Microstructure and Properties of Cold Sprayed NiCrAl Coating on AZ91D Magnesium Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Coating Preparation
2.2. Characterization
2.3. Sliding Wear Friction Test
2.4. Corrosion Resistance Test
3. Results and Discussion
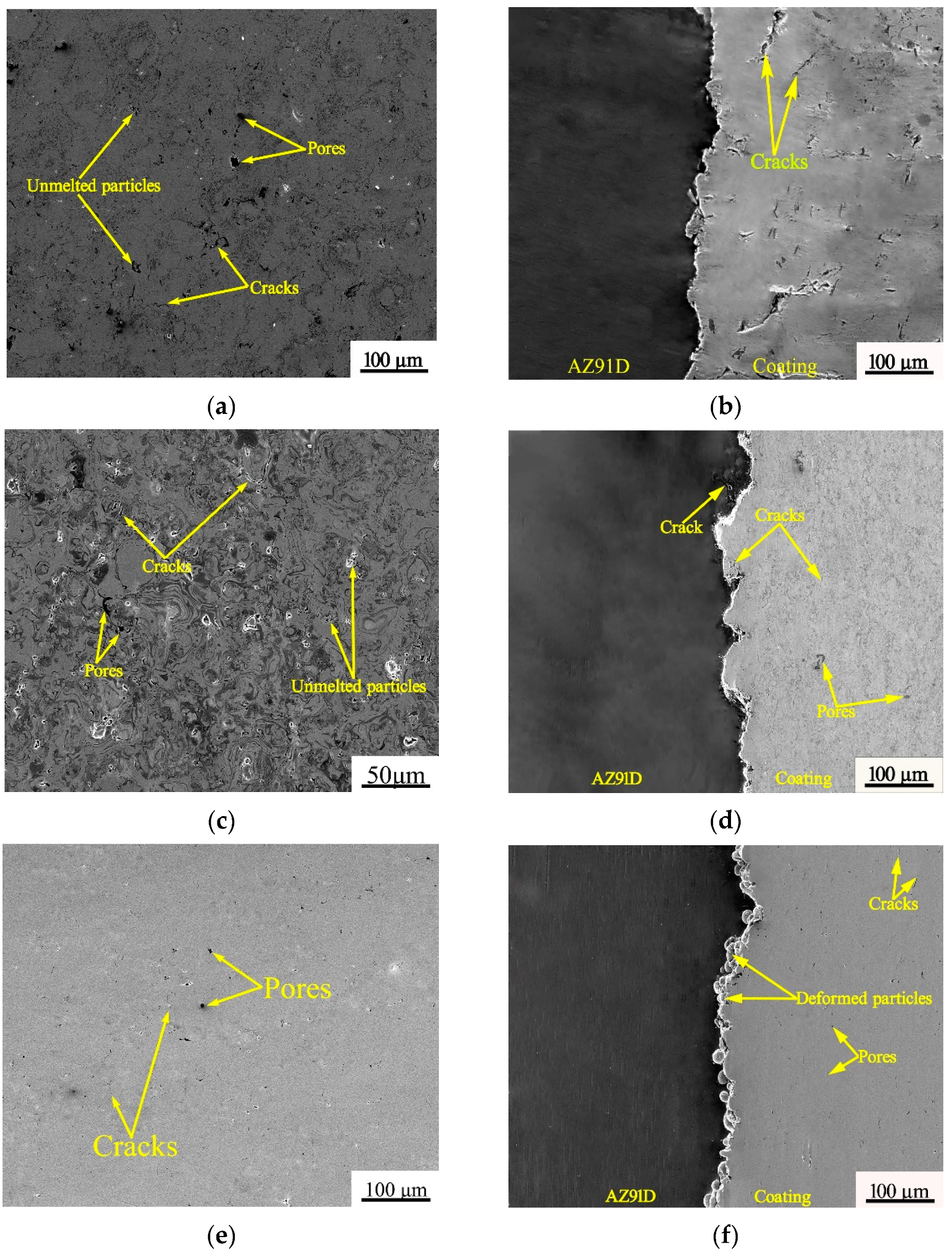
3.1. Microstructure of the Coatings
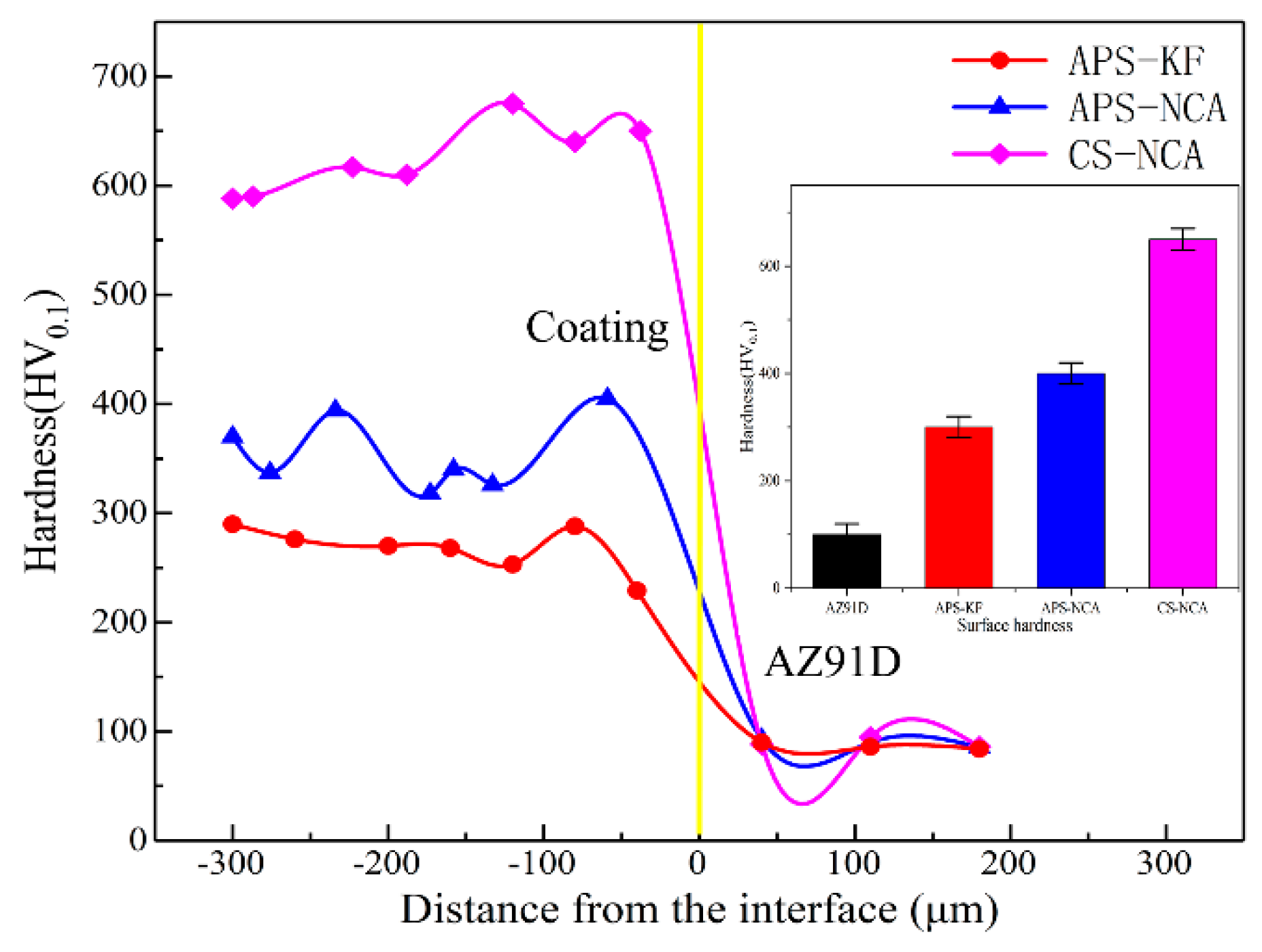
3.2. Mechanical Properties
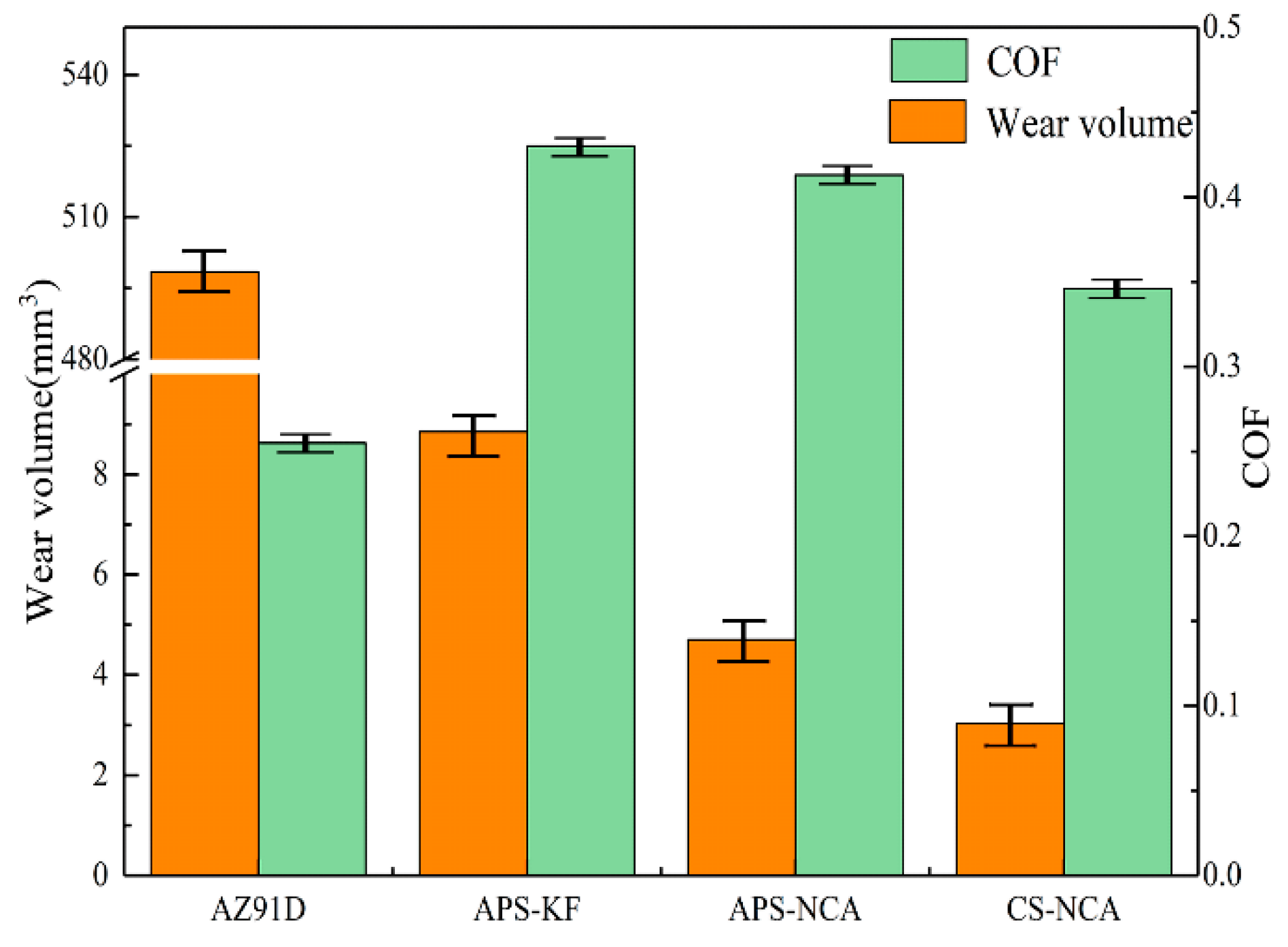
3.3. Wear Performance
- (a)
- Compared with thermal spraying, cold spraying can obtain higher coating density.
- (b)
- (c)
3.4. Electrochemical Results
4. Conclusions
- (a)
- The porosity of the two-thermal sprayed NiCrAl coatings is 3.21% and 2.66%, respectively, while that of the cold sprayed NiCrAl coating is only 0.68%; moreover, the hardness of the cold sprayed NiCrAl coating (650 HV0.1) is obviously higher than those of the two-thermal sprayed NiCrAl coatings (300 HV0.1, 400 HV0.1).
- (b)
- In the same sliding wear friction test environment, the three NiCrAl coatings have better wear resistance than AZ91D magnesium alloy; and among the three coatings, the cold sprayed NiCrAl coating has the lowest friction coefficient and the least wear amount. Therefore, the cold sprayed NiCrAl coating has superior wear resistance to the two-thermal sprayed NiCrAl coatings.
- (c)
- Electrochemical experiments in 3.5 wt.% NaCl solution show that the corrosion current density of the two-thermal sprayed NiCrAl coatings is two orders of magnitude lower than that of the substrate, while the corrosion current density of the cold sprayed NiCrAl coating is two orders of magnitude lower than those of the two-thermal sprayed NiCrAl coating. Therefore, the cold sprayed NiCrAl coating has superior corrosion resistance to the two-thermal sprayed NiCrAl coatings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, G.; Ibrahim, J.M.; Chu, P.K. Surface design of biodegradable magnesium alloys—A review. Surf. Coat. Technol. 2013, 233, 2–12. [Google Scholar] [CrossRef]
- Cao, F.; Songabc, G.-L.; Atrens, A. Corrosion and passivation of magnesium alloys. Corros. Sci. 2016, 111, 835–845. [Google Scholar] [CrossRef] [Green Version]
- Esmaily, M.; Svensson, J.; Fajardo, S.; Birbilis, N.; Frankel, G.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Liu, J.; Yu, H.; Chen, C.; Weng, F.; Dai, J. Research and development status of laser cladding on magnesium alloys: A review. Opt. Lasers Eng. 2017, 93, 195–210. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, H.; Chen, J.; Xia, W.; Su, B.; Li, X.; Huang, W.; Song, M. Effects of the β1’ precipitates on mechanical and damping properties of ZK60 magnesium alloy. Mater. Sci. Eng. A 2021, 804, 140730. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Q.; Sun, H.; Li, Y. Effect of heat treatment on corrosion behavior of AZ63 magnesium alloy in 3.5 wt.% sodium chloride solution. Corros. Sci. 2016, 111, 288–301. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Sha, L. Corrosion Resistance of AZ91D Magnesium alloy modified by rare earths-laser surface treatment. J. Rare Earths 2007, 25, 201–203. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.; Ma, C.; Guo, S. Improving mechanical properties and corrosion resistance of Mg6ZnMn magnesium alloy by rapid solidification. Mater. Lett. 2013, 92, 45–48. [Google Scholar] [CrossRef]
- Gray, J.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, S.; Jiang, F.; Wang, F. Cerium conversion coatings for AZ91D magnesium alloy in ethanol solution and its corrosion resistance. Corros. Sci. 2009, 51, 2916–2923. [Google Scholar] [CrossRef]
- Luo, D.; Liu, Y.; Yin, X.; Wang, H.; Han, Z.; Ren, L. Corrosion inhibition of hydrophobic coatings fabricated by micro-arc oxidation on an extruded Mg-5Sn-1Zn alloy substrate. J. Alloys Compd. 2018, 731, 731–738. [Google Scholar] [CrossRef]
- Gan, R.; Wang, N.; Xie, Z.-H.; He, L. Improving surface characteristic and corrosion inhibition of coating on Mg alloy by trace stannous (II) chloride. Corros. Sci. 2017, 123, 147–157. [Google Scholar] [CrossRef]
- Pardo, A.; Casajús, P.; Mohedano, M.; Coy, A.E.; Viejo, F.; Torres, B.; Matykina, E. Corrosion protection of Mg/Al alloys by thermal sprayed aluminium coatings. Appl. Surf. Sci. 2009, 255, 6968–6977. [Google Scholar] [CrossRef]
- Wei, X.; Liu, P.; Ma, S.; Li, Z.; Peng, X.; Deng, R.; Zhao, Q. Improvement on corrosion resistance and biocompability of ZK60 magnesium alloy by carboxyl ion implantation. Corros. Sci. 2020, 173, 108729. [Google Scholar] [CrossRef]
- Riquelme, A.; Rodrigo, P.; Escalera-Rodríguez, M.D.; Rams, J. Characterisation and mechanical properties of Al/SiC metal matrix composite coatings formed on ZE41 magnesium alloys by laser cladding. Results Phys. 2019, 13, 102160. [Google Scholar] [CrossRef]
- Jonda, E.; Łatka, L.; Pakieła, W. Microstructure and selected properties of Cr3C2–NiCr coatings obtained by HVOF on magnesium alloy substrates. J. Mater. 2020, 13, 2775. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Huang, M.; Wang, C.; Long, S.; Zha, J.; You, G. Research status and prospects of melt refining and purification technology of magnesium alloys. J. Magnes. Alloys 2019, 7, 370–380. [Google Scholar] [CrossRef]
- Gan, W.; Liu, C.; Lu, F. Study on surface modification and properties of the AZ91D magnesium alloy used for automobile engine. J. Mater. Res. Technol. 2020. [Google Scholar] [CrossRef]
- Tiamiyu, A.A.; Schuh, C.A. Particle flattening during cold spray: Mechanistic regimes revealed by single particle impact tests. Surf. Coat. Technol. 2020, 403, 126386. [Google Scholar] [CrossRef]
- Sample, C.M.; Champagne, V.K.; Nardi, A.T.; Lados, D.A. Factors governing static properties and fatigue, fatigue crack growth, and fracture mechanisms in cold spray alloys and coatings/repairs: A review. Addit. Manuf. 2020, 36, 101371. [Google Scholar] [CrossRef]
- Su, J.; Kang, J.; Yue, W.; Ma, G.-Z.; Fu, Z.-Q.; Zhu, L.-N.; She, D.-S.; Wang, H.-D.; Wang, C.-B. Comparison of tribological behavior of Fe-based metallic glass coatings fabricated by cold spraying and high velocity air fuel spraying. J. Non Cryst. Solids 2019, 522, 119582. [Google Scholar] [CrossRef]
- Xie, R.G.; Li, C.J.; Cui, H. Preparation of Cold Spraying-based NiAl /ZrC Bond Course of Thermal Barrier Coating. Surf. Technol. 2016, 45, 100119. [Google Scholar] [CrossRef]
- ASTM E2109-01 Standard Test Methods for Determining Area Percentage Porosity in Thermal Sprayed Coatings; ASTM International: West Conshohocken, PA, USA, 2014.
- Ding, C.; Zhou, Z.-Y.; Yuan, Z.-P.; Zhu, H.; Piao, Z.-Y. Study on the correlation between the running-in attractor and the wear particle group. Ind. Lubr. Tribol. 2019, 72, 681–686. [Google Scholar] [CrossRef]
- Mao, B.; Siddaiah, A.; Zhang, X.; Li, B.; Menezes, P.L.; Liao, Y. The influence of surface pre-twinning on the friction and wear performance of an AZ31B Mg alloy. Appl. Surf. Sci. 2019, 480, 998–1007. [Google Scholar] [CrossRef]
- García-Rodríguez, S.; Torres, B.; Maroto, A.; López, A.; Otero, E.; Rams, J. Dry sliding wear behavior of globular AZ91 magnesium alloy and AZ91/SiCp composites. Wear 2017, 390-391, 1–10. [Google Scholar] [CrossRef]
- Hu, H.-J.; Sun, Z.; Ou, Z.-W.; Wang, X.-Q. Wear behaviors and wear mechanisms of wrought magnesium alloy AZ31 fabricated by extrusion-shear. Eng. Fail. Anal. 2017, 72, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Alidokht, S.A.; Yue, S.; Chromik, R.R. Effect of WC morphology on dry sliding wear behavior of cold-sprayed Ni-WC composite coatings. Surf. Coat. Technol. 2019, 357, 849–863. [Google Scholar] [CrossRef]
- Qiu, X.; Tariq, N.U.H.; Qi, L.; Tang, J.-R.; Cui, X.-Y.; Du, H.; Wang, J.-Q.; Xiong, T.-Y. Influence of particulate morphology on microstructure and tribological properties of cold sprayed A380/Al2O3 composite coatings. J. Mater. Sci. Technol. 2020, 44, 9–18. [Google Scholar] [CrossRef]
- Alidokht, S.; Manimunda, P.; Vo, P.; Yue, S.; Chromik, R. Cold spray deposition of a Ni-WC composite coating and its dry sliding wear behavior. Surf. Coat. Technol. 2016, 308, 424–434. [Google Scholar] [CrossRef] [Green Version]
- Seiner, H.; Cizek, J.; Sedlák, P.; Huang, R.; Cupera, J.; Dlouhy, I.; Landa, M. Elastic moduli and elastic anisotropy of cold sprayed metallic coatings. Surf. Coat. Technol. 2016, 291, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-J.; Luo, X.-T.; Rashid, H.; Li, C.-J. A new approach to prepare fully dense Cu with high conductivities and anti-corrosion performance by cold spray. J. Alloys Compd. 2018, 740, 406–413. [Google Scholar] [CrossRef]
- Liu, T.; Vaudin, M.D.; Bunn, J.R.; Ungár, T.; Brewer, L.N. Quantifying dislocation density in Al-Cu coatings produced by cold spray deposition. Acta Mater. 2020, 193, 115–124. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Q.; Guo, Y.-B.; Liu, H.; Deng, Z. Atomic simulation of crystal orientation effect on coating surface generation mechanisms in cold spray. Comput. Mater. Sci. 2020, 184, 109859. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Haché, M.J.; Chu, X.; Irissou, E.; Zou, Y. Prediction of heterogeneous microstructural evolution in cold sprayed copper coatings using local Zener-Hollomon parameter and strain. Acta Mater. 2020, 193, 191–201. [Google Scholar] [CrossRef]
- Bhowmik, A.; Tan, A.W.-Y.; Sun, W.; Wei, Z.; Marinescu, I.; Liu, E. On the heat-treatment induced evolution of residual stress and remarkable enhancement of adhesion strength of cold sprayed Ti-6Al-4V coatings. Results Mater. 2020, 7, 100119. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Han, E.; Dong, J.; Ke, W. AC impedance spectroscopy study of the corrosion behavior of an AZ91 magnesium alloy in 0.1M sodium sulfate solution. Electrochim. Acta 2007, 52, 3299–3309. [Google Scholar] [CrossRef]
- Ge, Y.; Luo, X.T.; Li, C.J. Research Progress on Interface Bonding Formation Mechanism of Solid Particle Deposition in Cold Spray. Surf. Technol. 2020, 49, 60–67. [Google Scholar] [CrossRef]
- Wei, Y.-K.; Luo, X.-T.; Ge, Y.; Chu, X.; Huang, G.; Li, C.-J. Deposition of fully dense Al-based coatings via in-situ micro-forging assisted cold spray for excellent corrosion protection of AZ31B magnesium alloy. J. Alloys Compd. 2019, 806, 1116–1126. [Google Scholar] [CrossRef]
- Meng, G.; Li, Y.; Shao, Y.; Zhang, T.; Wang, Y.; Wang, F.; Cheng, X.; Dong, C.; Li, X. Effect of microstructures on corrosion behavior of nickel coatings: (II) Competitive effect of grain size and twins density on corrosion behavior. J. Mater. Sci. Technol. 2016, 32, 465–469. [Google Scholar] [CrossRef]
- Mahdavian, M.; Attar, M. Another approach in analysis of paint coatings with EIS measurement: Phase angle at high frequencies. Corros. Sci. 2006, 48, 4152–4157. [Google Scholar] [CrossRef]
- Calderón, J.; Jiménez, J.; Zuleta, A. Improvement of the erosion-corrosion resistance of magnesium by electroless Ni-P/Ni(OH)2-ceramic nanoparticle composite coatings. Surf. Coat. Technol. 2016, 304, 167–178. [Google Scholar] [CrossRef]
- Deng, N.; Dong, H.; Che, H.Y.; Li, S.F.; Zhou, Z.J. The Research Progress on Preparation of Metal Coatings by ColdSpraying and Its Application in Additive Manufacturing. Surf. Technol. 2020, 49, 57–66. [Google Scholar] [CrossRef]
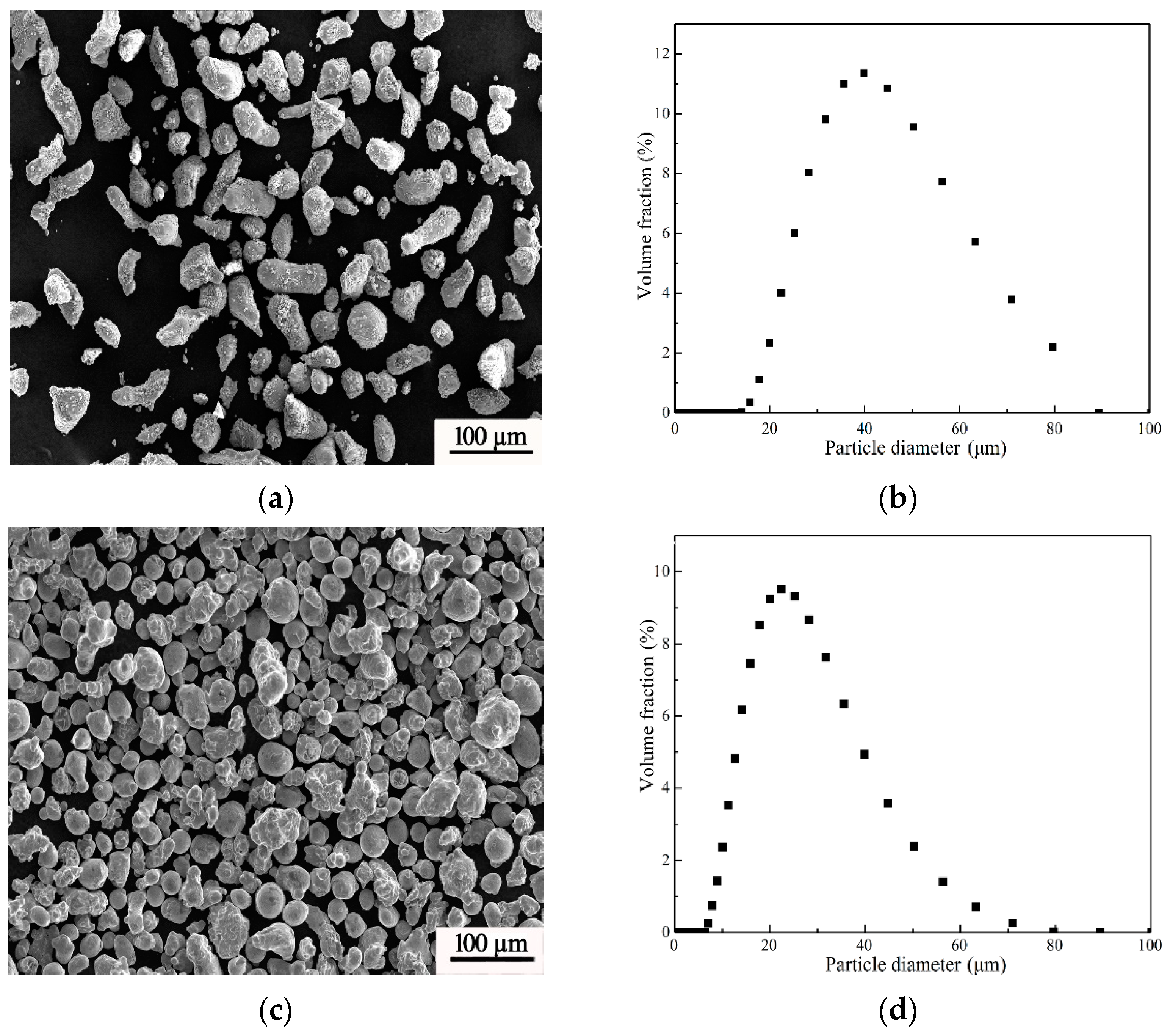





| Material | Mg | Ni | Cr | Al | Mn | Zn |
|---|---|---|---|---|---|---|
| AZ91D | Bal. | 0.01 | - | 9.3 | 0.32 | 0.95 |
| KF-110 powder (Beijing) | - | Bal. | 14.6 | 4.4 | - | - |
| NiCrAl powder (Nangong) | - | Bal. | 16 | 4 | - | - |
| Coatings Title | Power (KW) | Pressure (MPa) | Powder Feeding Rate (g/min) | Spraying Distance (mm) | Powder Feeding Amount (m3/h) | Carrier Gas Flow (L/min) | Spray Gun Moving Speed (mm/s) |
|---|---|---|---|---|---|---|---|
| APS-KF | 50 | 3 | - | 110 | 10 | 16 | - |
| APS-NCA | 42 | 3 | - | 130 | 30 | 25 | - |
| CS-NCA | - | 4 | 20 | 20 | - | - | 10 |
| Lubrication Conditions | Temperature | Diameter of Steel Ball (mm) | Stroke Length (mm) | Load (N) | Test Frequency (Hz) | Experiment Time (min) |
|---|---|---|---|---|---|---|
| Dry friction | 25 °C | 6 | 4 | 10 | 10 | 20 |
| Coatings | Ecorr (V vs. SCE) | Icorr (μA·cm−2) | Anodic Tafel Slope (mV/dec) | Cathodic Tafel Slope (mV/dec) |
|---|---|---|---|---|
| AZ91D | −1.601 | 2637 | 201.49 | 256.93 |
| APS-KF | −0.823 | 25.96 | 219.58 | 55.682 |
| APS-NCA | −0.851 | 26.98 | 182.94 | 150.82 |
| CS-NCA | −0.02 | 4.404 × 10−2 | 183.35 | 184.50 |
| Coatings | Rs (Ω·cm2) | Rct (Ω·cm2) | Qdl (Sn/cm2) | Rc (Ω·cm2) | Qs (Ω·cm2) | Rt (Sn/cm2) | L (H·cm2) |
|---|---|---|---|---|---|---|---|
| AZ91D | 12.19 | 91.43 | 0.87 | - | - | 119.5 | 30.39 |
| APS-KF | 12.8 | 225.9 | 0.79 | 87.19 | 0.97 | - | - |
| APS-NCA | 11.82 | 624.6 | 0.59 | 137.5 | 1 | - | - |
| CS-NCA | 10.55 | 900 | 0.79 | 11,440 | 0.75 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Dong, T.; Fu, B.; Li, G.; Liu, Q.; Li, Y. Microstructure and Properties of Cold Sprayed NiCrAl Coating on AZ91D Magnesium Alloy. Coatings 2021, 11, 193. https://doi.org/10.3390/coatings11020193
Zhao X, Dong T, Fu B, Li G, Liu Q, Li Y. Microstructure and Properties of Cold Sprayed NiCrAl Coating on AZ91D Magnesium Alloy. Coatings. 2021; 11(2):193. https://doi.org/10.3390/coatings11020193
Chicago/Turabian StyleZhao, Xiangwei, Tianshun Dong, Binguo Fu, Guolu Li, Qi Liu, and Yanjiao Li. 2021. "Microstructure and Properties of Cold Sprayed NiCrAl Coating on AZ91D Magnesium Alloy" Coatings 11, no. 2: 193. https://doi.org/10.3390/coatings11020193
APA StyleZhao, X., Dong, T., Fu, B., Li, G., Liu, Q., & Li, Y. (2021). Microstructure and Properties of Cold Sprayed NiCrAl Coating on AZ91D Magnesium Alloy. Coatings, 11(2), 193. https://doi.org/10.3390/coatings11020193





