Deposition of Zinc Oxide Coatings on Wood Surfaces Using the Solution Precursor Plasma Spraying Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Deposition
2.3. Characterization
3. Results and Discussion
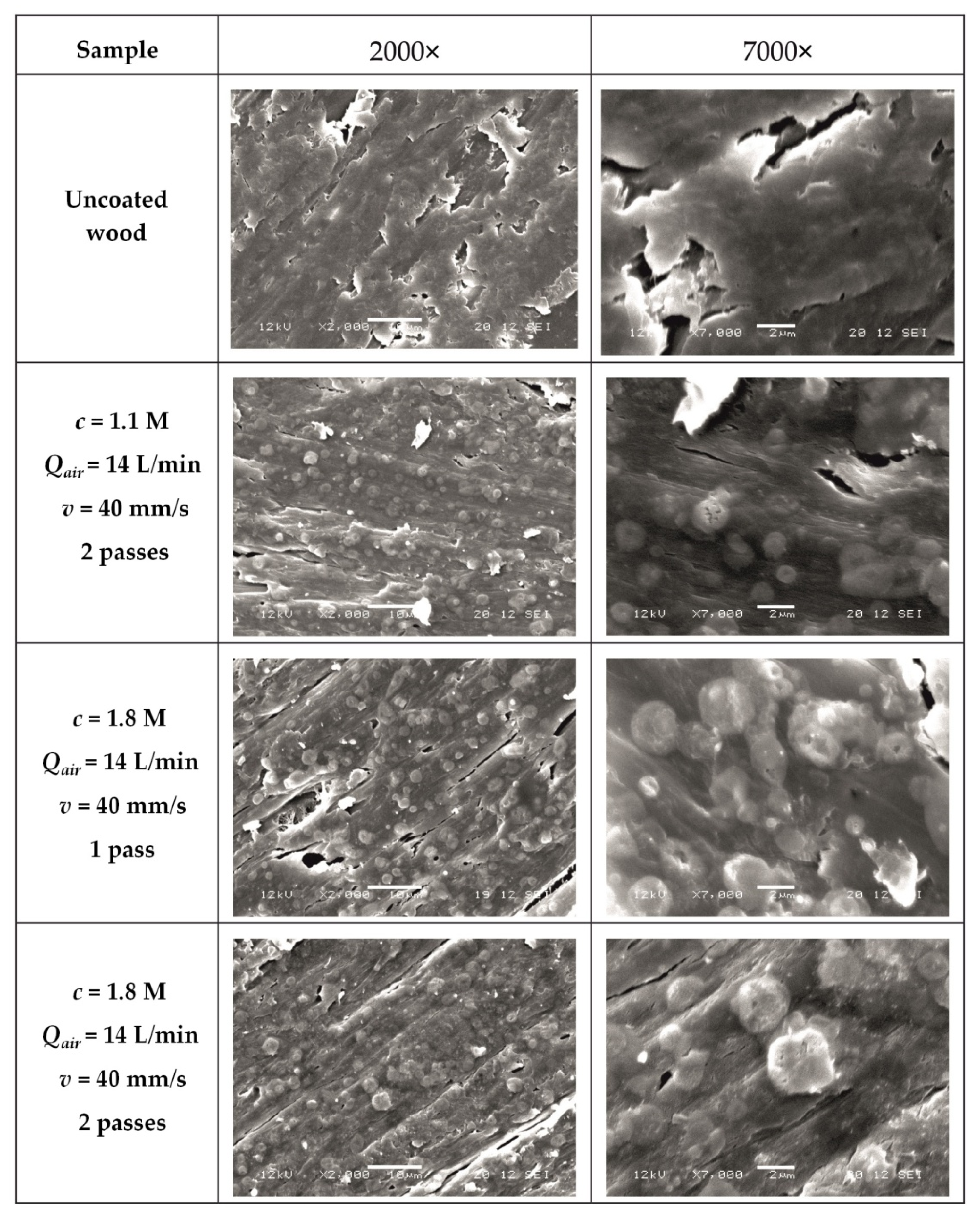
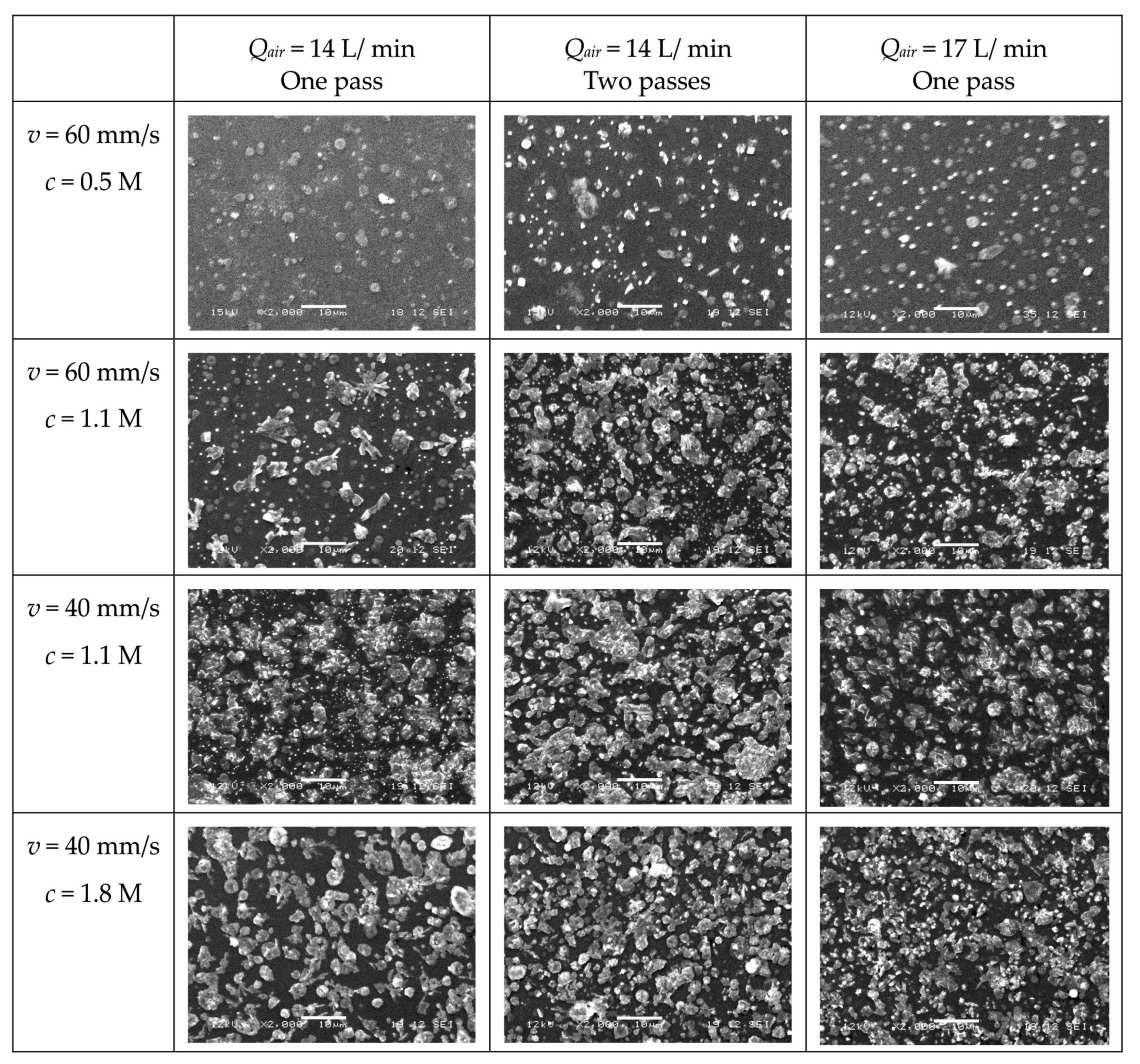
3.1. Characterization of Coatings by SEM
3.2. Characterization of Coatings by AFM
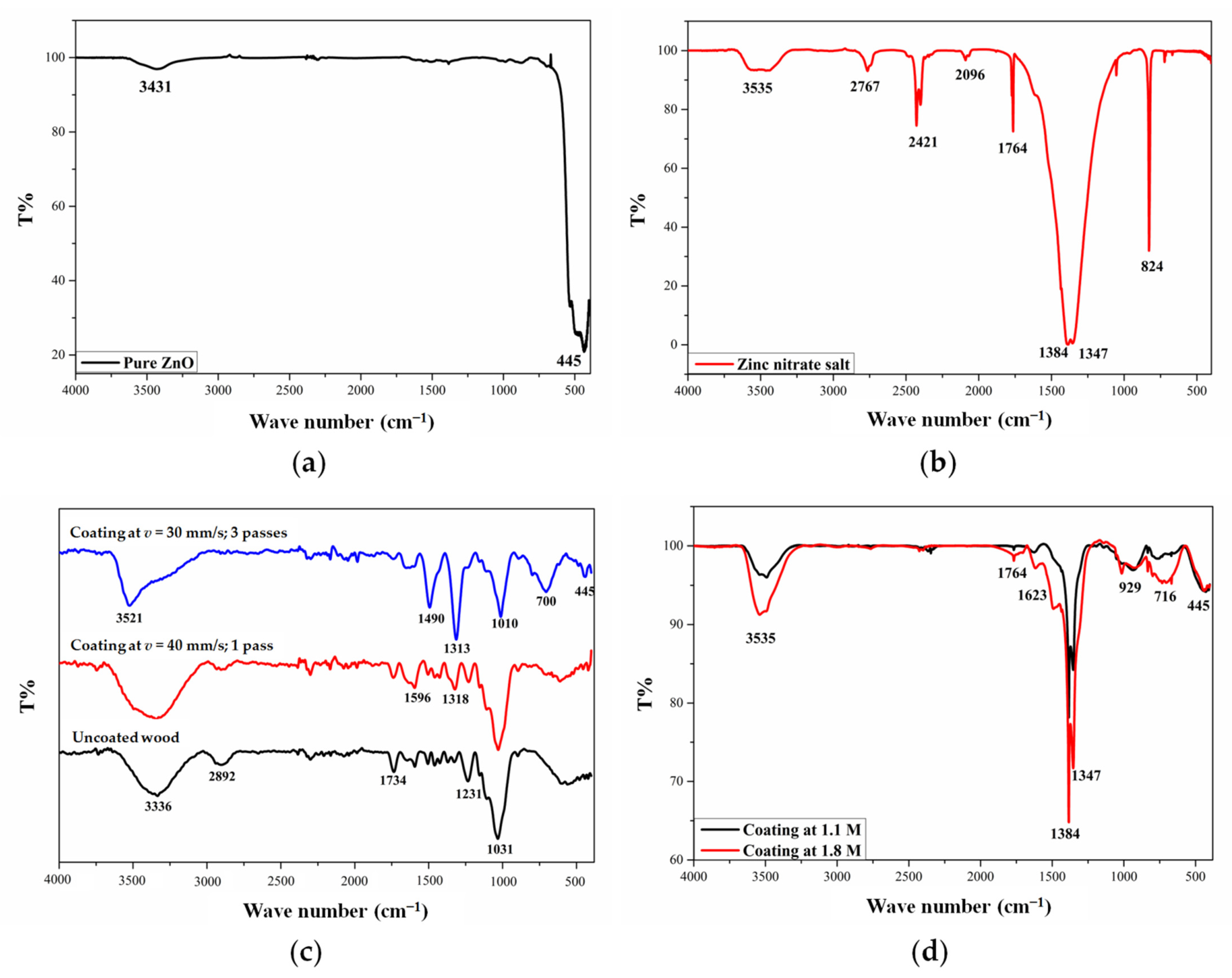
3.3. Characterization of Coatings by FTIR
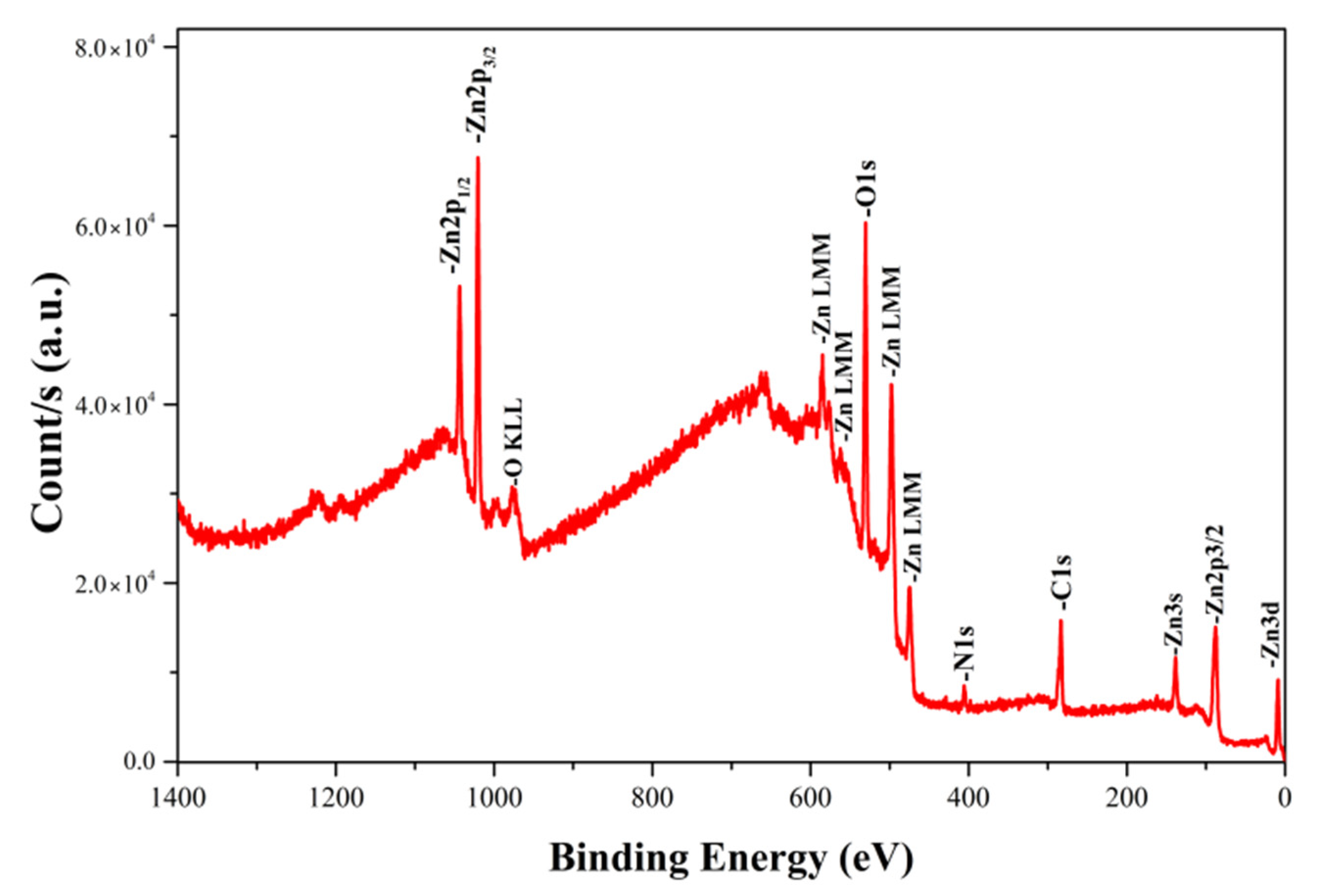
3.4. Characterization of Coatings on Wood by XPS
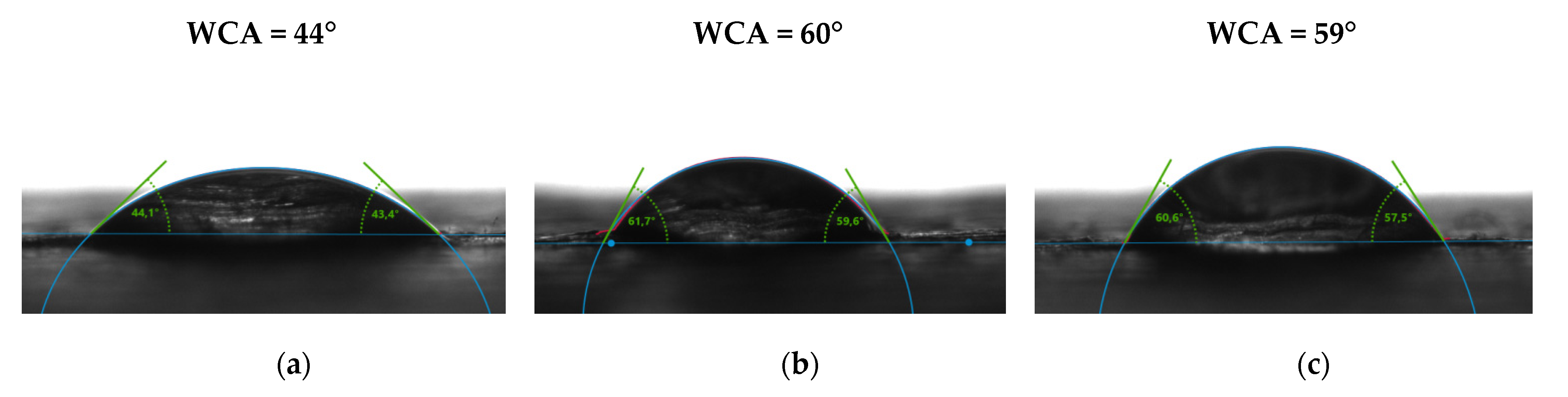
3.5. Contact Angle Measurements
3.6. UV Protection Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falk, B. Wood as a sustainable building material. For. Prod. J. 2009, 59, 6. [Google Scholar]
- Hon, D.N.S. Weathering and photochemistry of wood. In Wood and Cellulosic Chemistry; Hon, D.N.S., Shirashi, N., Eds.; Marcel Decker Ink.: New York, NY, USA, 2001; pp. 525–555. [Google Scholar]
- Pandey, K.K. A note on the influence of extractives on the photo-discoloration and photo-degradation of wood. Polym. Degrad. Stab. 2005, 87, 375–379. [Google Scholar] [CrossRef]
- Weichelt, F.; Beyer, M.; Emmler, R.; Flyunt, R.; Beyer, E.; Buchmeiser, M. Zinc oxide based coatings for the UV-protection of wood for outdoor applications. Macromol. Symp. 2011, 301, 23–30. [Google Scholar] [CrossRef]
- Wang, Z.L. Nanostructures of zinc oxide. Mater. Today 2004, 7, 26–33. [Google Scholar] [CrossRef]
- Favarim, H.R.; Leite, L.O. Performance of ZnO nanoparticles for fire retardant and UV protection of pine wood. BioResources 2018, 13, 6963–6969. [Google Scholar]
- Guo, H.; Fuchs, P.; Cabane, E.; Michen, B.; Hagendorfer, H.; Romanyuk, Y.E.; Burgert, I. UV-protection of wood surfaces by controlled morphology fine-tuning of ZnO nanostructures. Holzforschung 2016, 70, 699–708. [Google Scholar] [CrossRef]
- Salla, J.; Pandey, K.K.; Srinivas, K. Improvement of UV resistance of wood surfaces by using ZnO nanoparticles. Polym. Degrad. Stabil. 2012, 97, 592–596. [Google Scholar] [CrossRef]
- Nair, S.; Nagarajappa, G.B.; Pandey, K.K. UV stabilization of wood by nano metal oxides dispersed in propylene glycol. J. Photochem. Photobiol. B 2018, 183, 1–10. [Google Scholar] [CrossRef]
- De Peres, M.L.; de Avila Delucis, R.; Amico, S.C.; Gatto, D.A. Zinc oxide nanoparticles from microwave-assisted solvothermal process: Photocatalytic performance and use for wood protection against xylophagous fungus. Nanomater. Nanotechnol. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Fu, W.; Liu, Y.; Zhang, G.; Liu, Y.; Yu, H. Comparison of ZnO nanorod array coatings on wood and their UV prevention effects obtained by microwave-assisted hydrothermal and conventional hydrothermal synthesis. Holzforschung 2015, 69, 1009–1014. [Google Scholar] [CrossRef]
- Denes, A.R.; Young, R.A. Reduction of weathering degradation of wood through plasma–polymer coating. Holzforschung 1999, 53, 632–640. [Google Scholar] [CrossRef]
- Wallenhorst, L.; Gurau, L.; Gellerich, A.; Militz, H.; Ohms, G.; Viöl, W. UV-blocking properties of Zn/ZnO coatings on wood deposited by cold plasma spraying at atmospheric pressure. Appl. Surf. Sci. 2018, 434, 1183–1192. [Google Scholar] [CrossRef]
- Köhler, R.; Sauerbier, P.; Militz, H.; Viöl, W. Atmospheric pressure plasma coating of wood and mdf with polyester powder. Coatings 2017, 7, 171. [Google Scholar] [CrossRef] [Green Version]
- Jordan, E.H.; Jiang, C.; Gell, M. The solution precursor plasma spray (SPPS) process: A review with energy considerations. J. Therm. Spray Tech. 2015, 24, 1153–1165. [Google Scholar] [CrossRef]
- Unabia, R.; Candidato, R., Jr.; Pawłowski, L. Current progress in solution precursor plasma spraying of cermets: A review. Metals 2018, 8, 420. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.; Li, H.; Lien, S.; Chen, J.; Cheng, I.; Hsu, C. Deposition of ZnO thin films by an atmospheric pressure plasma jet-assisted process: The selection of precursors. IEEE Trans. Plasma Sci. 2015, 43, 670–674. [Google Scholar]
- Shutov, D.A.; Rybkin, V.V.; Ivanov, A.N. Synthesis of zinc oxide powders in plasma–solution systems. High Energy Chem. 2017, 51, 65–69. [Google Scholar] [CrossRef]
- Tummala, R.; Guduru, R.K.; Mohanty, P.S. Solution precursor plasma deposition of nanostructured ZnO coatings. Mater. Res. Bull. 2011, 46, 1276. [Google Scholar] [CrossRef]
- Dom, R.; Sivakumar, G.; Hebalkar, N.Y.; Joshi, S.V.; Borse, P.H. Deposition of nanostructured photocatalytic zinc ferrite films using solution precursor plasma spraying. Mater. Res. Bull. 2012, 47, 562–570. [Google Scholar] [CrossRef]
- Yu, Z.; Moussa, H.; Liu, M.; Schneider, R.; Moliere, M.; Liao, H. Solution precursor plasma spray process as an alternative rapid one-step route for the development of hierarchical ZnO films for improved photocatalytic degradation. Ceram. Int. 2018, 44, 2085–2092. [Google Scholar] [CrossRef]
- Candidato, R.T.; Ontolan, J.P.; Carpio, P.; Pawłowski, L.; Vequizo, R.M. Effects of precursor composition used in solution precursor plasma spray on the properties of ZnO coatings for CO2 and UV light sensing. Surf. Coat. Technol. 2019, 371, 395–400. [Google Scholar] [CrossRef]
- Galmiz, O.; Stupavska, M.; Wulff, H.; Kersten, H.; Brablec, A.; Cernak, M. Deposition of Zn-containing films using atmospheric pressure plasma jet. Open Chem. 2014, 13, 198–203. [Google Scholar] [CrossRef]
- Jnido, G.; Ohms, G.; Viöl, W. Deposition of TiO2 Thin films on wood substrate by an air atmospheric pressure plasma jet. Coatings. 2019, 9, 441. [Google Scholar] [CrossRef] [Green Version]
- Jnido, G.; Ohms, G.; Viöl, W. One-step deposition of polyester/tio2 coatings by atmospheric pressure plasma jet on wood surfaces for uv and moisture protection. Coatings 2020, 10, 184. [Google Scholar] [CrossRef] [Green Version]
- Nagaraju, G.; Nagabhushana, H.; Basavaraj, R.B.; Raghu, G.K.; Suresh, D.; Rajanaika, H.; Nagabhushana, H.; Sharma, S.C. Green, nonchemical route for the synthesis of ZnO superstructures, evaluation of its applications toward photocatalysis, photoluminescence, and biosensing. Cryst. Growth Des. 2016, 16, 6828–6840. [Google Scholar]
- Jain, G.; Macia-Montero, M.; Velasumy, T.; Maguire, P.; Mariotti, D. Porous zinc oxide nanocrystalline film deposition by atmospheric pressure plasma: Fabrication and energy band estimation. Plasma Processes Polym. 2017, 14, 1700052. [Google Scholar] [CrossRef]
- Singh, S.C. Effect of oxygen injection on the size and compositional evolution of ZnO/Zn(OH)2 nanocomposite synthesized by pulsed laser ablation in distilled water. J. Nanopart. Res. 2011, 13, 4143–4152. [Google Scholar] [CrossRef]
- Winiarski, J.; Tylus, W.; Winiarska, K.; Szczygieł, I.; Szczygieł, B. XPS and FT-IR characterization of selected synthetic corrosion products of zinc expected in neutral environment containing chloride ions. J. Spectrosc. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [Green Version]


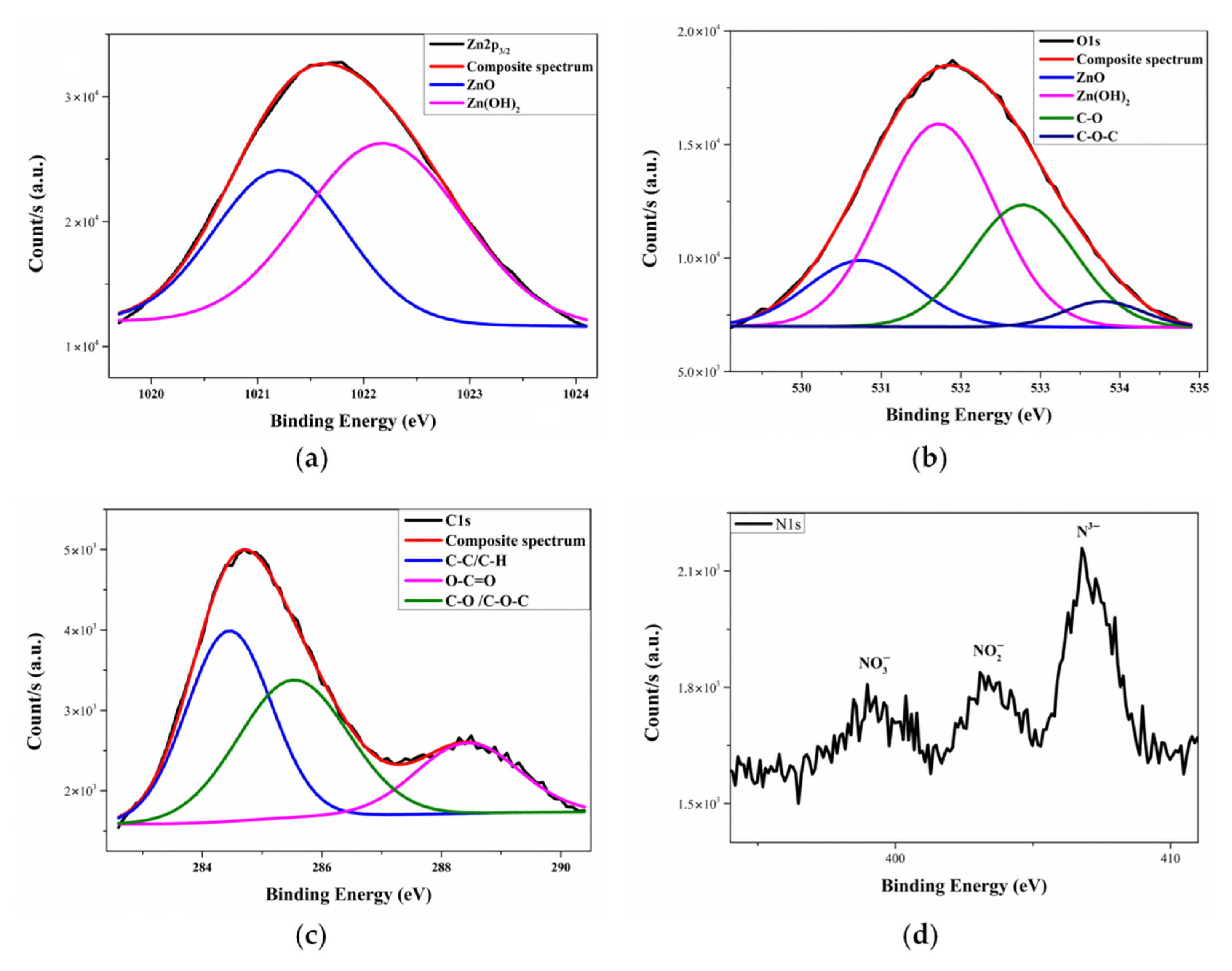



| Element | Assignment | Peak Position (eV) | Peak Area (%) | Atomic Percentage |
|---|---|---|---|---|
| Zn | Zn–O | 1021.21 | 41.1 | 7.1 |
| Zn(OH)2 | 1022.18 | 58.9 | 10.2 | |
| O | Zn–O | 530.75 | 15.9 | 7.1 |
| Zn(OH)2 | 531.72 | 51.1 | 22.9 | |
| C–O | 532.78 | 28.5 | 12.8 | |
| C-O-C | 533.79 | 4.5 | 2.0 | |
| C | C–C/C–H | 284.55 | 45.5 | 17.1 |
| C–O/C–O–C | 285.66 | 36.6 | 13.8 | |
| O–C=O | 288.52 | 17.9 | 6.7 | |
| N | N2 | 398 | - | 0.26 |
| NO2 | 403 | |||
| NO3 | 406 |
| Deposition Scan Speed | Precursor Concentration | 14 L/min One Pass | 14 L/min Two Passes | 17 L/min One Pass | 17 L/min Two Passes |
|---|---|---|---|---|---|
| 60 mm/s | 0.5 M | 2.3 | 2.6 | 2.7 | 3.2 |
| 1.1 M | 3.6 | 5.1 | 3.9 | - | |
| 40 mm/s | 1.1 M | 4.9 | 7.3 | 5.6 | - |
| 1.8 M | 7.6 | 10.2 | 6.4 | 12.2 |
| Sample | ΔL* | Δa* | Δb* | ΔE* | Protection Efficiency |
|---|---|---|---|---|---|
| Uncoated wood | −4.12 ± 0.52 | 1.39 ± 0.47 | 7.10 ± 0.42 | 8.34 ± 0.18 | - |
| c = 1.1 M; Qair = 14 L/min; v = 40 mm/s; 1 pass | −2.72 ± 0.43 | 1.17 ± 0.69 | 6.02 ± 0.59 | 6.74 ± 0.67 | 19% |
| c = 1.1 M; Qair = 14 L/min; v = 40 mm/s; 2 passes | −2.39 ± 0.27 | 1.35 ± 0.42 | 5.48 ± 0.70 | 6.16 ± 0.52 | 26% |
| c = 1.1 M; Qair = 17 L/min; v = 40 mm/s; 1 pass | −2.68 ± 0.05 | 1.33 ± 0.25 | 5.67 ± 0.32 | 6.41 ± 0.21 | 23% |
| c = 1.8 M; Qair = 17 L/min; v = 40 mm/s; 1 pass | −1.31 ± 0.37 | 0.37 ± 0.53 | 5.31 ± 0.11 | 5.51 ± 0.08 | 34% |
| c = 1.8 M; Qair = 17 L/min; v = 30 mm/s; 1 pass | −1.08 ± 0.75 | 0.49 ± 0.76 | 5.04 ± 0.68 | 5.25 ± 0.58 | 37% |
| c = 1.8 M; Qair = 14 L/min; v = 30 mm/s; 3 passes | 0.05 ± 1.62 | −0.18 ± 0.54 | 2.95 ± 0.09 | 3.19 ± 0.03 | 61% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jnido, G.; Ohms, G.; Viöl, W. Deposition of Zinc Oxide Coatings on Wood Surfaces Using the Solution Precursor Plasma Spraying Process. Coatings 2021, 11, 183. https://doi.org/10.3390/coatings11020183
Jnido G, Ohms G, Viöl W. Deposition of Zinc Oxide Coatings on Wood Surfaces Using the Solution Precursor Plasma Spraying Process. Coatings. 2021; 11(2):183. https://doi.org/10.3390/coatings11020183
Chicago/Turabian StyleJnido, Ghiath, Gisela Ohms, and Wolfgang Viöl. 2021. "Deposition of Zinc Oxide Coatings on Wood Surfaces Using the Solution Precursor Plasma Spraying Process" Coatings 11, no. 2: 183. https://doi.org/10.3390/coatings11020183
APA StyleJnido, G., Ohms, G., & Viöl, W. (2021). Deposition of Zinc Oxide Coatings on Wood Surfaces Using the Solution Precursor Plasma Spraying Process. Coatings, 11(2), 183. https://doi.org/10.3390/coatings11020183






