Characterization of Beeswax, Candelilla Wax and Paraffin Wax for Coating Cheeses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Waxes
2.2. Cheese
2.3. Characterization of Waxes
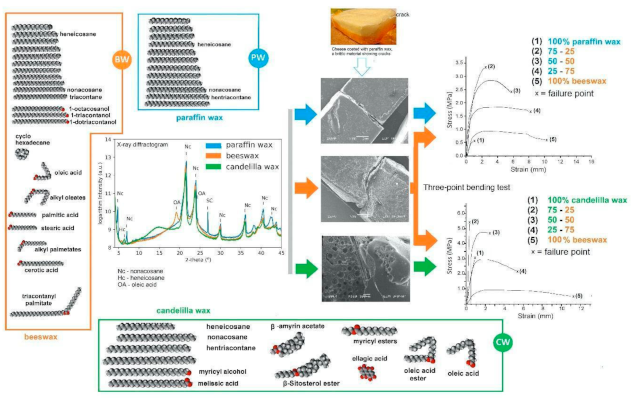
X-ray Diffraction (XRD)
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Raw Waxes Characterization by Thermal Analysis
2.6. Water Vapor Permeance (WVP) and Water Vapor Transmission Rate (WVTR)
2.7. Viscosity
2.8. Density of Waxes
2.9. Mechanical Properties of Waxes
2.10. Scanning Electron Microscopy (SEM)
2.11. Cheese Coatings
2.12. Degree of Cracking of Coatings during Cheese Storage
2.13. Statistical Analysis
3. Results and Discussion
3.1. DRX
3.2. Wax Characterization by Fourier Transform Infrared Spectroscopy
3.2.1. DSC and TGA
3.2.2. Viscosity
3.2.3. Density
3.2.4. Water Vapor Permeance and Water Vapor Transmission Rate
3.2.5. Weight Loss of Cheese
3.2.6. Image Analysis of Coatings of Cheeses
3.2.7. Mechanical Properties and Microstructure of Waxes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mansoori, A.G.; Barnes, H.; Webster, G.M. Petroleum waxes. In Fuels and Lubricants Handbook: Technology, Properties, Performance, and Testing, 2nd ed.; Totten, G.E., Ed.; American Society for Testing & Materials (ASTM): West Conshohocken, PA, USA, 2003; Volume 19, pp. 525–556. [Google Scholar]
- Karaman, A.D.; Özer, B.; Pascall, M.A.; Alvarez, V. Recent advances in dairy packaging. Food Rev. Int. 2005, 31, 295–318. [Google Scholar] [CrossRef]
- Kevelam, W.J.; Koopmans, W.C.; Meijer, W. Cheese Coating Compositions with Improved Wax Adhesion Properties. European Patent 1642505 B1, 11 November 2009. Available online: https://patents.google.com/patent/EP1642505B1/en (accessed on 18 November 2020).
- Poças, M.d.F.; Pintado, M. Packaging and the Shelf Life of Cheese. In Food Packaging and Shelf Life: A Practical Guide, 1st ed.; Robertson, G.L., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 103–126. [Google Scholar]
- Fröhlich-Wyder, M.T.; Bisig, W.; Guggisberg, D.; Jakob, E.; Turgay, M.; Wechsler, D. Cheeses with propionic acid fermentation: Chapter 35. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; McSweeney, P., Fox, P., Cotter, P., Everett, D., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 889–910. [Google Scholar]
- González-Córdova, A.; Yescas, F.; Ortiz-Estrada, C.; Hernández-Mendoza, A.; Vallejo-Cordoba, B. Invited review: Artisanal Mexican cheeses. J. Dairy Sci. 2016, 99, 3250–3262. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, V.B.; Pascall, M.A. Packaging. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 3124–3131. [Google Scholar]
- Schneider, Y.; Kluge, C.; Weiβ, U.; Rohm, H. Packaging Materials and Equipment. In The Technology of Cheese Making; Law, B.A., Tamime, A.Y., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 413–439. [Google Scholar]
- Hui, Y.H. Fermented Animal Products and Their Manufacture. In Handbook of Animal-Based Fermented Food and Beverage Technology; CRC Press: Boca Raton, FL, USA, 2012; pp. 3–30. [Google Scholar]
- Yilmaz, F.; Dagdemir, F.E. The effects of beeswax coating on quality of Kashar cheese during ripening. Int. J. Food Sci. Technol. 2012, 47, 2582–2589. [Google Scholar] [CrossRef]
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of edible films and coatings in cheese preservation: Opportunities and challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; Mc Sweeney, P.L. Fundamentals of Cheese Science, 4th ed.; Springer: Boston, MA, USA, 2017; pp. 185–229. [Google Scholar]
- Todaro, M.; Palmeri, M.; Cardamone, C.; Settanni, L.; Mancuso, I.; Mazza, F.; Scatassa, M.L.; Corona, O. Impact of packaging on the microbiological, physicochemical and sensory characteristics of a “pasta filata”cheese. Food Packag. Shelf 2018, 17, 85–90. [Google Scholar] [CrossRef]
- Wang, J.; Severtson, S.J.; Geil, P.H. Brittle–ductile transitions and the toughening mechanism in paraffin/organo-clay nanocomposites. Mater. Sci. Eng. A 2007, 467, 172–180. [Google Scholar] [CrossRef]
- Donhowe, G.; Fennema, O. Water vapor and oxygen permeability of wax films. J. Am. Oil Chem. Soc. 1993, 70, 867–873. [Google Scholar] [CrossRef]
- Irvine, O.R.; Bullock, D.H.; Edmond, R.E. Paraffin and Microcrystalline Waxes as Cheese Coating Materials. Can. J. Agric. Sci. 1955, 35, 153–167. [Google Scholar]
- Bernal, J.; Jimenez, J.J.; Del Nozal, M.J.; Toribio, L.; Martin, M.T. Physico-chemical parameters for the characterization of pure beeswax and detection of adulterations. Eur. J. Lipid Sci. Technol. Eur. 2005, 107, 158–166. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.; Alonzo-Macias, M.; González-Chávez, M.M. Thermal and Textural Properties of Organogels Developed by Candelilla Wax in Safflower Oil. J. Am. Oil Chem. Soc. 2007, 84, 989–1000. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, X.; Luo, X.; Wang, Y.; Li, Y.; Li, B.; Liu, S. Edible coating based on beeswax-in-water Pickering emulsion stabilized by cellulose nanofibrils and carboxymethyl chitosan. Food Chem. 2020, 331, 127108. [Google Scholar] [CrossRef]
- Oliveira, V.R.L.; Santos, F.K.G.; Leite, R.H.L.; Aroucha, E.M.M.; Silva, K.N.O. Use of biopolymeric coating hydrophobized with beeswax in post-harvest conservation of guavas. Food Chem. 2018, 259, 55–64. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv. Camarosa) under commercial storage conditions. LWT-Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, H.; Qian, L. Enhanced water vapour barrier and grease resistance of paper bilayer-coated with chitosan and beeswax. Carbohydr. Polym. 2014, 101, 401–406. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; Álvarez, B.; Ventura, J.M.; García-Galindo, J.O.; De León-Zapata, M.A.; Rojas, R.; Saucedo, S.; Aguilar, C.N. Edible Coatings and Films from Lipids, Waxes, and Resins. In Edible Food Packaging: Materials and Processing Technologies; Ribeiro Cerqueira, M.A.P., Correia Pereira, R.N., da Silva Ramos, O.L., Couto Teixeira, J.A., Vicente, A.A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 121–152. [Google Scholar]
- Aguirre-Joya, J.A.; Cerqueira, M.A.; Ventura-Sobrevilla, J.; Aguilar-Gonzalez, M.A.; Carbó-Argibay, E.; Castro, L.P.; Aguilar, C.N. Candelilla wax-based coatings and films: Functional and physicochemical characterization. Food Bioprocess Technol. 2019, 12, 1787–1797. [Google Scholar] [CrossRef]
- Paredes-Lopez, O.; Camargo-Rubio, E.; Gallardo-Navarro, Y. Use of coatings of candelilla wax for the preservation of limes. J. Sci. Food Agric. 1974, 25, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Molina, R.; De León-Zapata, M.A.; Saucedo-Pompa, S.; Aguilar-Gonzalez, M.A.; Aguilar, C.N. Chemical and structural characterization of Candelilla (Euphorbia antisyphilitica Zucc.). J. Med. Plants Res. 2013, 7, 702–705. [Google Scholar]
- Cervantes, F.; Villegas, A.; Cesín, A.; Espinoza, A. Los Quesos Mexicanos Genuinos; Mundi-Prensa México: Mexico City, Mexico, 2008. [Google Scholar]
- Hernández-Mendoza, A.; Reyes-Díaz, R.; Vallejo-Cordoba, B.; González-Córdova, A.F. Poro de Tabasco Cheese: Chemical Composition and Microbiological Quality during Its Artisanal Manufacturing Process. J. Dairy Sci. 2020, 103, 3025–3037. [Google Scholar] [CrossRef]
- Perez Pacheco, F.; Bucio, G.A. Microbial safety of raw milk cheese traditionally made at a pH below 4.7 and with other hurdles limiting pathogens growth. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Formatex Research Center: Badajoz, Spain, 2010; pp. 1205–1216. [Google Scholar]
- ICDD. Powder Diffraction File; International Centre for Diffraction Data: Newtown Square, PA, USA, 2007. [Google Scholar]
- Derrick, M.R.; Stulik, D.; Landry, J.M. Infrared Spectroscopy in Conservation Science; Getty Publications: Los Angeles, CA, USA, 2000. [Google Scholar]
- ASTM. Standard Test Method for Measurement of Transition Temperatures of Petroleum Waxes by Differential Scanning Calorimetry [DSC]; Designation D4419-90; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM. Standard Test Method for Transition Temperatures and Enthalpies of Fusion and Crystallization of Polymers by Differential Scanning Calorimetry; Designation D3418-15; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM. Standard Test Methods for Water Vapor Transmission of Organic Coating Films; Designation D1653-03; ASTM International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Steffe, J.F. Rheological Methods in Food Process Engineering, 2nd ed.; Freeman Press: East Lansing, MI, USA, 1996. [Google Scholar]
- ASTM. Standard Test Methods for Density and Specific Gravity [Relative Density] of Wood and Wood-Based Materials; Designation D2395-14; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Sward, G.G. Density, Specific Gravity, and Bulking Values. In Paint Testing Manual; ASTM International: West Conshohocken, PA, USA, 1972. [Google Scholar]
- ASTM. Standard Specification for ASTM Liquid-In-Glass Thermometers; Designation E1-14; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- ASTM. Standard Test Method for Evaluating Degree of Cracking of Exterior Paints; Designation D661-93; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- ASTM. Standard Test Method for Evaluating Degree of Checking of Exterior Paints; Designation D660-93; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Bennett, H. Industrial Waxes, Vol. 1; Chemical Publishing Company: Gloucester, MA, USA, 1963. [Google Scholar]
- Tulloch, A.P. The composition of BW and other waxes secreted by insects. Lipids 1970, 5, 247–258. [Google Scholar] [CrossRef]
- Chibnall, A.C.; Piper, S.H.; Pollard, A.; Williams, E.F.; Sahai, P.N. The constitution of the primary alcohols, fatty acids and paraffins present in plant and insect waxes. Biochem. J. 1934, 28, 2189–2208. [Google Scholar] [CrossRef]
- Hodge, W.H. The mexican candelilla wax plant and its wax. Econ. Bot. 1956, 10, 134–154. [Google Scholar] [CrossRef]
- Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.A.; Weiss, R.G.; Toro-Vazquez, J.F. Thermo-mechanical properties of candelilla wax and dotriacontane organogels in safflower oil. Eur. J. Lipid Sci. Technol. 2009, 111, 207–215. [Google Scholar] [CrossRef]
- Serrato-Palacios, L.L.; Toro-Vazquez, J.F.; Dibildox-Alvarado, E.; Aragón-Piña, A.; Morales-Armenta, M.R.; Ibarra-Junquera, V.; Pérez-Martínez, J.D. Phase Behavior and Structure of Systems Based on Mixtures of n-Hentriacontane and Melissic Acid. J. Am. Oil Chem Soc. 2015, 92, 533–540. [Google Scholar] [CrossRef]
- Dorset, D.L. The crystal structure of waxes. Acta Crystallogr. B Struct. Sci. 1995, 51, 1021–1028. [Google Scholar] [CrossRef] [Green Version]
- Dorset, D.L. Crystallography of the Polymethylene Chain: An Inquiry into the Structure of Waxes International Union of Crystallography Monography on Crystallography; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Zimnicka, B.; Hacura, A. An Investigation of Molecular Structure and Dynamics of Crude Beeswax by Vibrational Spectroscopy. Pol. J. Environ. Stud. 2006, 15, 112–114. [Google Scholar]
- Maia, M.; Barros, A.I.; Nunes, F.M. A novel, direct, reagent-free method for the detection of beeswax adulteration by single-reflection attenuated total reflectance mid- infrared spectroscopy. Talanta 2012, 107, 74–80. [Google Scholar] [CrossRef]
- Svečnjak, L.; Baranović, G.; Vinceković, M.; Prđun, S.; Bubalo, D.; Gajger, I.T. An approach for routine analytical detection of beeswax adulteration using FTIR-ATR spectroscopy. J. Apic. Sci. 2015, 59, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Edwards, H.G.M.; Falk, M.J.P. Fourier-transform Raman spectroscopic study of unsaturated and saturated waxes. Spectrochim. Acta A 1997, 53, 2685–2694. [Google Scholar] [CrossRef]
- Svečnjak, L.; Chesson, L.A.; Gallina, A.; Maia, M.; Martinello, M.; Mutinelli Necati Muz, M.; Nunes, F.M.; Saucy, F.; Tipple, B.J.; Wallner, K.; et al. Standard methods for Apis mellifera beeswax research. J. Apic. Res. 2019, 58, 1–108. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S. Food Analysis; Food Science Text Series; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.; Ayvaz, H.; Wehling, R.L. Infrared and Raman Spectroscopy. In Food Analysis, 5th ed.; Nielsen, S., Ed.; Food Science Text Series; Springer: Lafayette, IN, USA, 2017; pp. 107–128. [Google Scholar]
- Bernardini, F.; Tuniz, C.; Coppa, A.; Mancini, L.; Dreossi, D.; Eichert, D.; Turco, G.L.; Biasotto, M.; Terrasi, F.; De Cesare, N.; et al. Beeswax As Dental Filling On A Neolithic Human Tooth. PLoS ONE 2012, 7, e44904. [Google Scholar] [CrossRef]
- Buchwald, R.; Greenberg, A.R.; Breed, M.D. A biomehanical perspective on beeswax. Am. Entomol. 2005, 51, 39–41. [Google Scholar] [CrossRef] [Green Version]
- Masae, M.; Pitsuwan, P.; Sikong, L.; Kooptarnond, K.; Kongsong, P.; Phoempoon, P. Thermo-physical characterization of paraffin and beeswax on cotton fabric. Sci. Technol. Asia. 2014, pp. 69–77. Available online: https://www.tci-thaijo.org/index.php/SciTechAsia/article/view/41129 (accessed on 11 November 2019).
- Neidleman, S.L. Effects of temperature on lipid unsaturation. Biotechnol. Genet. Eng. Rev. 1987, 5, 245–268. [Google Scholar] [CrossRef] [Green Version]
- Lai, O.M.; Lo, S.K. Thermal Technique: Differential Scanning Calorimetry (DSC). In Modern Techniques for Food Authentication; Sun, D.-W., Ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 543–584. [Google Scholar]
- Shellhammer, T.H.; Rumsey, T.R.; Krochta, J.M. Viscoelastic properties of edible lipids. J. Food Eng. 1997, 33, 305–320. [Google Scholar] [CrossRef]
- Kamper, S.L.; Fennema, O. Water vapor permeability of edible bilayer films. J. Food Sci. 1984, 49, 1478–1481. [Google Scholar] [CrossRef]
- Kester, J.J.; Fennema, O. Resistance of lipid films to water vapor transmission. J. Am. Oil Chem. Soc. 1989, 66, 1139–1146. [Google Scholar] [CrossRef]
- Morillon, V.; Debeaufort, F.; Blond, G.; Capelle, M.; Voilley, A. Factors affecting the moisture permeability of lipid-based edible films: A review. Crit. Rev. Food Sci. 2002, 42, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Young, H.H.; Rubinstein, D. Wax Composition. U.S. Patent No. 2,394,833, 12 February 1946. Available online: https://patents.google.com/patent/US2394833A/en (accessed on 18 November 2020).
- Buchwald, R.; Breed, M.D.; Bjostad, L.; Hibbard, B.E.; Greenberg, A.R. The role of fatty acids in the mechanical properties of beeswax. Apidologie 2009, 40, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Cao, R. Micromechanism of Cleavage Fracture of Metals: A Comprehensive Microphysical Model for Cleavage Cracking in Metals; Butterworth-Heinemann: Waltham, MA, USA, 2015. [Google Scholar]
- Kotsiomiti, E.; Mccabe, J.F.J. Experimental wax mixtures for dental use. J. Oral Rehab. 1997, 24, 517–521. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering; John Wiley and Sons: New York, NY, USA, 2011. [Google Scholar]
- Perdiou, A.S.; Eldin, R.A.; Hajaj, K.R.; Rominu, M.; Sinescu, C.; Negrutiu, M.; Hajaj, T.A. Comparative Evaluation of Stress Resistance Between Nano-hybrid Composite and Ormocer Restorations on Posterior Teeth-in vitro Study. Mater. Plast. 2020, 57, 8–12. [Google Scholar] [CrossRef]






| Characteristics of Waxes | BW | CW | PW |
|---|---|---|---|
| Crystal phases identified by Χ-ray Diffraction * | - | - | - |
| C29H60 (straight-chain alkane), n-nonacosane (40-1997) | X | X | X |
| C21H44 (straight-chain alkane), n-heneicosane (31-1705) | X | X | X |
| C23H48, C25H52 (straight-chain alkanes), n-tricosane, n-pentacosane (53-1798) | X | - | X |
| C23H48 (straight-chain alkane), n-tricosane (43-1854) | X | - | X |
| C25H52 (straight-chain alkane) n-pentacosane (53-1793) | m | - | X |
| C33H68 (straight-chain alkane), n-tritriaacontane (54-1717) | - | X | - |
| C30H60O2 (straight-chain, saturated fatty acid), triacontanoic acid (34-1796) | - | X | - |
| C18H34O2 (nonlinear, saturated fatty acid), oleic acid (34-1810) | m | tr | - |
| C29H50O (terpene), β-sitosterol (10-0545) | - | X | - |
| Other Physical Characteristics | |||
| Peak melting temperature (°C) | 70 | 76 | 66 |
| Enthalpy (J/g) | 192 | 177.5 | 199.0 |
| Lower temperature limit for melting (°C) | 45.5 | 54.5 | 56 |
| Upper temperature limit for melting (°C) | 88.4 | 91.4 | 81.9 |
| Density 100 °C (g/cm3) | 0.82 | 0.90 | 0.78 |
| Density 25 °C (g/cm3) | 0.95 | 0.98 | 0.87 |
| Kinematic viscosity (centistokes) 100 °C | 8.9 | 16.2 | 4.6 |
| Kinematic Viscosity (mPa·s) at Various Temperatures | |||
| 100 °C | 4.7 | 8.30 | 1.9 |
| 95 °C | 5.3 | 10.1 | 2.1 |
| 90 °C | 6.3 | 10.9 | 2.4 |
| 85 °C | 7.0 | 13.1 | 2.8 |
| 80 °C | 8.2 | 15.7 | 3.2 |
| Conditions | 80/43% RH * (Dry Cup Method) | 43/80% RH * (Wet Cup Method) | ||||
|---|---|---|---|---|---|---|
| Wax | Thickness Mm | WVP | WVTR g/(m2day) | Thickness mm | WVP | WVTR g/(m2day) |
| BW | 0.63 | 3.22 | 19.42 | 0.46 | 1.24 | 7.00 |
| CW | 0.78 | 4.18 | 34.70 | 0.80 | 6.63 | 35.01 |
| PW | 0.58 | 0.89 | 5.73 | 0.55 | 1.17 | 7.96 |
| BW25/CW75 | 0.81 | 1.31 | 8.91 | 0.68 | 2.91 | 19.74 |
| BW50/CW50 | 0.74 | 1.78 | 12.73 | 0.50 | 1.77 | 13.37 |
| BW75/CW25 | 0.76 | 2.03 | 15.28 | 0.87 | 2.42 | 20.05 |
| Variables | Statistical Test * | Waxes | ||
|---|---|---|---|---|
| BW | CW | PW | ||
| Wax weight/cheese weight (% w/w) | ANOVA | 6.1 | 6.13 | 5.4 |
| Thickness of coatings (mm) | ANOVA | 1.0 | 1.0 | 0.98 |
| Weight change (%) of cheese after 5 weeks of storage | ANOVA | 3.2 A | 11.6 B | 11.2 B |
| Moisture (%)(at 0 weeks of storage) | ANOVA | 34.9 | 34.9 | 34.9 |
| Moisture (%) (at 5 weeks of storage) | ANOVA | 33.8 C | 24 A | 25.7 B |
| aw (at 0 weeks of storage) | ANOVA | 0.95 | 0.95 | 0.95 |
| aw (at 5 weeks of storage) | ANOVA | 0.94 C | 0.87 A | 0.91 B |
| Cracks (average of the length of cracks) | KW | 0.11 A | 132.8 B | 122.5 B |
| Area peeled by cracks (% in cheese area) | KW | 0.006 A | 7. 29 B | 7.19 B |
| Area reduction (%) | KW | 0.3 A | 5.58 B | 4.84 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucio, A.; Moreno-Tovar, R.; Bucio, L.; Espinosa-Dávila, J.; Anguebes-Franceschi, F. Characterization of Beeswax, Candelilla Wax and Paraffin Wax for Coating Cheeses. Coatings 2021, 11, 261. https://doi.org/10.3390/coatings11030261
Bucio A, Moreno-Tovar R, Bucio L, Espinosa-Dávila J, Anguebes-Franceschi F. Characterization of Beeswax, Candelilla Wax and Paraffin Wax for Coating Cheeses. Coatings. 2021; 11(3):261. https://doi.org/10.3390/coatings11030261
Chicago/Turabian StyleBucio, Adolfo, Rosario Moreno-Tovar, Lauro Bucio, Jessica Espinosa-Dávila, and Francisco Anguebes-Franceschi. 2021. "Characterization of Beeswax, Candelilla Wax and Paraffin Wax for Coating Cheeses" Coatings 11, no. 3: 261. https://doi.org/10.3390/coatings11030261






