Abstract
The influence of different wettability on explosive boiling exhibits a significant distinction, where the hydrophobic surface is beneficial for bubble nucleation and the hydrophilic surface enhances the critical heat flux. Therefore, to receive a more suitable surface for the explosive boiling, in this paper a hybrid hydrophobic–hydrophilic nanostructured surface was built by the method of molecular dynamics simulation. The onset temperatures of explosive boiling with various coating thickness, pillar width, and film thicknesses were investigated. The simulation results show that the hybrid nanostructure can decrease the onset temperature compared to the pure hydrophilic surface. It is attributed to the effect of hydrophobic coating, which promotes the formation of bubbles and causes a quicker liquid film break. Furthermore, with the increase of the hydrophobic coating thickness, the onset temperature of explosive boiling decreases. This is because the process of heat transfer between the liquid film and the hybrid nanostructured surface is inevitably enhanced. In addition, the onset temperature of explosive boiling on the hybrid wetting surface decreases with the increase of pillar width and liquid film thickness.
1. Introduction
Explosive boiling is a common natural phenomenon that can release a lot of latent heat of vaporization due to the high overheating degree of the surface [1,2,3]. A large number of homogeneous vapor bubble nucleations form a bubble cluster, and finally are ejected rapidly in the area near the surface. It can be found in many industrial applications, including laser cleaning of a surface [4], laser surgery [5] microelectronic devices [6,7,8,9,10], and so forth. The study of explosive boiling is important because it can enhance the energy efficiency of industrial processes.
With the development of both materials science and micro-nano manufacturing technology, the characteristics of the heating surface for the study of boiling behavior have been extensively studied. Both the shape of geometry and size of nanopatterns are two key parameters affecting the heat transfer effect of explosion boiling [11]. Many researchers have focused on the effects of the heating surface topographical features on the explosive boiling, such as sphere [12], conical [11,13,14], cuboid [15,16], cylindrical [17,18], nanochannel [19], and so on. These studies have revealed the mechanism of the nanostructure affecting the explosive boiling heat transfer.
In addition to the nanostructures, wettability also plays an important role in explosive boiling. For example, Zhang [20] studied the boiling phenomenon of ultra-thin argon on three different wettabilities of the aluminum surface. It is found that at 350 K, the explosive boiling occurs only on the lyophilic and neutral surfaces, which also indicates that the increase of the solid-liquid force causes the heat transfer rate to increase. Hasan [21] investigated the influence of the hydrophilic and hydrophobic wettability of three different substances surface of platinum, silver, and aluminum on the phenomenon of liquid film phase transition heat. On the surface of the three materials, the explosive boiling occurs only on the hydrophilic surface, which further shows that compared with the substrate material, the wettability of the substrate occupies a dominant role in the phase change heat. Shavik [22] studied the effect of wettability on explosive boiling on the platinum surface with rectangular nanostructures. At the same time, they draw the same conclusion that hydrophilic was more conducive to heat transfer.
Recently, some experimental studies have also reported the effect of mixed wettability on boiling nucleation behaviors. Betz [23] tested the performance of pool boiling on the hydrophilic surface with a hydrophobic hexagonal pattern and hydrophobic surface with a hexagonal pattern. The results showed that both could increase the heat transfer coefficient and the critical heat flux. Hydrophilic networks featuring hydrophobic islands have a better effect and can prevent the appearance of an insulating vapor layer. Jo [24] studied the nucleate boiling under different wetting conditions, namely hydrophilic (123°), hydrophobic (54°), and heterogeneous wetting surfaces. Their experimental results show that at very low heat flux, according to the bubble analysis, the hydrophilic surface with hydrophobic dots has the strongest heat-transfer ability. Moreover, both the position and number of hydrophobic dots affect the performance of heat transfer. Suroto et al. [25] investigated the influence of the length of PTFE hydrophobic spot on pool boiling. The results show that the mixed-wettability surface has better boiling heat transfer performance than the hydrophilic surface, and as the hydrophobic perimeter increases, the heat transfer capacity gradually increases. These works clearly demonstrated that the hybrid hydrophilic–hydrophobic surface enhances boiling heat transfer performance.
The above research mainly discussed the influence of nanostructure, wettability, and mixed wettability of the flat surface on boiling. It was found that both nanostructure and mixed wettability can enhance the boiling heat transfer. However, so far, there is no work to combine the above two factors for analysis. Moreover, it is very challenging and practical to design a mixed-wetting nanostructure surface that can achieve the best boiling effect. Therefore, this paper uses the molecular dynamics simulation method to construct a hybrid hydrophobic–hydrophilic nanostructured surface. It was verified that the onset temperature for explosive boiling of this surface was significantly lower than that of the pure hydrophilic nanostructured surface. The effects of coating thickness, pillar width, and film thicknesses on the onset temperature of explosive boiling are also studied. The results are further studied by snapshots of atom trajectories, heat flux, interaction potential energy, liquid atom numbers, and temperature. The achievement from this work can provide a valuable guide for the design of enhanced explosive boiling on hybrid wettability surfaces.
2. Simulation Method
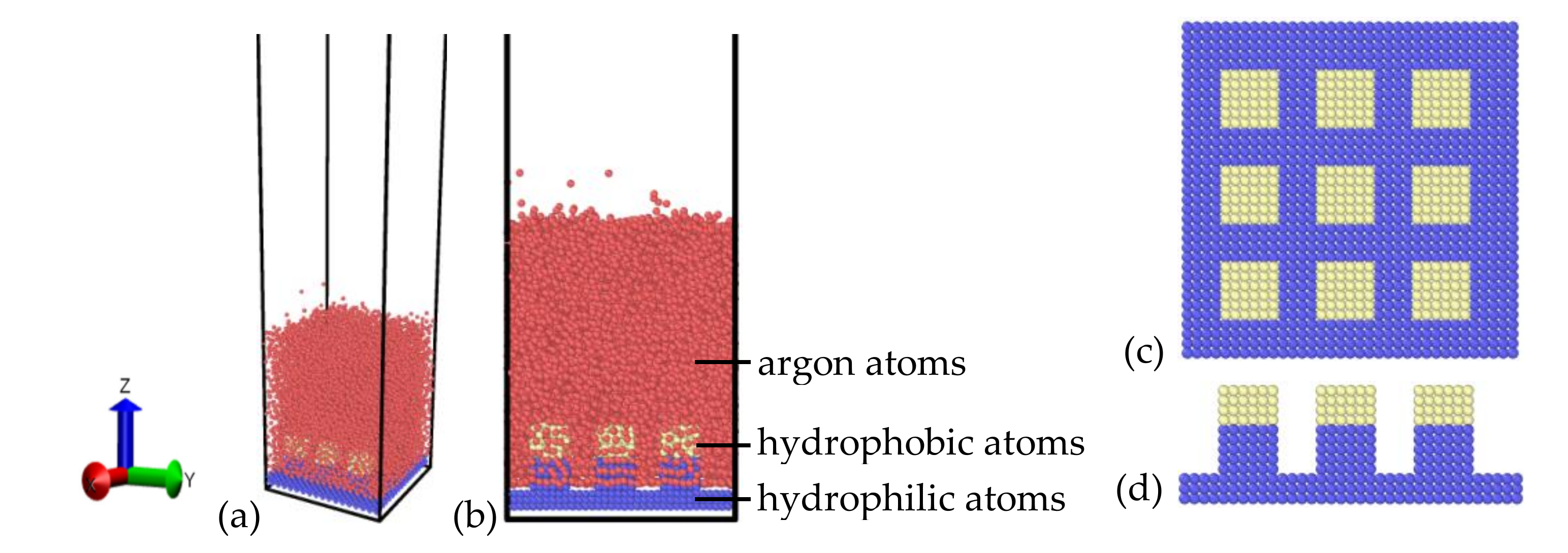
All the simulations in this work were performed using molecular dynamics (MD) simulation, which was supported by the Large-scale Atomic/Molecular Massively Parallel Simulator (LAMMPS) [26,27] package (Sandia National Laboratories, Albuquerque, New Mexico; Livermore, CA, USA). The explosive boiling process and evolutions of liquid film on various nanopillar surfaces with different hybrid hydrophobic–hydrophilic were studied. The simulation system is schematically shown in Figure 1a,b. To save computation resources and improve computation efficiency, argon atoms were used as the phase-change medium, which is a universal medium for the studies of liquid film heat transfer at the nanoscale [21,28]. Simpler gold-like atoms are used to build the substrate, which are created with face-center cubic unit (FCC) that has a constant lattice of 4.08 Å. As shown in Figure 1c,d, the substrate was constituted by a flat plate with a thickness of 0.816 nm and three square pillars with a thickness of 2.040 nm, among which the flat part is the basic substrate and the pillar part has hybrid wettability. The blue color atoms represent a more hydrophilic surface and the yellow atoms represent a more hydrophobic surface. Therefore, Figure 1 shows the hydrophilic nanopillar with hydrophobic coating. In this work, the pillar had two different widths with 0.816 and 1.224 nm, and three distinctive coating heights with 0.612, 1.020, and 1.428 nm, which represented three coating thickness ratios (rh) of 0.3, 0.5, and 0.7. The gold substrate was located at the bottom of the system, and the atoms in the lowest layer were kept fixed to avoid the movement of the plate. The argon film was placed on the substrate, which had two different thicknesses with 3 and 6 nm. The box horizontal area corresponds to 7.12 × 7.12 nm2 (x × y) with periodic boundary conditions, and the height was 80.00 nm (z) with fixed boundary condition, which will make the vapor atoms bounce back without any losses of kinetic energy and momentum once they collide with the top boundary.
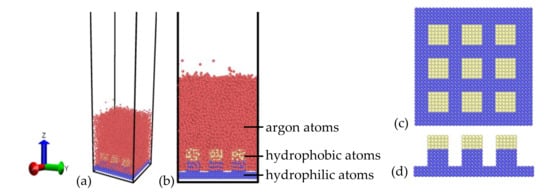
Figure 1.
The schematic diagrams of the simulation system with (a) orthogonal view and (b) front view. The nanostructured surface was constructed by a hydrophilic nanopillar with a hydrophobic coating with (c) top view and (d) front view.
The interactions between gold–gold, argon–argon, and argon–gold atoms were the same as those in our previous paper [29], where the embedded-atom (EAM) [30] and 12−6 Lennard-Jones (L-J) methods are employed. Furthermore, that paper [29] described in detail how to obtain different wettability surfaces, and finally achieved two typical contact angles with 42.7° and 106.9°, according to the method of computing the density contour of the droplet. In this work, the contact angles for hydrophilic and hydrophobic surfaces were also the same as Ref. 30, therefore, the detailed process is no longer repeated here.
When the simulation domain and interaction force field were prepared, both the argon film and the upper layers of gold atoms were first equilibrated in the canonical ensemble (NVT) with 90 K for 6 ns by using Nose–Hoover [31,32] thermostat, which is a simplified formulation of Nose dynamics [32]. Then the thermostat of argon was removed. The system continued to run for the equilibrium state in the microcanonical (NVE) ensembles for another 1.2 ns, where no external thermostat participated. However, the upper layers of gold atoms were still in the NVT ensemble to keep the desired temperature. After equilibration, the dynamics were started, where the gold atoms in the upper layers were suddenly heated to some high value to achieve the phenomenon of explosive boiling of the liquid film. Meanwhile, the argon atoms were still run in the NVE ensemble to ensure that they only absorbed energy from the heated wall.
For all simulations, a time step of 6 fs was considered, and a cut off of 12 Å was adopted beyond which the interaction potential was truncated. The positions and velocities of each particle were computed by the Velocity-Verlet algorithm and finally were recorded and analyzed by the software of Ovito every 1000 time-steps.
3. Results and Discussion
The onset temperature of explosive boiling on the hydrophilic nanostructured surface with the hydrophobic coating is discussed. It was found that the hybrid nanostructure could decrease the onset temperature compared to the pure hydrophilic surface. Furthermore, the coating thickness, pillar width (Lw), and liquid film thickness all affected the onset temperature of explosive boiling. The following parts will analyze the influences of these factors in detail, and reveal some hidden mechanisms in depth.
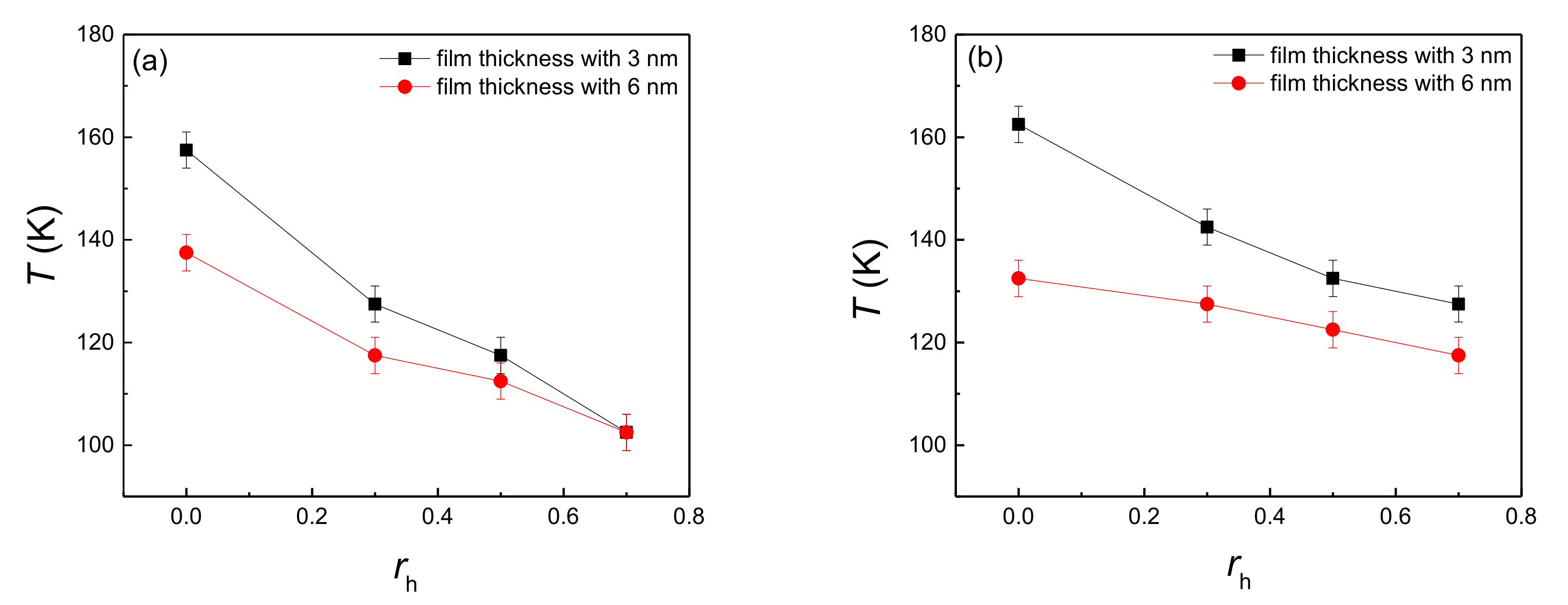
The onset temperature of explosive boiling on the hydrophilic nanostructured surface with the hydrophobic coating is shown in Figure 2, where both Figure 2a,b represent the pillar width of 1.224 nm and 0.816 nm, respectively. Here, rh = 0 means the coating thickness is 0 nm, that is to say, the nanostructured surface was pure hydrophilic. Therefore, for both pillar widths, the existence of hydrophobic coating can make the onset temperature of explosive boiling decrease, which means that hybrid wetting surfaces promotes the generation of explosive boiling.
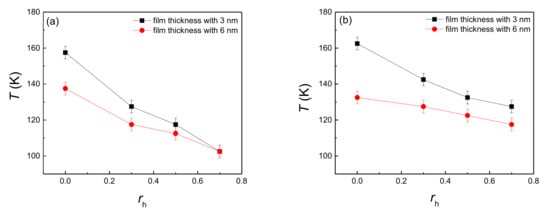
Figure 2.
The onset temperature of explosive boiling on hydrophilic nanostructured surfaces with a hydrophobic coating, whose pillar width are (a) 1.226 nm and (b) 0.816 nm.
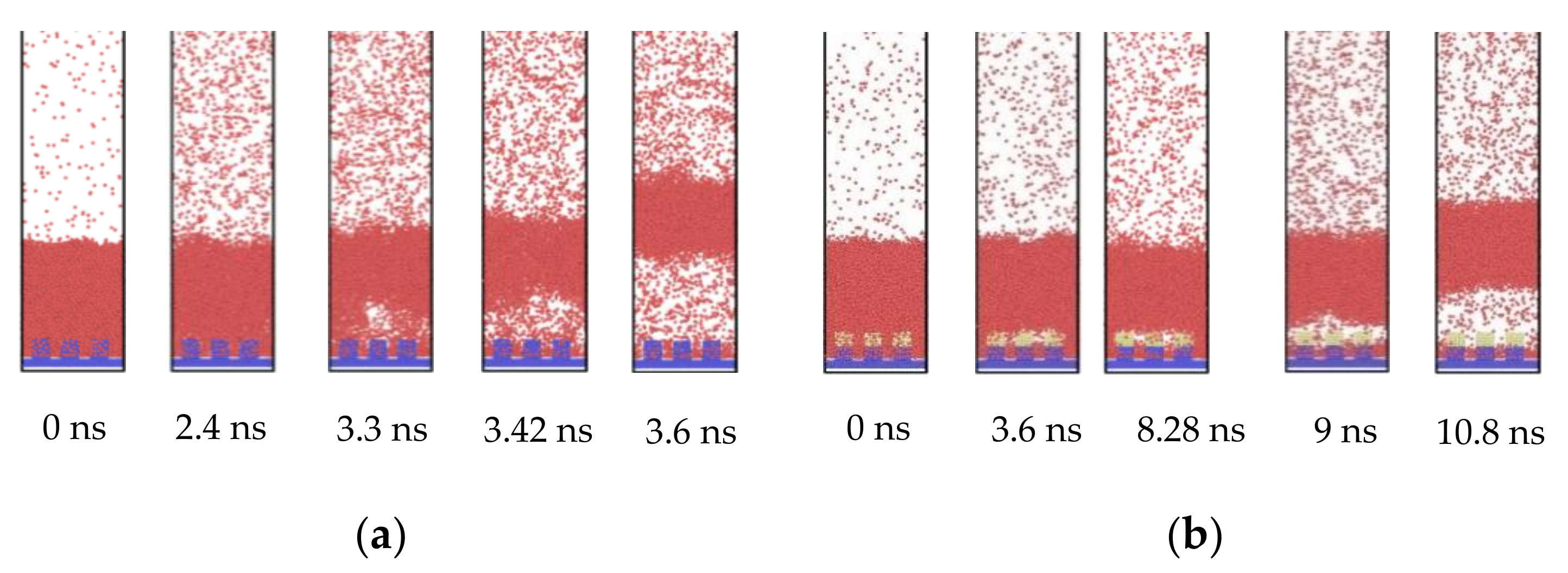
To explain the influence of the hydrophobic coating, a set of comparative snapshots are shown in Figure 3. Figure 3a exhibits the explosive boiling on the pure hydrophilic nanostructured surface, and Figure 3b shows the explosive boiling on the hydrophilic nanostructured surface with the hydrophobic coating. In these two cases, the pillar width with 1.224 nm and the film thickness with 6 nm were the same, and the coating thickness was 1.02 nm. By observing the initial status at t = 0 ns, it was found that despite the application of the hydrophobic coating, the liquid film was still in direct contact with the bottom plate, rather than being suspended at the top of the pillar. For the hydrophilic nanostructured surface, the bubble was observed above the pillar at t = 3.3 ns (Figure 3a). As the liquid film continued to absorb heat from the substrate, the bubble gradually grew and eventually formed an air layer between the liquid film and the bottom plate. It should be noted that the position of the initial bubble was some distance above the pillar, not at the nanostructure. However, when the top of the pillar was covered by a hydrophobic coating, the bubble had exactly occurred at the coatings. Furthermore, small bubbles also appeared on all three coating pillars, which could be found at t = 8.28 ns (Figure 3b). As these bubbles grew, they joined together to form one big bubble, which finally became the air layer between the liquid film and the bottom plate. Therefore, the presence of hydrophobic coating encouraged the formation of bubbles, which in turn accelerated the explosive boiling process.
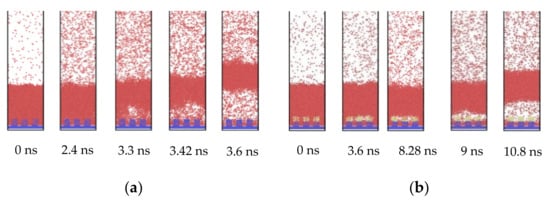
Figure 3.
Snapshots of the phase change of liquid films on (a) hydrophilic nanostructured surface and (b) hydrophilic nanostructured surface with hydrophobic coating.
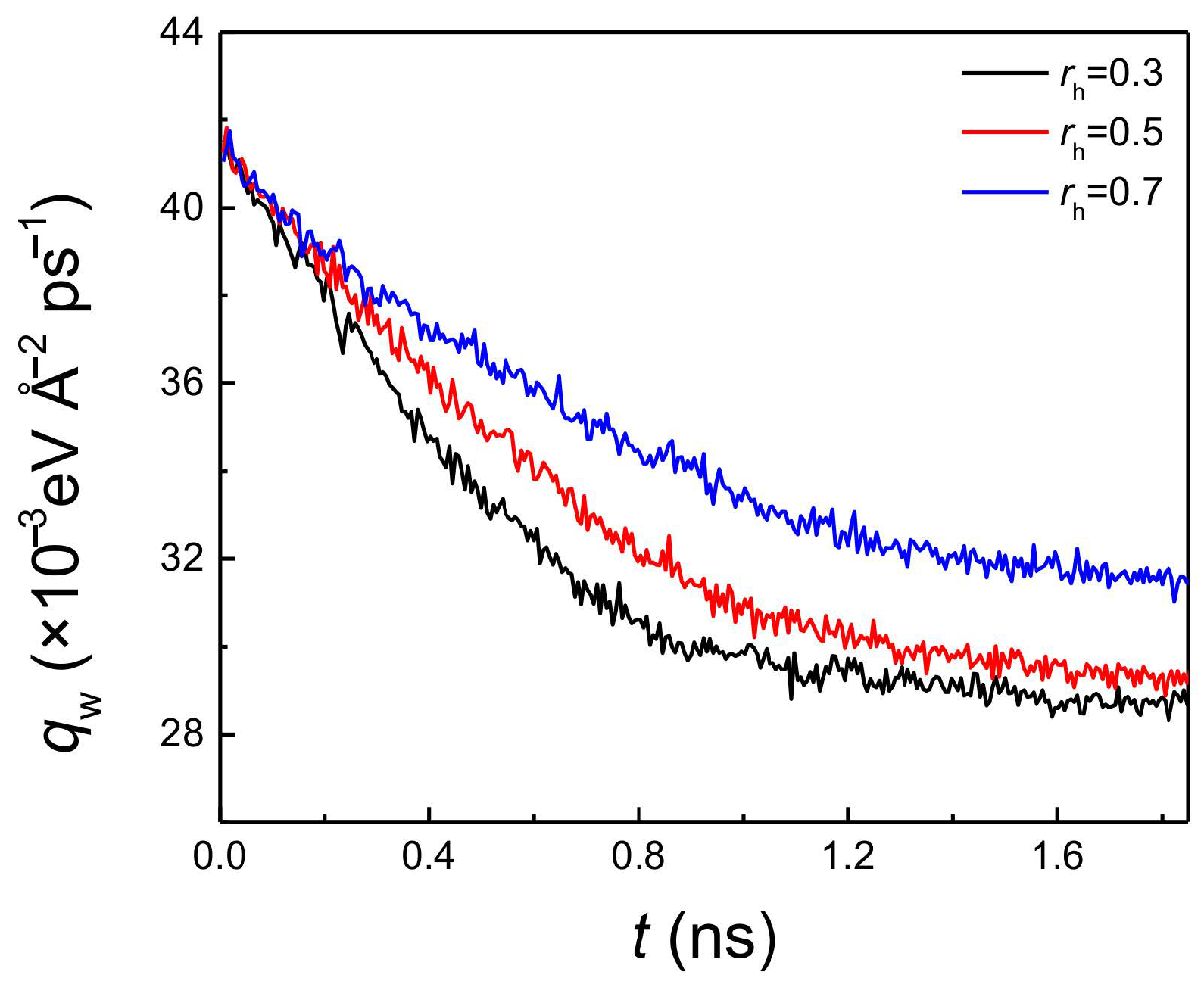
With the increase of the hydrophobic coating thickness, the onset temperature of explosive boiling decreased for both pillar widths (Figure 2). This indicated that the hydrophobic coating inevitably enhanced the process of heat transfer between the liquid film and the hybrid nanostructured surface. To quantitatively illustrate the effect of coating thickness, the average heat flux (qw) with a pillar width of 1.224 nm and film thickness of 3 nm on the hydrophilic nanostructured surface with three different hydrophobic coatings at 120 K were computed and shown in Figure 4. The trends on these three surfaces are similar, where the heat flux gradually decreases. Because the surface temperature was suddenly increased from 90 to 120 K at t = 0 ns, while the temperature of the liquid film was still its equilibrium value of 90 K, the temperature difference between the liquid film and the bottom plate reached the maximum at this time. Therefore, for these three surfaces, qw had its maximum value at the initial moment of the explosive boiling stage. Then, as the liquid film continued to absorb heat from the bottom plate, the temperature difference began to narrow, inducing the decline of qw. However, at the same time, the heat flux of the larger coating thickness ratio was higher (Figure 4). This indicates the heat transfer was greater when the hydrophobic coating was thicker, which means increasing the coating thickness can promote explosive boiling. Hence, this property also results in that the hybrid surface with thicker coating requires a lower temperature for explosive boiling. It should be noted that, if the film thickness increased to 6 nm, qw shows a similar trend to the situation of case of 3 nm thickness, which is shown in Supporting Material (Figure S1). This also illustrates that no matter what the film thickness is, the heat flux increases with the coating thickness.
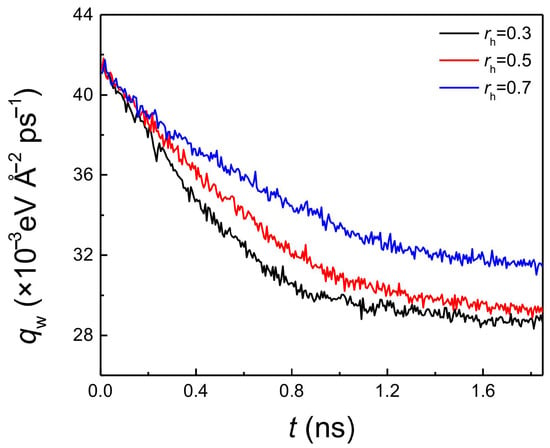
Figure 4.
The average heat flux with pillar width of 1.224 nm and film thickness of 3 nm on hydrophilic nanostructured surface with three different hydrophobic coating ratios at 120 K.
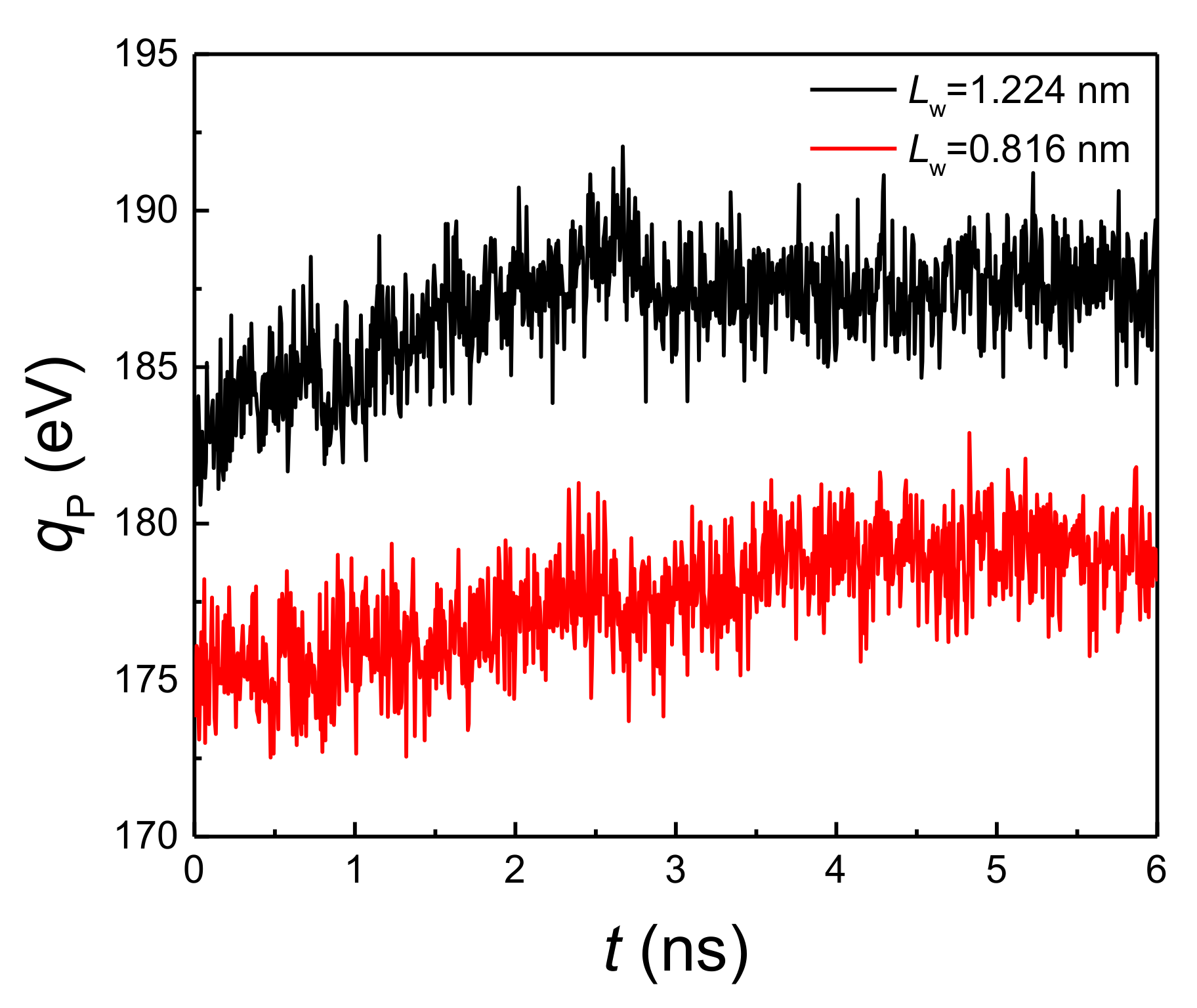
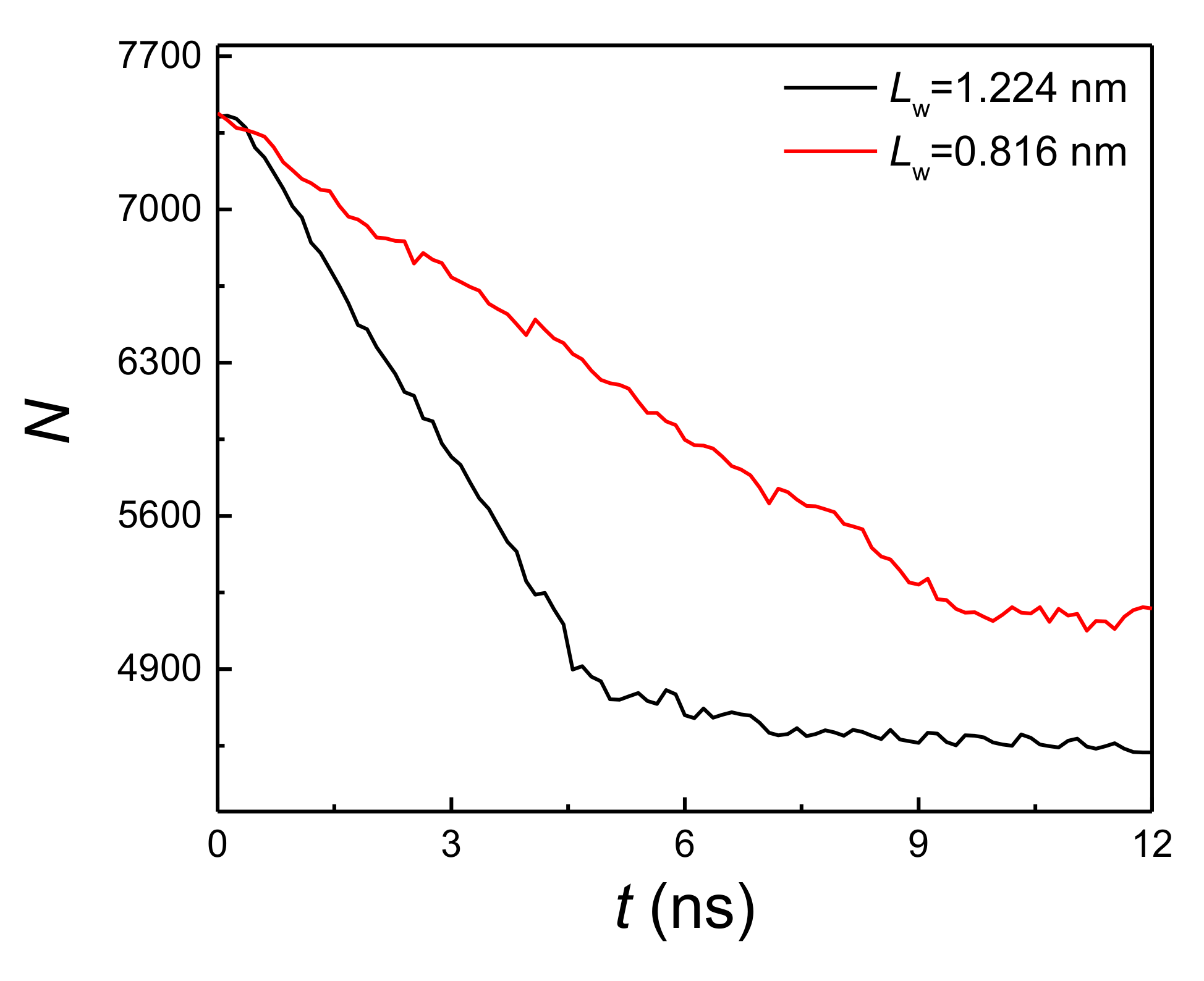
By comparing Figure 2a,b, it is found that when the liquid film thickness and coating thickness ratio are the same, the onset temperature of explosive boiling on the hybrid wetting surface with 1.224 nm pillar width is smaller than that on the surface with 0.816 nm pillar width. For investigating the reason for this result, the interaction potential energy (qp) between the liquid film and hybrid wetting nanostructure is calculated. The cases of film thickness with 6 nm, coating ratio with 0.5, and two different pillar widths at 125 K are chosen as examples, and the statistical results are shown in Figure 5. It is found from the figure that the potential energy with 1.224 nm pillar width is obviously greater than that with 0.816 nm pillar width, indicating that the adsorption capacity of the liquid film on hybrid wetting surface with 1.224 nm pillar width is stronger. Therefore, the time-dependent liquid molecular number in the liquid film on the surfaces mentioned above was computed, as shown in Figure 6. With the liquid film continuously absorbing heat from the bottom plate, the liquid molecules were evaporated, so that the number of liquid molecules gradually decreased. After explosive boiling occurs, the number of liquid molecules remains basically stable. As expected, when the pillar width was 1.224 nm, more argon molecules were evaporated into the upper space, making fewer molecules remain on the surface. Moreover, as the structure with 1.224 pillar width had a lower onset temperature of explosive boiling (115 K), it achieved this process earlier at the higher temperature (125 K).
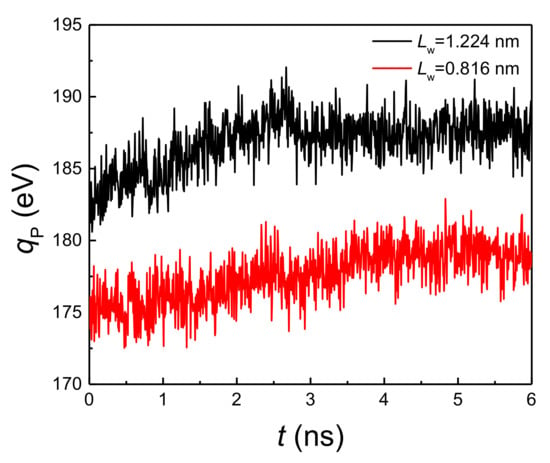
Figure 5.
The potential energy with a film thickness of 6 nm, coating ratio of 0.5, and two different pillar widths at 125 K.
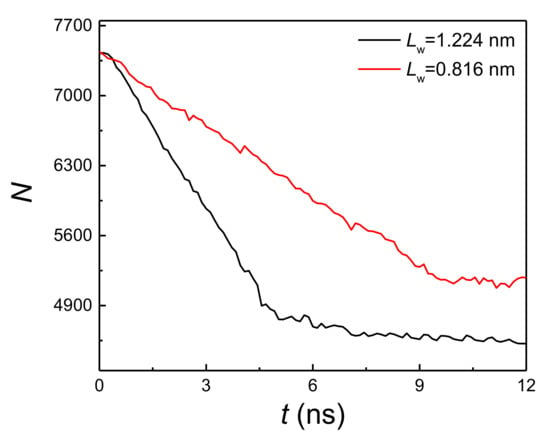
Figure 6.
The liquid molecular number with a film thickness of 6 nm, coating ratio of 0.5, and two different pillar widths at 125 K.
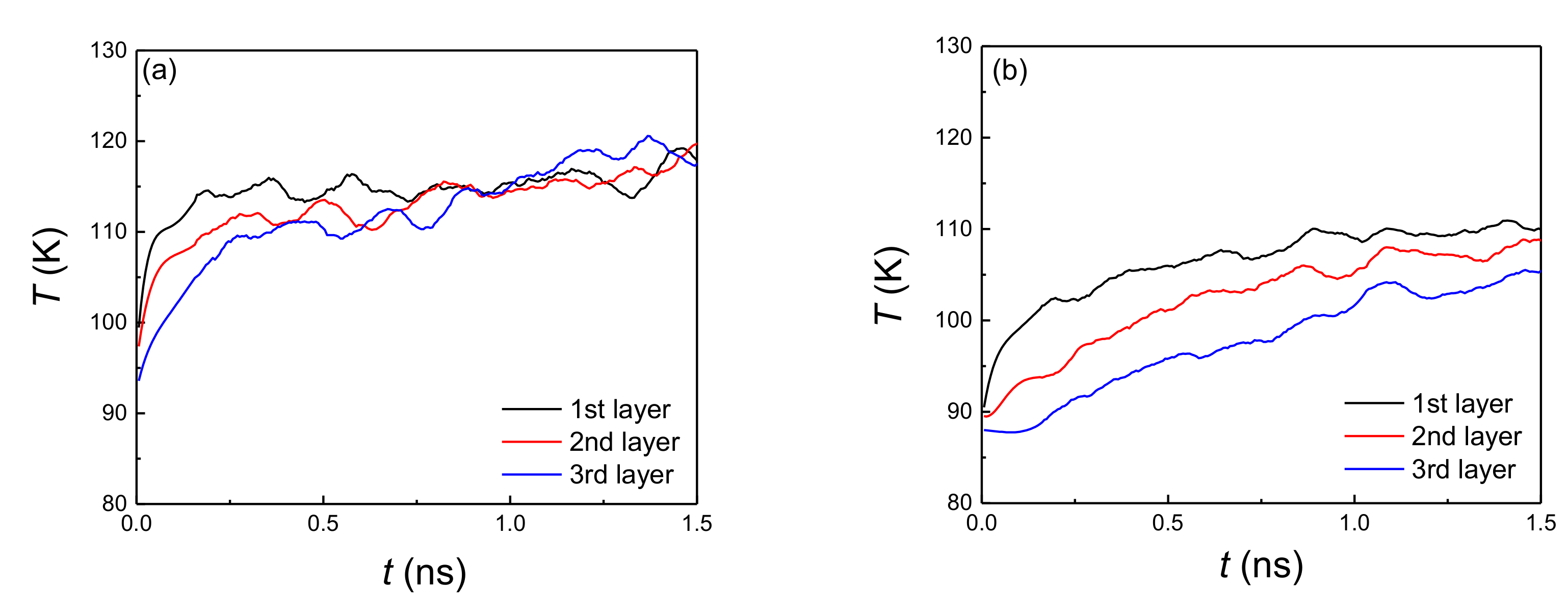
According to previous research [29,33,34], the onset temperature of explosive boiling decreases with the increase of the film thickness, where the surfaces are flat or pure hydrophobic or hydrophilic nanostructured. To verify the relationship of the above two factors on this hybrid hydrophobic–hydrophilic nanostructured surface, the trend of onset temperature with two film thicknesses is also discussed. Similar to the conclusion of explosive boiling on the pure hydrophilic or hydrophobic nanostructured surface, the onset temperature also decreases with the increase of liquid film thickness. To verify this variation trend, the temperature distribution in the liquid films with a thickness of 3 nm and 6 nm on the hydrophilic surface with a coating ratio of 0.3 and pillar width of 0.816 nm are calculated and discussed. It was divided into three layers for both 3 nm and 6 nm liquid films. The thicknesses of each layer for these two liquid films were 1 nm and 2 nm, respectively. Correspondingly, the locations of the first layers for these two liquid films are z = 0.8−1.8 Å and z = 0.8−2.8 Å. Figure 7a shows the evolution of the average temperature of each layer for 3 nm liquid film. It was found that at the early stage (before 0.5 ns), it showed a temperature difference for three layers. That is because the first layer, which contacted with the bottom surface, elevated its temperature to a high level quickly, while the second and third layers increased their temperature gradually. For the next stage (after 0.5 ns), there a was little temperature difference between these layers, indicating the heat absorbed from the plate could be fast transferred from the bottom layer to the top layer. Finally, the whole liquid film was heated and evaporated. Therefore, only the heated temperature was high enough, and the temperature difference between the top and bottom layers was obvious, with the reason that the heat could not be transferred from the bottom to the top immediately. However, when the liquid film thickness increased to 6 nm, the temperature exhibited a significant difference for various layers (Figure 7b). It indicates when the bottom layer received a high temperature, the top layer still remained at its low level, while this temperature distribution makes the atoms close to the bottom plate accumulate a high quantity of heat, promoting the bubble nucleation, followed by coalescence of the small bubbles with each other. Finally, a vapor layer is formed, which separates the upper liquid film from the plate. Actually, this is also the realization process of explosive boiling. Therefore, the larger temperature gradient is the primary reason for the thicker liquid film having a lower onset temperature, which benefits to trigger the bubble nucleation.
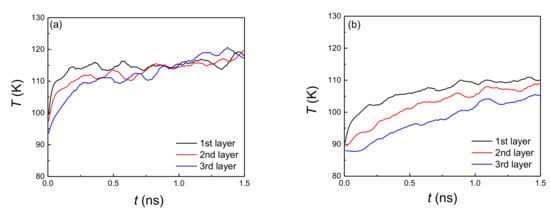
Figure 7.
Time evolution of temperature for three layers in the liquid films with thickness: (a) 3 nm and (b) 6 nm on a hydrophilic surface with coating ratio of 0.3 and pillar width of 0.816 nm.
4. Conclusions
Explosive boiling on the hydrophilic nanostructured surface with the hydrophobic coating at the nanoscale has been studied by molecular dynamics simulation. The onset temperatures of explosive boiling are summarized with a coating thickness of 0.612 nm, 1.02 nm, and 1.42 nm, a liquid film thickness of 3 nm and 6 nm, and two kinds of pillar width. In this paper, the effects of the hybrid hydrophobic–hydrophilic nanostructure were investigated in-depth. Furthermore, the coating thickness, pillar width, and liquid film thickness all affected the onset temperature of explosive boiling. The main conclusions are listed as follows.
- The hybrid nanostructure can decrease the onset temperature compared to the pure hydrophilic surface. By comparing the snapshots of the evolution of explosive boiling on the pure hydrophilic and hybrid nanostructured surfaces, it is found that the hydrophobic coating can promote the formation of bubbles and cause a quicker liquid film break, resulting in the onset temperature of explosive boiling decrease.
- With the increase of the hydrophobic coating thickness, the onset temperature of explosive boiling decreases for both pillar widths and liquid film thicknesses. The main reason is that the hydrophobic coating inevitably enhances the process of heat transfer between the liquid film and the hybrid nanostructured surface. Therefore, the average heat flux on the hydrophilic nanostructured surface with a thicker hydrophobic coating is larger.
- When the liquid film thickness and coating thickness ratio are the same, the onset temperature of explosive boiling on the hybrid wetting surface with 1.224 nm pillar width is smaller than that on the surface with 0.816 nm pillar width. This is because the potential energy with 1.224 nm pillar width is obviously greater than that with 0.816 nm pillar width, indicating that the adsorption capacity of the liquid film on hybrid wetting surface with 1.224 nm pillar width is stronger.
- The onset temperature of explosive boiling decreases with the increase of liquid film thickness, which is attributed to the temperature distribution in the liquid films. When the hydrophobic coating thickness and pillar width are the same, a larger temperature gradient is discovered with thicker liquid film, which benefits to trigger the bubble nucleation.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6412/11/2/212/s1, Figure S1: The average heat flux with pillar width of 1.224 nm and film thickness of 6 nm on hydrophilic nanostructured surface with three different hydrophobic coating ratios at 120 K.
Author Contributions
M.-J.L. wrote the manuscript. L.-Q.D. supervised and supported this study and critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Nature Science Foundation Projects of China, (No. 52076078 and No. 52090064) and the Science Fund for Creative Research Groups of the National Natural Science Foundation of China, No. 51821004.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frost, D.L. Dynamics of explosive boiling of a droplet. Phys. Fluids 1988, 31, 2554–2561. [Google Scholar] [CrossRef]
- Blander, M. Bubble nucleation in liquids. Adv. Colloid Interface Sci. 1979, 10, 1–32. [Google Scholar] [CrossRef]
- Gu, X.; Urbassek, H.M. Atomic dynamics of explosive boiling of liquid-argon films. Appl. Phys. B-Lasers Opt. 2005, 81, 675–679. [Google Scholar] [CrossRef]
- Tam, A.C.; Park, H.K.; Grigoropoulos, C.P. Laser cleaning of surface contaminants. Appl. Surf. Sci. 1998, 127, 721–725. [Google Scholar] [CrossRef]
- Juhasz, T.; Hu, X.H.; Turi, L.; Bor, Z. Dynamics of shock waves and cavitation bubbles generated by picosecond laser pulses in corneal tissue and water. Lasers Surg. Med. 1994, 15, 91–98. [Google Scholar] [CrossRef]
- Agostini, B.; Fabbri, M.; Park, J.E.; Wojtan, L.; Thome, J.R.; Michel, B. State of the art of high heat flux cooling technologies. Heat Transf. Eng. 2007, 28, 258–281. [Google Scholar] [CrossRef]
- Riofrío, M.C.; Caney, N.; Gruss, J.A. State of the art of efficient pumped two-phase flow cooling technologies. Appl. Therm. Eng. 2016, 104, 333–343. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, W.; Tang, Y.; Chen, J.; Li, Z. Enhanced flow boiling in an interconnected microchannel net at different inlet subcooling. Appl. Therm. Eng. 2016, 104, 659–667. [Google Scholar] [CrossRef]
- Jia, J.; Guo, Y.; Wang, W.; Zhou, S. Modeling and Experimental Research on Spray Cooling. In Proceedings of the 2008 Twenty-fourth Annual IEEE Semiconductor Thermal Measurement and Management Symposium, San Jose, CA, USA, 6–20 May 2008; pp. 118–123. [Google Scholar]
- Van Gils, R.W.; Danilov, D.; Notten, P.H.L.; Speetjens, M.F.M.; Nijmeijer, H. Battery thermal management by boiling heat-transfer. Energy Convers. Manag. 2014, 79, 9–17. [Google Scholar] [CrossRef]
- Seyf, H.R.; Zhang, Y. Effect of nanotextured array of conical features on explosive boiling over a flat substrate: A nonequilibrium molecular dynamics study. Int. J. Heat Mass Transf. 2013, 66, 613–624. [Google Scholar] [CrossRef]
- Seyf, H.R.; Zhang, Y. Molecular dynamics simulation of normal and explosive boiling on nanostructured surface. J. Heat Transfer 2013, 135, 121503. [Google Scholar] [CrossRef]
- Fu, T.; Mao, Y.; Tang, Y.; Zhang, Y.; Yuan, W. Molecular Dynamics Simulation on Rapid Boiling of Thin Water Films on Cone-Shaped Nanostructure Surfaces. Nanoscale Microscale Thermophys. Eng. 2015, 19, 17–30. [Google Scholar] [CrossRef]
- Qasemian, A.; Qanbarian, M.; Arab, B. Molecular dynamics simulation on explosive boiling of thin liquid argon films on cone-shaped Al–Cu-based nanostructures. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Fu, T.; Mao, Y.; Tang, Y.; Zhang, Y.; Yuan, W. Effect of nanostructure on rapid boiling of water on a hot copper plate: A molecular dynamics study. Heat Mass Transf. 2015, 52, 1469–1478. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Tian, C.; Meng, X. Numerical experiments on evaporation and explosive boiling of ultra-thin liquid argon film on aluminum nanostructure substrate. Nanoscale Res. Lett. 2015, 10, 158. [Google Scholar] [CrossRef]
- Morshed, A.K.M.M.; Paul, T.C.; Khan, J.A. Effect of nanostructures on evaporation and explosive boiling of thin liquid films: A molecular dynamics study. Appl. Phys. A 2011, 105, 445–451. [Google Scholar] [CrossRef]
- Zhanga, H.; Lia, C.; Wanga, Y.; Zhub, Y.; Wangb, W. Effect of heat source temperature, nanostructure, and wettability on explosive boiling of ultra-thin liquid argon film over graphene substrate: A molecular dynamics study. Curr. Nanosci. 2021, 17, 1–11. [Google Scholar]
- Zhang, S.; Hao, F.; Chen, H.; Yuan, W.; Tang, Y.; Chen, X. Molecular dynamics simulation on explosive boiling of liquid argon film on copper nanochannels. Appl. Therm. Eng. 2017, 113, 208–214. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Zhao, M.; Zhu, Y.; Wang, W. Influence of interface wettability on normal and explosive boiling of ultra-thin liquid films on a heated substrate in nanoscale: A molecular dynamics study. Micro Nano Lett. 2017, 12, 843–848. [Google Scholar] [CrossRef]
- Hens, A.; Agarwal, R.; Biswas, G. Nanoscale study of boiling and evaporation in a liquid Ar film on a Pt heater using molecular dynamics simulation. Int. J. Heat Mass Transf. 2014, 71, 303–312. [Google Scholar] [CrossRef]
- Shavik, S.M.; Hasan, M.N.; Monjur Morshed, A.K.M. Molecular dynamics study on explosive boiling of thin liquid argon film on nanostructured surface under different wetting conditions. J. Electron. Packag. 2016, 138, 010904. [Google Scholar] [CrossRef]
- Betz, A.R.; Xu, J.; Qiu, H.; Attinger, D. Do surfaces with mixed hydrophilic and hydrophobic areas enhance pool boiling? Appl. Phys. Lett. 2010, 97, 141909. [Google Scholar] [CrossRef]
- Jo, H.; Ahn, H.S.; Kang, S.; Kim, M.H. A study of nucleate boiling heat transfer on hydrophilic, hydrophobic and heterogeneous wetting surfaces. Int. J. Heat Mass Transf. 2011, 54, 5643–5652. [Google Scholar] [CrossRef]
- Suroto, B.J.; Tashiro, M.; Hirabayashi, S.; Hidaka, S.; Kohno, M.; Takata, Y. Effects of hydrophobic-spot periphery and subcooling on nucleate pool boiling from a mixed-wettability surface. J. Therm. Sci. Technol. 2013, 8, 294–308. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- LAMMPS Users Manual. Available online: http://lammps.sandia.gov (accessed on 10 February 2021).
- Rabbi, K.F.; Tamim, S.I.; Faisal, A.H.M.; Mukut, K.M.; Hasan, M.N. A molecular dynamics study on thin film liquid boiling characteristics under rapid linear boundary heating: Effect of liquid film thickness. AIP Conf. Proc. 2017, 1851, 020102. [Google Scholar]
- Liao, M.J.; Duan, L.Q. Explosive Boiling of Liquid Argon Films on Flat and Nanostructured Surfaces. Numer. Heat Transf. 2020, 78, 94–105. [Google Scholar] [CrossRef]
- Johnson, R.A. Alloy models with the embedded-atom method. Phys. Rev. B 1989, 39, 12554–12559. [Google Scholar] [CrossRef] [PubMed]
- Hoover, W. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Dou, Y.; Zhigilei, L.V.; Postawa, Z.; Winograd, N.; Garrison, B.J. Thickness effects of water overlayer on its explosive evaporation at heated metal surfaces. Nucl. Instrum. Meth. B. 2001, 180, 105–111. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, S.Y.; Lu, G.; Wang, X.D. Explosive boiling of nano-liquid argon films on high temperature platinum walls: Effects of surface wettability and film thickness. Int. J Therm. Sci. 2018, 132, 610–617. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).