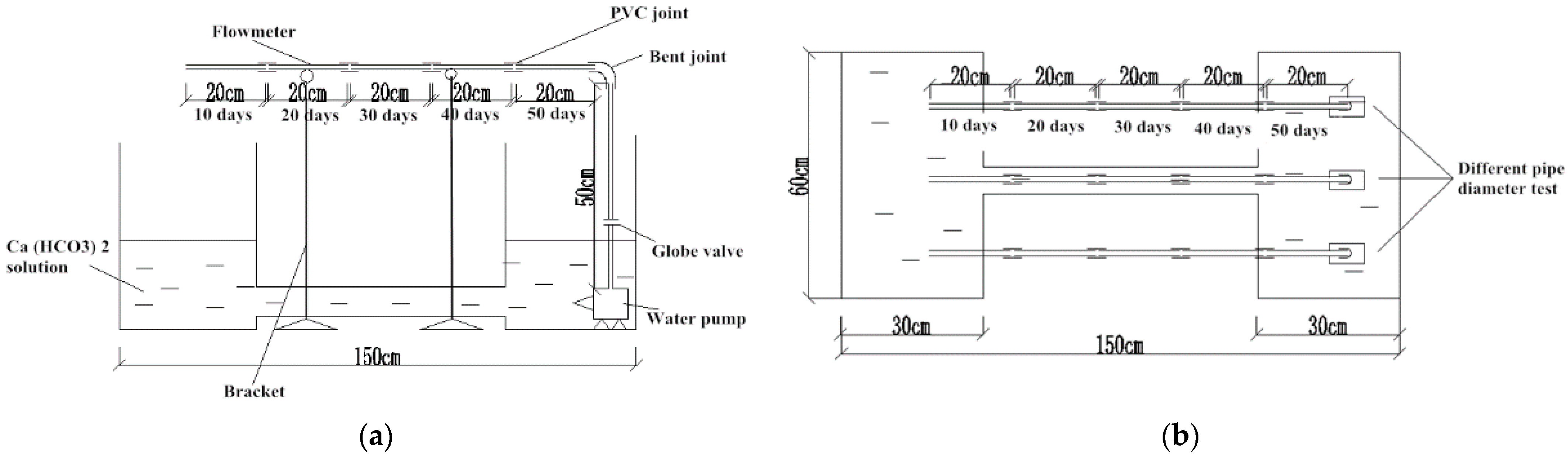
2.2. Test Scheme
2.3. Test Solution
3. Results
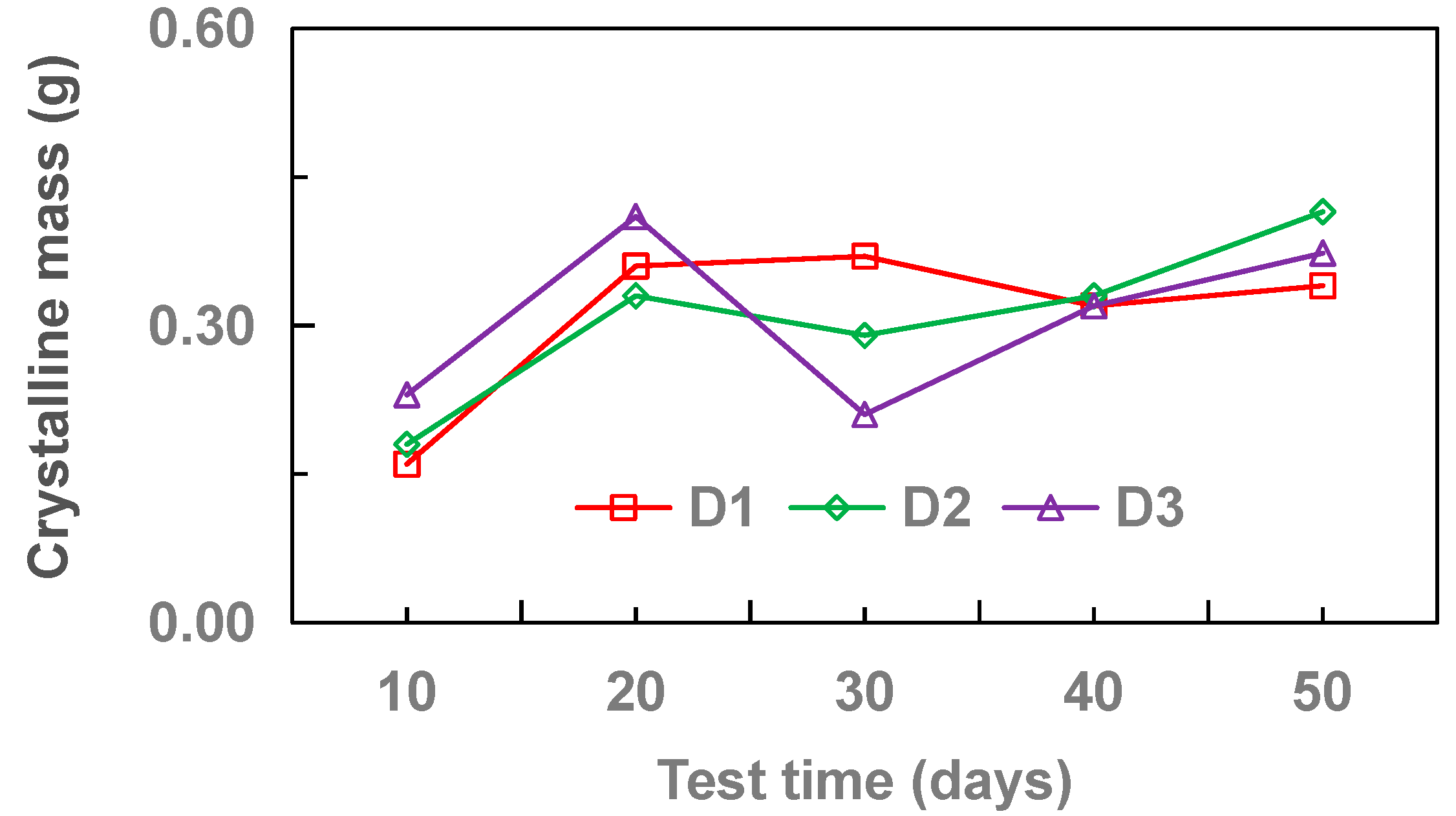
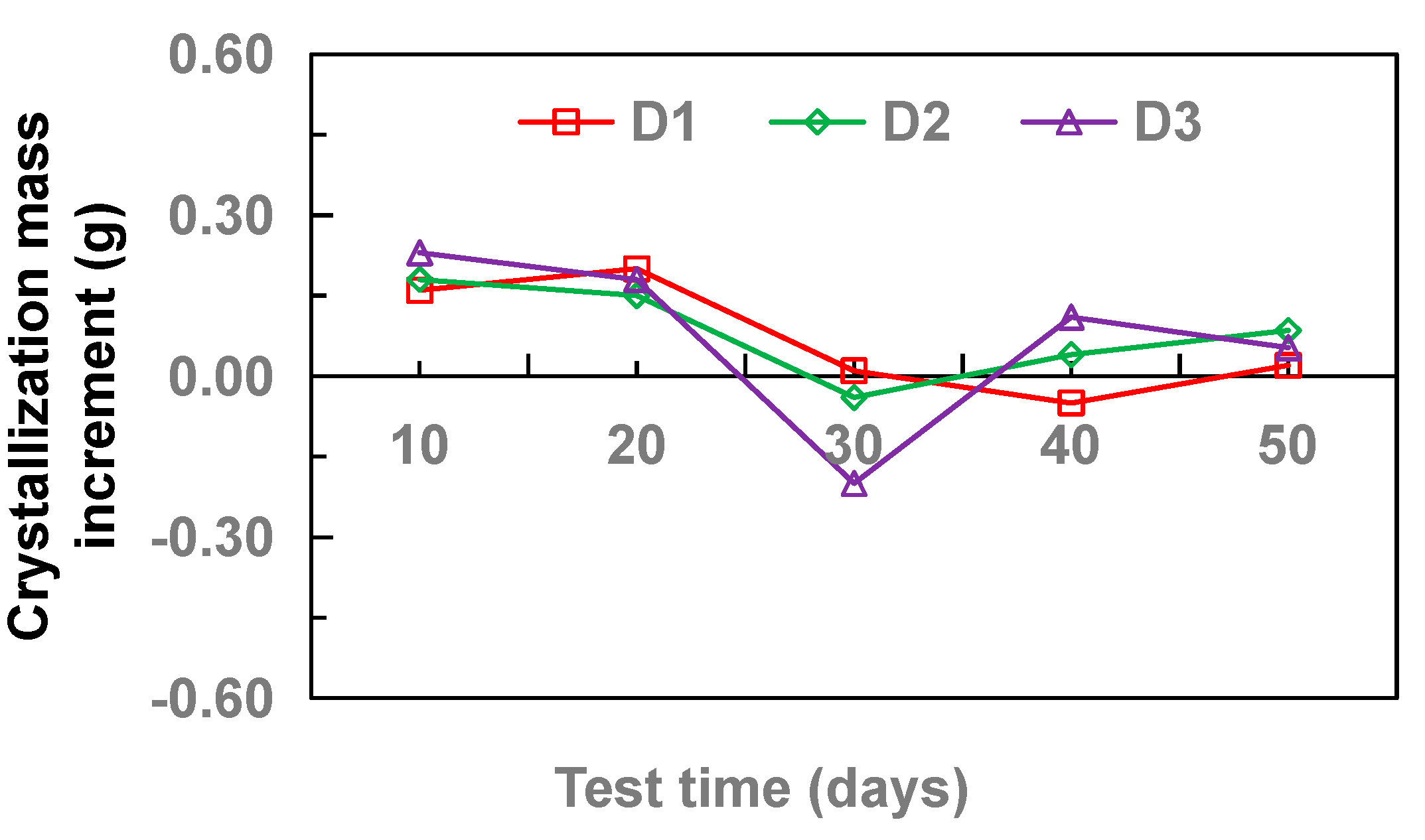
3.1. Effect of Pipe Diameter
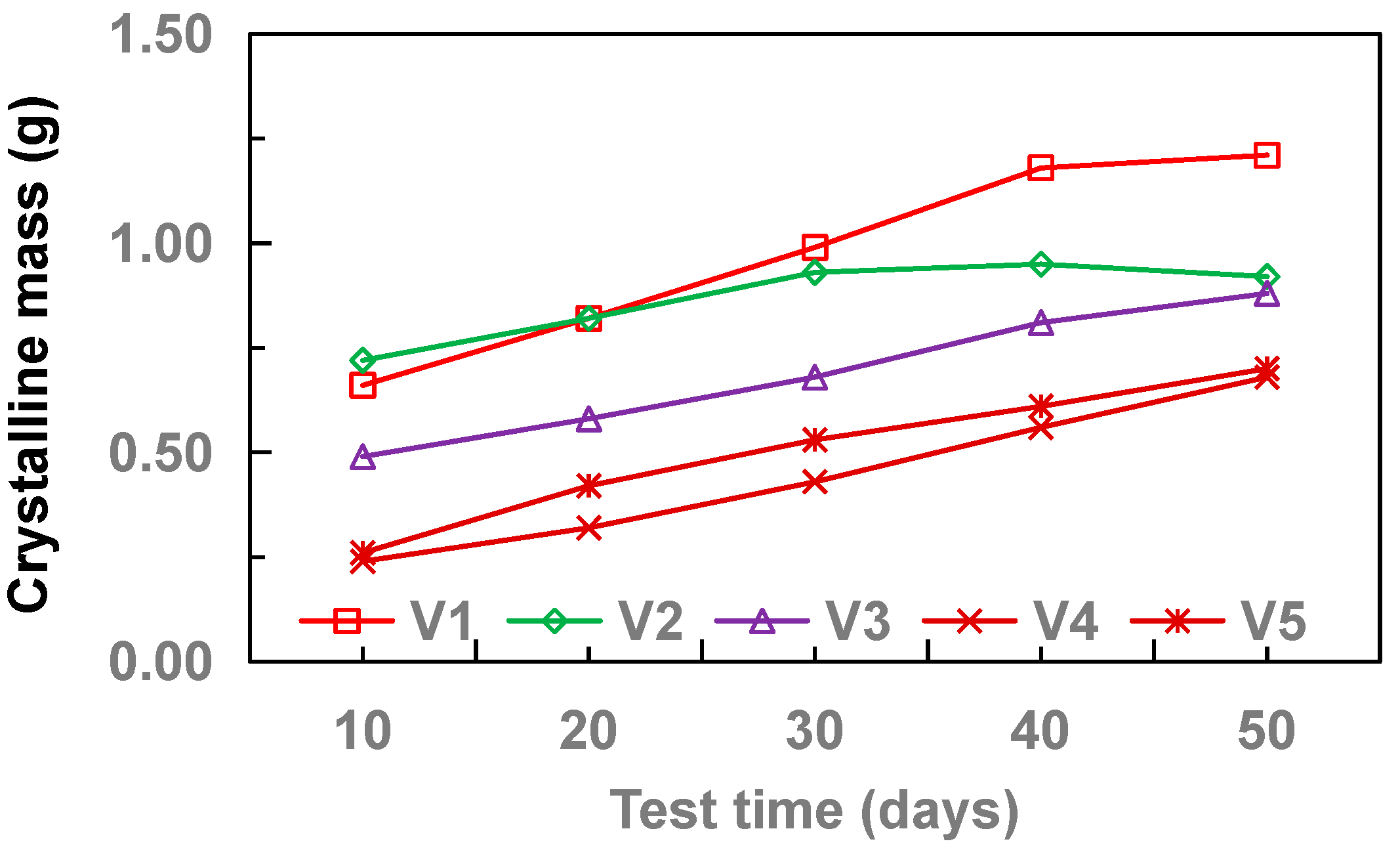
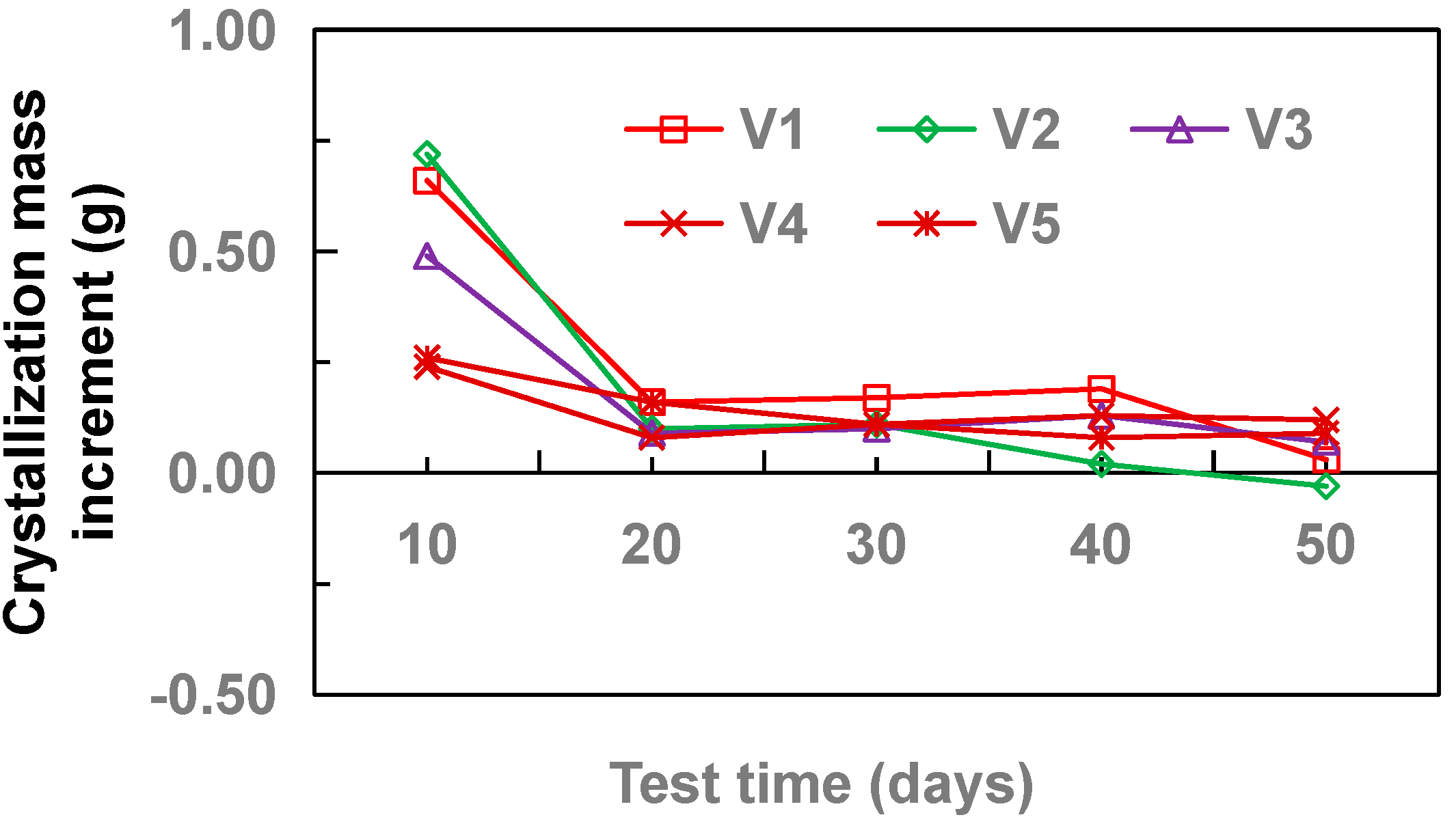
3.2. Effect of Flow Velocity
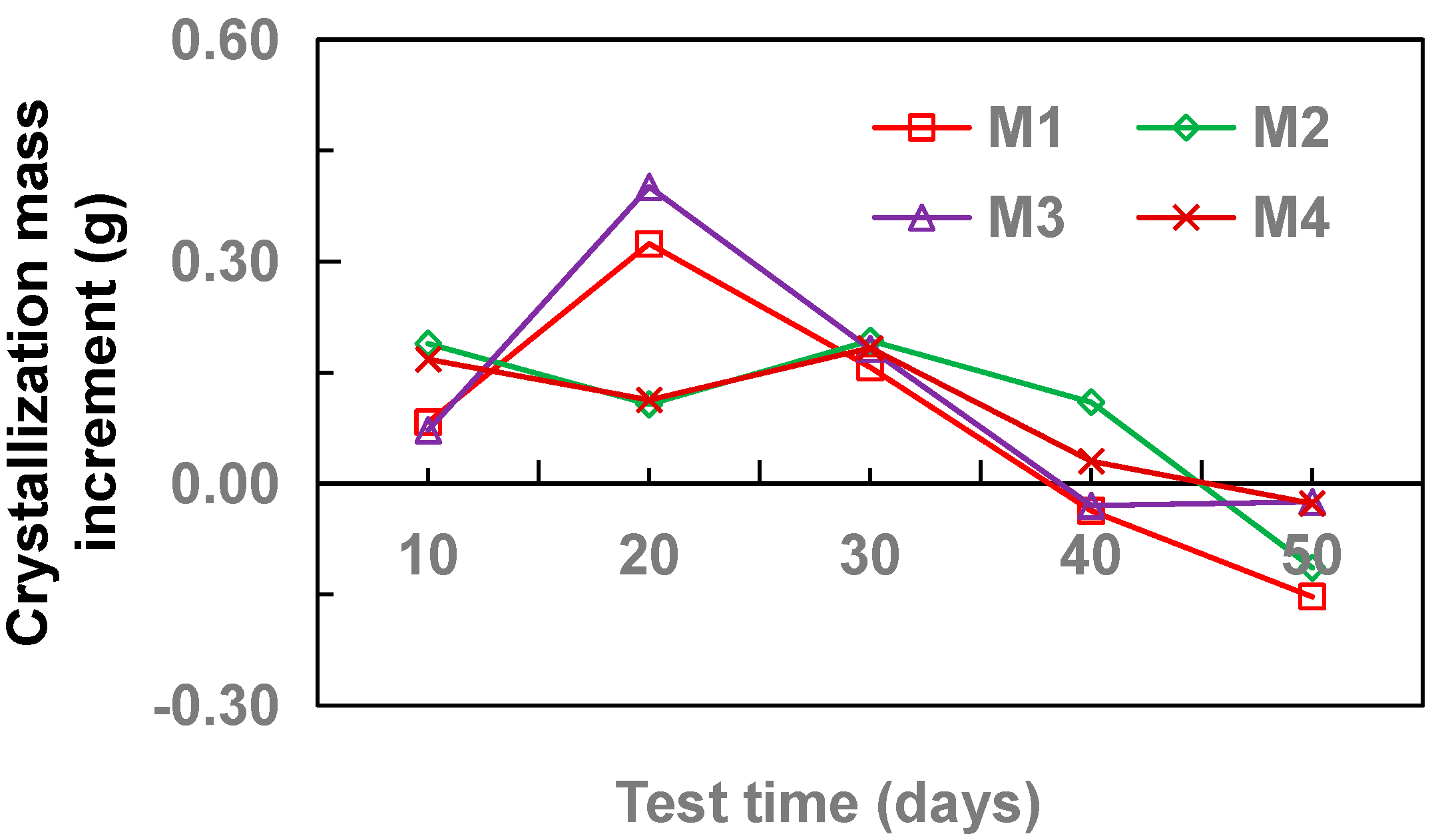
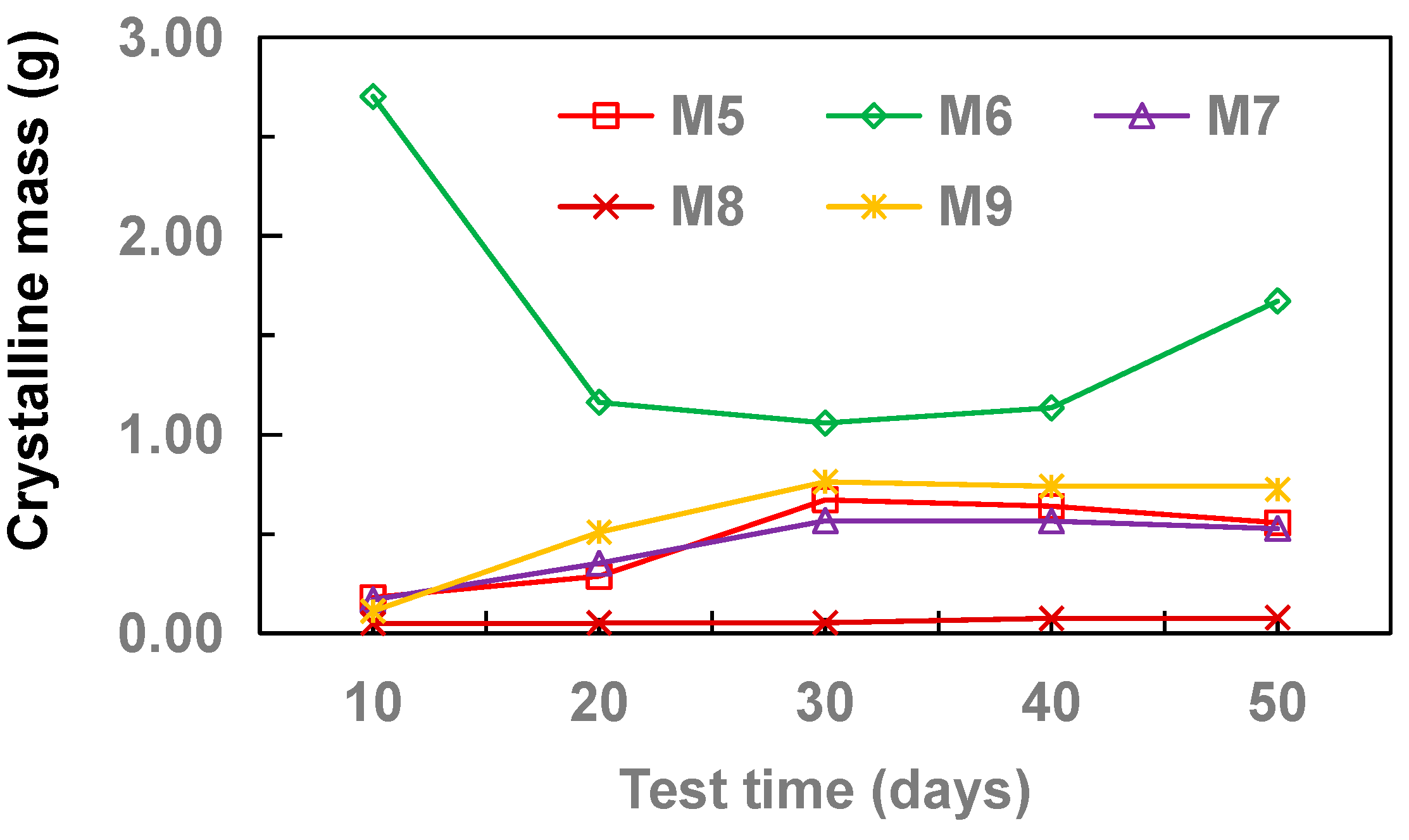
3.3. Effect of Pipe Material
4. Discussion
5. Conclusions
- (1)
- With the increase of drainage pipe diameter (20–32 mm), the crystallinity of drainage pipe increases first and then decreases gradually. With the increase of water velocity in the drainage pipe, the crystallinity of the drainage pipe decreases gradually. At different flow rates, the growth rate of crystallization increased first and then gradually stabilized. Without adding other materials, the crystallinity of drainage pipe is: M3 > M2 > M4 > M1. When other materials are added, the crystallinity of drainage pipe is M6 > M9 > M5 > M7 > M8. When the groundwater flow rate is 34.5 cm·s−1, M1 and M8 drainage pipes can be used in the tunnel to ensure that the drainage pipe will not be blocked by crystallization.
- (2)
- There is a 5-fold relationship between the diameter of indoor test (20–32 mm) and the diameter of field drainage pipe (80–160 mm). Therefore, whether there is a 5-fold relationship between the law obtained from indoor test and the actual crystallization change law needs to be further determined. Ca2+ and HCO3− were mainly selected as the ions in the test solution, while the actual groundwater on site also contains other ions that may affect crystallization, such as Mg2+, requiring a lot of further research work.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Zhang, M.; You, S.; Qiang, L.; Sun, H.; Wang, J.; Kongyun, W. Spatial and diurnal variations of geochemical indicators in a calcite-precipitating stream-Case study of Baishuitai. Yunnan. Geochimica 2004, 33, 269–278. [Google Scholar]
- Katsifaras, A.; Spanos, N. Effect of inorganic phosphate ions on the spontaneous precipitation of vaterite and the transformation of vaterite to calcite. J. Cryst. Growth 1999, 204, 183–190. [Google Scholar] [CrossRef]
- Lin, Y.; Fang, J.; Li, J. Quantitive study of the effect of a magnetic field on the precipitation of calcium carbonate aqueous solutions. Ind. Water Treat. 2002, 22, 16–18. [Google Scholar]
- Knez, S.; Pohar, C. The magnetic field influence on the polymorph composition of CaCO3 precipitated from carbonized aqueous solution. J. Colloid Interface Sci. 2005, 281, 377–388. [Google Scholar] [CrossRef]
- Parsons, S.; Wang, B.; Judd, S.; Stephenson, T. Magnetic treatment of Calcium Carbonate scale-effect of PH control. Water Res. 1997, 31, 339–342. [Google Scholar] [CrossRef]
- Dermitzaki, E.; Bauer, J.; Wunderle, B.; Michel, B. Diffusion of water in amorphous polymers at different temperatures using molecular dynamics simulation. In Proceedings of the 2006 1st Electronic Systemintegration Technology Conference, Dresden, Germany, 5–7 September 2006; Volume 2, pp. 762–772. [Google Scholar]
- You, X.; Zhang, X. Molecular dynamics simulation on an aqueous solution of calcite. J. North Univ. China 2013, 170–173. [Google Scholar]
- Wang, D.; Huang, L. Molecular dynamics simulation on an aclueous solution of calcium carbonate. Sci. Technol. Eng. 2009, 9, 6619–6623. [Google Scholar]
- Zhang, S.; Shi, W.; Lei, W.; Xia, M.; Wang, F. Molecular dynamics simulation of interaction between calcite crystal and water-soluble polymers. Acta Phys. Chim. Sin. 2005, 21, 1198–1204. [Google Scholar]
- Song, J. Experimental Study on the Impact of Magnetic Field on Calcium Carbonate Crystallization Process; Shandong University: Jinan, China, 2015. [Google Scholar]
- Chen, J. Manufacturing Water Treatment Devices Generating Alternating Magnetic Field and Experimental Study on Its Antiscaling Effect; Chongqing University: Chongqing, China, 2014. [Google Scholar]
- Huang, S.; Song, H. Study on the crystallization and precipitation of carbonate at different temperature conditions. Geoscience 1991, 442–449 and 475–476. [Google Scholar]
- Jiang, S.; Yu, H.; Liu, C. Research on the scaling dynamic model of the high salinity system. Appl. Chem. Ind. 2011, 40, 1623–1628. [Google Scholar]
- Zhang, J.; Li, S.; Zhou, Y. On bacterial and fun gal effects on karst process and its application. Carsologica Sin. 1997, 16, 362–369. [Google Scholar]
- Jakucs, L. Morphogenetics of Karst Regions: Variants of Karst Evolution; Adam Hilger: New York, NY, USA, 1977. [Google Scholar]
- Greenfield, L. Metabolism and concentration of calcium and magnesium and precipitation of calcium carbonate by a marine bacteria. Ann. N. Y. Acad. Sci. 1963, 109, 23–45. [Google Scholar] [CrossRef]
- Krumbein, W. Photolithotopic and chemoorganotrophic activity of bacteria and algae as related to beachrock formation and degradation (Gulf of Aqabu, Sinai). Deep. Sea Res. Part B Oceanogr. Lit. Rev. 1979, 1, 139–203. [Google Scholar]
- Gu, X.; Qiu, F.; Zhou, X.; Yang, D.; Guo, Q.; Guo, X. Synthesis and application of terpolymer scale inhibitor in the presence of β-cyclodextrins. J. Pet. Sci. Eng. 2013, 109, 177–186. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Liu, G.; Huang, J.; Yao, Q.; Ma, S.; Cao, K.; Liu, Y.; Wu, W.; Sun, W.; et al. Investigation of calcium carbonate precipitation in the presence of fluorescent-tagged scale inhibitor for cooling water systems. Desalination Water Treat. 2015, 53, 3491–3498. [Google Scholar] [CrossRef]
- Yoon, S.; Park, E.; Lee, J.; Chun, B. Laboratory test of molecular vibration for preventing drainage pipe blockage in deteriorated tunnel. J. Korean Geotech. Soc. 2012, 28, 69–77. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Zhang, B.; Zhou, Y.; Liu, S. Investigation and Analysis on Crystallization of Tunnel Drainage Pipes in Chongqing. Adv. Mater. Sci. Eng. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Guo, X. Crystallization mechanism and countermeasures of drainage system for railway tunnel. China Railw. Sci. 2020, 41, 71–77. [Google Scholar]
- Ye, F.; Tian, C.; He, B.; Zhao, M.; Wang, J.; Han, X.; Song, G. Experimental study on scaling and cloggingin drainage system of tunnels under construction. China J. Highw. Transp. 2021, 34, 159–170. [Google Scholar]
- Xiang, K.; Zhou, J.; Zhang, X.; Huang, C.; Song, L.; Liu, S. Experimental Study on Crystallization Rule of Tunnel Drainpipe in Alkaline Environment. Tunn. Constr. 2019, 39, 207–212. [Google Scholar]
- Tian, C.; Ye, F.; Song, G.; Wang, Q.; Zhao, M.; He, B.; Wang, J.; Han, X. On Mechanism of crystal blockage of tunnel drainage system and preventive countermeasures. Mod. Tunn. Technol. 2020, 57, 66–76. [Google Scholar]
- Lu, G.; Wang, P.; Yang, Y.; Mao, C.; Wu, Y.; Wu, J.; Dong, P.; Wu, J. Advance in mechanism of groundwater crystallization blockage in tunnel drainage pipe and scale inhibiting techniques in karst area. Mod. Tunn. Technol. 2021, 1–16. Available online: http://kns.cnki.net/kcms/detail/51.1600.U.20210818.1810.006.html (accessed on 25 November 2021).
- Mao, C.; Yang, Y.; Wu, J.; Dong, P.; Wu, J. Numerical simulation of crystal blockage in tunnel drainage pipe based on dynamic grid and level set. Carsologica Sin. 2021, 1–18. Available online: http://kns.cnki.net/kcms/detail/45.1157.P.20211025.1840.002.html (accessed on 25 November 2021).
- Higashitani, K.; Kage, A.; Katamura, S.; Imai, K.; Hatade, S. Effects of a Magnetic Field on the Formation of CaCO3 Particles. J. Colloid Interface Sci. 1993, 156, 90–95. [Google Scholar] [CrossRef]
- Alimi, F.; Tlili, M.; Amor, M.B.; Maurin, G.; Gabrielli, C. Influence of magnetic field on calcium carbonate precipitation in the presence of foreign ions. Surf. Eng. Appl. Electrochem. 2009, 45, 56–62. [Google Scholar] [CrossRef]




| Influencing Factor | Variable Value |
|---|---|
| Drainage pipe diameter | D1 = 20 mm; D2 = 25 mm; D3 = 32 mm |
| Flow velocity | V1 = 22.0 cm·s−1, V2 = 26.5 cm·s−1, V3 = 34.5 cm·s−1, V4 = 44.5 cm·s−1, V5 = 63.5 cm·s−1 |
| Drainage pipe material | M1 = PVC, M2 = PPR, M3 = PTFE, M4 = HDPE, M5 = PVC + hydrophobic antistatic self-cleaning agent, M6 = PVC + flocking film, M7 = PVC + silicone, M8 = PVC + magnetic field coil, M9 = PVC + PE powder |
| Ion Type | Ion | Concentration c (mmol·L−1) | Percentage (%) | Ion Type | Ion | Concentration c (mmol·L−1) | Percentage (%) |
|---|---|---|---|---|---|---|---|
| Cation | K++Na+ | 3.68 | 5.17 | Anion | Cl− | 1.8 | 0.69 |
| Ca2+ | 45.2 | 63.50 | SO42− | 51.9 | 19.83 | ||
| Mg2+ | 22.3 | 31.33 | HCO3− | 208 | 79.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, S.; Xiong, S.; Leng, H.; Chen, H.; Zhang, B.; Liu, Z. Laboratory Experimental Study on Influencing Factors of Drainage Pipe Crystallization in Highway Tunnel in Karst Area. Coatings 2021, 11, 1493. https://doi.org/10.3390/coatings11121493
Li H, Liu S, Xiong S, Leng H, Chen H, Zhang B, Liu Z. Laboratory Experimental Study on Influencing Factors of Drainage Pipe Crystallization in Highway Tunnel in Karst Area. Coatings. 2021; 11(12):1493. https://doi.org/10.3390/coatings11121493
Chicago/Turabian StyleLi, Huaming, Shiyang Liu, Shuai Xiong, Hao Leng, Huiqiang Chen, Bin Zhang, and Zhen Liu. 2021. "Laboratory Experimental Study on Influencing Factors of Drainage Pipe Crystallization in Highway Tunnel in Karst Area" Coatings 11, no. 12: 1493. https://doi.org/10.3390/coatings11121493
APA StyleLi, H., Liu, S., Xiong, S., Leng, H., Chen, H., Zhang, B., & Liu, Z. (2021). Laboratory Experimental Study on Influencing Factors of Drainage Pipe Crystallization in Highway Tunnel in Karst Area. Coatings, 11(12), 1493. https://doi.org/10.3390/coatings11121493






