Electro-Oxidation of Ammonia at Novel Ag2O?PrO2/?-Al2O3 Catalysts
Abstract
:1. Introduction
2. Catalyst Preparation
2.1. Materials
2.2. Synthesis of γ-Al2O3
2.3. Synthesis of Ag2O–PrO2/γ-Al2O3
3. Physical Characterization
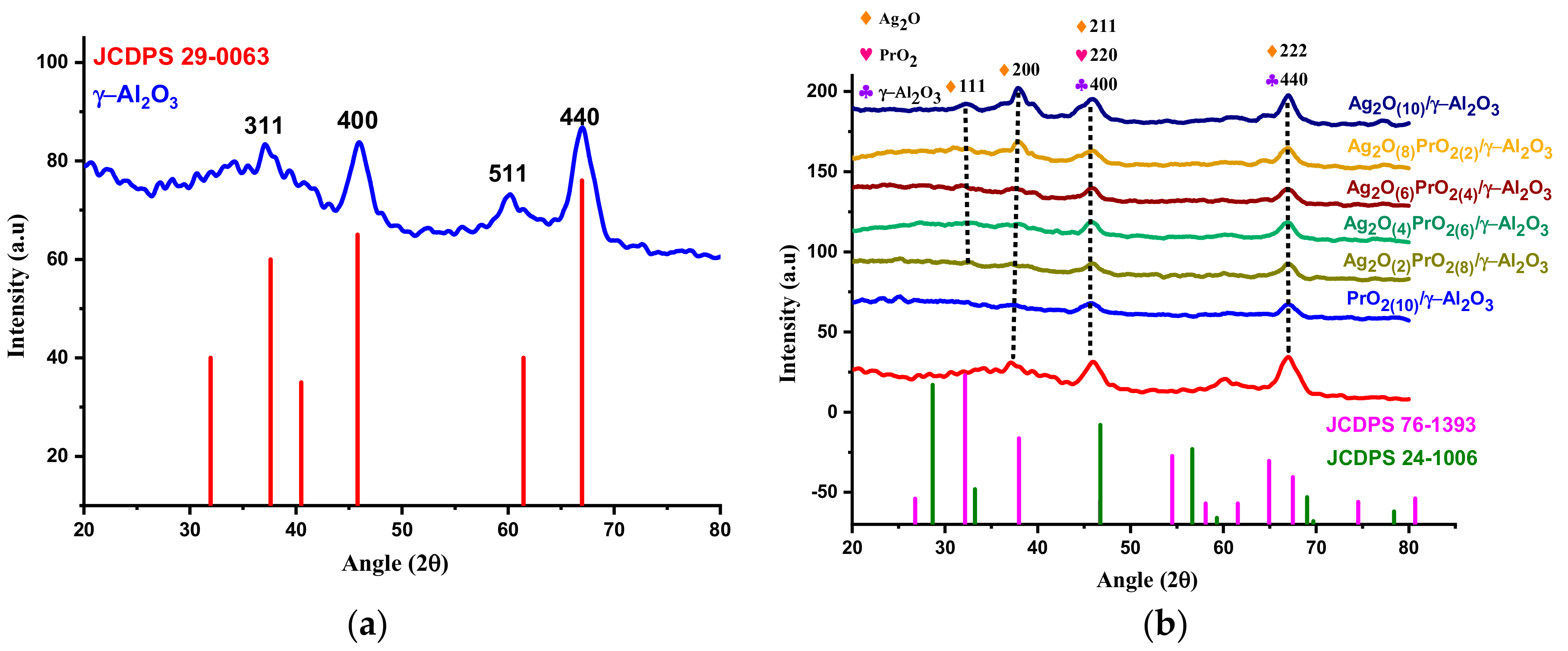
3.1. X-Ray Diffraction Analysis
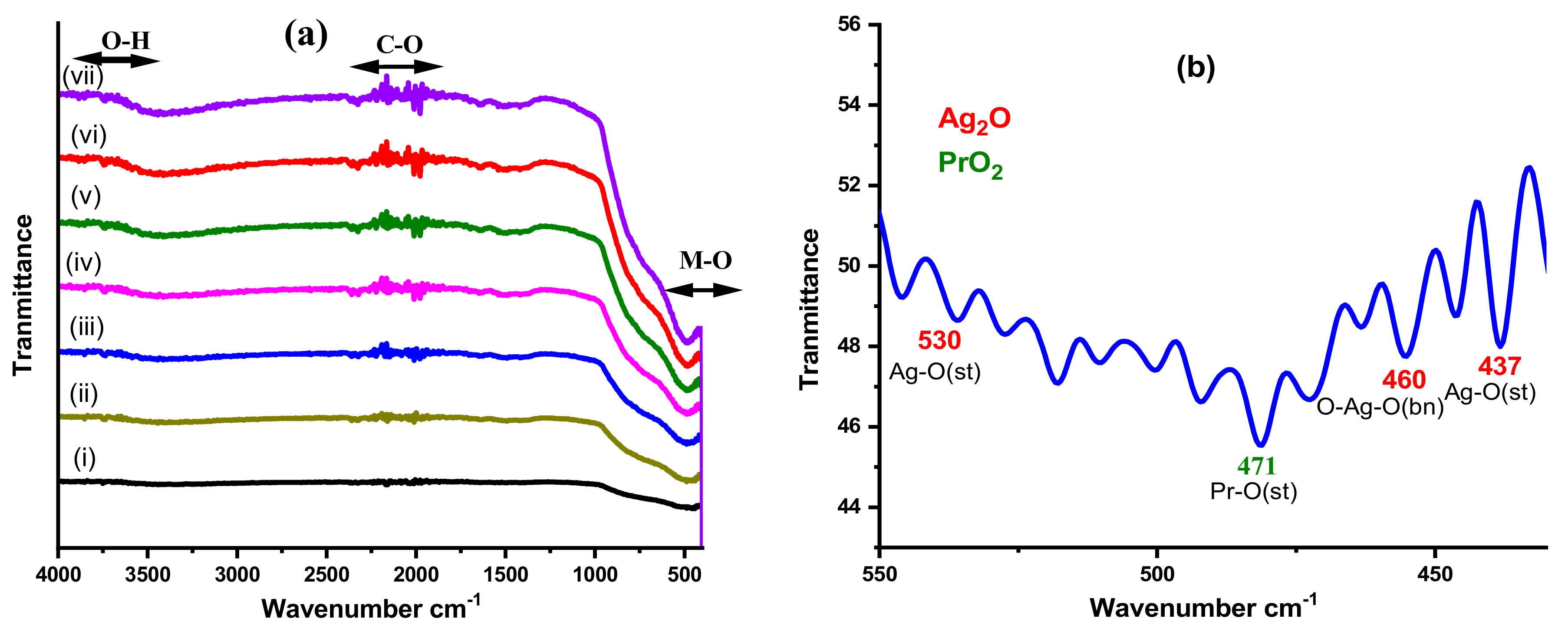
3.2. FTIR Analysis
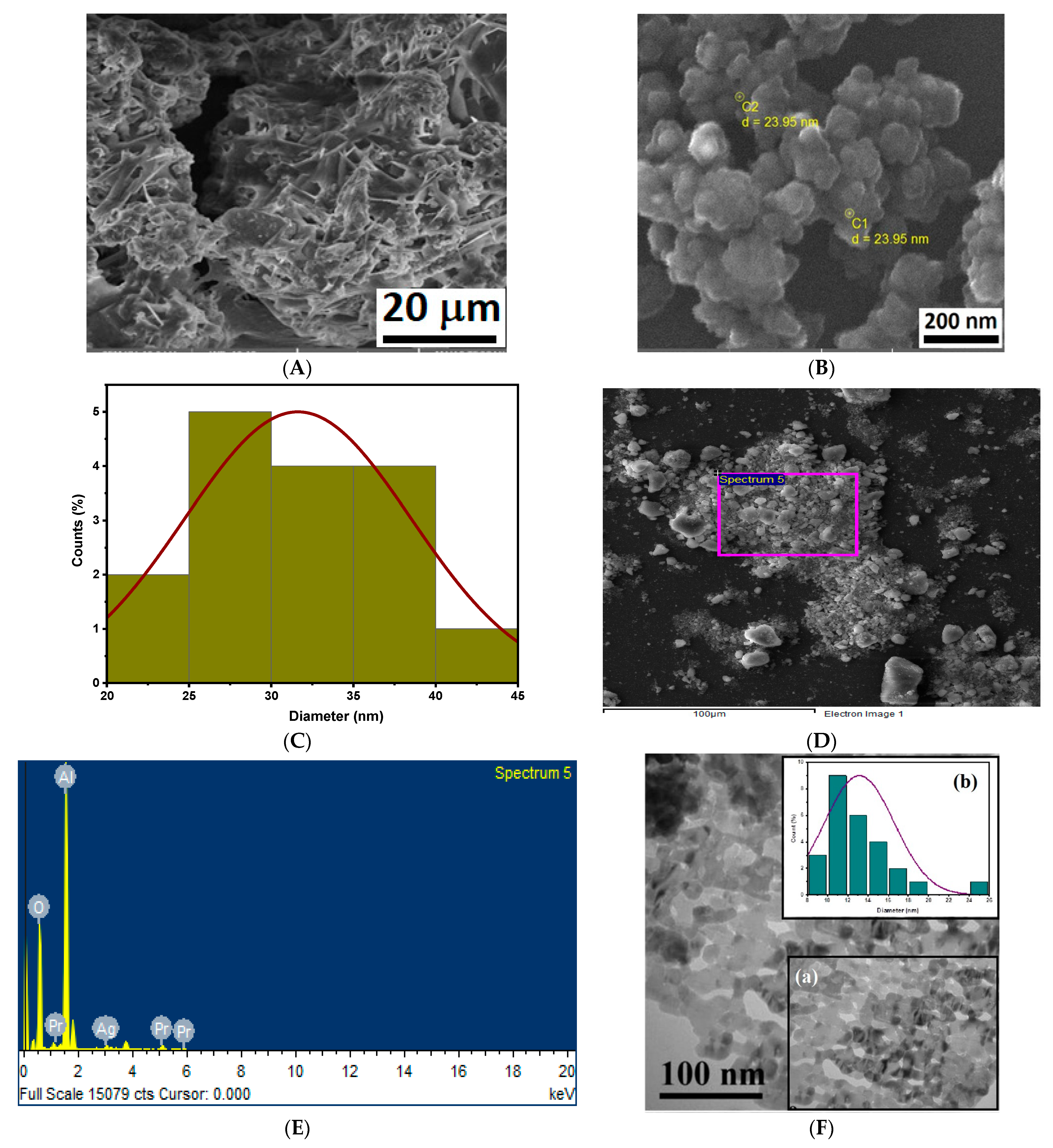
3.3. SEM, TEM and EDX Analysis
4. Electrochemical Characterization
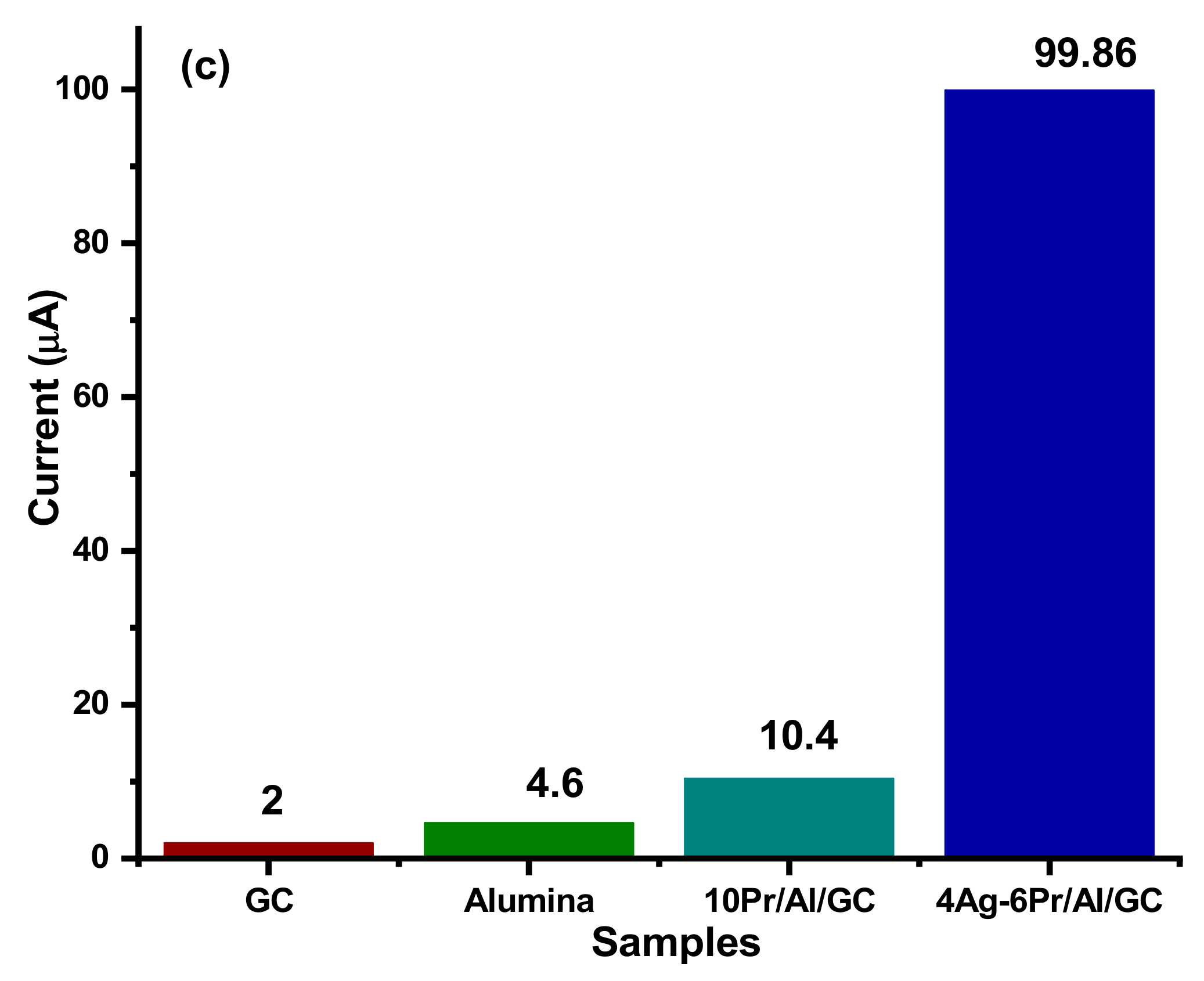
4.1. Estimation of Electrochemical Surface Area (ECSA) of As-Synthesized Electrocatalysts
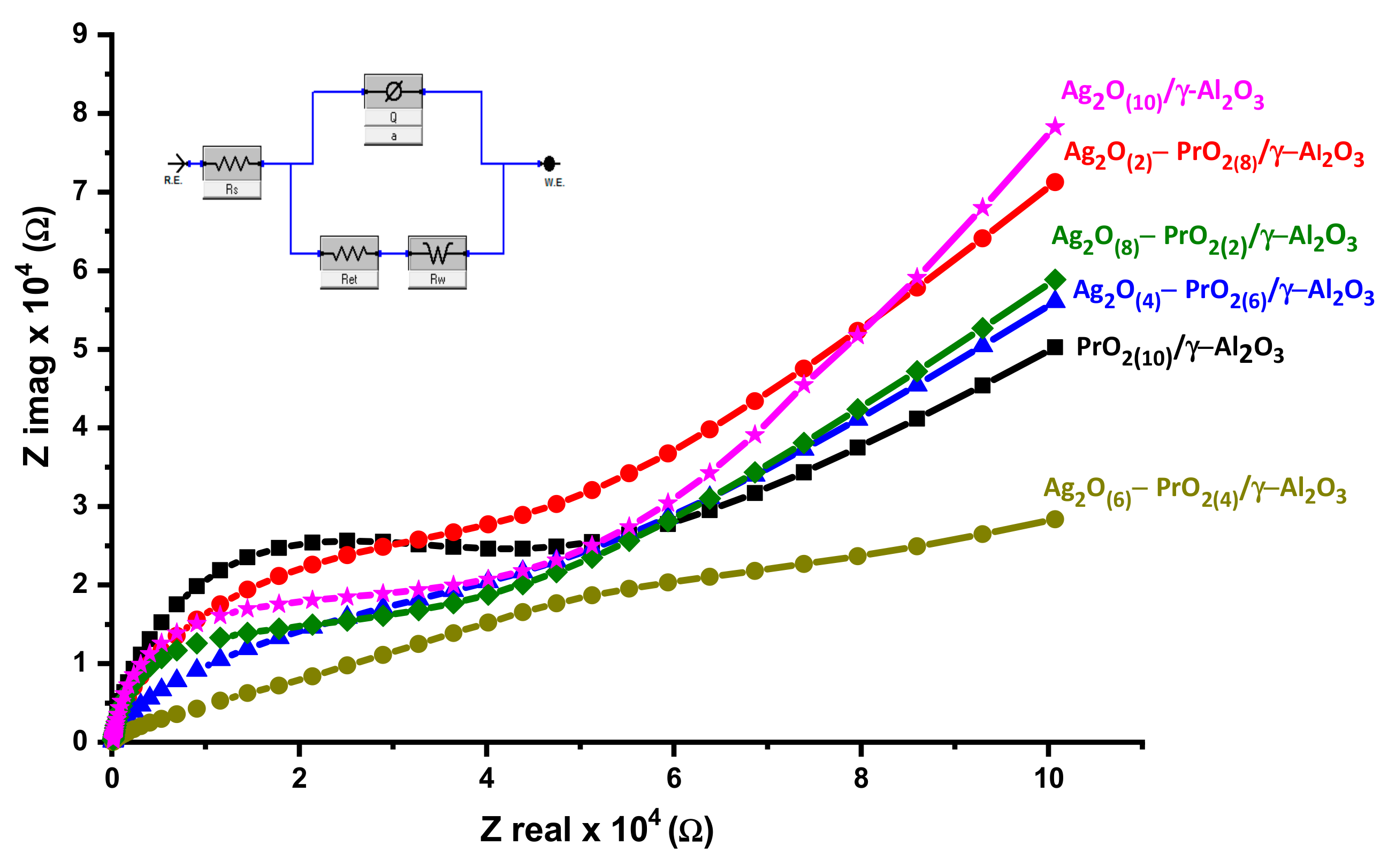
4.2. Electrochemical Impedance Spectroscopy EIS
5. Results and Discussion
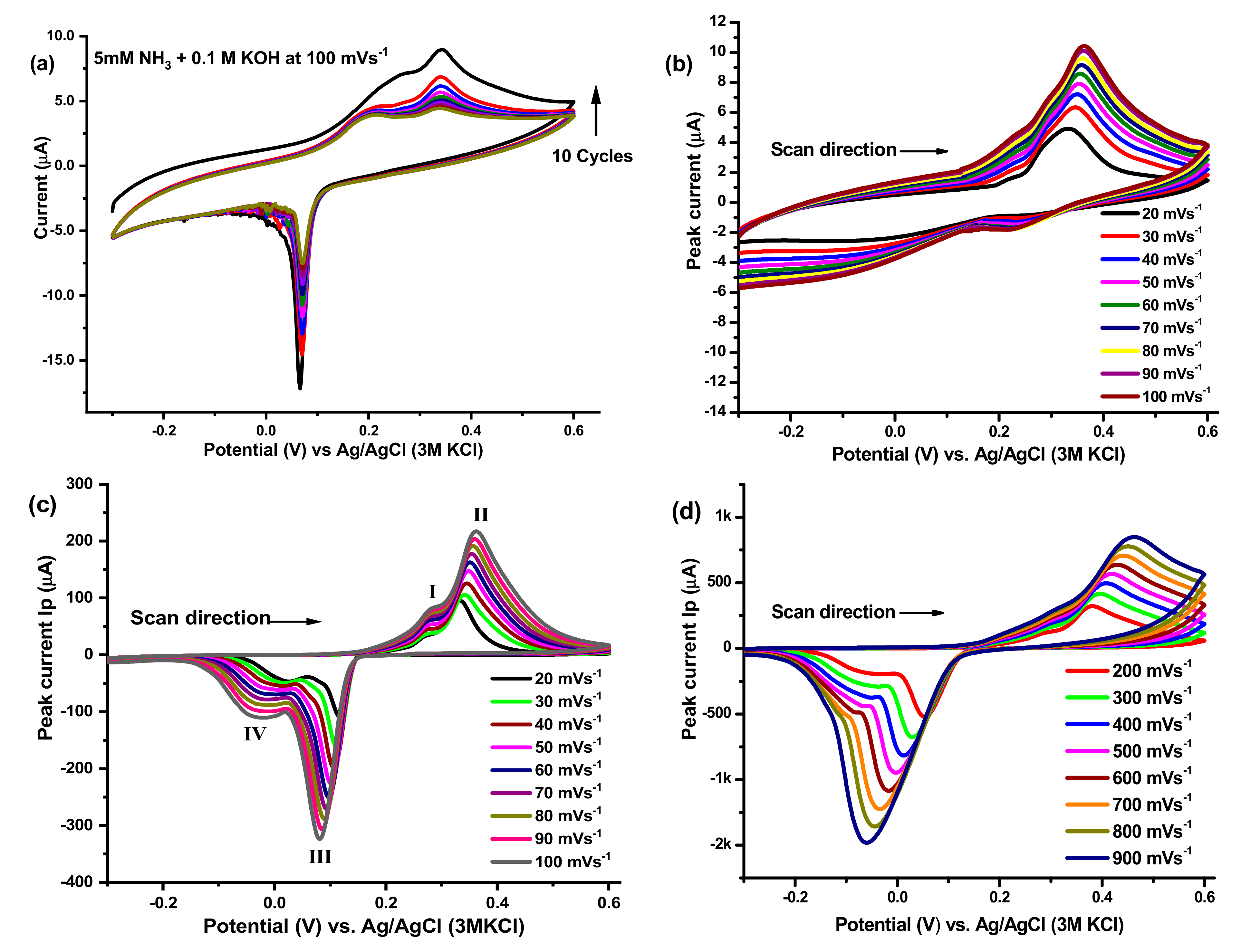
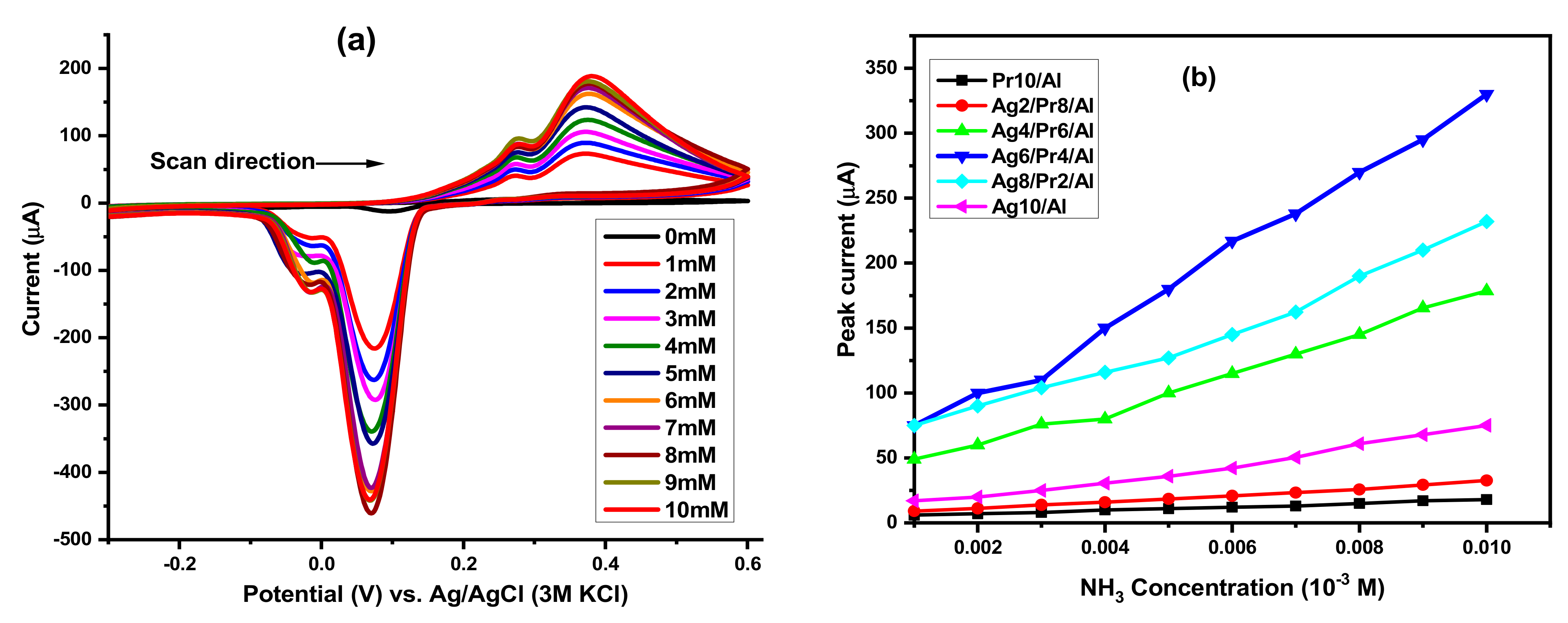
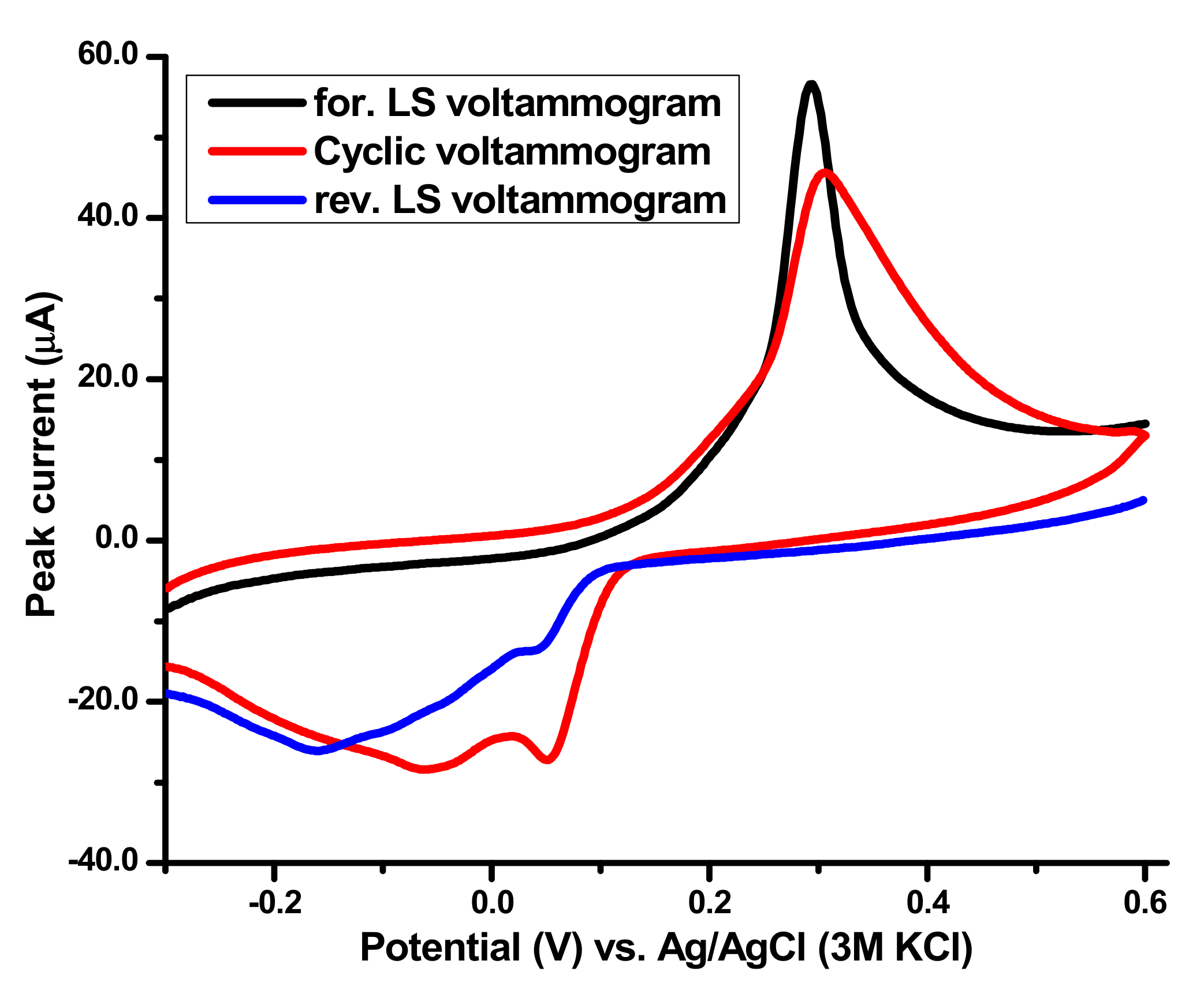
5.1. Ammonia Electro-Oxidation at Ag2O(x)–PrO2(y)/γ-Al2O3 Modified GC Electrodes
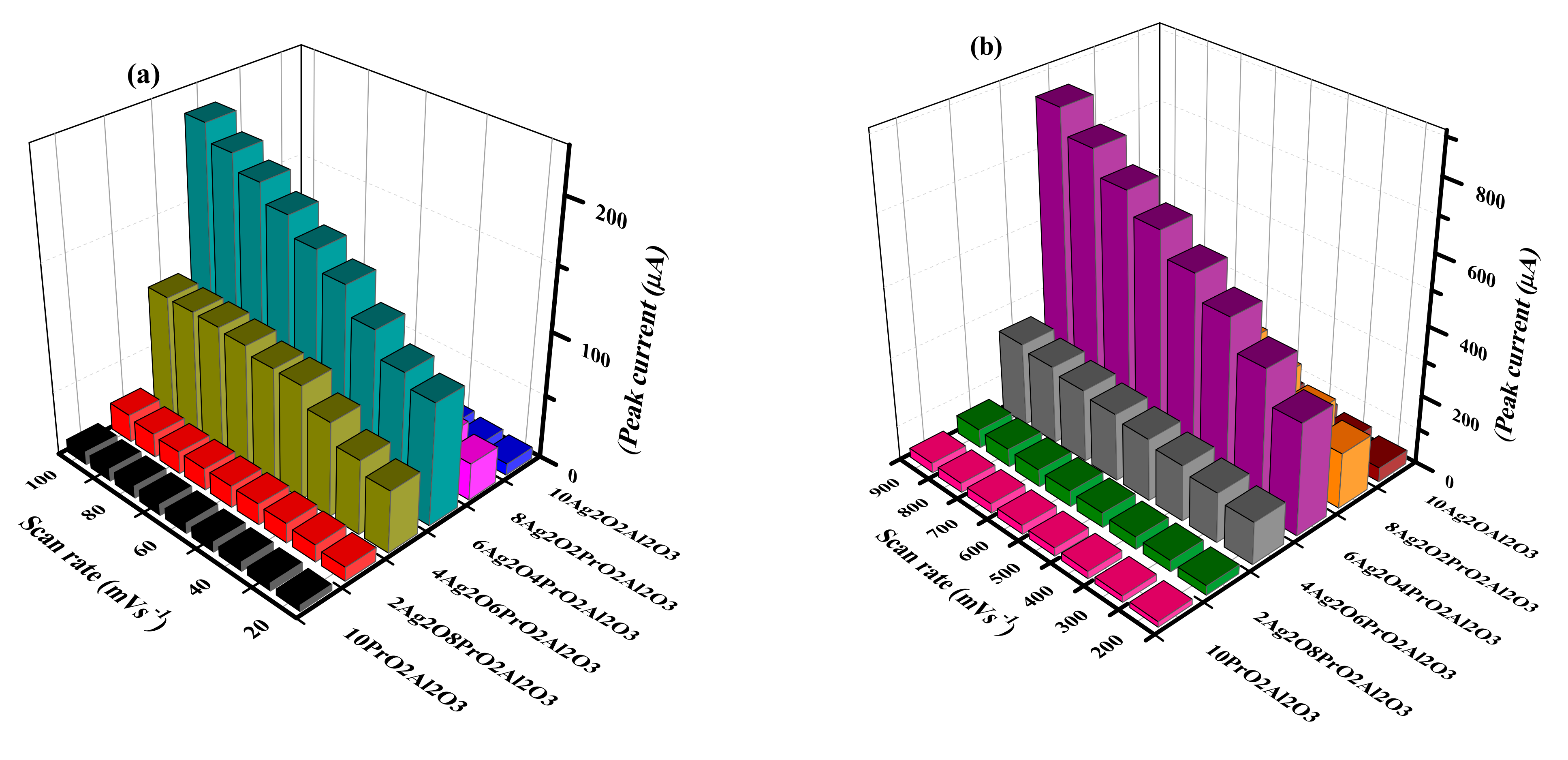
5.2. Kinetics of Ammonia Electro-Oxidation
5.3. Thermodynamic Studies for Ammonia Electro-Oxidation
5.4. Mechanism of Ammonia Oxidation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowotny, J.; Hoshino, T.; Dodson, J.; Atanacio, A.J.; Ionescu, M.; Peterson, V.; Prince, K.E.; Yamawaki, M.; Bak, T.; Sigmund, W.; et al. Towards sustainable energy. Generation of hydrogen fuel using nuclear energy. Int. J. Hydrogen Energy 2016, 41, 12812–12825. [Google Scholar] [CrossRef]
- Pervaiz, M.; Ahmad, I.; Yousaf, M.; Kirn, S.; Munawar, A.; Saeed, Z.; Adnan, A.; Gulzar, T.; Kamal, T.; Ahmad, A.; et al. Synthesis, spectral and antimicrobial studies of amino acid derivative Schiff base metal (Co, Mn, Cu, and Cd) complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Ngaini, Z.; Harry, A.V.; Vekariya, R.L.; Ahmad, A.; Zuo, Z.; Sahari, S.K.; Hussain, S.; Khan, Z.A.; Alarifi, A. An experimental and DFT study on novel dyes incorporated with natural dyes on titanium dioxide (TiO2) towards solar cell application. Appl. Phys. A 2020, 126, 1–13. [Google Scholar] [CrossRef]
- Ahmad, A.; Jini, D.; Aravind, M.; Parvathiraja, C.; Ali, R.; Kiyani, M.Z.; Alothman, A. A novel study on synthesis of egg shell based activated carbon for degradation of methylene blue via photocatalysis. Arab. J. Chem. 2020, 13, 8717–8722. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, A.J.; Arshad, M.; Javed, M.S.; Ahmad, A.; Shah, S.S.A.; Khan, M.R.; Akram, S.; Zulfiqar; Ali, S.; et al. Charge storage in binder-free 2D-hexagonal CoMoO4 nanosheets as a redox active material for pseudocapacitors. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Afif, A.; Radenahmad, N.; Cheok, Q.; Shams, S.; Kim, J.H.; Azad, A.K. Ammonia-fed fuel cells: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 60, 822–835. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Xu, P.H.; Liu, G.W.; Ahmad, A.; Chen, X.H.; Zhu, Y.L.; Alothman, A.; Hussain, S.; Qiao, G.J. Synthesis, Characterization and Wettability of Cu-Sn Alloy on the Si-Implanted 6H-SiC. Coatings 2020, 10, 906. [Google Scholar] [CrossRef]
- Aravind, M.; Ahmad, A.; Ahmad, I.; Amalanathan, M.; Naseem, K.; Mary, S.M.M.; Parvathiraja, C.; Hussain, S.; Algarni, T.S.; Pervaiz, M.; et al. Critical green routing synthesis of silver NPs using jasmine flower extract for biological activities and photocatalytical degradation of methylene blue. J. Environ. Chem. Eng. 2021, 9, 104877. [Google Scholar] [CrossRef]
- Naseem, K.; Rehman, M.Z.U.; Ahmad, A.; Dubal, D.; Algarni, T.S. Plant Extract Induced Biogenic Preparation of Silver Nanoparticles and Their Potential as Catalyst for Degradation of Toxic Dyes. Coatings 2020, 10, 1235. [Google Scholar] [CrossRef]
- Zhan, M.; Hussain, S.; Algarni, T.S.; Shah, S.; Liu, J.; Zhang, X.; Ahmad, A.; Javed, M.S.; Qiao, G.; Liu, G. Facet controlled polyhedral ZIF-8 MOF nanostructures for excellent NO2 gas-sensing applications. Mater. Res. Bull. 2021, 136, 111133. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Ahmad, A.; Karri, R.R.; Pakalapati, H. Functionalized multi-walled carbon nanotubes and hydroxyapatite nanorods reinforced with polypropylene for biomedical application. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Jafaar, E.; Sahari, S.K.; Low, F.W.; Hoa, N.D.; Ahmad, A.; Abbas, A.; Ngaini, Z.; Shafa, M.; Qurashi, A. Organic sensitization of graphene oxide and reduced graphene oxide thin films for photovoltaic applications. Int. J. Energy Res. 2021. [Google Scholar] [CrossRef]
- Vidal-Iglesias, F.J.; Solla-Gullón, J.; Montiel, V.; Feliu, J.; Aldaz, A. Screening of electrocatalysts for direct ammonia fuel cell: Ammonia oxidation on PtMe (Me: Ir, Rh, Pd, Ru) and preferentially oriented Pt(100) nanoparticles. J. Power Sources 2007, 171, 448–456. [Google Scholar] [CrossRef]
- Thou, C.Z.; Khan, F.S.A.; Mubarak, N.; Ahmad, A.; Khalid, M.; Jagadish, P.; Walvekar, R.; Abdullah, E.; Khan, S.; Khan, M.; et al. Surface charge on chitosan/cellulose nanowhiskers composite via functionalized and untreated carbon nanotube. Arab. J. Chem. 2021, 14, 103022. [Google Scholar] [CrossRef]
- Saleem, M.; Irfan, M.; Tabassum, S.; Albaqami, M.D.; Javed, M.S.; Hussain, S.; Pervaiz, M.; Ahmad, I.; Ahmad, A.; Zuber, M. Experimental and theoretical study of highly porous lignocellulose assisted metal oxide photoelectrodes for dye-sensitized solar cells. Arab. J. Chem. 2021, 14, 102937. [Google Scholar] [CrossRef]
- Zou, P.; Chen, S.; Lan, R.; Tao, S. Investigation of Perovskite Oxide SrCo0.8Cu0.1Nb0.1O3–δ as a Cathode Material for Room Temperature Direct Ammonia Fuel Cells. ChemSusChem 2019, 12, 2788–2794. [Google Scholar] [CrossRef] [Green Version]
- Adli, N.M.; Zhang, H.; Mukherjee, S.; Wu, G. Review—Ammonia Oxidation Electrocatalysis for Hydrogen Generation and Fuel Cells. J. Electrochem. Soc. 2018, 165, J3130–J3147. [Google Scholar] [CrossRef]
- Suzuki, S.; Muroyama, H.; Matsui, T.; Eguchi, K. Fundamental studies on direct ammonia fuel cell employing anion exchange membrane. J. Power Sources 2012, 208, 257–262. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Z.; Yan, Z.; Lu, G.; Rintoul, L. Catalytic ammonia decomposition over Ru/carbon catalysts: The importance of the structure of carbon support. Appl. Catal. A: Gen. 2007, 320, 166–172. [Google Scholar] [CrossRef]
- Schaper, H.; Doesburg, E.; Van Reijen, L. The influence of lanthanum oxide on the thermal stability of gamma alumina catalyst supports. Appl. Catal. 1983, 7, 211–220. [Google Scholar] [CrossRef]
- Mardkhe, M.K.; Huang, B.; Bartholomew, C.H.; Alam, T.M.; Woodfield, B.F. Synthesis and characterization of silica doped alumina catalyst support with superior thermal stability and unique pore properties. J. Porous Mater. 2015, 23, 475–487. [Google Scholar] [CrossRef]
- Jo, J.-O.; Trinh, Q.H.; Kim, S.H.; Mok, Y.S. Plasma-catalytic decomposition of nitrous oxide over γ-alumina-supported metal oxides. Catal. Today 2018, 310, 42–48. [Google Scholar] [CrossRef]
- Yang, X.; Sun, M.; Liu, B.; Lin, W. NOx removal by oxidation on alumina supported metal oxide catalysts followed by alkaline absorption. China Pet. Process. Petrochem. Technol. 2019, 21, 1–9. [Google Scholar]
- Sandupatla, A.S.; Ray, K.; Thaosen, P.; Sivananda, C.; Deo, G. Oxidative dehydrogenation of propane over alumina supported vanadia catalyst–Effect of carbon dioxide and secondary surface metal oxide additive. Catal. Today 2020, 354, 176–182. [Google Scholar] [CrossRef]
- Adil, S.F.; Assal, M.E.; Khan, M.; Shaik, M.R.; Kuniyil, M.; Sekou, D.; Dewidar, A.Z.; Al-Warthan, A.; Siddiqui, M.R.H. Eco-Friendly Mechanochemical Preparation of Ag2O–MnO2/Graphene Oxide Nanocomposite: An Efficient and Reusable Catalyst for the Base-Free, Aerial Oxidation of Alcohols. Catalysts 2020, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Xie, L.; Cao, S.; Xiao, Y.; Chen, G.; Jiang, Y.; Wei, W.; Wu, L. Ag/Ag2O confined visible-light driven catalyst for highly efficient selective hydrogenation of nitroarenes in pure water medium at room temperature. Chem. Eng. J. 2020, 394, 125036. [Google Scholar] [CrossRef]
- Tankov, I.; Arishtirova, K.; Bueno, J.M.; Damyanova, S. Surface and structural features of Pt/PrO2–Al2O3 catalysts for dry methane reforming. Appl. Catal. A: Gen. 2014, 474, 135–148. [Google Scholar] [CrossRef]
- Wachs, I.E. Recent conceptual advances in the catalysis science of mixed metal oxide catalytic materials. Catal. Today 2005, 100, 79–94. [Google Scholar] [CrossRef]
- Sharma, R.V.; Kumar, P.; Dalai, A.K. Selective hydrogenolysis of glycerol to propylene glycol by using Cu:Zn:Cr:Zr mixed metal oxides catalyst. Appl. Catal. A Gen. 2014, 477, 147–156. [Google Scholar] [CrossRef]
- Berenguer Betrián, R.; Quijada, C.; Morallón, E. The nature of the electro-catalytic response of mixed metal oxides: Pt-and Ru-doped SnO2 anodes. ChemElectroChem 2019, 6, 1057–1068. [Google Scholar] [CrossRef] [Green Version]
- Gangwar, J.; Gupta, B.K.; Kumar, P.; Tripathi, S.K.; Srivastava, A.K. Time-resolved and photoluminescence spectroscopy of θ-Al2O3nanowires for promising fast optical sensor applications. Dalton Trans. 2014, 43, 17034–17043. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Crespo, M.; Díaz-García, L.; De La Paz, M.C.; Zárate-Ramos, R.; Santes, V.; Pérez, G.L.; Arce-Estrada, E.; Torres-Huerta, A. Influence of Alumina Crystal Size on the Hydrotreating Activity of Supported NiMo Catalysts Using Real Feedstock. Pet. Sci. Technol. 2006, 24, 485–506. [Google Scholar] [CrossRef]
- Xu, L.; Wei, B.; Liu, W.; Zhang, H.; Su, C.; Che, J. Flower-like ZnO-Ag2O composites: Precipitation synthesis and photocatalytic activity. Nanoscale Res. Lett. 2013, 8, 536. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Wei, B.; Xu, L.; Lu, Z.; Zhang, H.; Gao, H.; Che, J. Ag2O–Bi2O3 composites: Synthesis, characterization and high efficient photocatalytic activities. CrystEngComm 2012, 14, 5705–5709. [Google Scholar] [CrossRef]
- Dhoondia, Z.H.; Chakraborty, H. Lactobacillus mediated synthesis of silver oxide nanoparticles. Nanomater. Nanotechnol. 2012, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Vorokh, A. Scherrer formula: Estimation of error in determining small nanoparticle size. Nanosyst. Physics, Chem. Math. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, M.; Dilawar, N.; Bandyopadhyay, A.K.; Raychaudhuri, A.K. Phonon dynamics of Zn(Mg,Cd)O alloy nanostructures and their phase segregation. J. Appl. Phys. 2009, 106, 084306. [Google Scholar] [CrossRef] [Green Version]
- Mahan, G.D.; Lucas, A.A. Collective vibrational modes of adsorbed CO. J. Chem. Phys. 1978, 68, 1344–1348. [Google Scholar] [CrossRef]
- Cheng, B.; Xiao, Y.; Wu, G.; Zhang, L. The vibrational properties of one-dimensional ZnO: Ce nanostructures. Appl. Phys. Lett. 2004, 84, 416–418. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan–silver oxide nanocomposite film: Preparation and antimicrobial activity. Bull. Mater. Sci. 2011, 34, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, G.I.N.; Bowmaker, G.A.; Metson, J.B. The thermal decomposition of silver (I, III) oxide: A combined XRD, FT-IR and Raman spectroscopic study. Phys. Chem. Chem. Phys. 2001, 3, 3838–3845. [Google Scholar] [CrossRef]
- González Hernández, N.N.; Contreras, J.L.; Pinto, M.; Zeifert, B.; Flores Moreno, J.L.; Fuentes, G.A.; Hernández-Terán, M.E.; Vázquez, T.; Salmones, J.; Jurado, J.M. Improved NOx reduction using C3H8 and H2 with Ag/Al2O3 catalysts promoted with Pt and WOx. Catalysts 2020, 10, 1212. [Google Scholar] [CrossRef]
- Yiğitalp, A. Nafion Modified NCA Cathode Synthesis for Superior Cycling Performance of Lithium-Ion Batteries. Ph.D. Thesis, Sabancı University, Istanbul, Turkey, 2019. [Google Scholar]
- Kashif, M.; Jaafar, E.; Bhadja, P.; Low, F.W.; Sahari, S.K.; Hussain, S.; Loong, F.K.; Ahmad, A.; Algarni, T.S.; Shafa, M.; et al. Effect of potassium permanganate on morphological, structural and electro-optical properties of graphene oxide thin films. Arab. J. Chem. 2021, 14, 102953. [Google Scholar] [CrossRef]
- Sharifi, E.; Salimi, A.; Shams, E. Electrocatalytic activity of nickel oxide nanoparticles as mediatorless system for NADH and ethanol sensing at physiological pH solution. Biosens. Bioelectron. 2013, 45, 260–266. [Google Scholar] [CrossRef]
- Sharifi, E.; Salimi, A.; Shams, E.; Noorbakhsh, A.; Amini, M.K. Shape-dependent electron transfer kinetics and catalytic activity of NiO nanoparticles immobilized onto DNA modified electrode: Fabrication of highly sensitive enzymeless glucose sensor. Biosens. Bioelectron. 2014, 56, 313–319. [Google Scholar] [CrossRef]
- Guo, D.J.; Li, H.L. Highly dispersed Ag nanoparticles on functional MWNT surfaces for methanol oxidation in alkaline solution. Carbon 2005, 43, 1259–1264. [Google Scholar] [CrossRef]
- Droog, J.M.; Huisman, F. Electrochemical formation and reduction of silver oxides in alkaline media. J. Electroanal. Chem. 1980, 115, 211–224. [Google Scholar] [CrossRef]
- Sun, Z.; Han, Y.; Gao, M.; Wei, X.; Hu, X. Cetyl trimethyl ammonium bromide-assisted electrochemical preparation of palladium-nickel bimetallic electrode. Int. J. Electrochem. Sci. 2011, 6, 5626–5638. [Google Scholar]
- Matinise, N.; Mayedwa, N.; Ikpo, C.O.; Hlongwa, N.W.; Ndipingwi, M.M.; Molefe, L.; Dywili, N.; Yonkeu, A.L.D.; Waryo, T.; Baker, P.G.L.; et al. Bimetallic Nanocomposites of Palladium (100) and Ruthenium for Electrooxidation of Ammonia. J. Nano Res. 2016, 44, 100–113. [Google Scholar] [CrossRef]
- Lomocso, T.L.; Baranova, E.A. Electrochemical oxidation of ammonia on carbon-supported bi-metallic PtM (M=Ir, Pd, SnOx) nanoparticles. Electrochim. Acta 2011, 56, 8551–8558. [Google Scholar] [CrossRef]
- Matsui, T.; Suzuki, S.; Katayama, Y.; Yamauchi, K.; Okanishi, T.; Muroyama, H.; Eguchi, K. In Situ Attenuated Total Reflection Infrared Spectroscopy on Electrochemical Ammonia Oxidation over Pt Electrode in Alkaline Aqueous Solutions. Langmuir 2015, 31, 11717–11723. [Google Scholar] [CrossRef]
- Katayama, Y.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. Electrochemical oxidation of ammonia over rare earth oxide modified platinum catalysts. J. Phys. Chem. C 2015, 119, 9134–9141. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wu, Z.; Leung, D.Y. An ammonia electrolytic cell with NiCu/C as anode catalyst for hydrogen production. Energy Procedia 2017, 142, 1539–1544. [Google Scholar] [CrossRef]
- Chrzescijanska, E.; Wudarska, E.; Kusmierek, E.; Rynkowski, J. Study of acetylsalicylic acid electroreduction behavior at platinum electrode. J. Electroanal. Chem. 2014, 713, 17–21. [Google Scholar] [CrossRef]
- Mujtaba, A.; Janjua, N.K. Fabrication and electrocatalytic application of CuO@Al2O3 hybrids. J. Electrochem. Soc. 2015, 162, H328. [Google Scholar] [CrossRef]
- Castilho, M.; Almeida, L.E.; Tabak, M.; Mazo, L.H. Voltammetric oxidation of dipyridamole in aqueous acid solutions. J. Braz. Chem. Soc. 2000, 11, 148–153. [Google Scholar] [CrossRef]
- Holze, R.; Brett, C.M.A.; Brett, A.M.O. Electrochemistry—Principles, Methods and Applications; Oxford University Press Inc.: New York, NY, USA, 1993. [Google Scholar]
- Soleymani, J.; Hasanzadeh, M.; Shadjou, N.; Jafari, M.K.; Gharamaleki, J.V.; Yadollahi, M.; Jouyban, A. A new kinetic–mechanistic approach to elucidate electrooxidation of doxorubicin hydrochloride in unprocessed human fluids using magnetic graphene based nanocomposite modified glassy carbon electrode. Mater. Sci. Eng. C 2016, 61, 638–650. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Zaborski, M. Electrooxidation of morin hydrate at a Pt electrode studied by cyclic voltammetry. Food Chem. 2014, 148, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Mazloum-Ardakani, M.; Taleat, Z. Investigation of electrochemistry behavior of hydroxylamine at glassy carbon electrode by indigocarmine. Int. J. Electrochem. Sci. 2009, 4, 694–706. [Google Scholar]
- Janjua, N.K.; Jabeen, M.; Islam, M.; Yaqub, A.; Sabahat, S.; Mehmood, S.; Abbas, G. Electrochemical properties of barium cerate doped with zinc for methanol oxidation. J. Chem. Soc. Pak. 2015, 37. [Google Scholar]
- Mu, Y.; Jia, D.; He, Y.; Miao, Y.; Wu, H.-L. Nano nickel oxide modified non-enzymatic glucose sensors with enhanced sensitivity through an electrochemical process strategy at high potential. Biosens. Bioelectron. 2011, 26, 2948–2952. [Google Scholar] [CrossRef] [PubMed]
- Kapałka, A.; Cally, A.; Neodo, S.; Comninellis, C.; Wächter, M.; Udert, K.M. Electrochemical behavior of ammonia at Ni/Ni(OH)2 electrode. Electrochem. Commun. 2010, 12, 18–21. [Google Scholar] [CrossRef]
- Diaz, L.A.; Valenzuela-Muñiz, A.; Muthuvel, M.; Botte, G.G. Analysis of ammonia electro-oxidation kinetics using a rotating disk electrode. Electrochim. Acta 2013, 89, 413–421. [Google Scholar] [CrossRef]
- Marcus, R.A. On the Theory of Electron-Transfer Reactions. VI. Unified Treatment for Homogeneous and Electrode Reactions. J. Chem. Phys. 1965, 43, 679–701. [Google Scholar] [CrossRef] [Green Version]
- Oswin, H.G.; Salomon, M. The anodic oxidation of ammonia at platinum black electrodes in aqueous koh electrolyte. Can. J. Chem. 1963, 41, 1686–1694. [Google Scholar] [CrossRef]





| Electrocatalysts | Davg(XRD) (nm) | Davg(SEM) (nm) |
|---|---|---|
| PrO2(10)/γ-Al2O3 | 41.3 | 26.02 |
| Ag2O(2)–PrO2(8)/γ-Al2O3 | 25.4 | 20.76 |
| Ag2O(4)–PrO2(6)/γ-Al2O3 | 28.9 | 29.41 |
| Ag2O(6)–PrO2(4)/γ-Al2O3 | 32.3 | 31.01 |
| Ag2O(8)–PrO2(2)/γ-Al2O3 | 20.3 | 23.02 |
| Ag2O(10)/γ-Al2O3 | 25.0 | 31.04 |
| Samples | wt.% Al | wt.% O | wt.% Pr | wt.% Ag |
|---|---|---|---|---|
| PrO2(10)/γ-Al2O3 | 26.90 | 69.90 | 9.20 | − |
| Ag2O(2)–PrO2(8)/γ-Al2O3 | 42.40 | 48.86 | 8.19 | 1.56 |
| Ag2O(4)–PrO2(6)/γ-Al2O3 | 42.57 | 51.88 | 5.87 | 3.45 |
| Ag2O(6)–PrO2(4)/γ-Al2O3 | 38.70 | 57.76 | 3.54 | 6.30 |
| Ag2O(8)–PrO2(2)/γ-Al2O3 | 35.24 | 61.00 | 1.54 | 7.39 |
| Ag2O(10)/γ-Al2O3 | 32.95 | 64.36 | − | 8.95 |
| Electrocatalysts | Rs (Ω) | Rct (kΩ) | CPE (µF) | A | Rw (Ω) | kapp/10−9 (cm·s−1) |
|---|---|---|---|---|---|---|
| PrO2(10)/γ-Al2O3 | 390.0 | 50.0 | 3.70 | 0.90 | 24.0 | 1.10 |
| Ag2O(2)–PrO2(8)/γ-Al2O3 | 389.0 | 40.4 | 3.00 | 0.89 | 29.0 | 1.30 |
| Ag2O(4)–PrO2(6)/γ-Al2O3 | 388.0 | 27.0 | 1.40 | 0.90 | 24.4 | 1.97 |
| Ag2O(6)–PrO2(4)/γ-Al2O3 | 243.0 | 24.7 | 43.0 | 0.90 | 21.9 | 2.20 |
| Ag2O(8)–PrO2(2)/γ-Al2O3 | 388.2 | 29.7 | 2.00 | 0.85 | 25.6 | 1.80 |
| Ag2O(10)/γ-Al2O3 | 388.3 | 36.5 | 2.20 | 0.92 | 29.7 | 1.50 |
| Sr. No. | Modified Electrode Systems | Oxidation Potential Range (V) | Reference |
|---|---|---|---|
| 1 | Carbon-supported Pt/HOPG electrode | 0.55–0.75 | [51] |
| 2 | Pt film electrode/Si prism | 0.45–0.85 | [52] |
| 3 | Pt disk electrode and Pt/PBI/MWNT | 0.45–0.90 | [53] |
| 4 | Ag−Pr/Al/GC electrode | 0.32–0.51 | This work |
| Electrocatalysts | α | (D°)/10−9 cm2·s−1 | (mT)/cm·s−1 | k°/10−3 cm·s−1 |
|---|---|---|---|---|
| PrO2(10)/γ-Al2O3 | 0.5 | 0.063 | 0.0005 | 0.40 |
| Ag2O(2)–PrO2(8)/γ-Al2O3 | 0.5 | 0.140 | 0.0007 | 0.72 |
| Ag2O(4)–PrO2(6)/γ-Al2O3 | 0.7 | 11.00 | 0.0070 | 6.10 |
| Ag2O(6)–PrO2(4)/γ-Al2O3 | 0.6 | 36.50 | 0.0120 | 7.40 |
| Ag2O(8)–PrO2(2)/γ-Al2O3 | 0.4 | 4.040 | 0.0040 | 5.20 |
| Ag2O(10)/γ-Al2O3 | 0.4 | 0.300 | 0.0011 | 1.60 |
| Electrocatalysts | (ΔH)/kJ·mol−1 | (ΔS)/kJ·mol−1K−1 | (ΔG)/kJ·mol−1 |
|---|---|---|---|
| PrO2(10)/γ-Al2O3 | −1.24 | −0.119 | 32.0 |
| Ag2O(2)–PrO2(8)/γ-Al2O3 | −1.30 | −0.100 | 29.0 |
| Ag2O(4)–PrO2(6)/γ-Al2O3 | −1.23 | −0.080 | 23.6 |
| Ag2O(6)–PrO2(4)/γ-Al2O3 | −1.22 | −0.082 | 23.0 |
| Ag2O(8)–PrO2(2)/γ-Al2O3 | −1.23 | −0.085 | 24.0 |
| Ag2O(10)/γ-Al2O3 | −1.24 | −0.094 | 27.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.; Janjua, N.K.; Khan, S.; Qazi, I.; Ali, S.; Saad Algarni, T. Electro-Oxidation of Ammonia at Novel Ag2O?PrO2/?-Al2O3 Catalysts. Coatings 2021, 11, 257. https://doi.org/10.3390/coatings11020257
Khan M, Janjua NK, Khan S, Qazi I, Ali S, Saad Algarni T. Electro-Oxidation of Ammonia at Novel Ag2O?PrO2/?-Al2O3 Catalysts. Coatings. 2021; 11(2):257. https://doi.org/10.3390/coatings11020257
Chicago/Turabian StyleKhan, Mariam, Naveed Kausar Janjua, Safia Khan, Ibrahim Qazi, Shafaqat Ali, and Tahani Saad Algarni. 2021. "Electro-Oxidation of Ammonia at Novel Ag2O?PrO2/?-Al2O3 Catalysts" Coatings 11, no. 2: 257. https://doi.org/10.3390/coatings11020257
APA StyleKhan, M., Janjua, N. K., Khan, S., Qazi, I., Ali, S., & Saad Algarni, T. (2021). Electro-Oxidation of Ammonia at Novel Ag2O?PrO2/?-Al2O3 Catalysts. Coatings, 11(2), 257. https://doi.org/10.3390/coatings11020257






