Galvanic Corrosion Behaviour of Different Types of Coatings Used in Safety Systems Manufacturing
Abstract
:1. Introduction
- -
- carbon steel-coated samples: if the carbon steel is not completely covered with the deposited layers;
- -
- duralumin–steel/coated samples and aluminium bronze–steel/coated samples: because even if the carabiner is made of carbon steel, the rivets or the locking system are made of a different material.
2. Materials and Methods
2.1. Material
2.2. Sample Preparation
2.3. Methods
3. Results and Discussion
3.1. Microstructural Analysis
3.2. Galvanic Couples in Saltwater
3.3. Galvanic Couples in Fire Extinguishing Solution
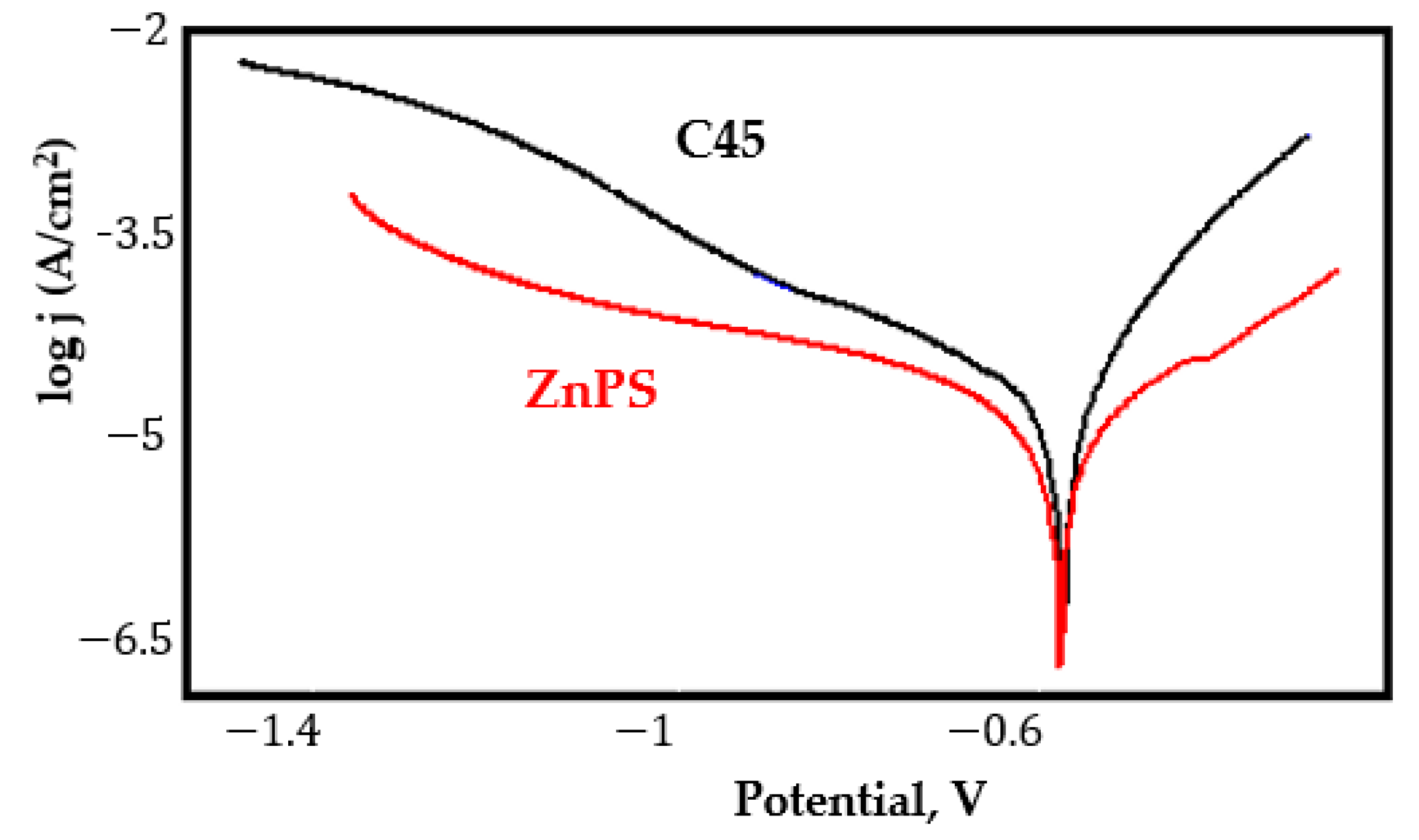
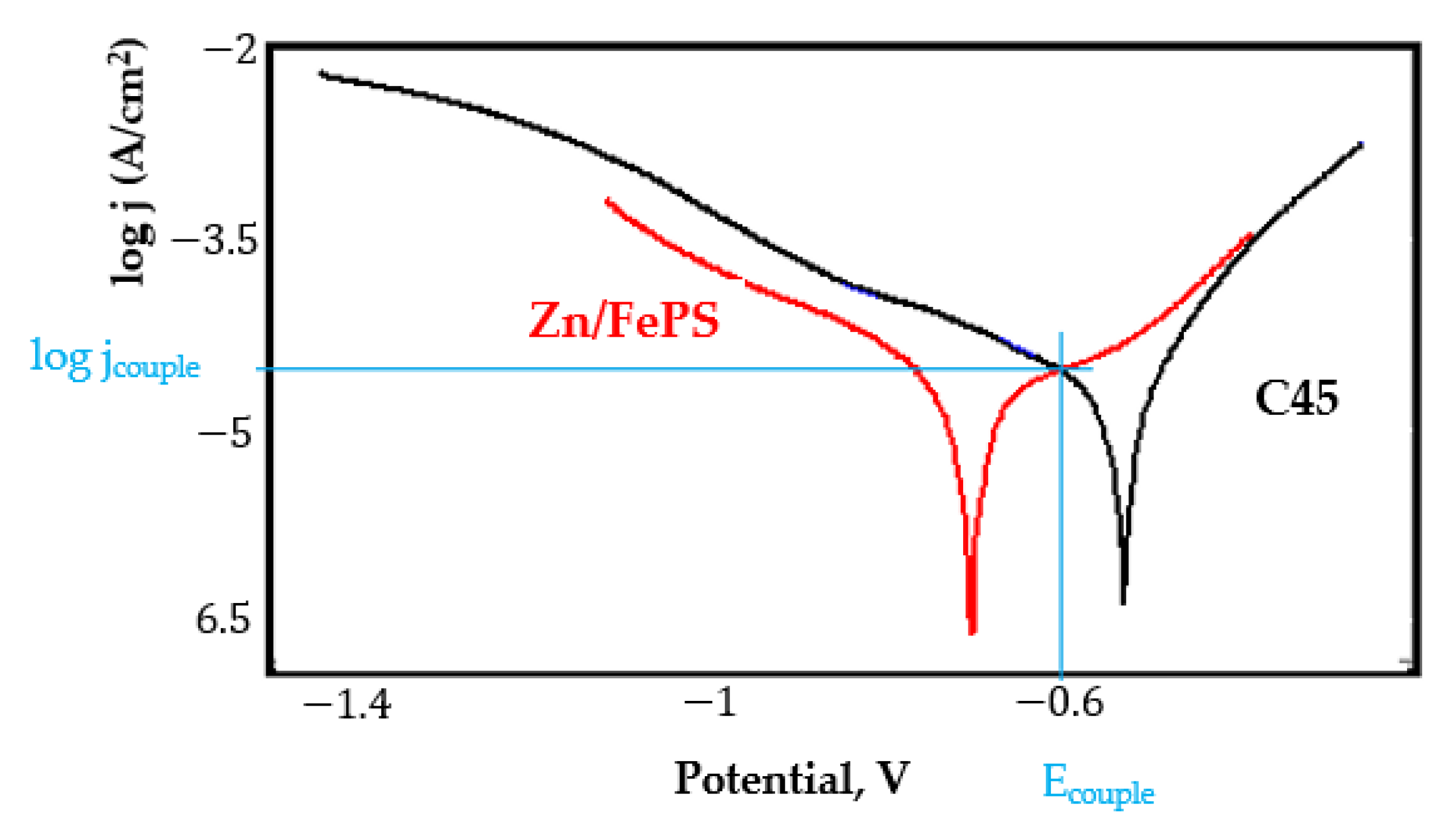
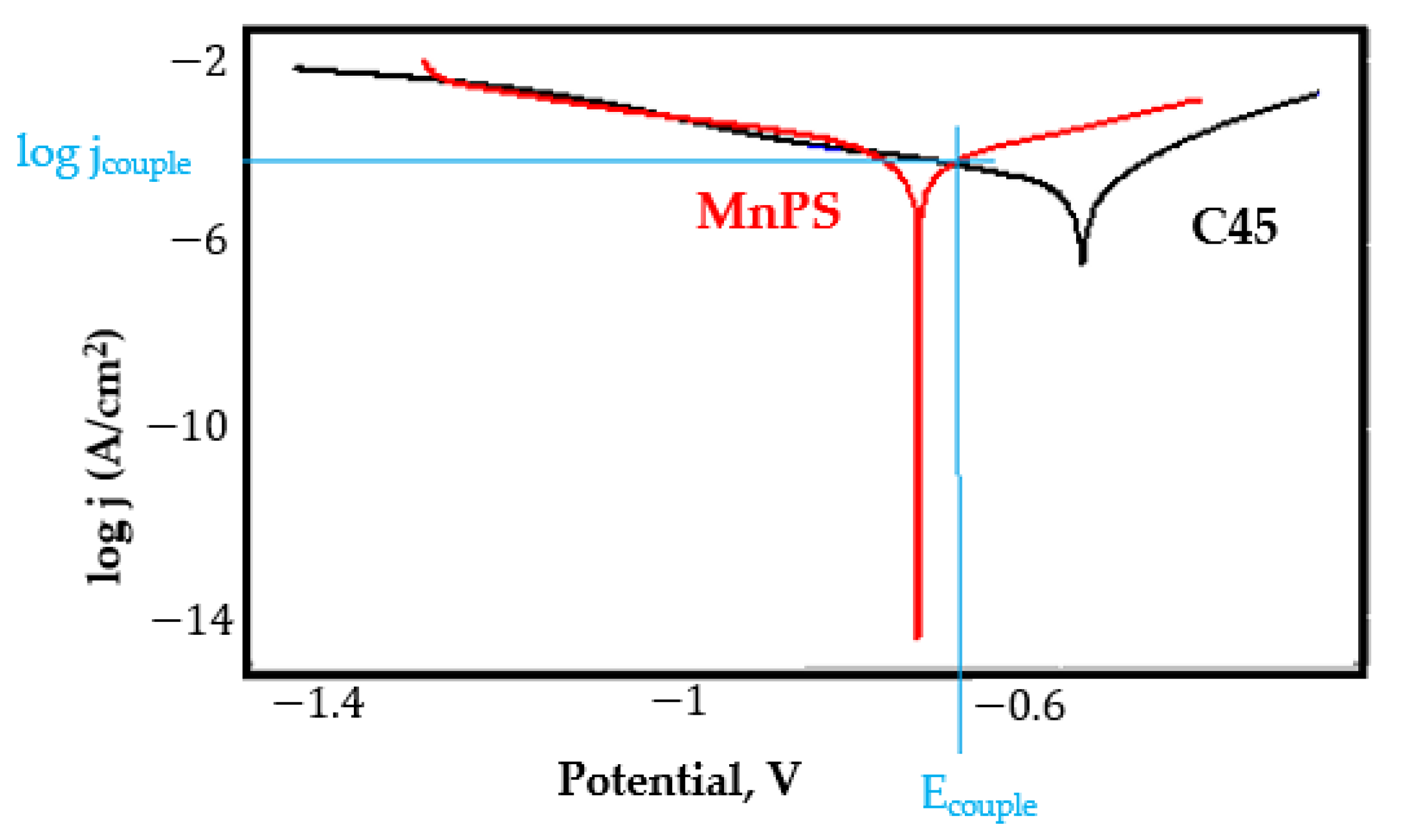
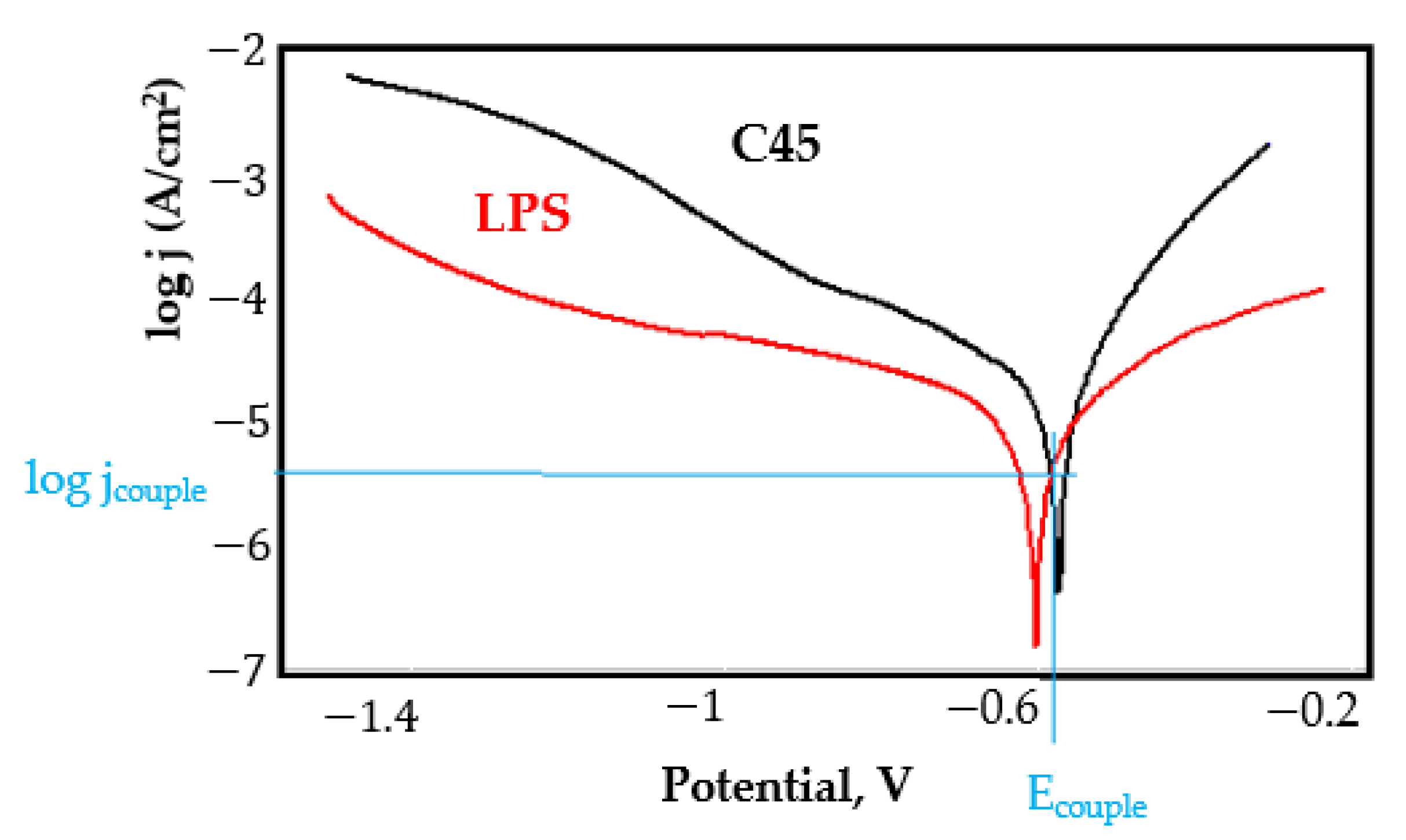
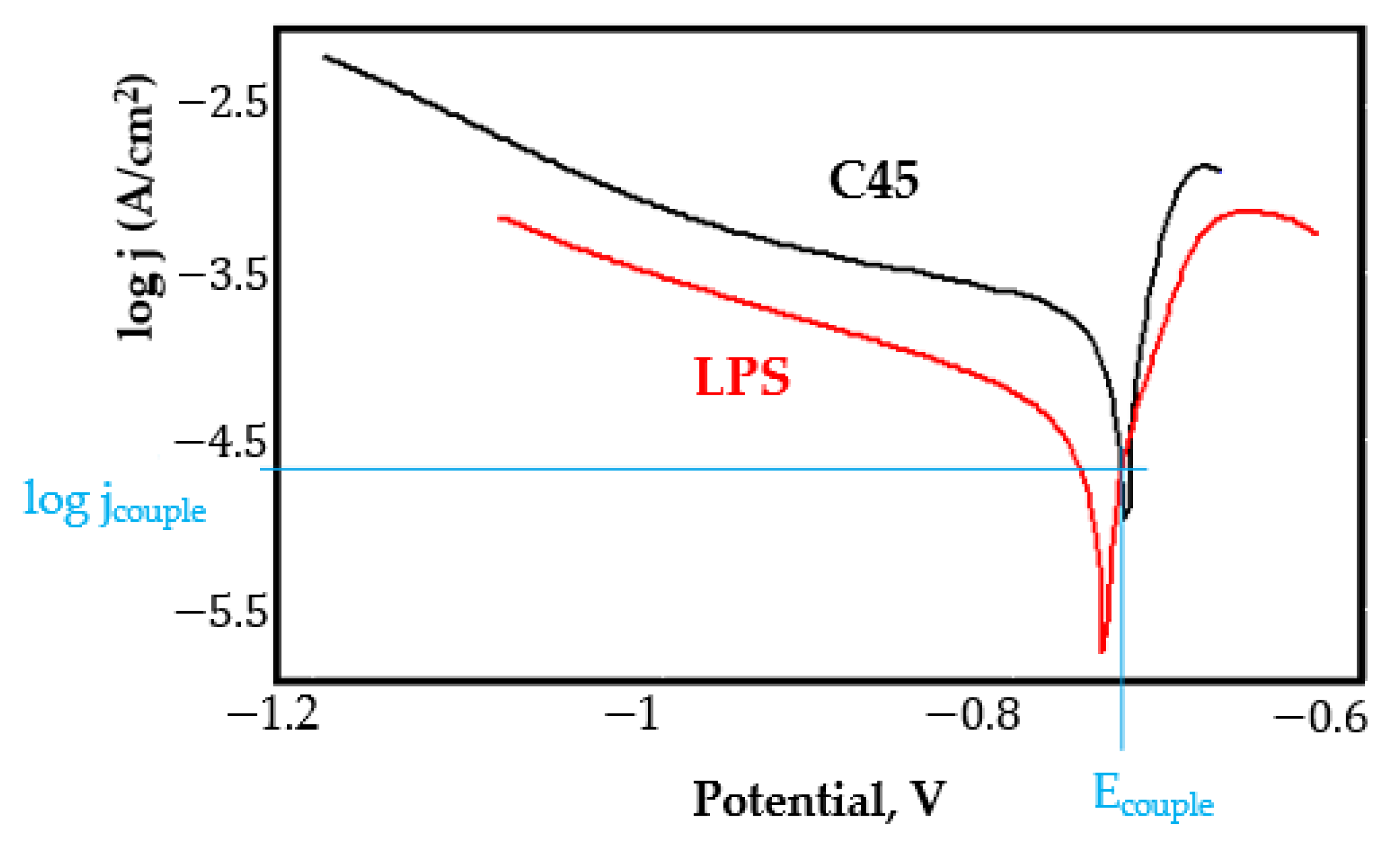
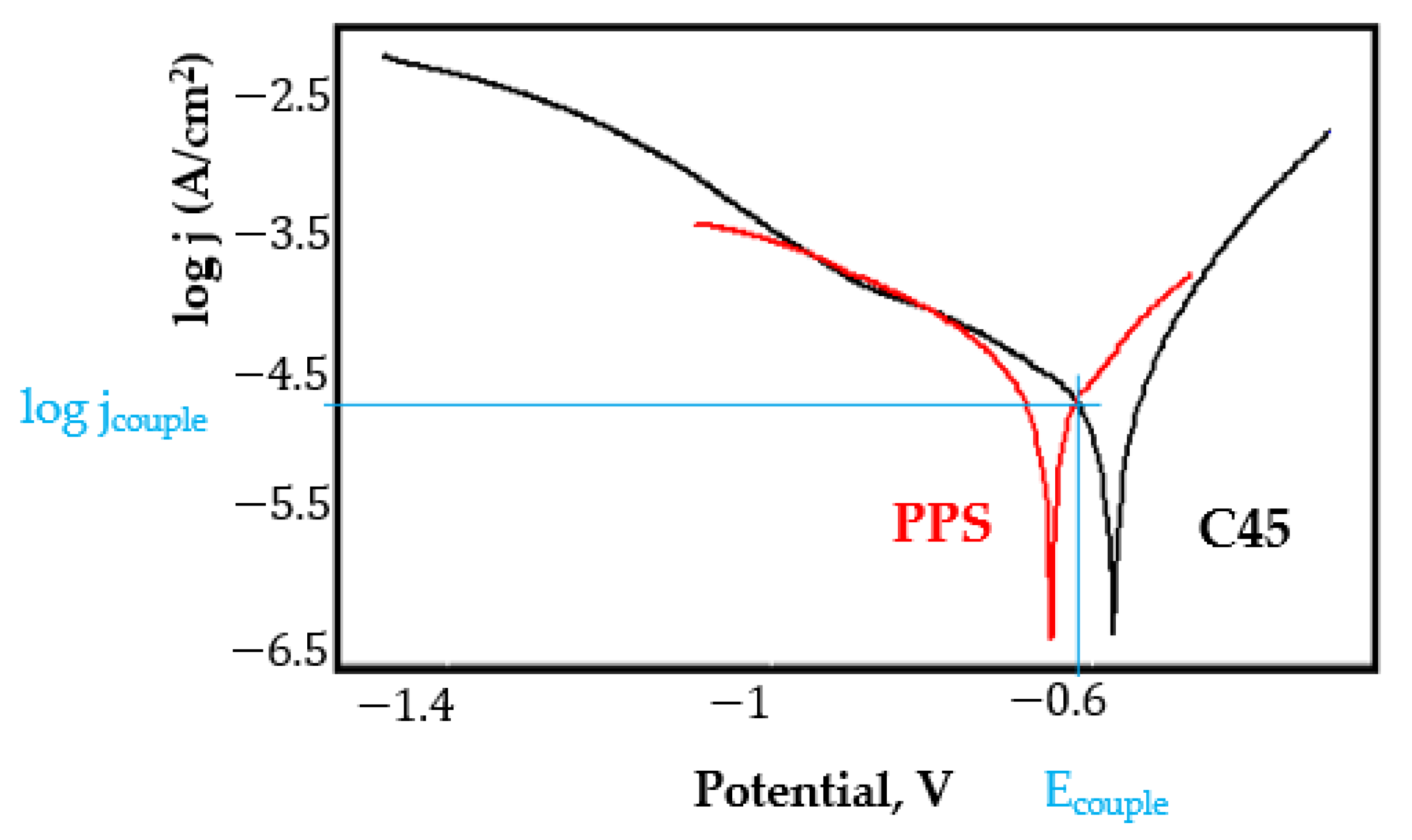
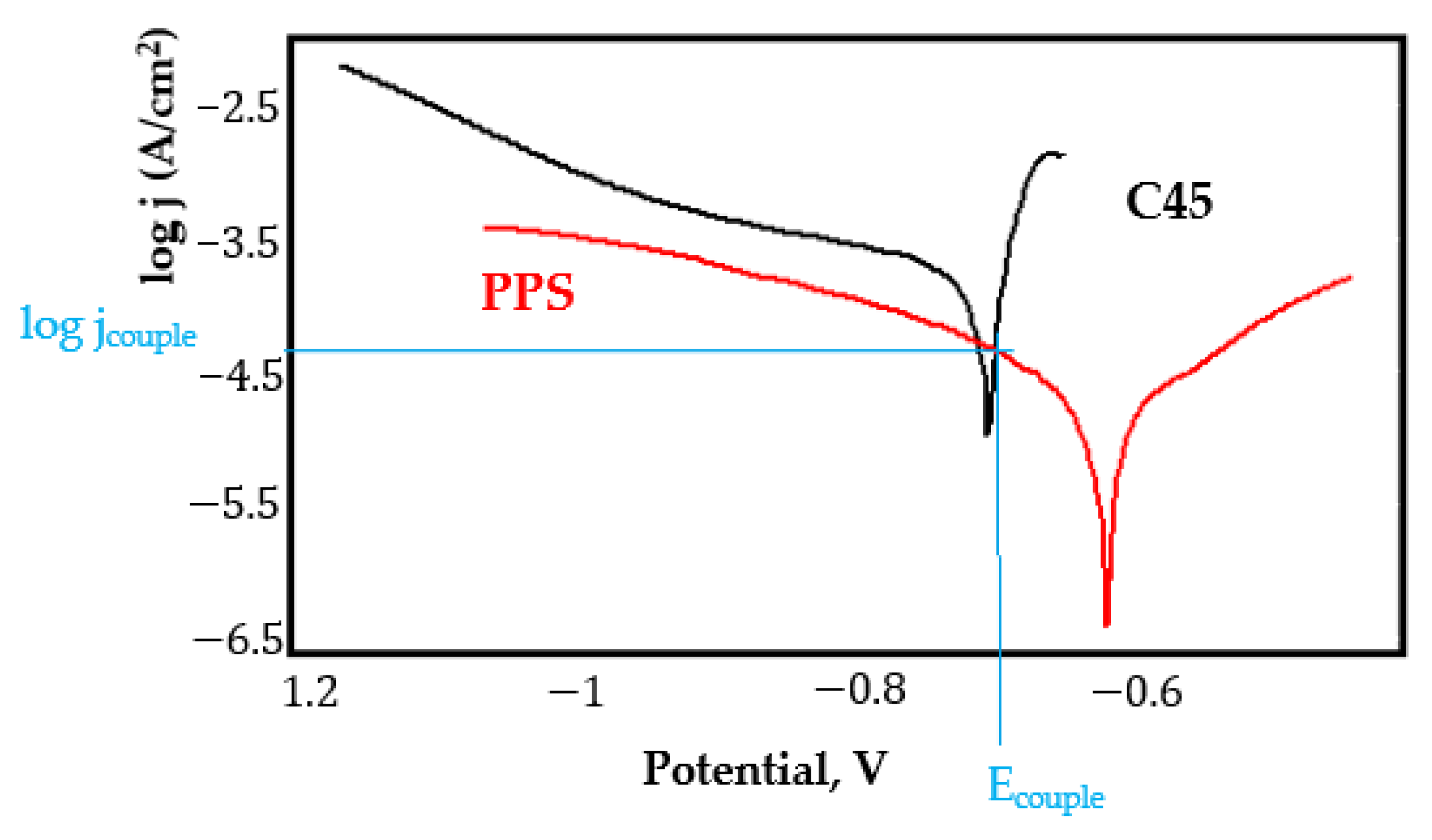
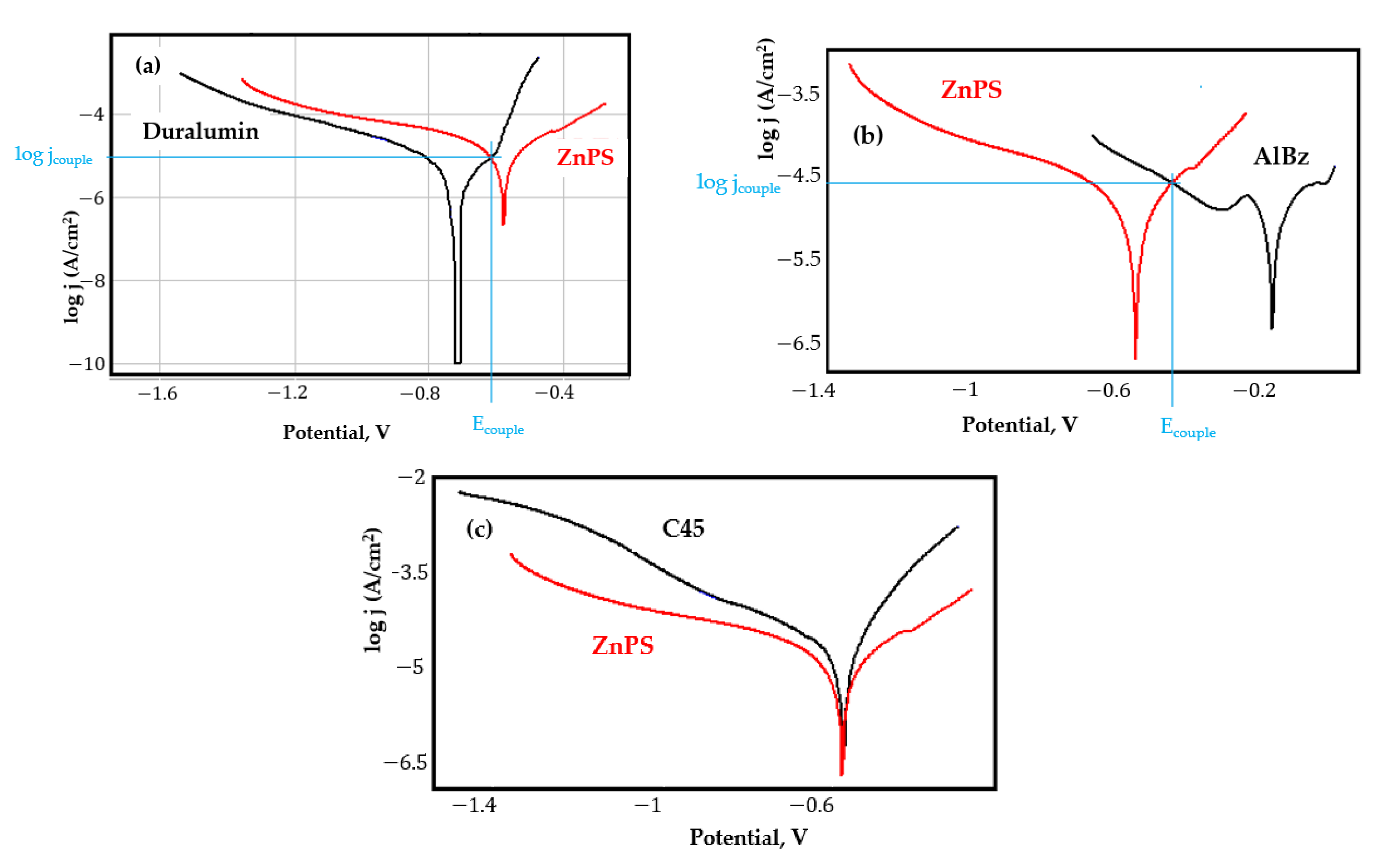
- In the figures presented in Appendix A, the polarisation curves are very close, almost overlapping, so that the couple potential is very close to the corrosion potential of the samples, for samples C45, Zn/FePS, MnPS, and LPS, the differences being in the limit of experimental errors and only for samples ZnPS (Figure 5a) and PPS couple potential is shifted to more negative values and duralumin corrodes at a higher speed. These results can be explained by correlating with the previous study results [15]. Comparing all three phosphate samples from the point of view of the polarisation resistance, the MnPS samples has the lower corrosion resistance, while the ZnPS samples have the best corrosion resistance, due to the clogging of pores resulting in the passivation of the ZnPS sample.
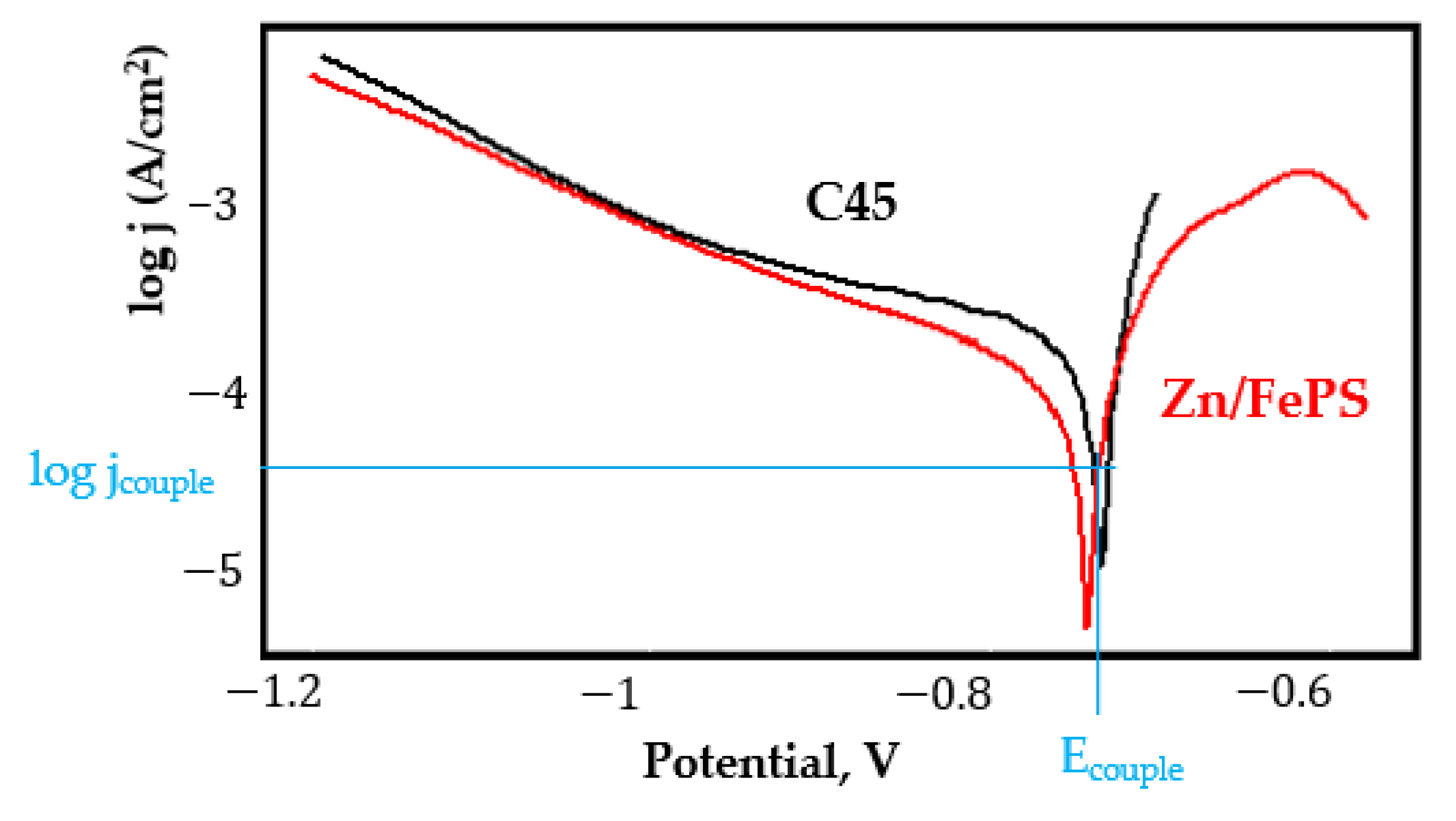
- Regarding the couple current density and therefore the corrosion rate, regardless of the value of the couple potential, the corrosion rate of C45, phosphate samples, and the lubricated sample is considerably reduced by coupling with duralumin in FES;
- In the case of the painted sample by coupling with duralumin, its corrosion rate increases even if the corrosion potential is shifted to more negative values. In the EIS results were observed that the PPS sample has a lower corrosion resistance compared with the ZnPS sample, due to the fact that a layer of iron oxide was formed under the paint layer [16].
- For all galvanic couplings the corrosion potentials are shifted relatively slightly to more positive values than the corrosion potentials of uncoupled samples;
- Although in all couples the aluminium bronze is the nobler alloy, the couple current density changes randomly, i.e., it does not change in the same direction for all couples. Thus, in the case of galvanic couplings between aluminium bronze and C45, ZnPS, and MnPS, the couple potential and corrosion rate decrease by coupling, while for couplings with Zn/FePS, LPS, and PPS the couple potential and corrosion rate increase by coupling in FES. From a qualitative point of view, this anomaly could be explained based on important changes in the surface quality of the samples in FES, which has a complex composition and has a high adsorption capacity in the pores of the coating layers. The polarisation curves for the galvanic coupling aluminium bronze/ZnPS are presented in Figure 5b.
4. Conclusions
- The duralumin acts as an anode for the galvanic coupling between C45, ZnPS, and LPS with duralumin leading to a decrease in the corrosion rate of the studied samples;
- In the case of galvanic couplings between aluminium bronze and studied sample, the aluminium bronze is the nobler alloy and acts as the cathode, leading to an increase of 2–3 times the corrosion rate of the studied samples.
- Even if it is the most aggressive corrosive agent, the galvanic couplings with duralumin and aluminium bronze do not lead to an increase of the corrosion rate, furthermore with some exceptions the corrosion rate is decreased;
- Similar to the galvanic corrosion behaviour in saltwater, in the galvanic couplings between duralumin and the coated sample, the latter is the more electropositive alloy and duralumin corrodes;
- In saltwater, the C45 is the nobler alloy, but the electronegativity difference is relatively small (for C45/ZnPS galvanic couple the linear polarization curves overlap, galvanic corrosion, in this case, being zero).
- In fire extinguishing solution the couple current density is lower, highlighting passivation (except for PPS sample).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A

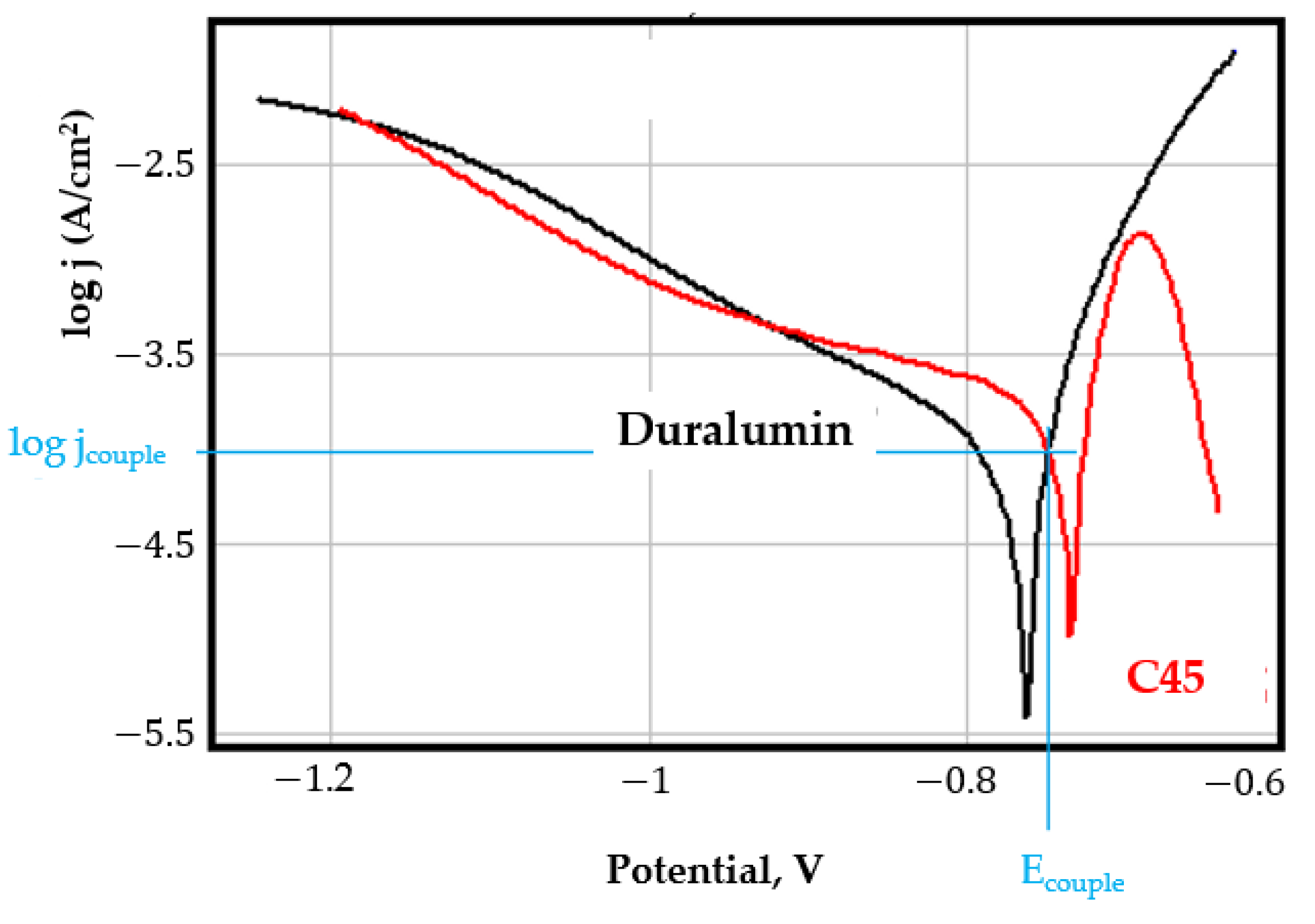
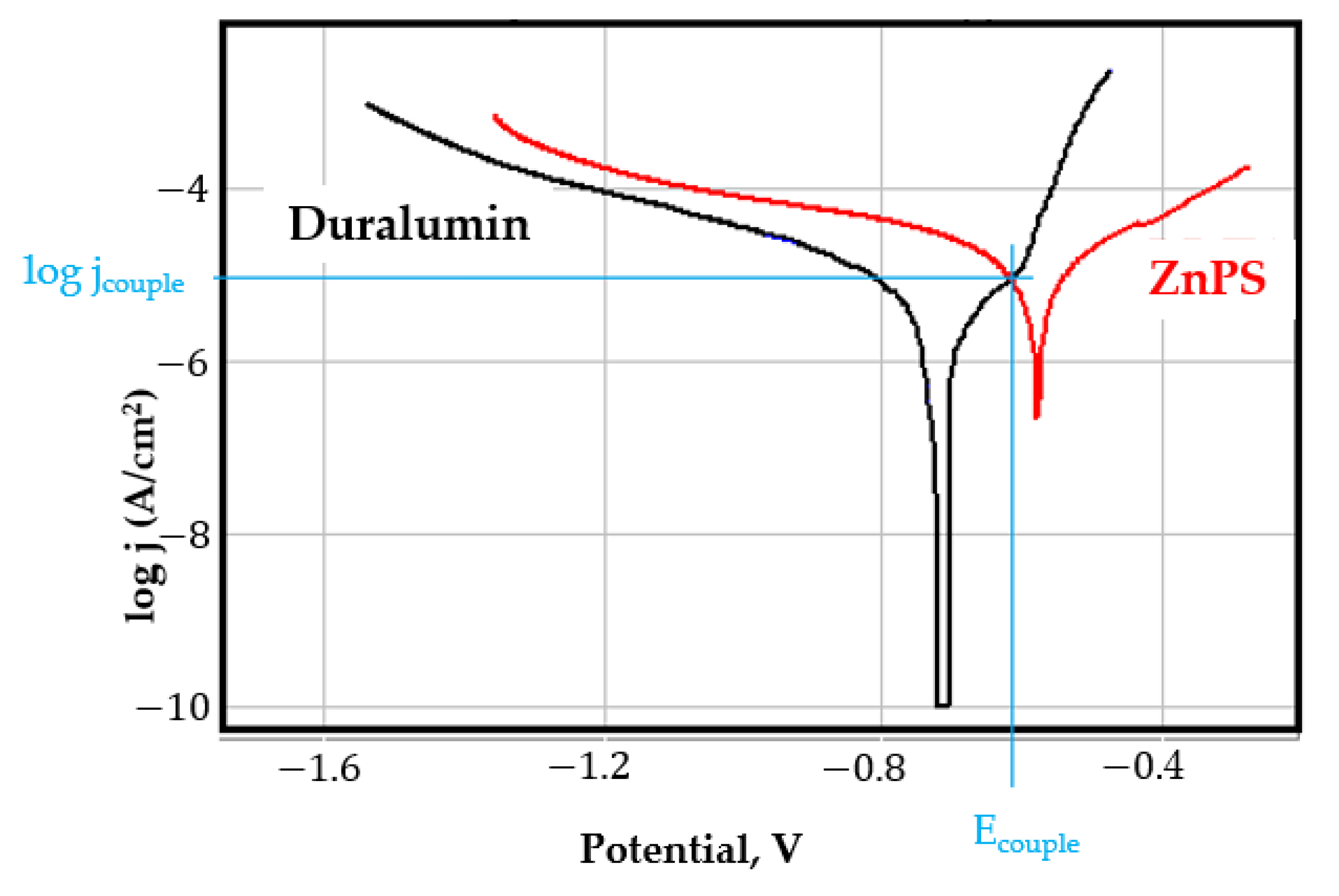

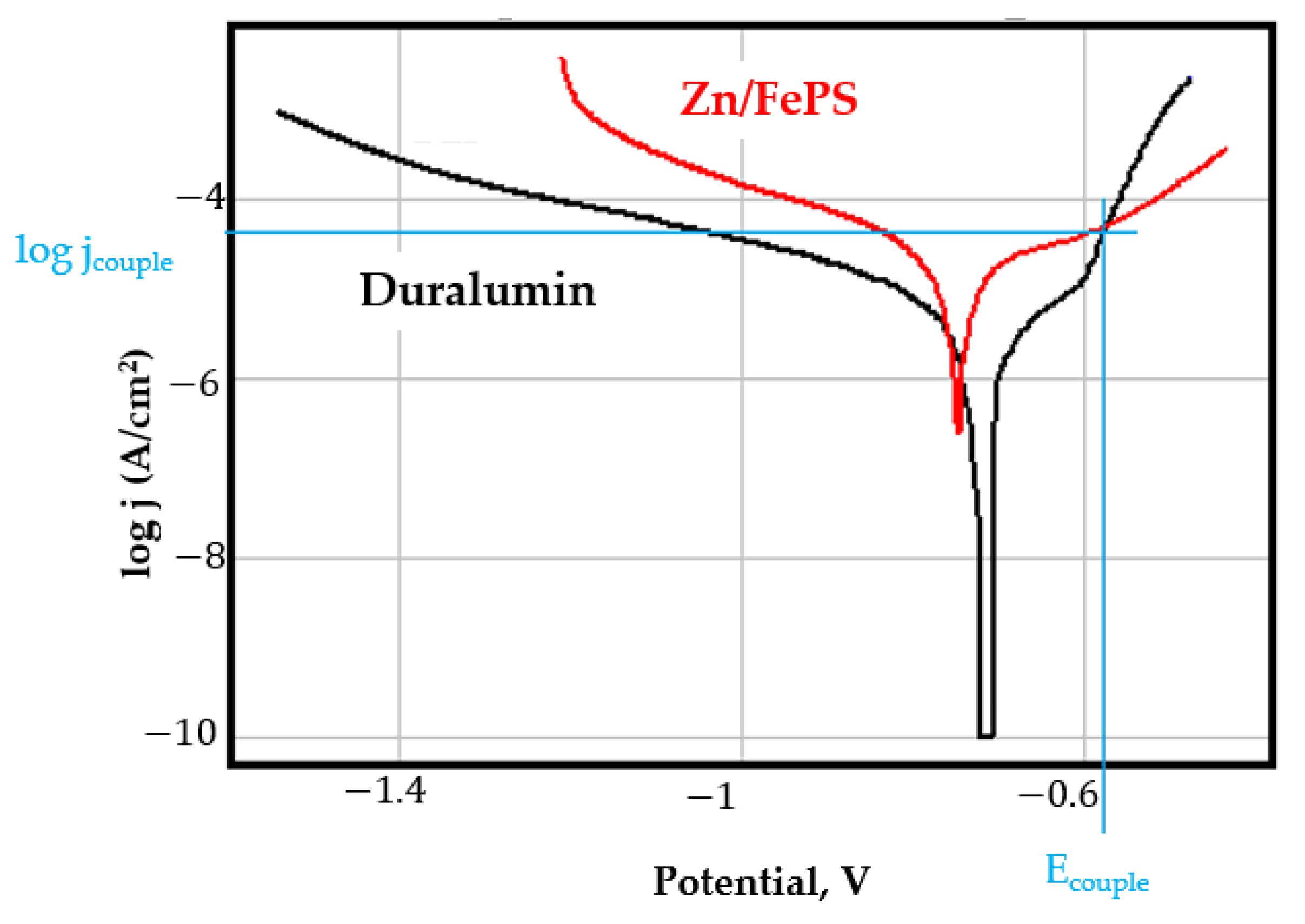
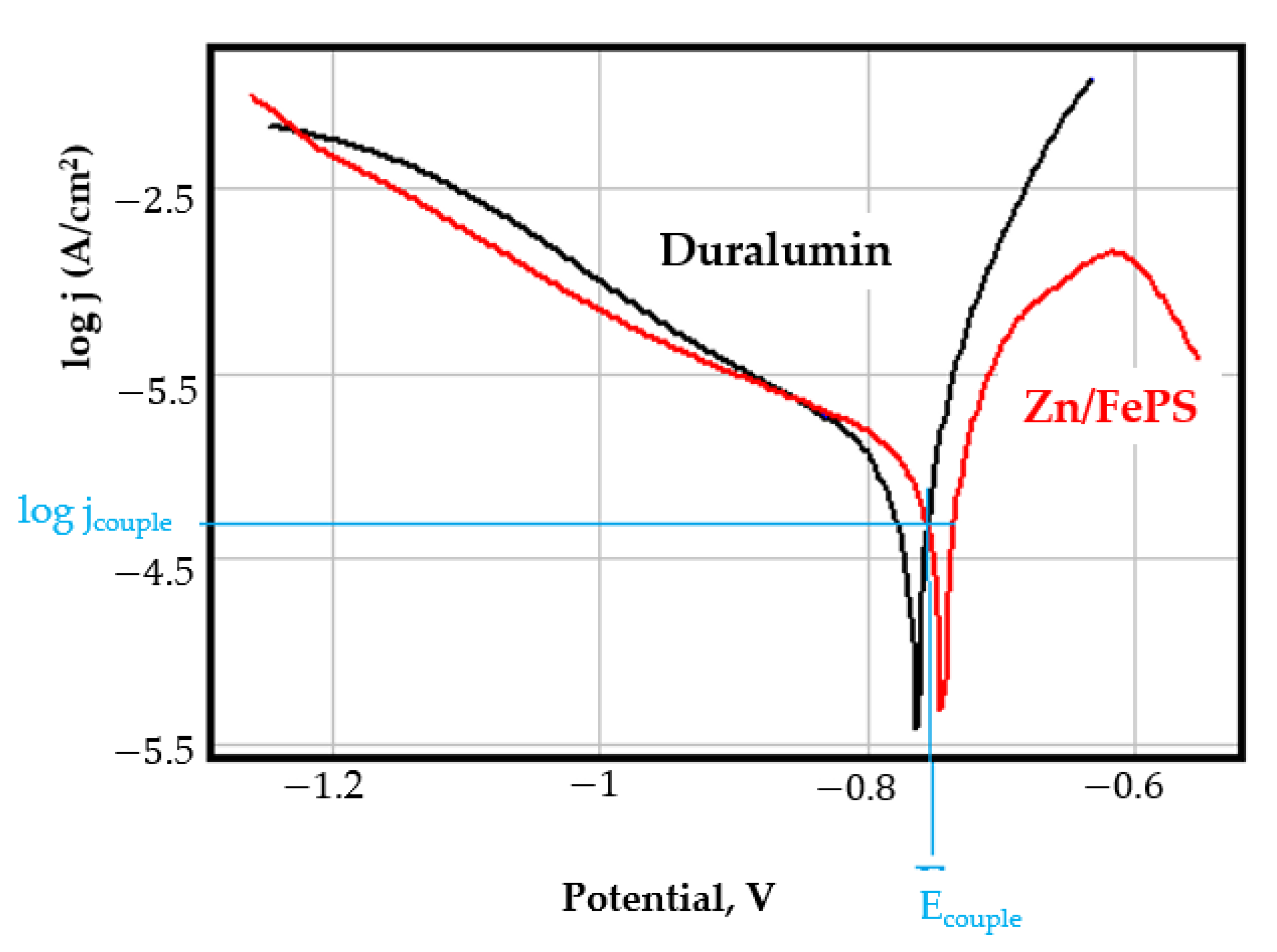
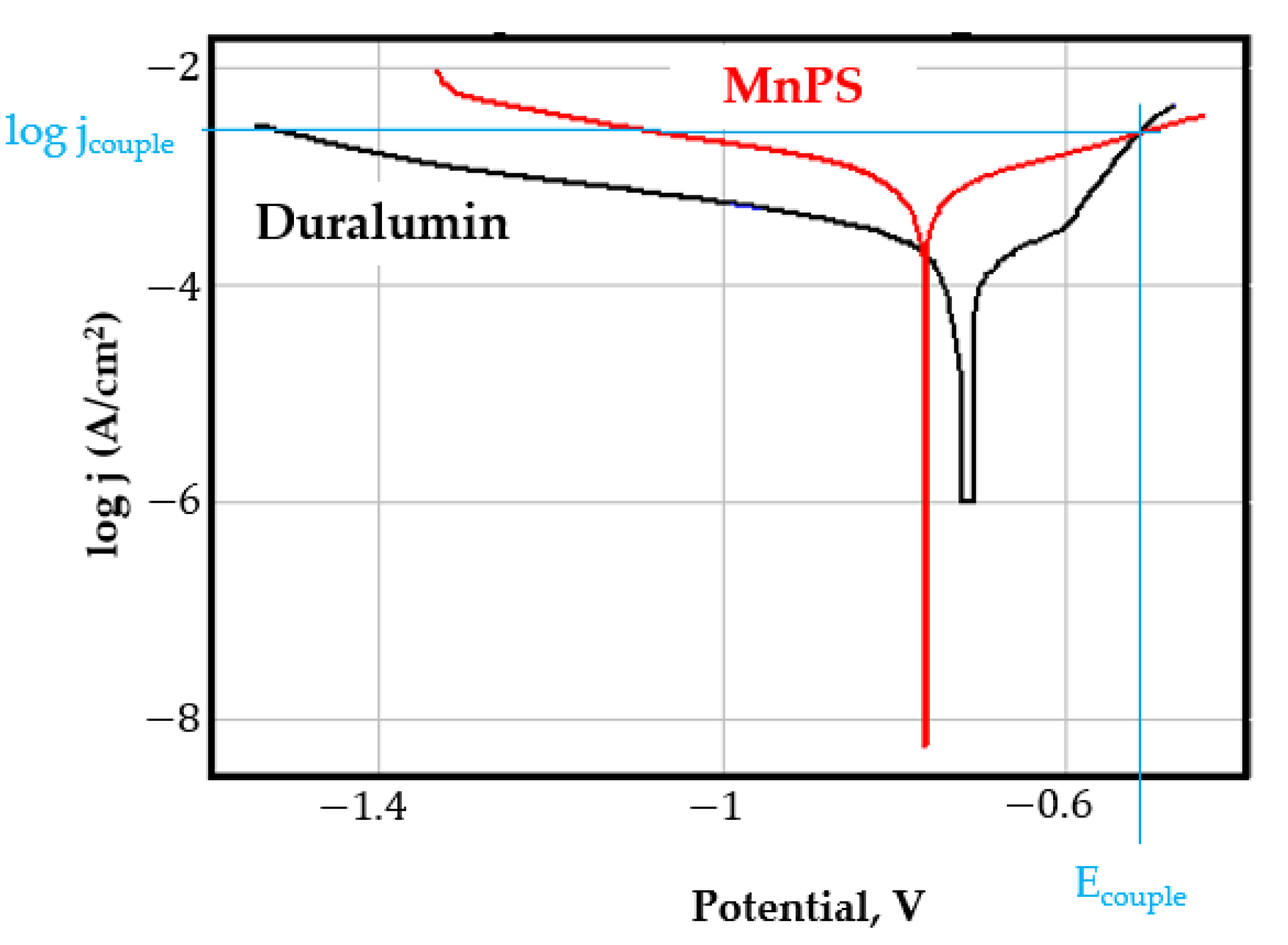
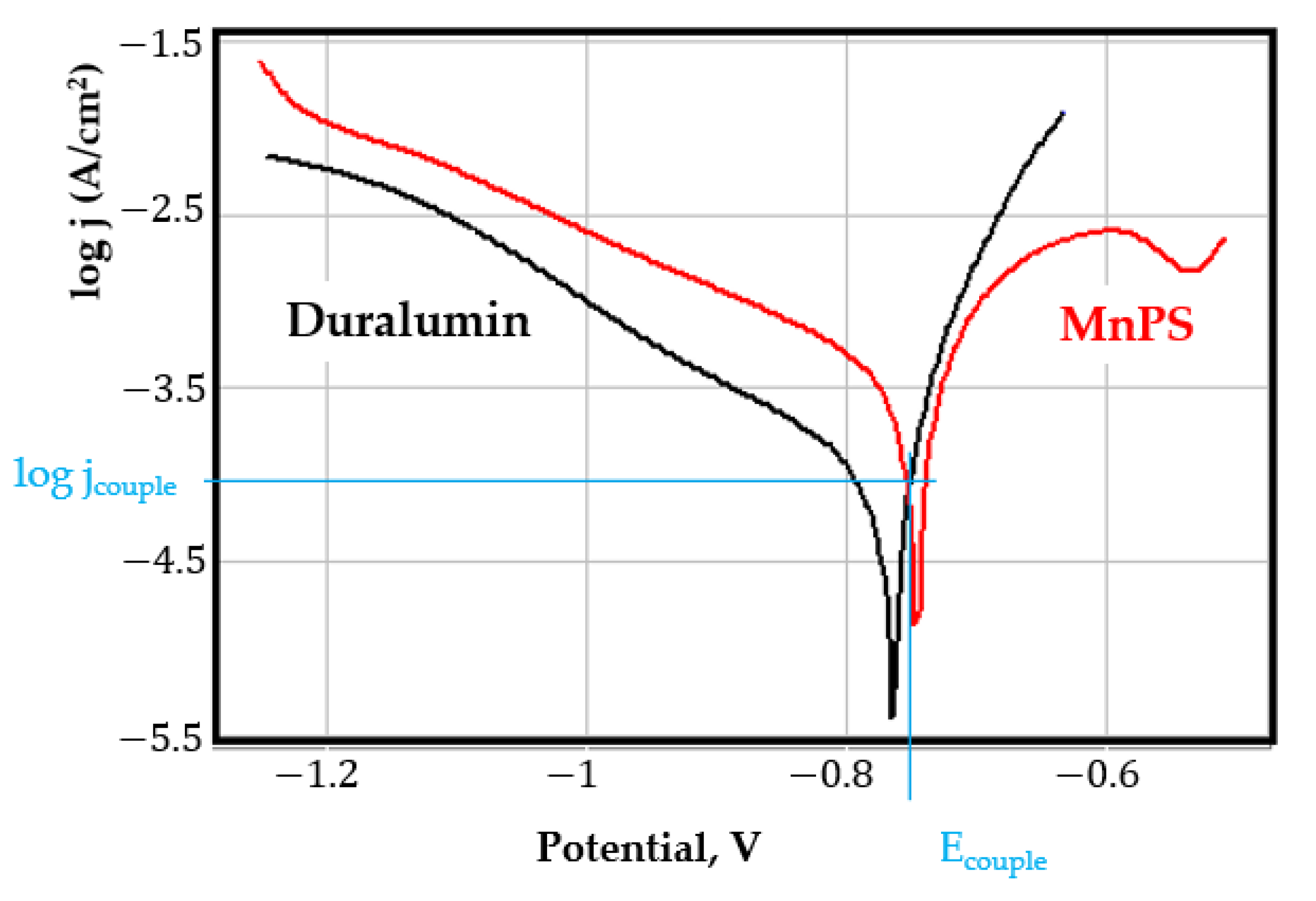
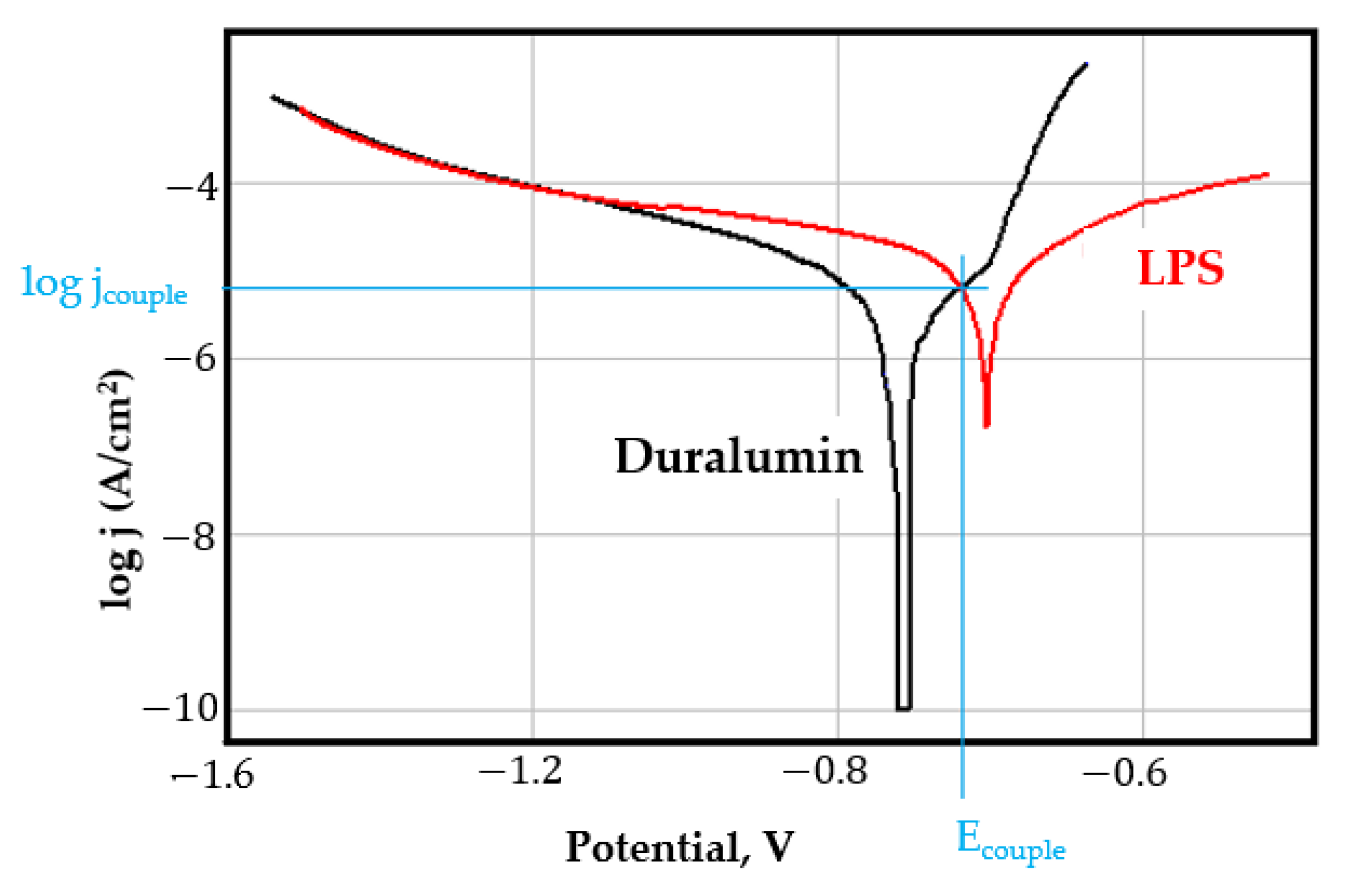
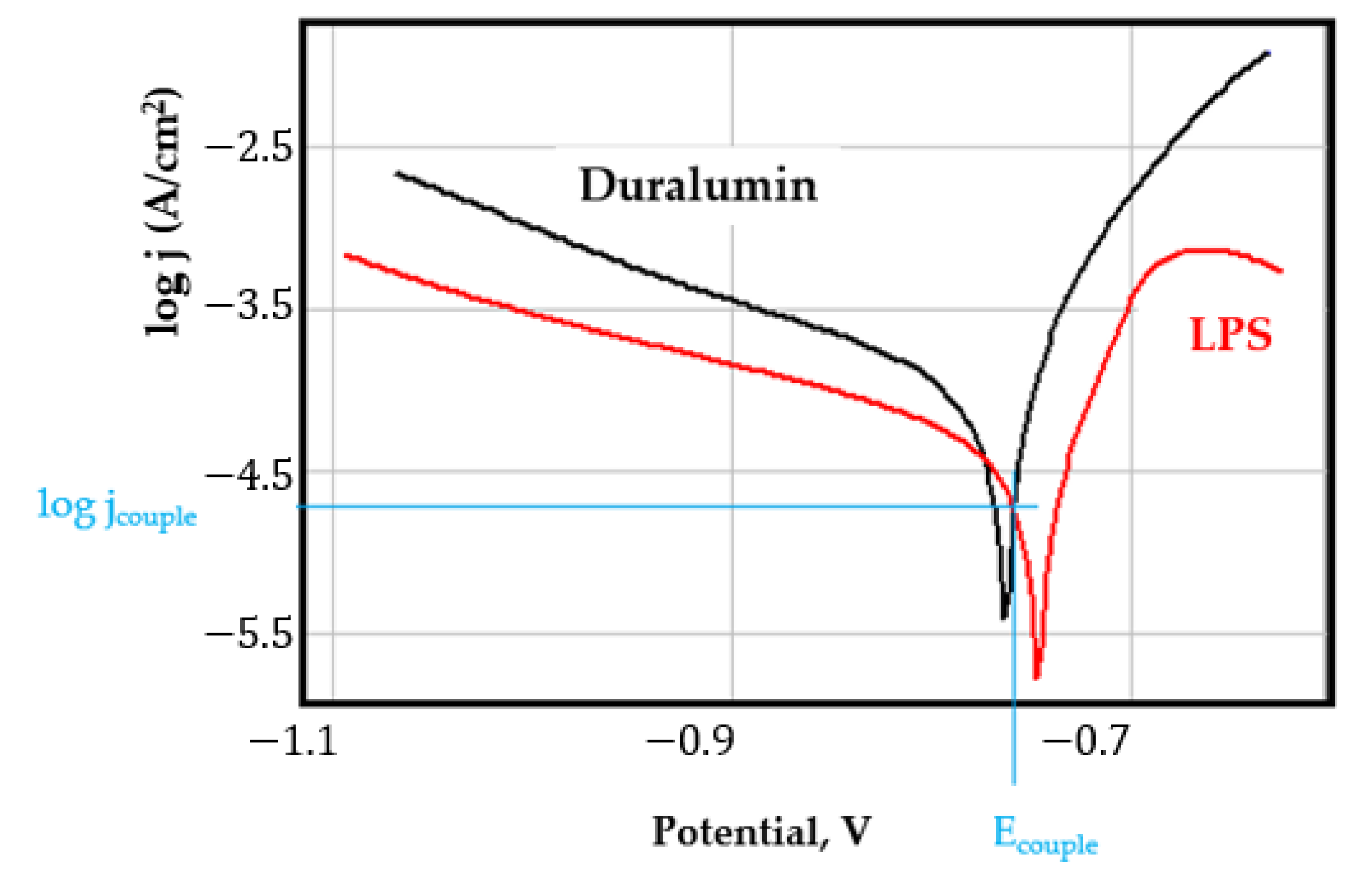
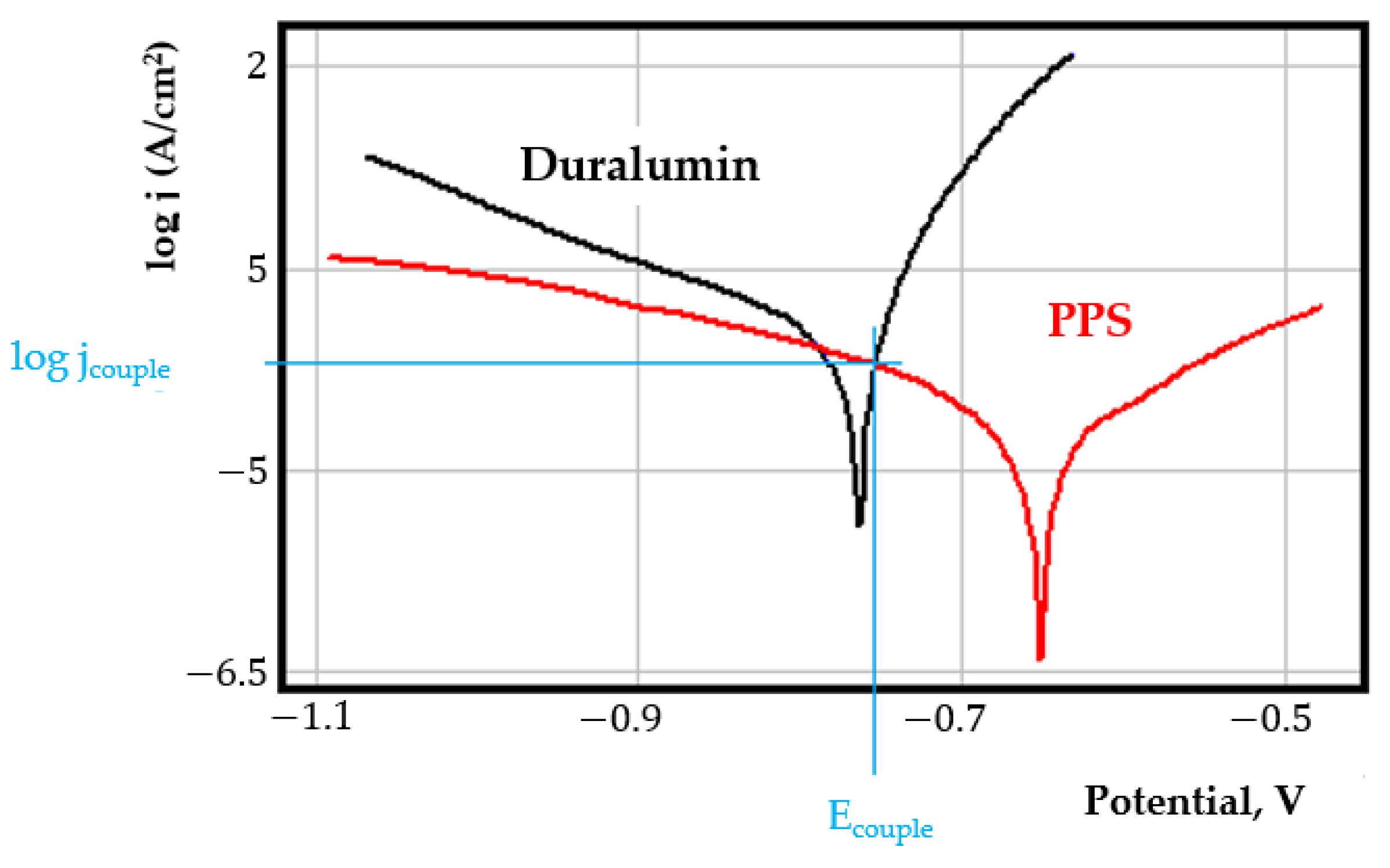



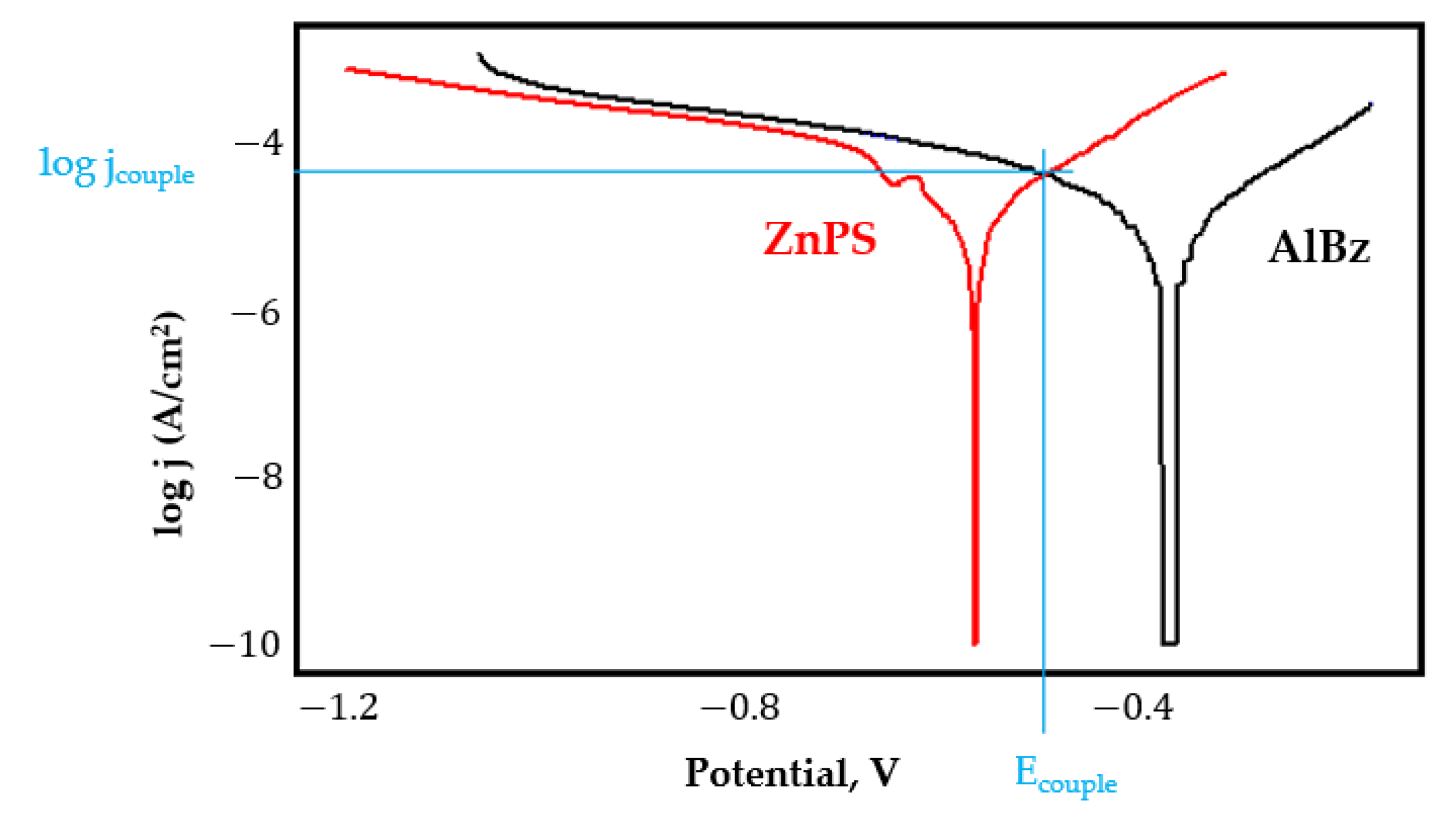
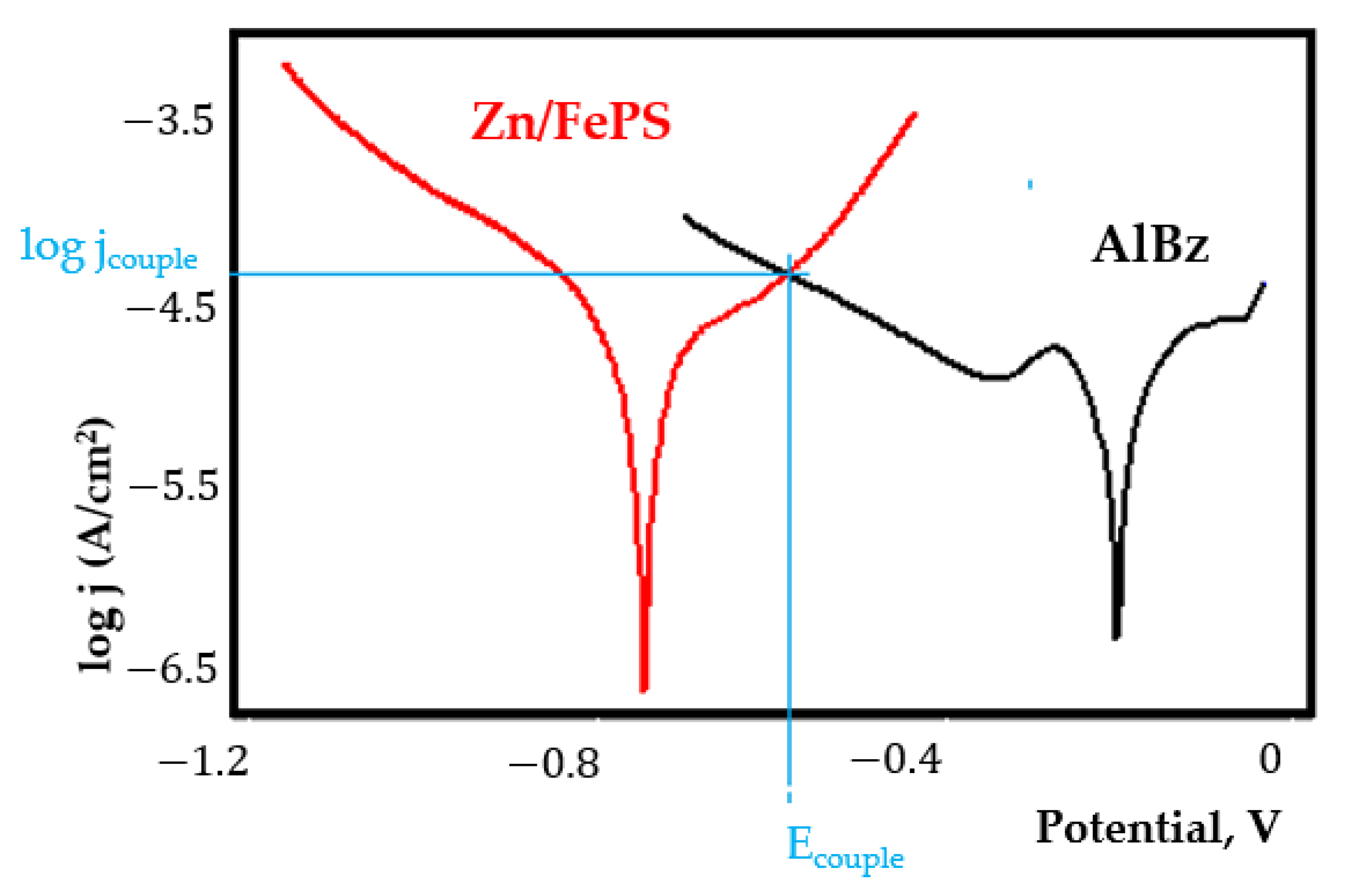
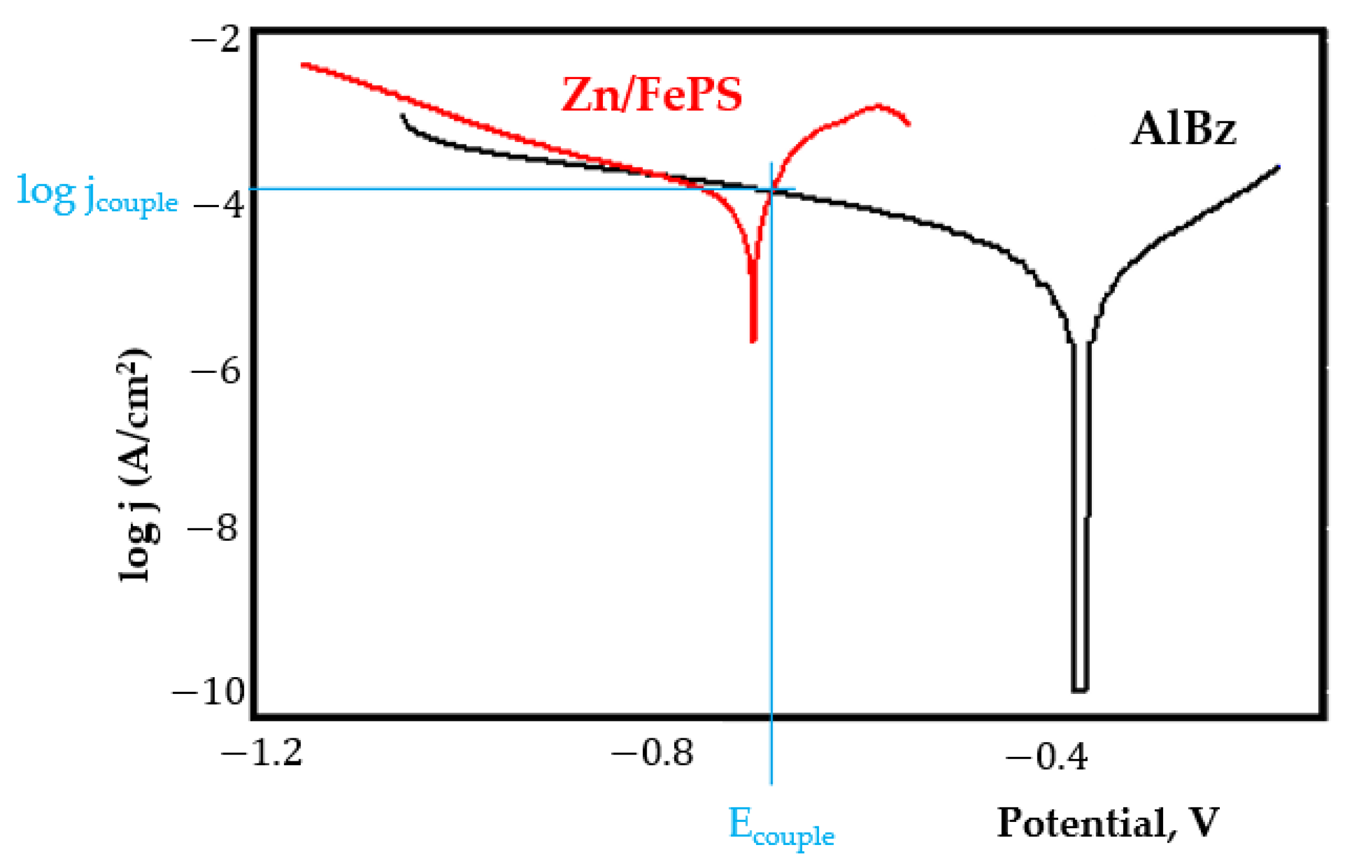
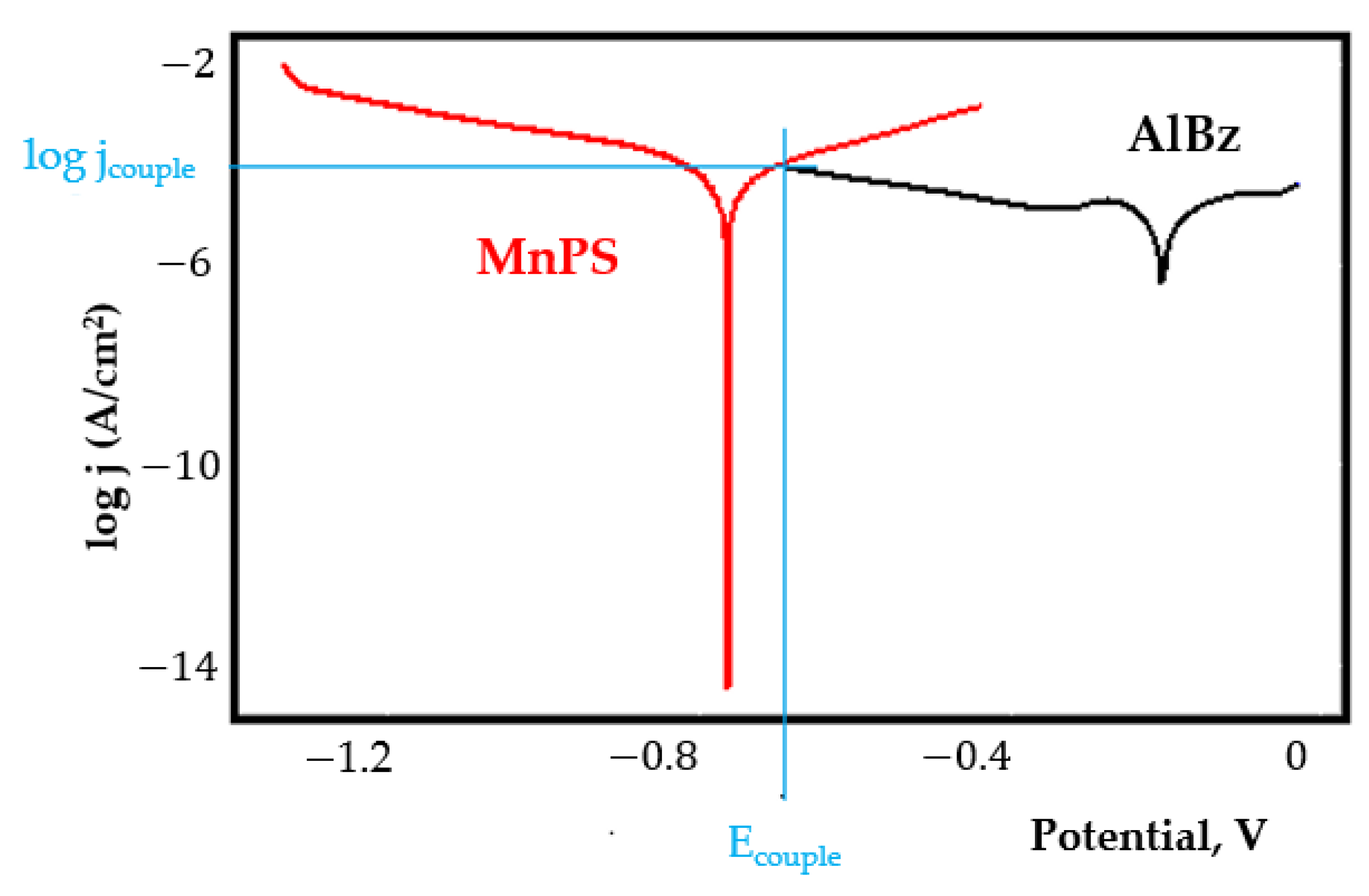
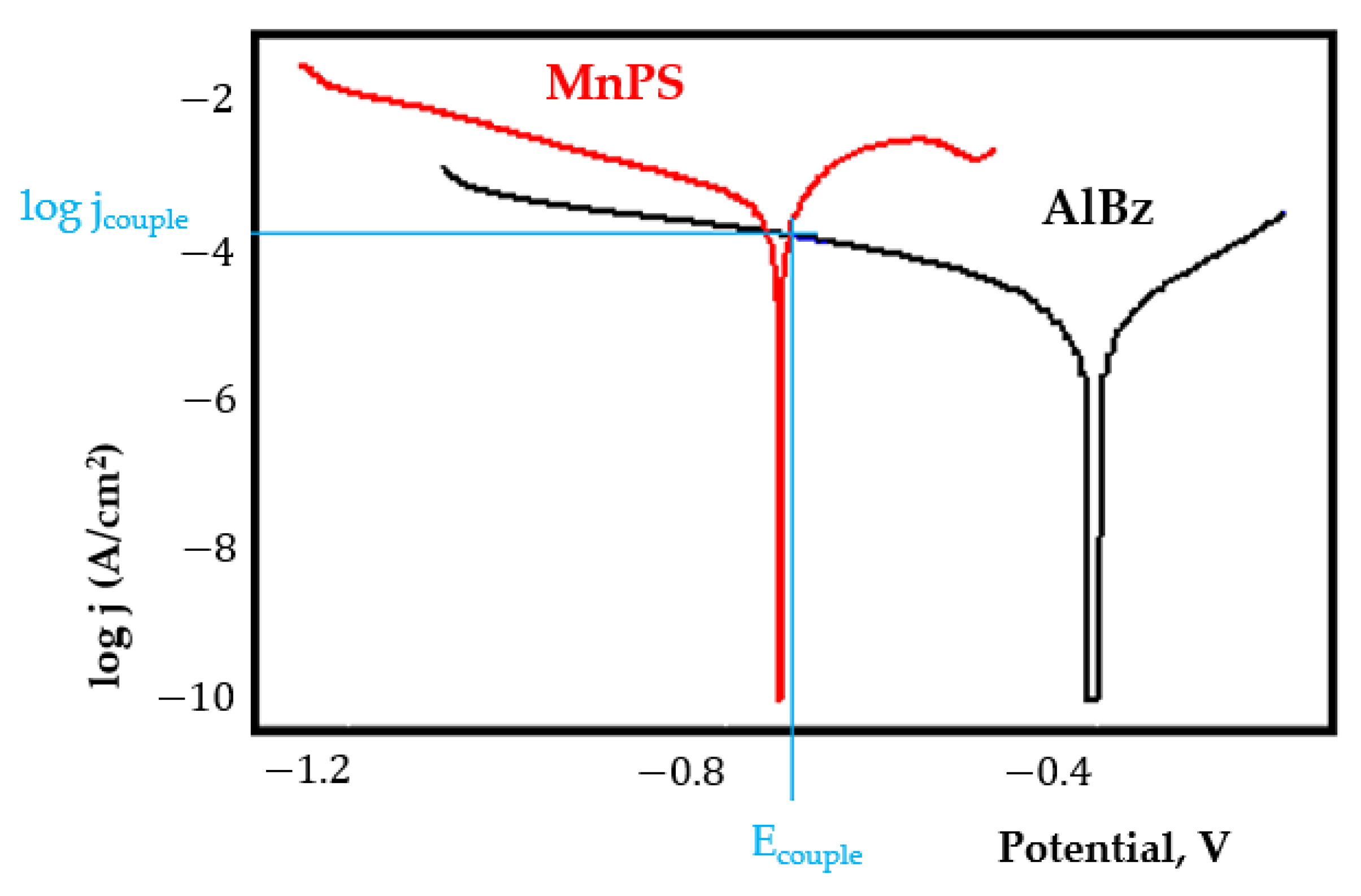


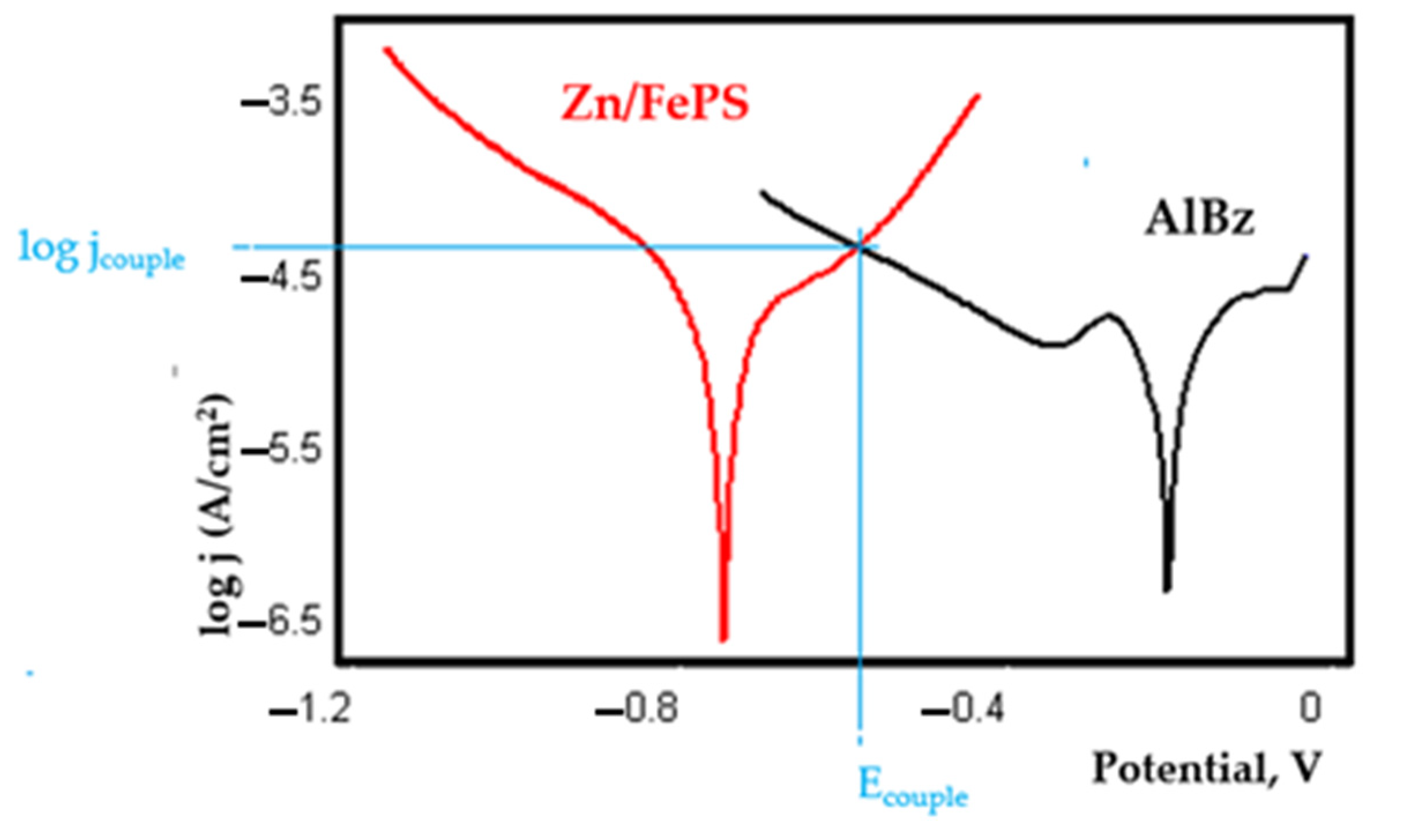
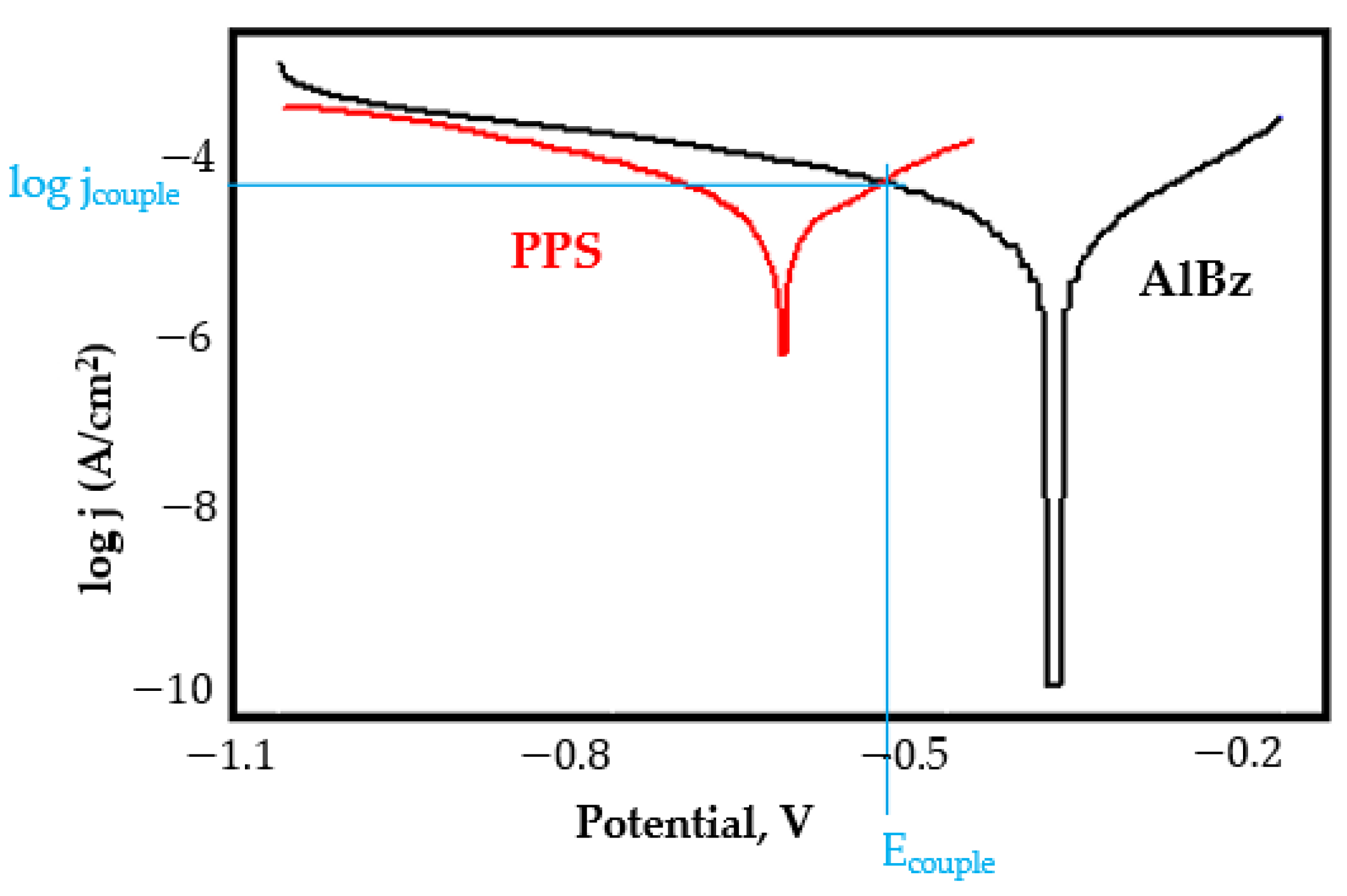


References
- Bejinariu, C.; Darabont, D.C.; Baciu, E.R.; Georgescu, I.S.; Bernevig-Sava, M.A.; Baciu, C. Considerations on Applying the Method for Assessing the Level of Safety at Work. Sustainability 2017, 9, 1263. [Google Scholar] [CrossRef] [Green Version]
- Whitacre, D.F. Additional Techniques and Safety Hints for Climbing Tall Trees, and Some Equipment and Information Sources. Biotropica 1981, 13, 286. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.P.; Baciu, C.; Vizureanu, P.; Lohan, N.M.; Bejinariu, C. Materials Types and Selection for Carabiners Manufacturing: A Review. In IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2019; Volume 572. [Google Scholar]
- Accidents at Work Statistics-Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Accidents_at_work_statistics (accessed on 16 October 2021).
- Scott, V. Design of a Composite Carabiner for Rock Climbing; Final Report; Allen Institute for AI: London, UK, 2008. [Google Scholar]
- Aliha, M.R.M.; Bahmani, A.; Akhondi, S. Fracture and Fatigue Analysis for a Cracked Carabiner Using 3D Finite Element Simulations. Strength Mater. 2015, 47, 890–902. [Google Scholar] [CrossRef]
- Kane, B.; Ryan, H.D. Residual Strength of Carabiners Used by Tree Climbers|Request PDF. Arboric. Urban For. 2009, 35, 75–79. [Google Scholar]
- Lehner, S.; Senner, V. Evaluation of Ergonomics of a New Effort Saving Via-Ferrata Carabiner—Child vs. Adult Use. In Procedia Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 60, pp. 319–324. [Google Scholar]
- Jackovics, P. Analysis with applied statistics of the safety use of rope rescue equipment. Int. J. Occup. Saf. Ergon. 2019, 26, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Qian, J.; Ji, Z.; Dai, X.; Li, Z. The Protective Effect of Magnesium Phosphate Cement on Steel Corrosion. Constr. Build. Mater. 2020, 255, 119422. [Google Scholar] [CrossRef]
- Fedosov, S.; Roumyantseva, V.; Konovalova, V. Phosphate Coatings as a Way to Protect Steel Reinforcement from Corrosion. MATEC Web Conf. 2019, 298, 00126. [Google Scholar] [CrossRef]
- Guenbour, A.; Benbachir, A.; Kacemi, A. Evaluation of the Corrosion Performance of Zinc-Phosphate-Painted Carbon Steel. Surf. Coat. Technol. 1999, 113, 36–43. [Google Scholar] [CrossRef]
- Teixeira, C.H.S.B.; Alvarenga, E.A.; Vasconcelos, W.L.; Lins, V.F.C. Effect of Porosity of Phosphate Coating on Corrosion Resistance of Galvanized and Phosphated Steels Part I: Measurement of Porosity of Phosphate. Mater. Corros. 2011, 62, 771–777. [Google Scholar] [CrossRef]
- Bejinariu, C.; Burduhos-Nergis, D.-P.; Cimpoesu, N. Immersion Behavior of Carbon Steel, Phosphate Carbon Steel and Phosphate and Painted Carbon Steel in Saltwater. Materials 2021, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Burduhos-Nergis, D.P.; Vizureanu, P.; Sandu, A.V.; Bejinariu, C. Evaluation of the Corrosion Resistance of Phosphate Coatings Deposited on the Surface of the Carbon Steel Used for Carabiners Manufacturing. Appl. Sci. 2020, 10, 2753. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.P.; Vasilescu, G.D.; Burduhos-Nergis, D.D.; Cimpoesu, R.; Bejinariu, C. Phosphate Coatings: EIS and SEM Applied to Evaluate the Corrosion Behavior of Steel in Fire Extinguishing Solution. Appl. Sci. 2021, 11, 7802. [Google Scholar] [CrossRef]
- Urbikain, G.; Perez, J.M.; López de Lacalle, L.N.; Andueza, A. Combination of friction drilling and form tapping processes on dissimilar materials for making nutless joints. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2016, 232, 1007–1020. [Google Scholar] [CrossRef]
- Song, G.; Johannesson, B.; Hapugoda, S.; StJohn, D. Galvanic corrosion of magnesium alloy AZ91D in contact with an aluminium alloy, steel and zinc. Corros. Sci. 2004, 46, 955–977. [Google Scholar] [CrossRef]
- Okonkwo, B.O.; Ming, H.; Wang, J.; Han, E.H.; Rahimi, E.; Davoodi, A.; Hosseinpour, S. A new method to determine the synergistic effects of area ratio and microstructure on the galvanic corrosion of LAS A508/309 L/308 L SS dissimilar metals weld. J. Mater. Sci. Technol. 2021, 78, 38–50. [Google Scholar] [CrossRef]
- Kwok, C.T.; Fong, S.L.; Cheng, F.T.; Man, H.C. Pitting and galvanic corrosion behavior of laser-welded stainless steels. J. Mater. Process. Technol. 2006, 176, 168–178. [Google Scholar] [CrossRef]
- Zhou, H.; Fu, F.; Dai, Z.; Qiao, Y.; Chen, J.; Liu, W. Effect of Laser Power on Microstructure and Micro-Galvanic Corrosion Behavior of a 6061-T6 Aluminum Alloy Welding Joints. Metals 2020, 11, 3. [Google Scholar] [CrossRef]
- Zhou, Y.; Qi, J.; Engelberg, D.L. On the application of bipolar electrochemistry for simulating galvanic corrosion behaviour of dissimilar stainless steels. Electrochem. Commun. 2021, 126, 107023. [Google Scholar] [CrossRef]
- Nejneru, C.; Cristina Perju, M.; Doru Burduhos Nergis, D.; Victor Sandu, A.; Bejinariu, C. Galvanic Corrosion Behaviour of Phosphate Nodular Cast Iron in Different Types of Residual Waters and Couplings. Rev. Chim. 2019, 70, 3597–3602. [Google Scholar] [CrossRef]
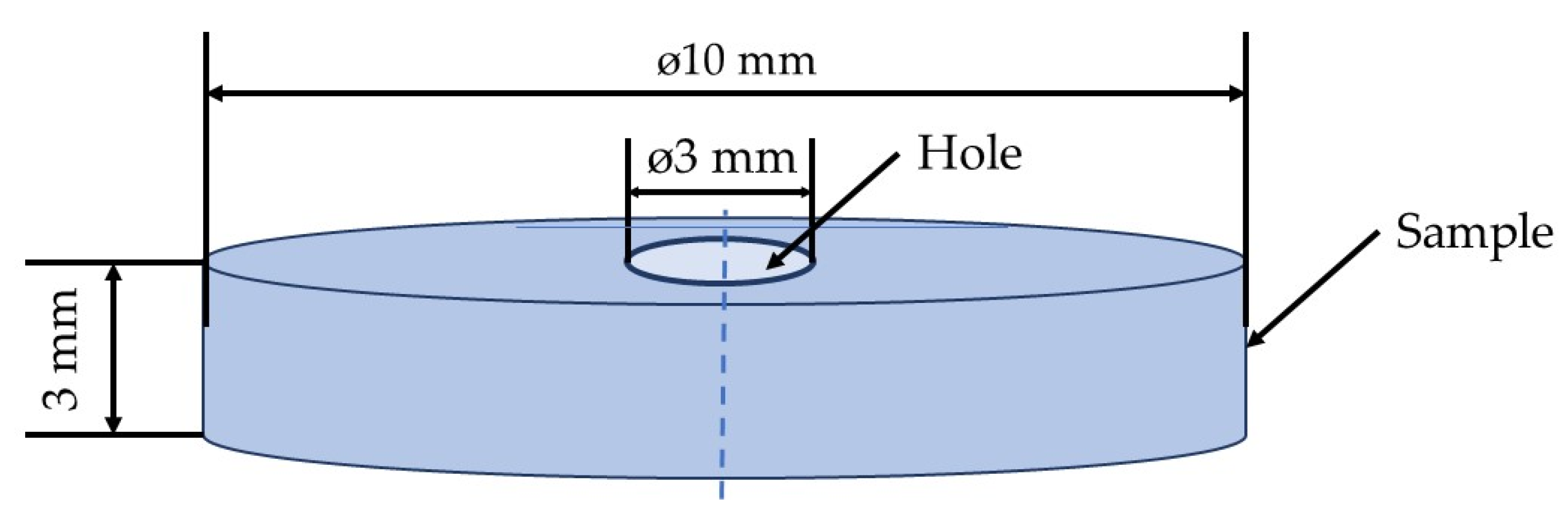
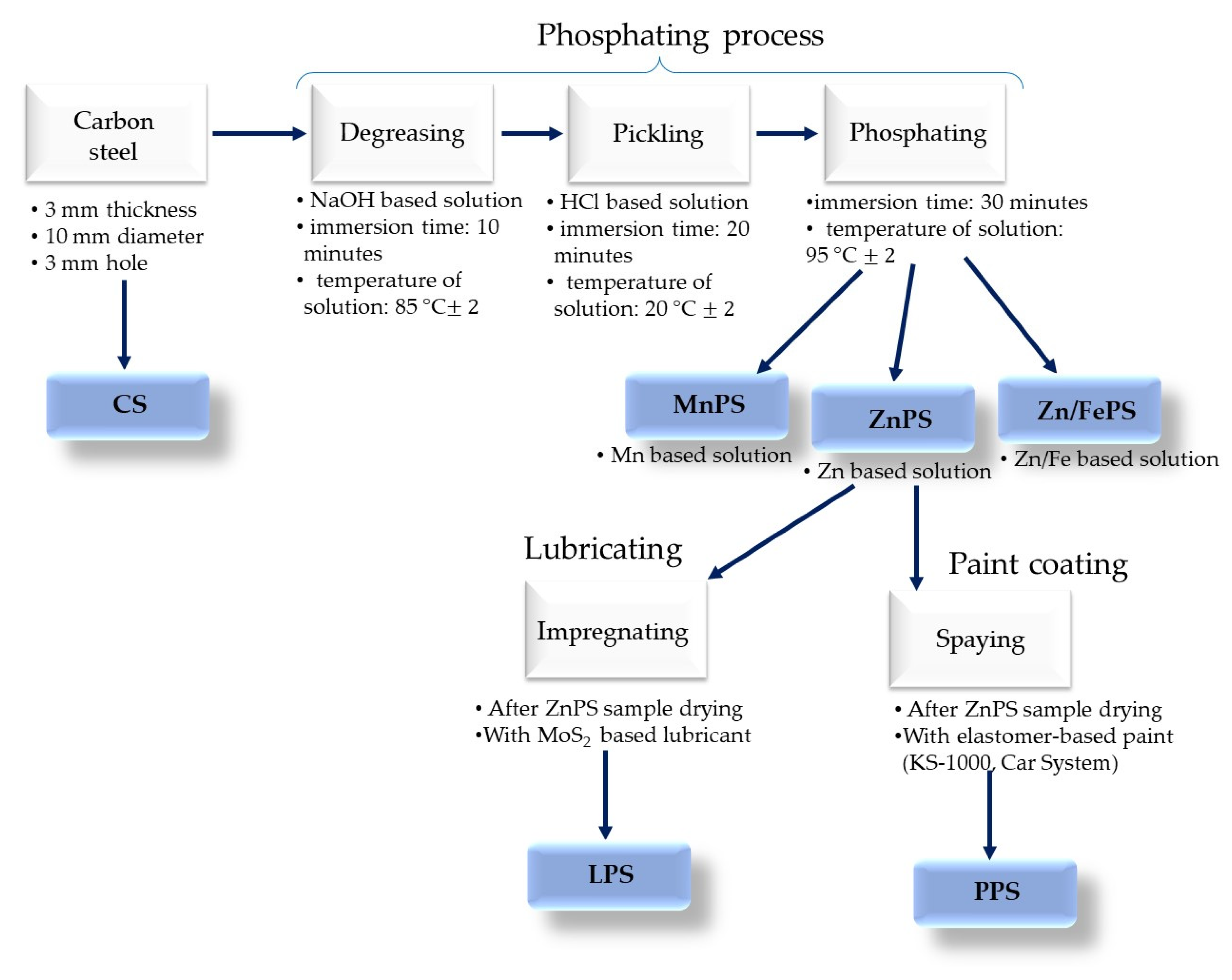

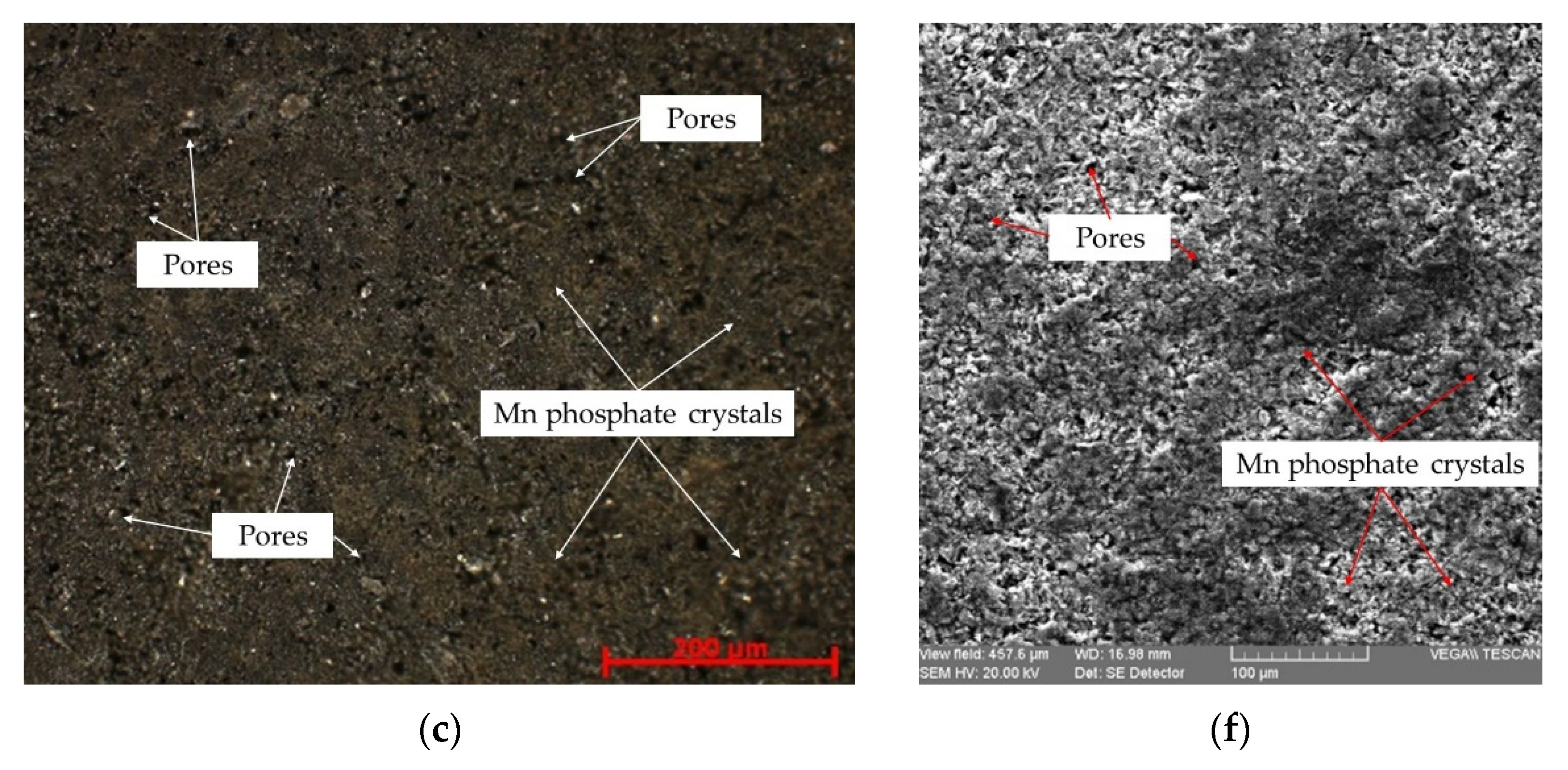

| Sample ID | Sample Description |
|---|---|
| CS | C45 carbon steel sample |
| ZnPS | Phosphate carbon steel sample obtained using a zinc-based solution |
| Zn/FePS | Phosphate carbon steel sample obtained using a zinc-iron based solution |
| MnPS | Phosphate carbon steel sample obtained using a manganese-based solution |
| PPS | ZnPS sample on which a layer of elastomer-based paint was deposited |
| LPS | ZnPS sample impregnated by MoS2 based lubricant |
| Duralumin | Aluminium Bronze | ||
|---|---|---|---|
| Element | wt.% | Element | wt.% |
| Aluminium (Al) | 90.3 | Copper (Cu) | 82.42 |
| Zinc (Zn) | 5.41 | Aluminium (Al) | 9.95 |
| Magnesium (Mg) | 2.26 | Nickel (Ni) | 4.15 |
| Copper (Cu) | 1.50 | Iron (Fe) | 2.13 |
| Other elements | 0.53 | Manganese (Mn) | 1.35 |
| Sample | Ecor mV | Ecouple mV | ΔE mV | jcor µA/cm2 | jcouple µA/cm2 | vcor (Uncoupled) µm/year | vcor (Coupled) µm/year |
|---|---|---|---|---|---|---|---|
| C45 | −578 | −600 | −22 | 15.97 | 11.99 | 194.1 | 135.73 |
| ZnPS | −580 | −615 | −35 | 18.41 | 9.162 | 223.9 | 111.43 |
| Zn/FePS | −748 | −573 | +175 | 15.94 | 50.12 | 193.8 | 609.36 |
| MnPS | −766 | −517 | +249 | 51.26 | 601.1 | 623.5 | 7311 |
| LPS | −581 | −637 | −56 | 9.38 | 6.43 | 114.0 | 78.15 |
| PPS | −163 | - | - | 57.91 | - | 704.4 | - |
| Sample | Ecor mV | Ecouple mV | ΔE mV | jcor µA/cm2 | jcouple µA/cm2 | vcor (Uncoupled) µm/year | vcor (Coupled) µm/year |
|---|---|---|---|---|---|---|---|
| C45 | −578 | −529 | +49 | 15.97 | 34.43 | 194.1 | 418.5 |
| ZnPS | −580 | −479 | +101 | 18.41 | 25.35 | 223.9 | 674.9 |
| Zn/FePS | −748 | −584 | +164 | 15.94 | 45.81 | 193.8 | 557.0 |
| MnPS | −766 | −693 | +73 | 51.26 | 95.94 | 623.5 | 1167 |
| LPS | −581 | −482 | +99 | 9.38 | 26.79 | 114.0 | 326.9 |
| PPS | −163 | - | - | 57.91 | - | 704.4 | - |
| Sample | Ecor mV | Ecouple mV | ΔE mV | jcor µA/cm2 | jcouple µA/cm2 | vcor (Uncoupled) µm/year | vcor (Coupled) µm/year |
|---|---|---|---|---|---|---|---|
| ZnPS | −580 | −580 | 0 | 18.41 | 18.41 | 223.9 | 223.9 |
| Zn/FePS | −748 | −644 | +104 | 15.94 | 28.18 | 193.8 | 342.6 |
| MnPS | −766 | −737 | +29 | 51.26 | 59.84 | 623.5 | 727.9 |
| LPS | −581 | −582 | −1 | 9.38 | 3.17 | 114.0 | 38.52 |
| PPS | −163 | −619 | −456 | 57.91 | 20.46 | 704.4 | 248.9 |
| Sample | Ecor mV | Ecouple mV | ΔE mV | jcor µA/cm2 | jcouple µA/cm2 | vcor (Uncoupled) µm/year | vcor (Coupled) µm/year |
|---|---|---|---|---|---|---|---|
| C45 | −743 | −748 | −5 | 183.4 | 100.0 | 2230 | 1216 |
| ZnPS | −605 | −749 | −144 | 146.3 | 100.2 | 1779 | 1218 |
| Zn/FePS | −748 | −746 | +2 | 107.9 | 48.42 | 1312 | 588.8 |
| MnPS | −749 | −751 | −2 | 355.4 | 77.09 | 4323 | 936.6 |
| LPS | −752 | −759 | −7 | 42.49 | 18.97 | 516 | 230.4 |
| PPS | −655 | −753 | −98 | 20.02 | 60.26 | 243.4 | 732.6 |
| Sample | Ecor mV | Ecouple mV | ΔE mV | jcor µA/cm2 | jcouple µA/cm2 | vcor (Uncoupled) µm/year | vcor (Coupled) µm/year |
|---|---|---|---|---|---|---|---|
| C45 | −743 | −719 | +24 | 183.4 | 142.9 | 2230 | 1737 |
| ZnPS | −605 | −528 | +77 | 146.3 | 42.27 | 1779 | 519.8 |
| Zn/FePS | −748 | −716 | +32 | 107.9 | 136.1 | 1312 | 1652 |
| MnPS | −749 | −731 | +18 | 355.4 | 141.6 | 4323 | 1722 |
| LPS | −752 | −710 | +42 | 42.49 | 130.6 | 516 | 1586 |
| PPS | −655 | −557 | +98 | 20.02 | 53.83 | 243.4 | 654.5 |
| Sample | Ecor mV | Ecouple mV | ΔE mV | jcor µA/cm2 | jcouple µA/cm2 | vcor (Uncoupled) µm/year | vcor (Coupled) µm/year |
|---|---|---|---|---|---|---|---|
| ZnPS | −605 | −728 | −123 | 146.3 | 82.79 | 1779 | 1006 |
| Zn/FePS | −748 | −738 | +10 | 107.9 | 29.51 | 1312 | 358.8 |
| MnPS | −749 | −740 | +9 | 355.4 | 45.50 | 4323 | 553.5 |
| LPS | −752 | −738 | +14 | 42.49 | 20.25 | 516 | 245.9 |
| PPS | −655 | −730 | +25 | 20.02 | 45.81 | 243.4 | 557.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burduhos-Nergis, D.-P.; Burduhos-Nergis, D.-D.; Bejinariu, C. Galvanic Corrosion Behaviour of Different Types of Coatings Used in Safety Systems Manufacturing. Coatings 2021, 11, 1542. https://doi.org/10.3390/coatings11121542
Burduhos-Nergis D-P, Burduhos-Nergis D-D, Bejinariu C. Galvanic Corrosion Behaviour of Different Types of Coatings Used in Safety Systems Manufacturing. Coatings. 2021; 11(12):1542. https://doi.org/10.3390/coatings11121542
Chicago/Turabian StyleBurduhos-Nergis, Diana-Petronela, Dumitru-Doru Burduhos-Nergis, and Costica Bejinariu. 2021. "Galvanic Corrosion Behaviour of Different Types of Coatings Used in Safety Systems Manufacturing" Coatings 11, no. 12: 1542. https://doi.org/10.3390/coatings11121542
APA StyleBurduhos-Nergis, D.-P., Burduhos-Nergis, D.-D., & Bejinariu, C. (2021). Galvanic Corrosion Behaviour of Different Types of Coatings Used in Safety Systems Manufacturing. Coatings, 11(12), 1542. https://doi.org/10.3390/coatings11121542









