Nanotubular Oxide Layer Formed on Helix Surfaces of Dental Screw Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Specimens
2.3. Electrochemical Oxidation
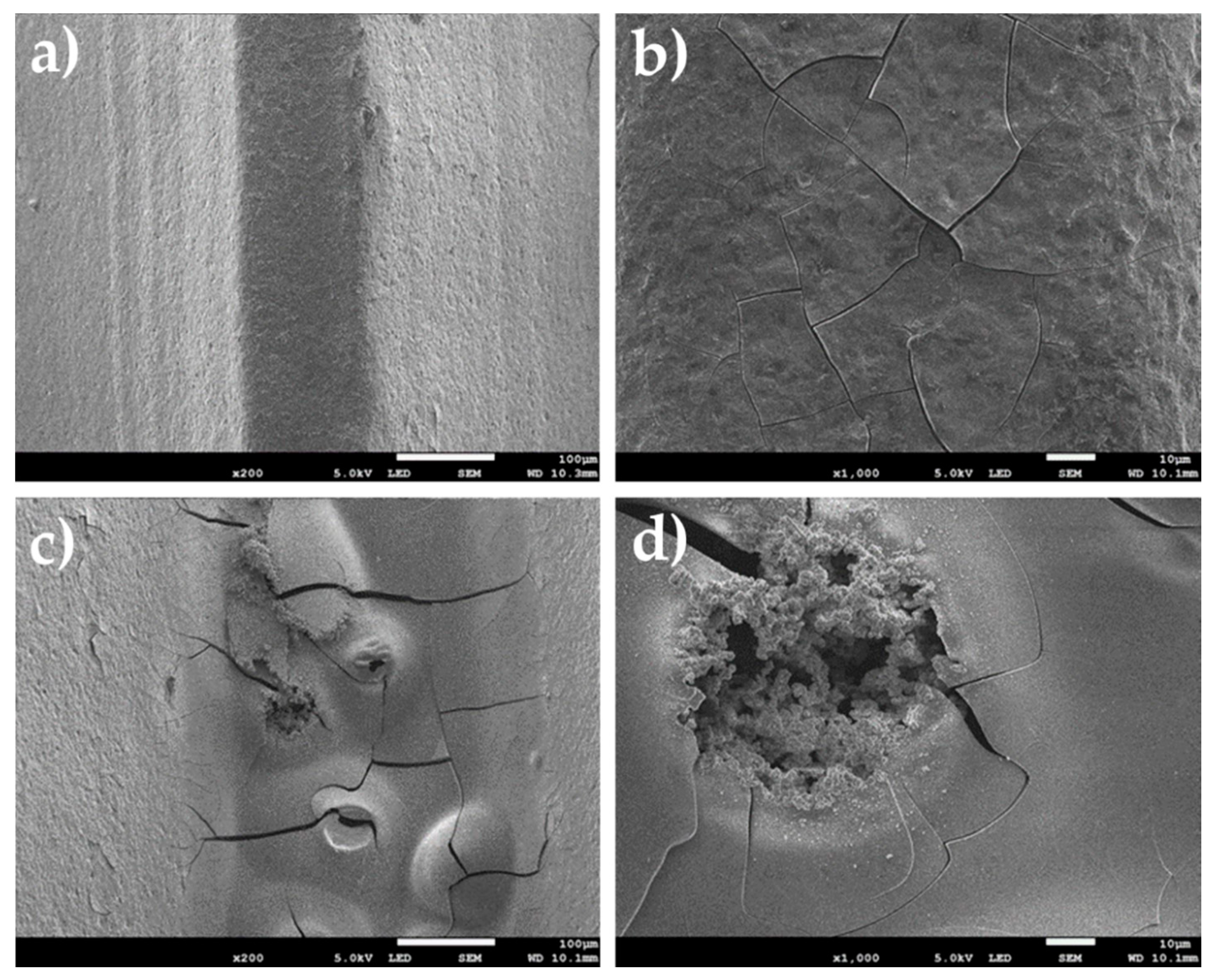
2.4. SEM Surface Examination
2.5. Light Microscopy Assessment of Roughness
2.6. Computer Tomography
2.7. Corrosion Examinations in Simulated Body Fluid
3. Results and Discussion
3.1. Substrate Specimens
3.2. Oxidized Specimens
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osman, R.B.; Swain, M.V. A critical review of dental implant materials with an emphasis on titanium versus zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Arnau, A.; Vallecillo-Capilla, M.F.; Cabrerizo-Vílchez, M.Á.; Rosales-Leal, J.I. Topographic characterisation of dental implants for commercial use. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e631–e636. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Mazare, A.; Schmuki, P.; Iglič, A. Biomaterial Surface Modification Of Titanium and Titanium Alloys for Medical Applications. In Nanomedicine; One Central Press: Altrincham, UK, 2014; pp. 111–136. [Google Scholar]
- Ellingsen, J.E.; Johansson, C.B.; Wennerberg, A.; Holmen, A. Improved retention and bone-tolmplant contact with fluoride-modified titanium implants. Int. J. Oral Maxillofac. Implant. 2004, 19, 659–666. [Google Scholar]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Bedi, G.; Garg, A. Implant surface modifications: A review. J. Clin. Diagn. Res. 2012, 6, 319–324. [Google Scholar]
- Gehrke, S.A.; Taschieri, S.; Massimo, D.P.; Coelho, P.G. The positive biomechanical effects of titanium oxide for sandblasting implant surface as an alternative to aluminium oxide. J. Oral Implantol. 2015, 41, 515–522. [Google Scholar] [CrossRef]
- Majkowska-Marzec, B.; Tęczar, P.; Bartmański, M.; Bartosewicz, B.; Jankiewicz, B.J. Mechanical and Corrosion Properties of Laser Surface-Treated Ti13Nb13Zr Alloy with MWCNTs Coatings. Materials 2020, 13, 3991. [Google Scholar] [CrossRef]
- Pawłowski, Ł.; Bartmański, M.; Strugała, G.; Mielewczyk-Gryń, A.; Jażdżewska, M.; Zieliński, A. Electrophoretic Deposition and Characterization of Chitosan/Eudragit E 100 Coatings on Titanium Substrate. Coatings 2020, 10, 607. [Google Scholar] [CrossRef]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef]
- Scopelliti, P.E.; Borgonovo, A.; Indrieri, M.; Giorgetti, L.; Bongiorno, G.; Carbone, R.; Podestà, A.; Milani, P. The effect of surface nanometre-scale morphology on protein adsorption. PLoS ONE 2010, 5, e11862. [Google Scholar] [CrossRef] [PubMed]
- Zinger, O.; Anselme, K.; Denzer, A.; Habersetzer, P.; Wieland, M.; Jeanfils, J.; Hardouin, P.; Landolt, D. Time-dependent morphology and adhesion of osteoblastic cells on titanium model surfaces featuring scale-resolved topography. Biomaterials 2004, 25, 2695–2711. [Google Scholar] [CrossRef] [PubMed]
- Kohavi, D.; Badihi Hauslich, L.; Rosen, G.; Steinberg, D.; Sela, M.N. Wettability versus electrostatic forces in fibronectin and albumin adsorption to titanium surfaces. Clin. Oral Implant. Res. 2013, 24, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Brett, P.M.; Harle, J.; Salih, V.; Mihoc, R.; Olsen, I.; Jones, F.H.; Tonetti, M. Roughness response genes in osteoblasts. Bone 2004, 35, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ejiofor, J.U. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef]
- Pinheiro, F.A.L.; de Almeida Barros Mourão, C.F.; Diniz, V.S.; Silva, P.C.; Meirelles, L.; Junior, E.S.; Schanaider, A. In-vivo bone response to titanium screw implants anodized in sodium sulfate1. Acta Cir. Bras. 2014, 29, 376–382. [Google Scholar] [CrossRef]
- Ogawa, T.; Saruwatari, L.; Takeuchi, K.; Aita, H.; Ohno, N. Ti nano-nodular structuring for bone integration and regeneration. J. Dent. Res. 2008, 87, 751–756. [Google Scholar] [CrossRef]
- Sugita, Y.; Ishizaki, K.; Iwasa, F.; Ueno, T.; Minamikawa, H.; Yamada, M.; Suzuki, T.; Ogawa, T. Effects of pico-to-nanometer-thin TiO 2 coating on the biological properties of microroughened titanium. Biomaterials 2011, 32, 8374–8384. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Tailor, S.; Rakoch, A.G.; Gladkova, A.A.; Van Truong, P.; Strekalina, D.M.; Sourkouni, G.; Manjunath, S.Y.; Takagi, T. Kinetic features of wear-resistant coating growth by plasma electrolytic oxidation. Surf. Innov. 2018, 6, 150–158. [Google Scholar] [CrossRef]
- Aydogan, D.T.; Muhaffel, F.; Acar, O.K.; Topcuoglu, E.N.; Kulekci, H.G.; Kose, G.T.; Baydogan, M.; Cimenoglu, H. Surface modification of Ti6Al4V by micro-arc oxidation in AgC2H3O2-containing electrolyte. Surf. Innov. 2018, 6, 277–285. [Google Scholar] [CrossRef]
- Webster, T.J.; Yao, C. Anodization: A Promising Nano-Modification Technique of Titanium-based Implants for Orthopedic Appl. In Surface Engineered Surgical Tools and Medical Devices; Jackson, M.J., Ahmed, W., Eds.; Springer US: Boston, MA, USA, 2007; pp. 21–47. [Google Scholar]
- Li, G.; Qu, S.; Ren, Z.; Li, X. Surface Modification Layer of Ti-6Al-4V Produced By Surface Rolling and Thermal Oxidation. Surf. Innov. 2017, 5, 1–29. [Google Scholar] [CrossRef]
- Sul, Y.T. The significance of the surface properties of oxidized titanium to the bone response: Special emphasis on potential biochemical bonding of oxidized titanium implant. Biomaterials 2003, 24, 3893–3907. [Google Scholar] [CrossRef]
- Sul, Y.T.; Johansson, C.B.; Jeong, Y.; Wennerberg, A.; Albrektsson, T. Resonance frequency and removal torque analysis of implants with turned and anodized surface oxides. Clin. Oral Implant. Res. 2002, 13, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y.T.; Johansson, C.B.; Petronis, S.; Krozer, A.; Jeong, Y.; Wennerberg, A.; Albrektsson, T. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown: The oxide thickness, micropore configurations, surface roughness, crystal structure and chemical composition. Biomaterials 2002, 23, 491–501. [Google Scholar] [CrossRef]
- Sul, Y.-T.; Johansson, C.B.; Albrektsson, T. Oxidized titanium screws coated with calcium ions and their performance in rabbit bone. Int. J. Oral Maxillofac. Implant. 2002, 17, 625–634. [Google Scholar]
- Choi, J.W.; Heo, S.J.; Koak, J.Y.; Kim, S.K.; Lim, Y.J.; Kim, S.H.; Lee, J.B. Biological responses of anodized titanium implants under different current voltages. J. Oral Rehabil. 2006, 33, 889–897. [Google Scholar] [CrossRef]
- Elias, C.N. Titanium dental implant surfaces. Rev. Mater. 2010, 15, 138–142. [Google Scholar] [CrossRef]
- Shayganpour, A.; Rebaudi, A.; Cortella, P.; Diaspro, A.; Salerno, M. Electrochemical coating of dental implants with anodic porous titania for enhanced osteointegration. Beilstein J. Nanotechnol. 2015, 6, 2183–2192. [Google Scholar] [CrossRef]
- Van Vuuren, D.J.; Laubscher, R.F. Surface Friction Behaviour of Anodized Commercially Pure Titanium Screw Assemblies. Procedia CIRP 2016, 45, 251–254. [Google Scholar] [CrossRef]
- Nasir, M.; Abdul Rahman, H. Mechanical Evaluation of Pure Titanium Dental Implants Coated with a Mixture of Nano Titanium Oxide and Nano Hydroxyapatite. J. Baghdad Coll. Dent. 2016, 28, 38–43. [Google Scholar] [CrossRef]
- Portan, D.V.; Nikolopoulou, F.; Bairami, V.; Mouzakis, D.; Papanicolaou, G.C.; Deligianni, D.D. Electrochemical Surface Processing Applied for the Functionalization of Titanium Screw Type Implants. J. Mater. Sci. Surf. Eng. 2016, 4, 376–382. [Google Scholar]
- Huang, J.; Zhang, X.; Yan, W.; Chen, Z.; Shuai, X.; Wang, A.; Wang, Y. Nanotubular topography enhances the bioactivity of titanium implants. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1913–1923. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Arruda, I.R.S.; Silva, G.M.M.; Machado, G.; Coelho, L.C.B.B.; Correia, M.T.S. Functionalization of titanium dioxide nanotubes with biomolecules for biomedical applications. Mater. Sci. Eng. C 2017, 81, 597–606. [Google Scholar] [CrossRef]
- Weszl, M.; Tóth, K.L.; Kientzl, I.; Nagy, P.; Pammer, D.; Pelyhe, L.; Vrana, N.E.; Scharnweber, D.; Wolf-Brandstetter, C.; Joób, F.Á.; et al. Investigation of the mechanical and chemical characteristics of nanotubular and nano-pitted anodic films on grade 2 titanium dental implant materials. Mater. Sci. Eng. C 2017, 78, 69–78. [Google Scholar] [CrossRef]
- Suchanek, K.; Hajdyła, M.; Maximenko, A.; Zarzycki, A.; Marszałek, M.; Jany, B.R.; Krok, F. The influence of nanoporous anodic titanium oxide substrates on the growth of the crystalline hydroxyapatite coatings. Mater. Chem. Phys. 2017, 186, 167–178. [Google Scholar] [CrossRef]
- Yazici, H.; Habib, G.; Boone, K.; Urgen, M.; Utku, F.S.; Tamerler, C. Self-assembling antimicrobial peptides on nanotubular titanium surfaces coated with calcium phosphate for local therapy. Mater. Sci. Eng. C 2019, 94, 333–343. [Google Scholar] [CrossRef]
- Wang, L.-N.; Jin, M.; Zheng, Y.; Guan, Y.; Lu, X.; Luo, J.-L. Surface modification of metallic implants with anodic oxide nanotubular arrays via electrochemical anodization techniques. In Nanomedicine; Seifalian, A., de Mel, A., Kalaskar, D.M., Eds.; One Central Press Ltd.: Manchester, UK, 2015; pp. 313–332. [Google Scholar]
- Ossowska, A.; Sobieszczyk, S.; Supernak, M.; Zielinski, A. Morphology and properties of nanotubular oxide layer on the “Ti-13Zr-13Nb” alloy. Surf. Coat. Technol. 2014, 258, 1239–1248. [Google Scholar] [CrossRef]
- Ossowska, A.; Beutner, R.; Scharnweber, D.; Zielinski, A. Properties of composite oxide layers on the Ti13Nb13Zr alloy. Surf. Eng. 2017, 33, 841–848. [Google Scholar] [CrossRef]
- EN ISO 10993-15:2009. Biological Evaluation of Medical Devices. Identification and Quantification of Degradation Products from Metals and Alloys; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Ehlert, M.; Radtke, A.; Jędrzejewski, T.; Roszek, K.; Bartmański, M.; Piszczek, P. In Vitro Studies on Nanoporous, Nanotubular and Nanosponge-Like Titania Coatings, with the Use of Adipose-Derived Stem Cells. Materials 2020, 13, 1574. [Google Scholar] [CrossRef]
- Alam, F.; Balani, K. Adhesion force of staphylococcus aureus on various biomaterial surfaces. J. Mech. Behav. Biomed. Mater. 2017, 65, 872–880. [Google Scholar] [CrossRef]
- Macak, J.M.; Schmuki, P. Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes. Electrochim. Acta 2006, 52, 1258–1264. [Google Scholar] [CrossRef]
- Klimas, J.; Dudek, A.; Klimas, M. Surface Refinement of Titanium Alloy TI6AL4V ELI. Eng. Biomater. 2012, 15, 52–54. [Google Scholar]
- Bartmanski, M.; Zielinski, A.; Majkowska-Marzec, B.; Strugala, G. Effects of solution composition and electrophoretic deposition voltage on various properties of nanohydroxyapatite coatings on the Ti13Zr13Nb alloy. Ceram. Int. 2018, 44, 19236–19246. [Google Scholar] [CrossRef]






| Element | Zr | Nb | O | C | N | Ti |
|---|---|---|---|---|---|---|
| wt.% | 13.0 | 13.0 | 0.11 | 0.04 | 0.019 | remainder |
| Element | Wt. Pct. | At. Pct. |
|---|---|---|
| O | 48.49 | 71.65 |
| Zr | 5.72 | 2.07 |
| P | 19.29 | 14.72 |
| Nb | 6.91 | 1.76 |
| K | 0.68 | 0.41 |
| Ca | 0.84 | 0.49 |
| Ti | 17.77 | 8.77 |
| Fe | 0.30 | 0.13 |
| Specimen | Sa Parameters (µm) | |
|---|---|---|
| Tip of the Helix | Bottom of the Helix | |
| Reference Ti-13Zr13Nb (before sand blasting) | 1.81 ± 1.11 | 9.10 ± 4.62 |
| Ti-13Zr-13Nb (after sand blasting) | 1.63 ± 1.40 | 9.94 ± 5.51 |
| Oxidized Ti-13Zr-13Nb | 1.39 ± 0.79 | 5.69 ± 2.98 |
| Specimen | Current Density (nA/cm2) | Corrosion Potential (mV) |
|---|---|---|
| Reference Ti-13Zr-13Nb | 503.25 | −392.46 |
| Oxidized Ti-13Zr-13Nb | 1451.00 | −1174.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jażdżewska, M.; Bartmański, M. Nanotubular Oxide Layer Formed on Helix Surfaces of Dental Screw Implants. Coatings 2021, 11, 115. https://doi.org/10.3390/coatings11020115
Jażdżewska M, Bartmański M. Nanotubular Oxide Layer Formed on Helix Surfaces of Dental Screw Implants. Coatings. 2021; 11(2):115. https://doi.org/10.3390/coatings11020115
Chicago/Turabian StyleJażdżewska, Magdalena, and Michał Bartmański. 2021. "Nanotubular Oxide Layer Formed on Helix Surfaces of Dental Screw Implants" Coatings 11, no. 2: 115. https://doi.org/10.3390/coatings11020115
APA StyleJażdżewska, M., & Bartmański, M. (2021). Nanotubular Oxide Layer Formed on Helix Surfaces of Dental Screw Implants. Coatings, 11(2), 115. https://doi.org/10.3390/coatings11020115






