The Role of Carbon Quantum Dots in Organic Photovoltaics: A Short Overview
Abstract
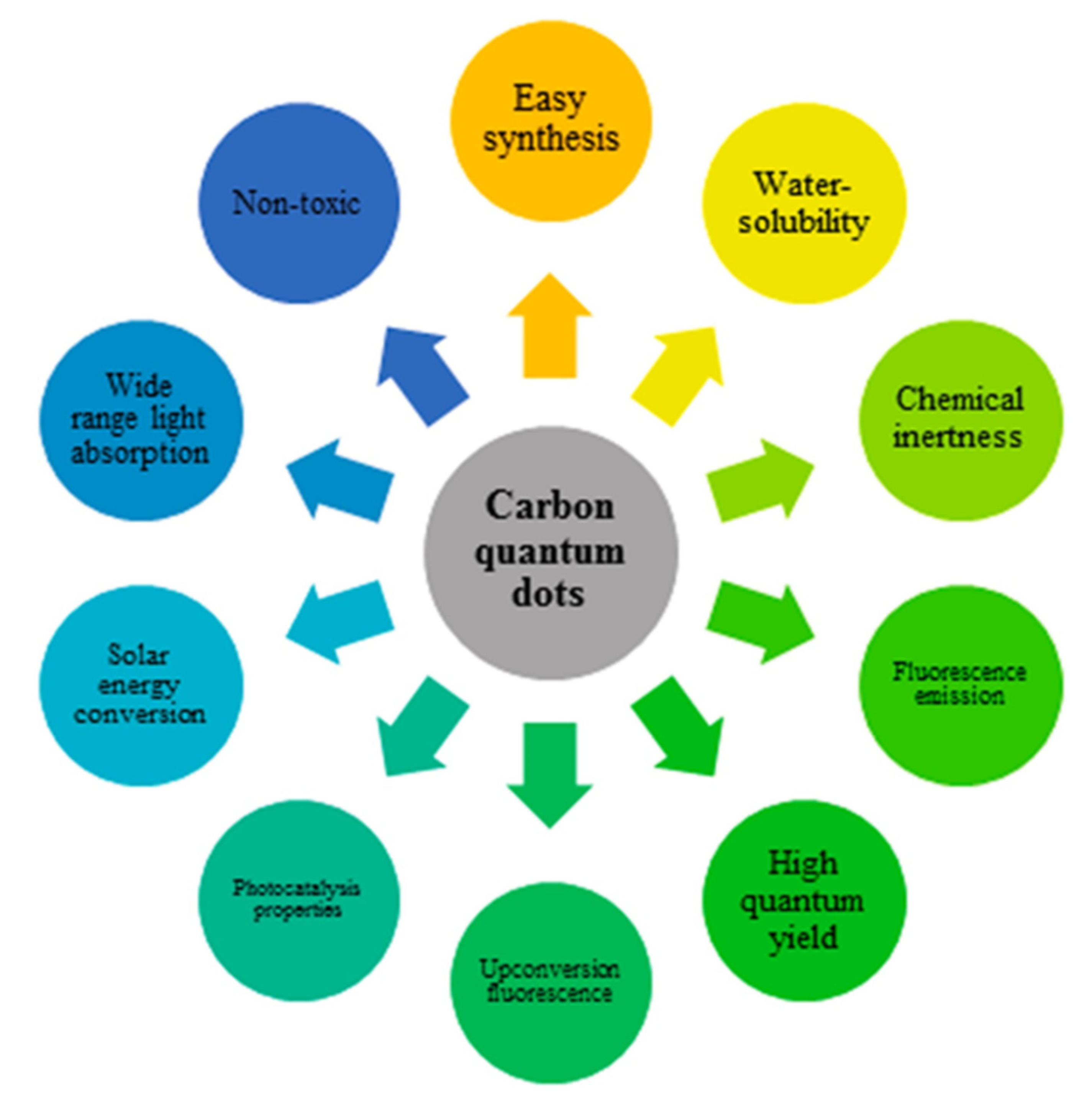
:1. Introduction
2. Structure Engineering and Optical Properties
3. Organic Photovoltaics (OPVs)
3.1. Bulk Heterojunction (BHJ) Organic Solar Cells
3.1.1. Electron Donors/Acceptors
3.1.2. Hole Extraction Layer
3.1.3. Electron Transport Layer (ETL)
3.2. Dye Sensitized Solar Cells (DSSCs)
3.2.1. Sensitizers
3.2.2. Counter Electrode
4. Final Remarks and Perspectives
Funding
Conflicts of Interest
References
- Grätzel, M. Recent Advances in Sensitized Mesoscopic Solar Cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Min, J. A Cost Analysis of Fully Solution-Processed ITO-Free Organic Solar Modules. Adv. Energy Mater. 2019, 9, 1802521. [Google Scholar] [CrossRef]
- Glogic, E.; Weyand, S.; Tsang, M.P.; Young, S.B.; Schebek, L.; Sonnemann, G. Life cycle assessment of organic photovoltaic charger use in Europe: The role of product use intensity and irradiation. J. Clean. Prod. 2019, 233, 1088–1096. [Google Scholar] [CrossRef]
- Gan, Q.; Bartoli, F.J.; Kafafi, Z.H. Plasmonic-Enhanced Organic Photovoltaics: Breaking the 10% Efficiency Barrier. Adv. Mater. 2013, 25, 2385–2396. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Mintz, K.J.; Cheng, L.; Chen, J.; Ferreira, B.C.; Hettiarachchi, S.D.; Liyanage, P.Y.; Seven, E.S.; Miloserdov, N.; Pandey, R.R.; et al. Direct conjugation of distinct carbon dots as Lego-like building blocks for the assembly of versatile drug nanocarriers. J. Colloid Interface Sci. 2020, 576, 412–425. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, H.; Zhang, X.; Jiang, H.; Geng, X.; Li, S.; Tu, H.; Cheng, X.; Yang, P.; Wan, Y. High-absorption solar steam device comprising Au@Bi2MoO6-CDs: Extraordinary desalination and electricity generation. Nano Energy 2020, 68, 104298. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, T.; Zhou, Y.; Li, S.; Li, J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Healthc. Mater. 2020, 9, e1901495. [Google Scholar] [CrossRef]
- Dou, X.; Zheng, Y.; Uchiyama, K.; Lin, J.-M. Fluorescent carbon nanoparticles: Mimicking hydrogen peroxide properties in a chemiluminescence system. Chem. Commun. 2016, 52, 14137–14140. [Google Scholar] [CrossRef]
- Zhao, H.X.; Liu, L.Q.; De Liu, Z.; Wang, Y.; Zhao, X.J.; Huang, C.Z. Highly selective detection of phosphate in very complicated matrixes with an off–on fluorescent probe of europium-adjusted carbon dots. Chem. Commun. 2011, 47, 2604–2606. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, D.; Shah, S.N.A.; Li, H.; Lin, J.-M. Ultra-Weak chemiluminescence enhanced by facilely synthesized nitrogen-rich quantum dots through chemiluminescence resonance energy transfer and electron hole injection. Chem. Commun. 2017, 53, 5657–5660. [Google Scholar] [CrossRef]
- Zhou, W.; Dong, S.; Lin, Y.; Lu, C. Insights into the role of nanostructure in the sensing properties of carbon nanodots for improved sensitivity to reactive oxygen species in living cells. Chem. Commun. 2017, 53, 2122–2125. [Google Scholar] [CrossRef]
- Dong, S.; Yuan, Z.; Zhang, L.; Lin, Y.; Lu, C. Rapid Screening of Oxygen States in Carbon Quantum Dots by Chemiluminescence Probe. Anal. Chem. 2017, 89, 12520–12526. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.-L.; Wang, J.-H. Inner filter effect-based fluorescent sensing systems: A review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar] [CrossRef]
- Feng, J.; Wang, W.-J.; Hai, X.; Yu, Y.-L.; Wang, J.-H. Green preparation of nitrogen-doped carbon dots derived from silkworm chrysalis for cell imaging. J. Mater. Chem. B 2015, 4, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, X.; Rider, A.E.; Furman, S.A.; Ostrikov, K. Fast, energy-efficient synthesis of luminescent carbon quantum dots. Green Chem. 2014, 16, 2566–2570. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.Q.; Yang, T.; Li, R.S.; Zou, H.Y.; Li, Y.F.; Guo, J.; Liu, X.D.; Huang, C.Z. A functional preservation strategy for the production of highly photoluminescent emerald carbon dots for lysosome targeting and lysosomal pH imaging. Nanoscale 2018, 10, 14705–14711. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.X.; Yang, L.; Zheng, Y.; Yang, X.X.; Zou, H.Y.; Han, J.; Liu, Z.X.; Li, Y.F.; Huang, C.Z. “Click” on Alkynylated Carbon Quantum Dots: An Efficient Surface Functionalization for Specific Biosensing and Bioimaging. Chem. A Eur. J. 2017, 23, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J. The optical properties and solar energy conversion applications of carbon quantum dots: A review. Sol. Energy 2020, 196, 549–566. [Google Scholar] [CrossRef]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene Quantum Dots. Part. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Hutton, G.A.M.; Martindale, B.C.M.; Reisner, E. Carbon dots as photosensitisers for solar-driven catalysis. Chem. Soc. Rev. 2017, 46, 6111–6123. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.-L.; Zhang, Z.-L.; Huang, B.-H.; Peng, J.; Zhang, M.; Pang, D.-W. Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite. Chem. Commun. 2008, 5116–5118. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, C.; Hu, C.; Yang, W.; Zhao, C.; Wang, S.; Zhang, M.; Zhao, J.; Wang, X.; Qiu, J. Solvothermal conversion of coal into nitrogen-doped carbon dots with singlet oxygen generation and high quantum yield. Chem. Eng. J. 2017, 320, 570–575. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Y.; Wu, W.; Hu, C.; Wang, X.; Zheng, J.; Li, Z.; Jiang, B.; Qiu, J. Preparation of functionalized water-soluble photoluminescent carbon quantum dots from petroleum coke. Carbon 2014, 78, 480–489. [Google Scholar] [CrossRef]
- Jaiswal, A.; Ghosh, S.S.; Chattopadhyay, A. One step synthesis of C-dots by microwave mediated caramelization of poly(ethylene glycol). Chem. Commun. 2012, 48, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhai, J.; Dong, S. Bifunctional fluorescent carbon nanodots: Green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction. Chem. Commun. 2012, 48, 9367–9369. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Kundu, S.; Patra, A. Nanoscale Strategies for Light Harvesting. Chem. Rev. 2016, 117, 712–757. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Cui, X.; Li, B.; Li, L.-S. Large, Solution-Processable Graphene Quantum Dots as Light Absorbers for Photovoltaics. Nano Lett. 2010, 10, 1869–1873. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, W.; Yu, S.; Yan, X. Three dimensional carbogenic dots/TiO2 nanoheterojunctions with enhanced visible light-driven photocatalytic activity. Carbon 2014, 79, 369–379. [Google Scholar] [CrossRef]
- Kim, H.; Kwon, W.; Choi, M.; Rhee, S.-W.; Yong, K. Photoelectrochemical Hydrogen Generation Using C-dot/ZnO Hierarchical Nanostructure as an Efficient Photoanode. J. Electrochem. Soc. 2015, 162, H366–H370. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-X.; Xiao, J.; Yang, Z.; Tang, H.; Zhu, Z.-T.; Zhao, M.; Liu, Y.; Zhang, C.; Zhang, H.-L. Rational design of nitrogen and sulfur co-doped carbon dots for efficient photoelectrical conversion applications. J. Mater. Chem. A 2015, 3, 11287–11293. [Google Scholar] [CrossRef]
- Wang, X.; Ling, D.; Wang, Y.; Long, H.; Sun, Y.; Shi, Y.; Chen, Y.; Jing, Y.; Sun, Y.; Dai, Y. N-doped graphene quantum dots-functionalized titanium dioxide nanofibers and their highly efficient photocurrent response. J. Mater. Res. 2014, 29, 1408–1416. [Google Scholar] [CrossRef]
- Pan, D.; Jiao, J.; Li, Z.; Guo, Y.; Feng, C.; Liu, Y.; Wang, L.; Wu, M. Efficient Separation of Electron–Hole Pairs in Graphene Quantum Dots by TiO2 Heterojunctions for Dye Degradation. ACS Sustain. Chem. Eng. 2015, 3, 2405–2413. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Liu, Y.; Wang, X.; Shen, M.; Lee, S.-T.; Kang, Z. Opto-electronic conversion logic behaviour through dynamic modulation of electron/energy transfer states at the TiO2–carbon quantum dot interface. Nanoscale 2013, 5, 1831–1835. [Google Scholar] [CrossRef]
- Mihalache, I.; Radoi, A.; Mihaila, M.; Munteanu, C.; Marin, A.; Danila, M.; Kusko, M.; Kusko, C. Charge and energy transfer interplay in hybrid sensitized solar cells mediated by graphene quantum dots. Electrochim. Acta 2015, 153, 306–315. [Google Scholar] [CrossRef]
- Sun, M.; Ma, X.; Chen, X.; Sun, Y.; Cui, X.; Lin, Y. A nanocomposite of carbon quantum dots and TiO2nanotube arrays: Enhancing photoelectrochemical and photocatalytic properties. RSC Adv. 2014, 4, 1120–1127. [Google Scholar] [CrossRef]
- Kokal, R.K.; Kumar, P.N.; Deepa, M.; Srivastava, A.K. Lead selenide quantum dots and carbon dots amplify solar conversion capability of a TiO2/CdS photoanode. J. Mater. Chem. A 2015, 3, 20715–20726. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Liu, P.; Li, Y.; Yang, H.G.; An, T.; Wong, P.-K.; Wang, D.; Tang, Z.; Zhao, H. A fluorescent quenching performance enhancing principle for carbon nanodot-sensitized aqueous solar cells. Nano Energy 2015, 13, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Dang, Y.; Zhang, X.; Chen, X.; Kang, B.; Silva, S.R.P. Heterojunction solar cells with improved power conversion efficiency using graphene quantum dots. RSC Adv. 2016, 6, 110493–110498. [Google Scholar] [CrossRef]
- Kwon, W.; Lee, G.; Do, S.; Joo, T.; Rhee, S.-W. Size-Controlled Soft-Template Synthesis of Carbon Nanodots toward Versatile Photoactive Materials. Small 2013, 10, 506–513. [Google Scholar] [CrossRef]
- Li, F.; Kou, L.; Chen, W.; Wu, C.; Guo, T. Enhancing the short-circuit current and power conversion efficiency of polymer solar cells with graphene quantum dots derived from double-walled carbon nanotubes. NPG Asia Mater. 2013, 5, e60. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Zhao, Y.; Shi, G.; Deng, L.; Hou, Y.; Qu, L. An Electrochemical Avenue to Green-Luminescent Graphene Quantum Dots as Potential Electron-Acceptors for Photovoltaics. Adv. Mater. 2010, 23, 776–780. [Google Scholar] [CrossRef]
- Anilkumar, P.; Wang, X.; Cao, L.; Sahu, S.; Liu, J.-H.; Wang, P.; Korch, K.; Ii, K.N.T.; Parenzan, A.; Sun, Y.-P. Toward quantitatively fluorescent carbon-based “quantum” dots. Nanoscale 2011, 3, 2023–2027. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, A.; Soriano, M.; Carrillocarrion, C.; Valcárcel, M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2016, 52, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Essner, J.B.; Baker, G.A. The emerging roles of carbon dots in solar photovoltaics: A critical review. Environ. Sci. Nano 2017, 4, 1216–1263. [Google Scholar] [CrossRef]
- LeCroy, G.E.; Messina, F.; Sciortino, A.; Bunker, C.E.; Wang, P.; Fernando, K.A.S.; Sun, Y.-P. Characteristic Excitation Wavelength Dependence of Fluorescence Emissions in Carbon “Quantum” Dots. J. Phys. Chem. C 2017, 121, 28180–28186. [Google Scholar] [CrossRef]
- Van Dam, B.; Nie, H.; Ju, B.; Marino, E.; Paulusse, J.M.J.; Schall, P.; Li, M.; Dohnalová, K. Carbon Dots: Excitation-Dependent Photoluminescence from Single-Carbon Dots. Small 2017, 13, 1702098. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.-Y.; Nguyen, B.-S.; Yeh, T.-F.; Lee, Y.-L.; Chen, S.-J.; Teng, H. Roles of nitrogen functionalities in enhancing the excitation-independent green-color photoluminescence of graphene oxide dots. Nanoscale 2017, 9, 8256–8265. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Barati, A.; Karami, S. Long-wavelength, multicolor, and white-light emitting carbon-based dots: Achievements made, challenges remaining, and applications. Carbon 2017, 124, 429–472. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, F.; Li, X.; Li, Y.; Zhong, H.; Fanglong, Y.; Yang, S. 53% Efficient Red Emissive Carbon Quantum Dots for High Color Rendering and Stable Warm White-Light-Emitting Diodes. Adv. Mater. 2017, 29, 1702910. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jing, P.; Sun, L.; An, Y.; Shan, X.; Lu, X.; Zhou, D.; Han, D.; Shen, D.; Zhai, Y.; et al. Near-Infrared Excitation/Emission and Multiphoton-Induced Fluorescence of Carbon Dots. Adv. Mater. 2018, 30, e1705913. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.; Gonzalez-Rodriguez, R.; Ryan, C.; Faerber, N.; Coffer, J.L.; Naumov, A.V. Photo and Electroluminescence from Nitrogen Doped and Nitrogen Sulfur Codoped Graphene Quantum Dots. Adv. Funct. Mater. 2018, 28, 1804337. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Zhang, S.; Fu, Y.; Wang, L.; Zhao, X.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Lu, S.; Xiao, G.; Sui, L.; Feng, T.; Yong, X.; Zhu, S.; Li, B.; Liu, Z.; Zou, B.; Jin, M.; et al. Piezochromic Carbon Dots with Two-photon Fluorescence. Angew. Chem. Int. Ed. 2017, 56, 6187–6191. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, G.; Yang, M.; Zou, B.; Zhang, Z.-L.; Pang, D.-W. Mechanofluorochromic Carbon Nanodots: Controllable Pressure-Triggered Blue- and Red-Shifted Photoluminescence. Angew. Chem. Int. Ed. 2017, 57, 1893–1897. [Google Scholar] [CrossRef]
- Niu, X.; Li, Y.; Shu, H.; Wang, J. Revealing the underlying absorption and emission mechanism of nitrogen doped graphene quantum dots. Nanoscale 2016, 8, 19376–19382. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Cheng, H.; Lin, H.; Humphrey, M.G.; Zhang, C. A hydrothermal route to water-stable luminescent carbon dots as nanosensors for pH and temperature. Carbon 2015, 82, 87–95. [Google Scholar] [CrossRef]
- Kalytchuk, S.; Poláková, K.; Wang, Y.; Froning, J.P.; Cepe, K.; Rogach, A.L.; Zbořil, R. Carbon Dot Nanothermometry: Intracellular Photoluminescence Lifetime Thermal Sensing. ACS Nano 2017, 11, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.M.; Ananthanarayanan, A.; Huang, L.; Lim, K.H.; Chen, P. Revealing the tunable photoluminescence properties of graphene quantum dots. J. Mater. Chem. C 2014, 2, 6954–6960. [Google Scholar] [CrossRef]
- Zhu, B.; Sun, S.; Wang, Y.; Deng, S.; Qian, G.; Wang, M.; Hu, A. Preparation of carbon nanodots from single chain polymeric nanoparticles and theoretical investigation of the photoluminescence mechanism. J. Mater. Chem. C 2013, 1, 580–586. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, Z.-L.; Tian, Z.-Q.; Zhang, L.; Liu, C.; Lin, Y.; Qi, B.; Pang, D.-W. Electrochemical Tuning of Luminescent Carbon Nanodots: From Preparation to Luminescence Mechanism. Adv. Mater. 2011, 23, 5801–5806. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Liu, M.; Yang, B.; Feng, L.; Ji, Y.; Tao, L.; Wei, Y. Size tunable fluorescent nano-graphite oxides: Preparation and cell imaging applications. Phys. Chem. Chem. Phys. 2013, 15, 19013–19018. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Zhou, N.; Lin, X.; Jianpeng, L.; Chi, Y.; Chen, G. Extraction of Electrochemiluminescent Oxidized Carbon Quantum Dots from Activated Carbon. Chem. Mater. 2010, 22, 5895–5899. [Google Scholar] [CrossRef]
- Liu, H.; Ye, T.; Mao, C. Fluorescent Carbon Nanoparticles Derived from Candle Soot. Angew. Chem. Int. Ed. 2007, 46, 6473–6475. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.-T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Vinci, J.C.; Ferrer, I.M.; Seedhouse, S.J.; Bourdon, A.K.; Reynard, J.M.; Foster, B.A.; Bright, F.V.; Colón, L.A. Hidden Properties of Carbon Dots Revealed After HPLC Fractionation. J. Phys. Chem. Lett. 2012, 4, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, L.; Yang, S.T.; Lu, F.S.; Meziani, M.J.; Tian, L.L.; Sun, K.W.; Bloodgood, M.A.; Sun, Y.P. Bandgap-Like Strong Fluorescence in Functionalized Carbon Nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 5310–5314. [Google Scholar] [CrossRef] [PubMed]
- Tetsuka, H.; Asahi, R.; Nagoya, A.; Okamoto, K.; Tajima, I.; Ohta, R.; Okamoto, A. Optically Tunable Amino-Functionalized Graphene Quantum Dots. Adv. Mater. 2012, 24, 5333–5338. [Google Scholar] [CrossRef] [PubMed]
- Tetsuka, H.; Nagoya, A.; Fukusumi, T.; Matsui, T. Molecularly Designed, Nitrogen-Functionalized Graphene Quantum Dots for Optoelectronic Devices. Adv. Mater. 2016, 28, 4632–4638. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, Q.; Long, Y.; Zhang, H.; Huang, X.; Zhu, R. Enhancing the luminescence of carbon dots with a reduction pathway. Chem. Commun. 2011, 47, 10650–10652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Meng, X.; Ding, T.; Xu, Y.; Xu, H.; Ren, Y.; Liu, B.; Huang, J.; Yang, J.; et al. Graphenol defects induced blue emission enhancement in chemically reduced graphene quantum dots. Phys. Chem. Chem. Phys. 2015, 17, 22361–22366. [Google Scholar] [CrossRef]
- Mandal, B.; Sarkar, S.; Sarkar, P. Theoretical Studies on Understanding the Feasibility of Porphyrin-Sensitized Graphene Quantum Dot Solar Cell. J. Phys. Chem. C 2015, 119, 3400–3407. [Google Scholar] [CrossRef]
- Rigodanza, F.; Dordevich, L.; Arcudi, F.; Prato, M. Customizing the Electrochemical Properties of Carbon Nanodots by Using Quinones in Bottom-Up Synthesis. Angew. Chem. Int. Ed. 2018, 57, 5062–5067. [Google Scholar] [CrossRef]
- Yang, G.; Wu, C.; Luo, X.; Liu, X.; Gao, Y.; Wu, P.; Cai, C.; Saavedra, S.S. Exploring the Emissive States of Heteroatom-Doped Graphene Quantum Dots. J. Phys. Chem. C 2018, 122, 6483–6492. [Google Scholar] [CrossRef]
- Holá, K.; Sudolská, M.; Kalytchuk, S.; Nachtigallová, D.; Rogach, A.L.; Otyepka, M.; Zbořil, R. Graphitic Nitrogen Triggers Red Fluorescence in Carbon Dots. ACS Nano 2017, 11, 12402–12410. [Google Scholar] [CrossRef]
- Fernando, K.A.S.; Sahu, S.; Liu, Y.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.-P. Carbon Quantum Dots and Applications in Photocatalytic Energy Conversion. ACS Appl. Mater. Interfaces 2015, 7, 8363–8376. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.; Yang, Y.; Liu, X.; Chen, Y.; Xu, B. P3HT/Dodecylamine Functioned Carbon Microspheres Composite Films for Polymer Solar Cells. Full Nanotub. Carbon Nanostruct. 2014, 23, 549–556. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, Y.; Yan, L.; Zhang, Y.; He, Y.; Yang, Y.; Liu, X. Low-Temperature Hydrothermal Synthesis of Green Luminescent Carbon Quantum Dots (CQD), and Optical Properties of Blends of the CQD with Poly(3-hexylthiophene). J. Electron. Mater. 2015, 44, 3436–3443. [Google Scholar] [CrossRef]
- Bin Yang, H.; Dong, Y.Q.; Wang, X.; Khoo, S.Y.; Liu, B. Cesium Carbonate Functionalized Graphene Quantum Dots as Stable Electron-Selective Layer for Improvement of Inverted Polymer Solar Cells. ACS Appl. Mater. Interfaces 2013, 6, 1092–1099. [Google Scholar] [CrossRef]
- Bin Yang, H.; Dong, Y.Q.; Wang, X.; Khoo, S.Y.; Liu, B.; Li, C.M. Graphene quantum dots-incorporated cathode buffer for improvement of inverted polymer solar cells. Sol. Energy Mater. Sol. Cells 2013, 117, 214–218. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.-H.; Liu, C.-Y.; Ma, D.-G. White light-emitting devices based on carbon dots’ electroluminescence. Chem. Commun. 2011, 47, 3502–3504. [Google Scholar] [CrossRef]
- Xie, C.; Nie, B.; Zeng, L.; Liang, F.-X.; Wang, M.-Z.; Luo, L.; Feng, M.; Yu, Y.; Wu, C.-Y.; Wu, Y.; et al. Core–Shell Heterojunction of Silicon Nanowire Arrays and Carbon Quantum Dots for Photovoltaic Devices and Self-Driven Photodetectors. ACS Nano 2014, 8, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ding, K.; Wang, Y.; Ruan, K.; Diao, S.; Zhang, Q.; Sun, B.; Jie, J. Crystalline Si/Graphene Quantum Dots Heterojunction Solar Cells. J. Phys. Chem. C 2014, 118, 5164–5171. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Li, Z.; Guo, J.; Shen, L.; Guo, W.; Zhang, L.; Ruan, S.; Long, Y. An easily prepared carbon quantum dots and employment for inverted organic photovoltaic devices. Chem. Eng. J. 2017, 315, 621–629. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, M.J.; Kim, S.J.; Wang, N.H.; Cho, S.P.; Bae, S.; Park, J.H.; Hong, B.H. Balancing Light Absorptivity and Carrier Conductivity of Graphene Quantum Dots for High-Efficiency Bulk Heterojunction Solar Cells. ACS Nano 2013, 7, 7207–7212. [Google Scholar] [CrossRef]
- Gupta, V.; Chaudhary, N.; Srivastava, R.; Sharma, G.D.; Bhardwaj, R.; Chand, S. Luminscent Graphene Quantum Dots for Organic Photovoltaic Devices. J. Am. Chem. Soc. 2011, 133, 9960–9963. [Google Scholar] [CrossRef]
- Privitera, A.; Righetto, M.; Mosconi, D.; Lorandi, F.; Isse, A.A.; Moretto, A.; Bozio, R.; Ferrante, C.; Franco, L. Boosting carbon quantum dots/fullerene electron transfer via surface group engineering. Phys. Chem. Chem. Phys. 2016, 18, 31286–31295. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wen, X.; Toh, Y.-R.; Lee, Y.-C.; Huang, K.-Y.; Huang, S.; Shrestha, S.; Conibeer, G.; Tang, J. Efficient electron transfer in carbon nanodot–graphene oxide nanocomposites. J. Mater. Chem. C 2014, 2, 2894. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Lu, F.; Meziani, M.J.; Li, H.; Qi, G.; Zhou, B.; Harruff, B.A.; Kermarrec, F.; Sun, Y.-P. Photoinduced electron transfers with carbon dots. Chem. Commun. 2009, 3774–3776. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Seth, S.K.; Gupta, P.; Purkayastha, P. Ultrafast Photoinduced Electron Transfer between Carbon Nanoparticles and Cyclometalated Rhodium and Iridium Complexes. J. Phys. Chem. C 2015, 119, 25122–25128. [Google Scholar] [CrossRef]
- Ding, Z.; Hao, Z.; Meng, B.; Xie, Z.; Liu, J.; Dai, L. Few-layered graphene quantum dots as efficient hole-extraction layer for high-performance polymer solar cells. Nano Energy 2015, 15, 186–192. [Google Scholar] [CrossRef]
- Li, M.; Ni, W.; Kan, B.; Wan, X.; Zhang, L.; Zhang, Q.; Long, G.; Zuo, Y.; Chen, Y. Graphene quantum dots as the hole transport layer material for high-performance organic solar cells. Phys. Chem. Chem. Phys. 2013, 15, 18973–18978. [Google Scholar] [CrossRef]
- Lim, H.C.; Min, S.H.; Lee, E.; Jang, J.; Kim, S.H.; Hong, J.-I. Self-Assembled Poly(3,4-ethylene dioxythiophene): Poly(styrenesulfonate)/Graphene Quantum Dot Organogels for Efficient Charge Transport in Photovoltaic Devices. ACS Appl. Mater. Interfaces 2015, 7, 11069–11073. [Google Scholar] [CrossRef]
- Samal, M.; Barange, N.; Ko, D.-H.; Yun, K. Graphene Quantum Rings Doped PEDOT:PSS Based Composite Layer for Efficient Performance of Optoelectronic Devices. J. Phys. Chem. C 2015, 119, 19619–19627. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, L.; Ji, G.; Wang, C.; Gu, H.; Luo, Q.; Chen, Q.; Chen, L.; Yang, Y.; Ma, C.-Q.; et al. Synthesis of N,S-Doped Carbon Quantum Dots for Use in Organic Solar Cells as the ZnO Modifier to Eliminate the Light-Soaking Effect. ACS Appl. Mater. Interfaces 2019, 11, 2243–2253. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Liu, C.; Guo, J.; Cui, H.; Shen, L.; Guo, W. Toward Efficient Carbon-Dots-Based Electron-Extraction Layer Through Surface Charge Engineering. ACS Appl. Mater. Interfaces 2018, 10, 40255–40264. [Google Scholar] [CrossRef]
- Lim, H.; Liu, Y.; Kim, H.Y.; Son, D.I. Facile synthesis and characterization of carbon quantum dots and photovoltaic applications. Thin Solid Films 2018, 660, 672–677. [Google Scholar] [CrossRef]
- Shalini, S.; Balasundaraprabhu, R.; Kumar, T.S.; Prabavathy, N.; Senthilarasu, S.; Prasanna, S. Status and outlook of sensitizers/dyes used in dye sensitized solar cells (DSSC): A review. Int. J. Energy Res. 2016, 40, 1303–1320. [Google Scholar] [CrossRef]
- Murakami, T.N.; Grätzel, M. Counter electrodes for DSC: Application of functional materials as catalysts. Inorg. Chim. Acta 2008, 361, 572–580. [Google Scholar] [CrossRef]
- Jeon, S.S.; Kim, C.; Ko, J.; Im, S.S. Spherical polypyrrole nanoparticles as a highly efficient counter electrode for dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 8146–8151. [Google Scholar] [CrossRef]
- Zang, J.; Li, C.M.; Bao, S.-J.; Cui, X.; Bao, Q.; Sun, C.Q. Template-Free Electrochemical Synthesis of Superhydrophilic Polypyrrole Nanofiber Network. Macromolecules 2008, 41, 7053–7057. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Fan, L.; Lan, Z.; Li, P.; Lin, J.; Hao, S. High-performance polypyrrole nanoparticles counter electrode for dye-sensitized solar cells. J. Power Sources 2008, 181, 172–176. [Google Scholar] [CrossRef]
- Skompska, M. Hybrid conjugated polymer/semiconductor photovoltaic cells. Synth. Met. 2010, 160, 1–15. [Google Scholar] [CrossRef]
- Lu, M.-N.; Chang, C.-Y.; Wei, T.-C.; Lin, J.-Y. Recent Development of Graphene-Based Cathode Materials for Dye-Sensitized Solar Cells. J. Nanomater. 2016, 2016, 1–21. [Google Scholar] [CrossRef]
- Dou, D.; Duan, J.; Zhao, Y.; He, B.; Tang, Q. Cubic carbon quantum dots for light-harvesters in mesoscopic solar cells. Electrochim. Acta 2018, 275, 275–280. [Google Scholar] [CrossRef]
- Efa, M.T.; Imae, T. Effects of carbon dots on ZnO nanoparticle-based dye-sensitized solar cells. Electrochim. Acta 2019, 303, 204–210. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Y.; Sun, W.; Wang, M.; He, B.; Chen, H.; Tang, Q. Biomass converted carbon quantum dots for all-weather solar cells. Electrochim. Acta 2017, 257, 259–266. [Google Scholar] [CrossRef]
- Briscoe, J.; Marinovic, A.; Sevilla, M.; Dunn, S.; Titirici, M. Biomass-Derived Carbon Quantum Dot Sensitizers for Solid-State Nanostructured Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 4463–4468. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zeng, L.; Cao, X.; Wang, Q.; Wang, X.; Sun, R. Sustainable carbon quantum dots from forestry and agricultural biomass with amplified photoluminescence by simple NH4OH passivation. J. Mater. Chem. C 2014, 2, 9760–9766. [Google Scholar] [CrossRef]
- Qi, H.; Teng, M.; Liu, M.; Liu, S.; Li, J.; Yu, H.; Teng, C.; Huang, Z.; Liu, H.; Shao, Q.; et al. Biomass-derived nitrogen-doped carbon quantum dots: Highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J. Colloid Interface Sci. 2019, 539, 332–341. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, J.; He, B.; Jiao, Z.; Tang, Q. Improved charge extraction with N-doped carbon quantum dots in dye-sensitized solar cells. Electrochim. Acta 2018, 282, 255–262. [Google Scholar] [CrossRef]
- Riaz, R.; Ali, M.; Maiyalagan, T.; Anjum, A.S.; Lee, S.Y.; Ko, M.J.; Jeong, S.H. Dye-sensitized solar cell (DSSC) coated with energy down shift layer of nitrogen-doped carbon quantum dots (N-CQDs) for enhanced current density and stability. Appl. Surf. Sci. 2019, 483, 425–431. [Google Scholar] [CrossRef]
- Chen, L.; Guo, C.X.; Zhang, Q.; Lei, Y.; Xie, J.; Ee, S.; Guai, G.; Song, Q.; Li, C.M. Graphene Quantum-Dot-Doped Polypyrrole Counter Electrode for High-Performance Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2013, 5, 2047–2052. [Google Scholar] [CrossRef]
- Lee, K.; Cho, S.; Kim, M.; Kim, J.; Ryu, J.; Shin, K.-Y.; Jang, J. Highly porous nanostructured polyaniline/carbon nanodots as efficient counter electrodes for Pt-free dye-sensitized solar cells. J. Mater. Chem. A 2015, 3, 19018–19026. [Google Scholar] [CrossRef]
- Dinari, M.; Momeni, M.M.; Goudarzirad, M. Dye-sensitized solar cells based on nanocomposite of polyaniline/graphene quantum dots. J. Mater. Sci. 2016, 51, 2964–2971. [Google Scholar] [CrossRef]
- Wang, C.-C.; Lu, S.-Y. Carbon black-derived graphene quantum dots composited with carbon aerogel as a highly efficient and stable reduction catalyst for the iodide/tri-iodide couple. Nanoscale 2015, 7, 1209–1215. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; He, B.; Tang, Q. Efficiency enhancement of bifacial dye-sensitized solar cells through bi-tandem carbon quantum dots tailored transparent counter electrodes. Electrochim. Acta 2018, 278, 204–209. [Google Scholar] [CrossRef]
- Lee, J.; Ko, S.-J.; Lee, H.; Huang, J.; Zhu, Z.; Seifrid, M.; Vollbrecht, J.; Brus, V.V.; Karki, A.; Wang, H.; et al. Side-Chain Engineering of Nonfullerene Acceptors for Near-Infrared Organic Photodetectors and Photovoltaics. ACS Energy Lett. 2019, 4, 1401–1409. [Google Scholar] [CrossRef]
- Karki, A.; Vollbrecht, J.; Gillett, A.J.; Selter, P.; Lee, J.; Peng, Z.; Schopp, N.; Dixon, A.L.; Schrock, M.; Nádaždy, V.; et al. Unifying Charge Generation, Recombination, and Extraction in Low-Offset Non-Fullerene Acceptor Organic Solar Cells. Adv. Energy Mater. 2020, 10, 2001203. [Google Scholar] [CrossRef]
- Brus, V.V.; Lee, H.K.; Proctor, C.M.; Ford, M.; Liu, X.; Burgers, M.A.; Lee, J.; Bazan, G.C.; Nguyen, T.-Q. Balance Between Light Absorption and Recombination Losses in Solution-Processed Small Molecule Solar Cells with Normal or Inverted Structures. Adv. Energy Mater. 2018, 8, 1801807. [Google Scholar] [CrossRef]
- Ji, J.; Zhou, H.; Eom, Y.K.; Kim, C.H.; Kim, H.K. Dye-Sensitized Solar Cells: 14.2% Efficiency Dye-Sensitized Solar Cells by Co-sensitizing Novel Thieno[3,2-b]indole-Based Organic Dyes with a Promising Porphyrin Sensitizer. Adv. Energy Mater. 2020, 10, 2000124. [Google Scholar] [CrossRef]


















| HEL | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|
| pristine | 11.97 | 0.58 | 0.54 | 3.77 |
| ogG0.2 | 16.08 | 0.59 | 0.50 | 4.74 |
| ogG0.5 | 14.59 | 0.58 | 0.48 | 4.08 |
| Device | Voc (V) | Jsc (mA cm−2) | FF /%) | PCE (%) |
|---|---|---|---|---|
| ITO/PEI | 0.73 | 16.46 | 60.56 | 7.25 |
| ITO/PFN | 0.74 | 18.17 | 61.93 | 8.29 |
| ITO/ZnO | 0.74 | 16.20 | 67.15 | 8.04 |
| ITO/TiO2 | 0.80 | 16.26 | 60.52 | 7.90 |
| ITO/C-dots@PEI | 0.73 | 18.37 | 71.14 | 9.53 |
| Device | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| CQD 0 | 0.73 ± 0.012 | 13.5 ± 0.018 | 74.52 ± 0.25 | 7.3 ± 0.051 |
| CQD 1 | 0.73 ± 0.011 | 14.0 ± 0.013 | 74.46 ± 0.24 | 7.6 ± 0.054 |
| CQD 2 | 0.73 ± 0.013 | 14.5 ± 0.019 | 74.42 ± 0.26 | 7.8 ± 0.052 |
| CQD 3 | 0.73 ± 0.010 | 15.0 ± 0.018 | 75.29 ± 0.24 | 8.2 ± 0.053 |
| CQD 4 | 0.73 ± 0.012 | 14.7 ± 0.017 | 74.90 ± 0.24 | 8.0 ± 0.055 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vercelli, B. The Role of Carbon Quantum Dots in Organic Photovoltaics: A Short Overview. Coatings 2021, 11, 232. https://doi.org/10.3390/coatings11020232
Vercelli B. The Role of Carbon Quantum Dots in Organic Photovoltaics: A Short Overview. Coatings. 2021; 11(2):232. https://doi.org/10.3390/coatings11020232
Chicago/Turabian StyleVercelli, Barbara. 2021. "The Role of Carbon Quantum Dots in Organic Photovoltaics: A Short Overview" Coatings 11, no. 2: 232. https://doi.org/10.3390/coatings11020232
APA StyleVercelli, B. (2021). The Role of Carbon Quantum Dots in Organic Photovoltaics: A Short Overview. Coatings, 11(2), 232. https://doi.org/10.3390/coatings11020232






