Electro-Oxidation of Ammonia over Copper Oxide Impregnated γ-Al2O3 Nanocatalysts
Abstract
1. Introduction
2. Experimental
2.1. Preparation of CuO/γ-Al2O3 Electrocatalysts
2.2. Electrochemical Investigations
3. Results and Discussion
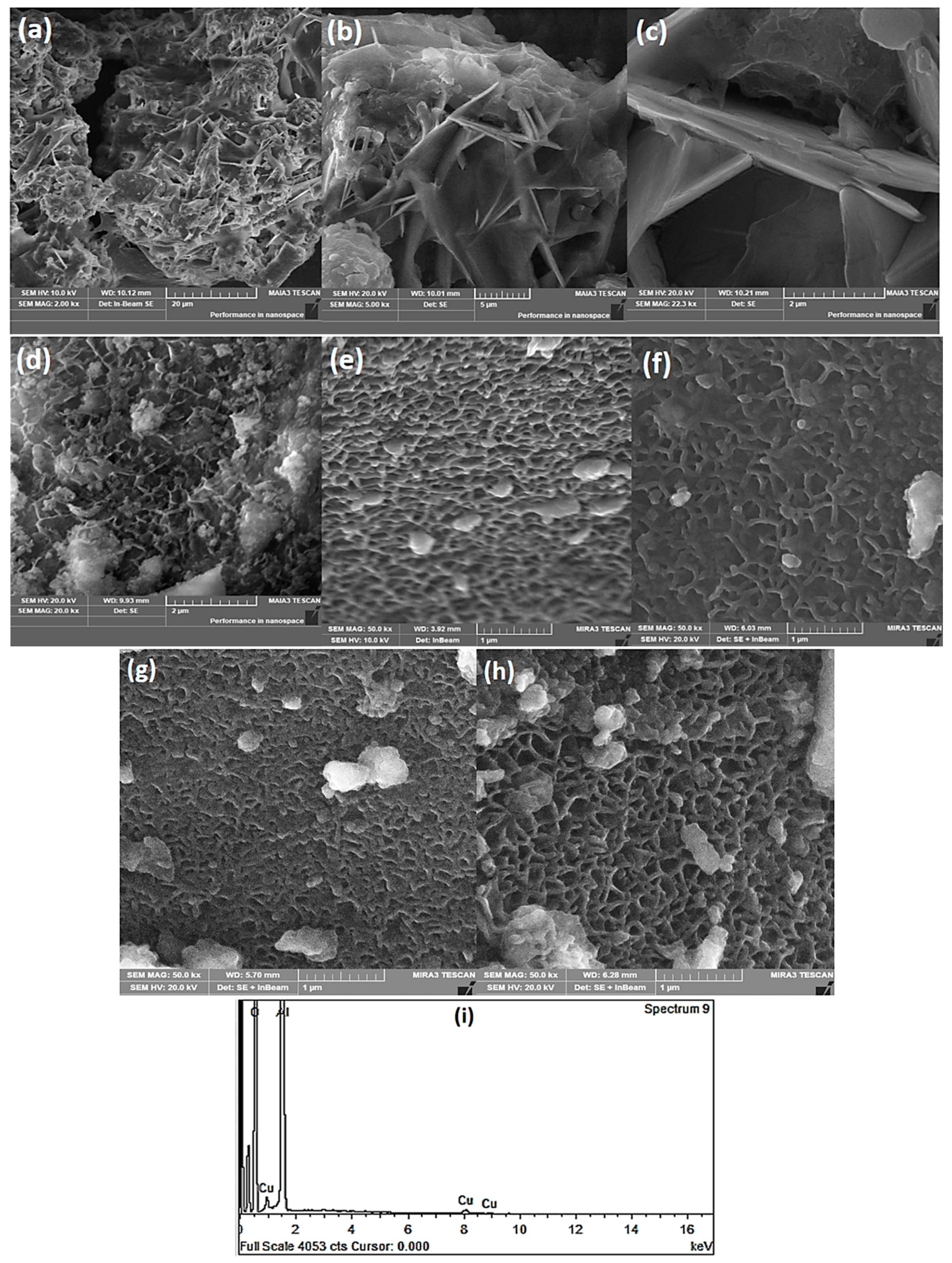
3.1. Structural Characterization
3.2. Surface Characterization
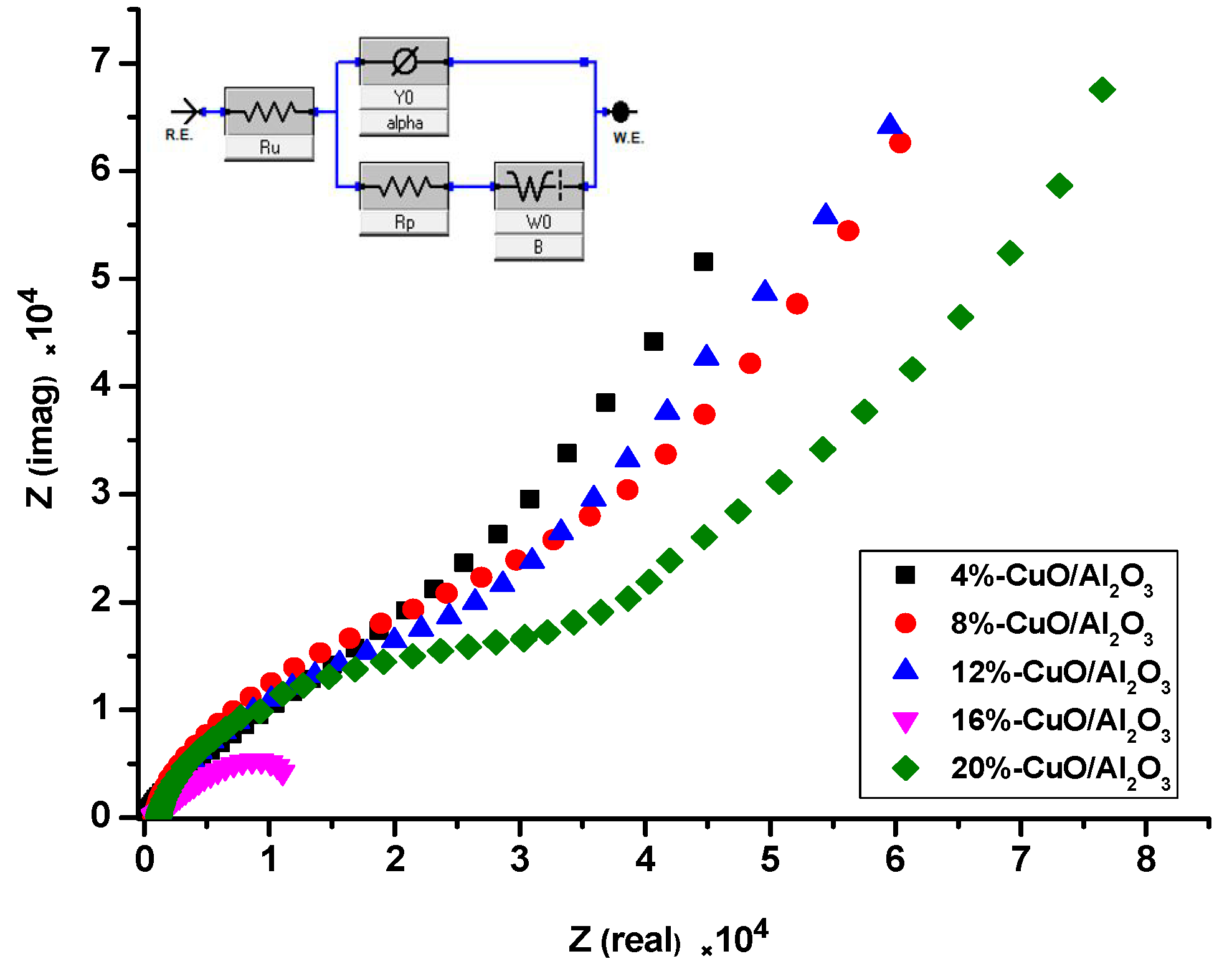
3.3. Electrochemical Impedance Spectroscopy EIS
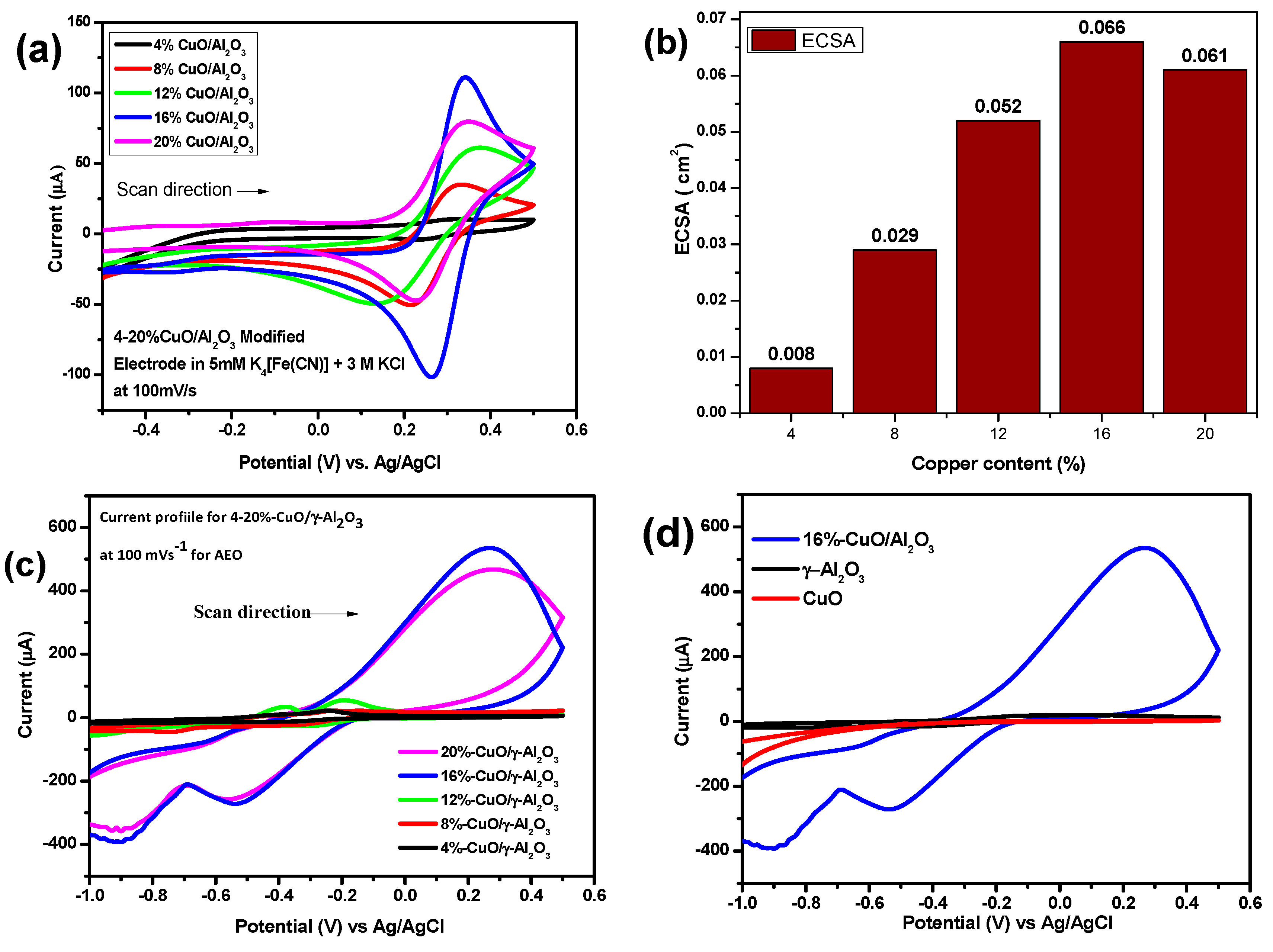
3.4. Active Surface Area of the CuO/γ-Al2O3 Modified Electrodes
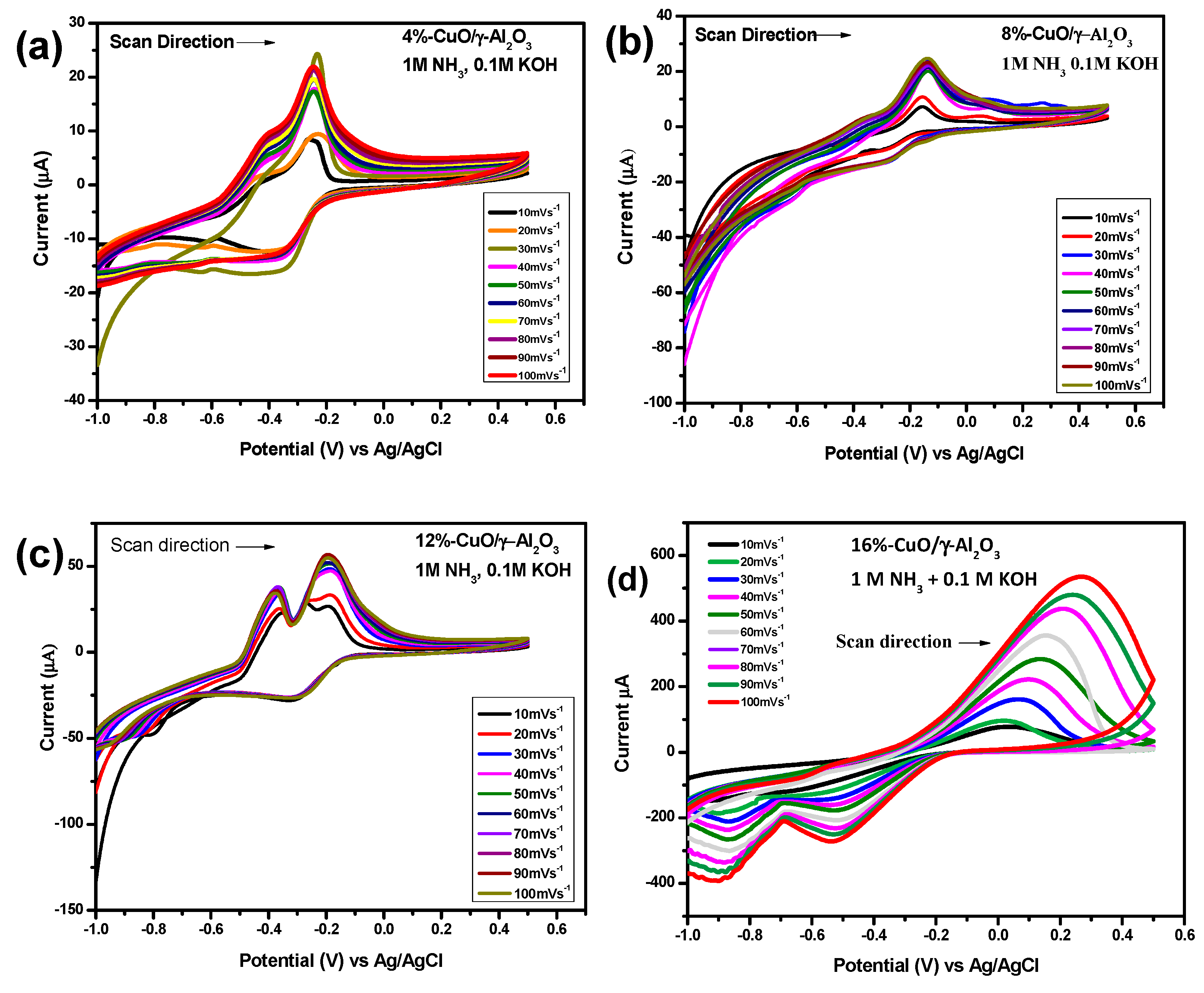
3.5. Electrochemical Studies
3.6. Diffusion Coefficients for AEO on CuO/γ-Al2O3 Modified Electrodes
3.7. Estimation of Bandgap Values of CuO/γ-Al2O3 for Ammonia Electro-Oxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, C.-D.; Chen, C.-W.; Kao, C.-M.; Hung, C.-M. Synthesis, characterization, and application of CuO-modified TiO2 electrode exemplified for ammonia electro-oxidation. Process Saf. Environ. Prot. 2017, 112, 243–253. [Google Scholar] [CrossRef]
- Yuan, M.-H.; Chen, Y.-H.; Tsai, J.-Y.; Chang, C.-Y. Ammonia removal from ammonia-rich wastewater by air stripping using a rotating packed bed. Process Saf. Environ. Prot. 2016, 102, 777–785. [Google Scholar] [CrossRef]
- Sheng, Y.; Fang, L.; Zhang, L.; Wang, Y. Aimed at building a healthy living environment: An analysis of performance of Clean-Air Heat Pump system for ammonia removal. Build. Environ. 2020, 171, 106639. [Google Scholar] [CrossRef]
- Ulu, F.; Kobya, M. Ammonia removal from wastewater by air stripping and recovery struvite and calcium sulphate precipitations from anesthetic gases manufacturing wastewater. J. Water Process. Eng. 2020, 38, 101641. [Google Scholar] [CrossRef]
- Barbosa, J.R.; Leon, M.N.; Fernandes, C.M.; Antoniassi, R.M.; Alves, Q.C.; Ponzio, E.A.; Silva, J.C.M. PtSnO2/C and Pt/C with preferential (100) orientation: High active electrocatalysts for ammonia electro-oxidation reaction. Appl. Catal. B Environ. 2020, 264, 118458. [Google Scholar] [CrossRef]
- Katsounaros, I.; Figueiredo, M.C.; Calle-Vallejo, F.; Li, H.; Gewirth, A.A.; Markovic, N.M.; Koper, M.T. On the mechanism of the electrochemical conversion of ammonia to dinitrogen on Pt (1 0 0) in alkaline environment. J. Catal. 2018, 359, 82–91. [Google Scholar] [CrossRef]
- Diaz, L.A.; Botte, G.G. Mathematical modeling of ammonia electrooxidation kinetics in a Polycrystalline Pt rotating disk electrode. Electrochimica Acta 2015, 179, 519–528. [Google Scholar] [CrossRef]
- Bayati, M.; Liu, X.; Abellan, P.; Pocock, D.; Dixon, M.; Scott, K. Synergistic Coupling of a Molybdenum Carbide Nanosphere with Pt Nanoparticles for Enhanced Ammonia Electro-Oxidation Activity in Alkaline Media. ACS Appl. Energy Mater. 2020, 3, 843–851. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wu, Z.; Leung, D.Y. An ammonia electrolytic cell with NiCu/C as anode catalyst for hydrogen production. Energy Procedia 2017, 142, 1539–1544. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y. Ammonia removal in electrochemical oxidation: Mechanism and pseudo-kinetics. J. Hazard. Mater. 2009, 161, 1010–1016. [Google Scholar] [CrossRef]
- Marinčić, L.; Leitz, F.B. Electro-oxidation of ammonia in waste water. J. Appl. Electrochem. 1978, 8, 333–345. [Google Scholar] [CrossRef]
- Botte, G.G.; Benedetti, L.; Gonzalez, J. Electrolysis of ammonia: An in situ hydrogen production process. In Proceedings of the 2005 AIChE Annual Meeting, Cincinnati, OH, USA, 30 October–4 November 2005. [Google Scholar]
- Michels, N.-L.; Kapałka, A.; Abd-El-Latif, A.; Baltruschat, H.; Comninellis, C. Enhanced ammonia oxidation on BDD induced by inhibition of oxygen evolution reaction. Electrochem. Commun. 2010, 12, 1199–1202. [Google Scholar] [CrossRef]
- Mahvi, A.H.; Ebrahimi, S.J.A.D.; Mesdaghinia, A.; Gharibi, H.; Sowlat, M.H. Performance evaluation of a continuous bipolar electrocoagulation/electrooxidation–electroflotation (ECEO–EF) reactor designed for simultaneous removal of ammonia and phosphate from wastewater effluent. J. Hazard. Mater. 2011, 192, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Allagui, A.; Sarfraz, S.; Ntais, S.; Al Momani, F.; Baranova, E.A. Electrochemical behavior of ammonia on Ni98Pd2 nano-structured catalyst. Int. J. Hydrog. Energy 2014, 39, 41–48. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; Xia, F.; Zhang, Y.; Wang, H.; Ning, P. Promoting effects of acid enhancing on N2 selectivity for selectivity catalytic oxidation of NH3 over RuOx/TiO2: The mechanism study. Appl. Surf. Sci. 2020, 500, 144044. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Tawalbeh, M. Electrochemical oxidation of ammonia on nickel oxide nanoparticles. Int. J. Hydrog. Energy 2020, 45, 10398–10408. [Google Scholar] [CrossRef]
- Kapałka, A.; Cally, A.; Neodo, S.; Comninellis, C.; Wächter, M.; Udert, K.M. Electrochemical behavior of ammonia at Ni/Ni(OH)2 electrode. Electrochem. Commun. 2010, 12, 18–21. [Google Scholar] [CrossRef]
- Xu, W.; Lan, R.; Du, D.; Humphreys, J.; Walker, M.; Wu, Z.; Wang, H.; Tao, S. Directly growing hierarchical nickel-copper hydroxide nanowires on carbon fibre cloth for efficient electrooxidation of ammonia. Appl. Catal. B Environ. 2017, 218, 470–479. [Google Scholar] [CrossRef]
- HHerron, J.A.; Ferrin, P.; Mavrikakis, M. Electrocatalytic oxidation of ammonia on transition-metal surfaces: A first-principles study. J. Phys. Chem. C 2015, 119, 14692–14701. [Google Scholar] [CrossRef]
- Kum, J.M.; Yoo, S.H.; Ali, G.; Cho, S.O. Photocatalytic hydrogen production over CuO and TiO2 nanoparticles mixture. Int. J. Hydrog. Energy 2013, 38, 13541–13546. [Google Scholar] [CrossRef]
- Hua, L.; Ma, H.; Zhang, L. Degradation process analysis of the azo dyes by catalytic wet air oxidation with catalyst CuO/γ-Al2O3. Chemosphere 2013, 90, 143–149. [Google Scholar] [CrossRef]
- Hosseini, S.R.; Kamali-Rousta, M. Preparation of electro-spun CuO nanoparticle and its application for hydrazine hydrate electro-oxidation. Electrochim. Acta 2016, 189, 45–53. [Google Scholar] [CrossRef]
- Firdous, N.; Janjua, N.K. CoPtx/γ-Al2O3 bimetallic nanoalloys as promising catalysts for hydrazine electrooxidation. Heliyon 2019, 5, e01380. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Wang, R.; Wang, H.; Lv, W.; Ji, S. Ultrathin willow-like CuO nanoflakes as an efficient catalyst for electro-oxidation of hydrazine. J. Power Sources 2015, 289, 22–25. [Google Scholar] [CrossRef]
- Mujtaba, A.; Janjua, N.K. Fabrication and electrocatalytic application of CuO@ Al2O3 hybrids. J. Electrochem. Soc. 2015, 162, H328–H337. [Google Scholar] [CrossRef]
- Firdous, N.; Janjua, N.K.; Qazi, I.; Wattoo, M.H.S. Optimal Co–Ir bimetallic catalysts supported on γ-Al2O3 for hydrogen generation from hydrous hydrazine. Int. J. Hydrog. Energy 2016, 41, 984–995. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, D.; Shi, L.; Wang, L.; Jin, Y.; Tian, L.; Li, Z.; Wang, G.; Zhao, L.; Yan, Y. A review of phase change heat transfer in shape-stabilized phase change materials (ss-PCMs) based on porous supports for thermal energy storage. Renew. Sustain. Energy Rev. 2021, 135, 110127. [Google Scholar] [CrossRef]
- López, M.; Palacio, R.; Mamede, A.-S.; Fernández, J.J.; Royer, S. Hydrodeoxygenation of guaiacol into cyclohexane over mesoporous silica supported Ni–ZrO2 catalyst. Microporous Mesoporous Mater. 2020, 309, 110452. [Google Scholar] [CrossRef]
- Ramesh, A.; Tamizhdurai, P.; Krishnan, P.S.; Ponnusamy, V.K.; Sakthinathan, S.; Shanthi, K. Catalytic transformation of non-edible oils to biofuels through hydrodeoxygenation using Mo-Ni/mesoporous alumina-silica catalysts. Fuel 2020, 262, 116494. [Google Scholar] [CrossRef]
- Thou, C.Z.; Khan, F.S.A.; Mubarak, N.M.; Ahmad, A.; Khalid, M.; Jagadish, P.; Walvekar, R.; Abdullah, E.C.; Khan, S.; Khan, M.; et al. Surface charge on chitosan/cellulose nanowhiskers composite via functionalized and untreated carbon nanotube. Arab. J. Chem. 2021, 14, 103022. [Google Scholar] [CrossRef]
- Kashif, M.; Ngaini, Z.; Harry, A.V.; Vekariya, R.L.; Ahmad, A.; Zuo, Z.; Sahari, S.K.; Hussain, S.; Khan, Z.A.; Alarifi, A. An experimental and DFT study on novel dyes incorporated with natural dyes on titanium dioxide (TiO2) towards solar cell application. Appl. Phys. A 2020, 126, 1–13. [Google Scholar] [CrossRef]
- Paranjpe, K.Y. Alpha, Beta and Gamma Alumina as Catalyst. Pharma. Innov. J. 2017, 6, 236–238. [Google Scholar]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a support for catalysts: A review of fundamental aspects. Eur. J. Inorg. Chem. 2005, 3393–3403. [Google Scholar] [CrossRef]
- Badri, M.T.; Barati, M.; Rasa, S.H. A kinetic study of acetone acidic oxidation with KMnO4 in the absence and presence of CuO/-Al2O3 as a heterogeneous nano-catalyst. Sci. Iran. 2020, 27, 1234–1242. [Google Scholar]
- Bradu, C.; Frunza, L.; Mihalche, N.; Avramescu, S.-M.; Neaţă, M.; Udrea, I. Removal of Reactive Black 5 azo dye from aqueous solutions by catalytic oxidation using CuO/Al2O3 and NiO/Al2O3. Appl. Catal. B Environ. 2010, 96, 548–556. [Google Scholar] [CrossRef]
- Firdous, N.; Janjua, N.K.; Wattoo, M.H.S. Promoting effect of ruthenium, platinum and palladium on alumina supported cobalt catalysts for ultimate generation of hydrogen from hydrazine. Int. J. Hydrog. Energy 2020, 45, 21573–21587. [Google Scholar] [CrossRef]
- Minakshi; Pundir, C.S. Construction of an amperometric enzymic sensor for triglyceride determination. Sens. Actuators B Chem. 2008, 133, 251–255. [Google Scholar] [CrossRef]
- Hooda, V.; Gahlaut, A. Amperometric cholesterol determination using HRP incorporated carbon paste electrode. Biosci. Biotechnol. Res. Asia 2020, 17, 53–64. [Google Scholar] [CrossRef]
- Odeyemi, C.S.; Awodugba, A.O. Improving the efficiency of titanium dioxide based dye sensitized solar cell (DSSC) using silver surface counter electrode. Futa J. Eng. Eng. Technol. 2018, 12, 1–6. [Google Scholar]
- Xu, W.; Du, D.; Lan, R.; Humphreys, J.; Miller, D.N.; Walker, M.; Wu, Z.; Irvine, J.T.; Tao, S. Electrodeposited NiCu bimetal on carbon paper as stable non-noble anode for efficient electrooxidation of ammonia. Appl. Catal. B Environ. 2018, 237, 1101–1109. [Google Scholar] [CrossRef]
- Strehlitz, B.; Gründig, B.; Kopinke, H. Sensor for amperometric determination of ammonia and ammonia-forming enzyme reactions. Anal. Chim. Acta 2000, 403, 11–23. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, W.; Li, J.; Li, Q.; Liu, S.; Lei, Z. A highly efficient Pt-NiO/C electrocatalyst for ammonia electro-oxidation. J. Electrochem. Soc. 2017, 164, F958. [Google Scholar] [CrossRef]
- Schechner, P.; Kroll, E.; Bubis, E.; Chervinsky, S.; Zussman, E. Silver-plated electrospun fibrous anode for glucose alkaline fuel cells. J. Electrochem. Soc. 2007, 154, B942–B948. [Google Scholar] [CrossRef]
- Luo, M.F.; Fang, P.; He, M.; Xie, Y.L. In situ XRD, Raman, and TPR studies of CuO/Al2O3 catalysts for CO oxidation. J. Mol. Catal. A Chem. 2005, 239, 243–248. [Google Scholar] [CrossRef]
- Bagtache, R.; Saib, F.; Abdmeziem, K.; Trari, M. A new hetero-junction p-CuO/Al2O3 for the H2 evolution under visible light. Int. J. Hydrog. Energy 2019, 44, 22419–22424. [Google Scholar] [CrossRef]
- Azam, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and-negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef]
- Li, Y.; Liang, J.; Tao, Z.; Chen, J. CuO particles and plates: Synthesis and gas-sensor application. Mater. Res. Bull. 2008, 43, 2380–2385. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Baqer, A.A.; Matori, K.A.; Al-Hada, N.M.; Shaari, A.H.; Kamari, H.M.; Saion, E.; Chyi, J.L.Y.; Abdullah, C.A.C. Synthesis and characterization of binary (CuO)0.6(CeO2)0.4 nanoparticles via a simple heat treatment method. Results Phys. 2018, 9, 471–478. [Google Scholar] [CrossRef]
- Gholami, T.; Salavati-Niasari, M. Effects of copper: Aluminum ratio in CuO/Al2O3 nanocomposite: Electrochemical hydrogen storage capacity, band gap and morphology. Int. J. Hydrog. Energy 2016, 41, 15141–15148. [Google Scholar] [CrossRef]
- Strohmeier, B.R.; Levden, D.E.; Field, R.S.; Hercules, D.M. Surface spectroscopic characterization of CuAl2O3 catalysts. J. Catal. 1985, 94, 514–530. [Google Scholar] [CrossRef]
- Keshavarz, A.R.; Rezaei, M.; Yaripour, F. Nanocrystalline gamma-alumina: A highly active catalyst for dimethyl ether synthesis. Powder Technol. 2010, 199, 176–179. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, D.; Han, X.; Bao, X.; Frandsen, W.; Wang, D.; Su, D. Hydrothermal synthesis of microscale boehmite and gamma nanoleaves alumina. Mater. Lett. 2008, 62, 1297–1301. [Google Scholar] [CrossRef]
- Schmücker, M.; Albers, W.; Schneider, H. Mullite formation by reaction sintering of quartz and α-Al2O3—A TEM study. J. Eur. Ceram. Soc. 1994, 14, 511–515. [Google Scholar] [CrossRef]
- Rozita, Y.; Brydson, R.; Scott, A.J. An investigation of commercial gamma-Al2O3 nanoparticles. J. Phys. Conf. Ser. 2010, 241, 012096. [Google Scholar] [CrossRef]
- Nie, M.-X.; Li, X.-Z.; Liu, S.-R.; Guo, Y. ZnO/CuO/Al2O3 composites for chloroform detection. Sens. Actuators B Chem. 2015, 210, 211–217. [Google Scholar] [CrossRef]
- Duan, N.; Liu, J.; Li, Q.; Xiao, H. Enhanced adsorption performance of CuO-Al2O3 composite derived from cotton template. Can. J. Chem. Eng. 2015, 93, 2015–2023. [Google Scholar] [CrossRef]
- Maximiano, E.M.; de Lima, F.; Cardoso, C.A.L.; Arruda, G.J. Incorporation of thermally activated zeolite into carbon paste electrodes for voltammetric detection of carbendazim traces in milk samples. J. Appl. Electrochem. 2016, 46, 713–723. [Google Scholar] [CrossRef]
- Muhammad, S.; Banin Zahra, U.; Ahmad, A.; Shah, L.A.; Muhammad, A. Understanding the basics of electron transfer and cyclic voltammetry of potassium ferricyanide-an outer sphere heterogeneous electrode reaction. J. Chem. Soc. Pak. 2020, 42, 813. [Google Scholar]
- Cossar, E.; Houache, M.S.; Zhang, Z.; Baranova, E.A. Comparison of electrochemical active surface area methods for various nickel nanostructures. J. Electroanal. Chem. 2020, 870, 114246. [Google Scholar] [CrossRef]
- Zhan, T.; Sun, X.; Wang, X.; Sun, W.; Hou, W. Application of ionic liquid modified carbon ceramic electrode for the sensitive voltammetric detection of rutin. Talanta 2010, 82, 1853–1857. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Han, S.; Yan, J.-M.; Shioyama, H.; Kuriyama, N.; Kobayashi, T.; Xu, Q. Electrochemical oxidation of ammonia borane on gold electrode. Int. J. Hydrog. Energy 2009, 34, 174–179. [Google Scholar] [CrossRef]
- Le Vot, S.; Roué, L.; Bélanger, D. Synthesis of Pt–Ir catalysts by coelectrodeposition: Application to ammonia electrooxidation in alkaline media. J. Power Sources 2013, 223, 221–231. [Google Scholar] [CrossRef]
- Li, Z.-F.; Wang, Y.; Botte, G.G. Revisiting the electrochemical oxidation of ammonia on carbon-supported metal nanoparticle catalysts. Electrochim. Acta 2017, 228, 351–360. [Google Scholar] [CrossRef]
- Matinise, N.; Mayedwa, N.; Ikpo, C.O.; Hlongwa, N.W.; Ndipingwi, M.M.; Molefe, L.; Dywili, N.; Yonkeu, A.L.D.; Waryo, T.; Baker, P.G.L.; et al. Bimetallic nanocomposites of palladium (100) and ruthenium for electrooxidation of ammonia. J. Nano Res. 2016, 44, 100–113. [Google Scholar] [CrossRef]
- Álvarez-Ruiz, B.; Gómez, R.; Orts, J.M.; Feliu, J.M. Role of the metal and surface structure in the electro-oxidation of hydrazine in acidic media. J. Electrochem. Soc. 2002, 149, D35–D45. [Google Scholar] [CrossRef]
- Zhou, L.; Cheng, Y. Catalytic electrolysis of ammonia on platinum in alkaline solution for hydrogen generation. Int. J. Hydrog. Energy 2008, 33, 5897–5904. [Google Scholar] [CrossRef]
- Zhu, P.; Zhao, Y. Effects of electrochemical reaction and surface morphology on electroactive surface area of porous copper manufactured by Lost Carbonate Sintering. RSC Adv. 2017, 7, 26392–26400. [Google Scholar] [CrossRef]
- da Silva, S.W.; Navarro, E.M.O.; Rodrigues, M.A.S.; Bernardes, A.M.; Pérez-Herranz, V. The role of the anode material and water matrix in the electrochemical oxidation of norfloxacin. Chemosphere 2018, 210, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.; Cattaneo, C.; Mishima, H.; De Mishima, B.A.L.; Silvetti, S.P.; Rodriguez, J.L.; Pastor, E. Ammonia oxidation on electrodeposited Pt–Ir alloys. J. Solid State Electrochem. 2008, 12, 583–589. [Google Scholar] [CrossRef]
- Yao, K.; Cheng, Y. Electrodeposited Ni–Pt binary alloys as electrocatalysts for oxidation of ammonia. J. Power Sources 2007, 173, 96–101. [Google Scholar] [CrossRef]
- Khan, M.; Janjua, N.K.; Khan, S.; Qazi, I.; Ali, S.; Saad Algarni, T. Electro-oxidation of ammonia at novel Ag2O-PrO2/γ-Al2O3 Catalysts. Coatings 2021, 11, 257. [Google Scholar] [CrossRef]
- Diaz, L.A.; Valenzuela-Muñiz, A.; Muthuvel, M.; Botte, G.G. Analysis of ammonia electro-oxidation kinetics using a rotating disk electrode. Electrochim. Acta 2013, 89, 413–421. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Yang, S.-H.; Hsu, C.-S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Admassie, S.; Inganäs, O.; Mammo, W.; Perzon, E.; Andersson, M.R. Electrochemical and optical studies of the band gaps of alternating polyfluorene copolymers. Synth. Met. 2006, 156, 614–623. [Google Scholar] [CrossRef]
- Loganathan, K.; Pickup, P.G. Poly(Δ4,4′-dicyclopenta[2,1-b:3,4-b′] dithiophene–co-3,4-ethylenedioxythiophene): Electrochemically generated low band gap conducting copolymers. Electrochim. Acta 2005, 51, 41–46. [Google Scholar] [CrossRef]
- Jessop, I.; Zamora, P.P.; Díaz, F.R.; del Valle, M.A.; Leiva, A.; Cattin, L.; Makha, M.; Bernède, J.C. New polymers based on 2, 6-di (thiophen-2-yl) aniline and 2, 2’-(thiophen-2, 5-diyl) dianiline monomers. preparation, characterization and thermal, optical, electronic and photovoltaic properties. Int. J. Electrochem. Sci. 2012, 7, 9502–9517. [Google Scholar]
- Lohrman, J.; Zhang, C.; Zhang, W.; Ren, S. Semiconducting single-wall carbon nanotube and covalent organic polyhedron-C60 nanohybrids for light harvesting. Chem. Commun. 2012, 48, 8377–8379. [Google Scholar] [CrossRef]
- Rehman, S.; Mumtaz, A.; Hasanain, S.K. Size effects on the magnetic and optical properties of CuO nanoparticles. J. Nanoparticle Res. 2011, 13, 2497–2507. [Google Scholar] [CrossRef]
- Phoka, S.; Laokul, P.; Swatsitang, E.; Promarak, V.; Seraphin, S.; Maensiri, S. Synthesis, structural and optical properties of CeO2 nanoparticles synthesized by a simple polyvinyl pyrrolidone (PVP) solution route. Mater. Chem. Phys. 2009, 115, 423–428. [Google Scholar] [CrossRef]
- Nasir, M.H.; Janjua, N.K.; Santoki, J. Electrochemical performance of carbon modified LiNiPO4 as Li-ion battery cathode: A combined experimental and theoretical study. J. Electrochem. Soc. 2020, 167, 130526. [Google Scholar] [CrossRef]
- Butt, T.M.; Janjua, N.K.; Mujtaba, A.; Zaman, S.A.; Ansir, R.; Rafique, A.; Sumreen, P.; Mukhtar, M.; Pervaiz, M.; Yaqub, A.; et al. B-site doping in lanthanum cerate nanomaterials for water electrocatalysis. J. Electrochem. Soc. 2020, 167, 026503. [Google Scholar] [CrossRef]





| Electrocatalyst | Dav (nm) |
|---|---|
| CuO | 14.6 |
| γ-Al2O3 | 2.5 |
| 4%-CuO/γ-Al2O3 | 7.1 |
| 8%-CuO/γ-Al2O3 | 9.1 |
| 12%-CuO/γ-Al2O3 | 11.5 |
| 16%-CuO/γ-Al2O3 | 15.1 |
| 20%-CuO/γ-Al2O3 | 16.2 |
| Electrocatalysts | Rs (Ω) | Rct (kΩ) | CPE (µF) | α | Wo (Ω) | kapp/10−9 (cms−1) |
|---|---|---|---|---|---|---|
| 4%-CuO/γ-Al2O3 | 816.0 | 23.92 | 2.57 | 0.80 | 17.0 | 10.4 |
| 8%-CuO/γ-Al2O3 | 770.6 | 22.7 | 2.12 | 0.87 | 16.7 | 8.81 |
| 12%-CuO/γ-Al2O3 | 968.0 | 22.2 | 2.26 | 0.82 | 16.0 | 10.8 |
| 16%-CuO/γ-Al2O3 | 736.0 | 3.92 | 55.4 | 0.56 | 13.9 | 61.2 |
| 20%-CuO/γ-Al2O3 | 934.2 | 27.9 | 4.77 | 0.84 | 23.6 | 8.73 |
| Electrocatalyst | DNH3 10−9 (cm2s−1) |
|---|---|
| 4%-CuO/γ-Al2O3 | 1.0 |
| 8%-CuO/γ-Al2O3 | 2.6 |
| 12%-CuO/γ-Al2O3 | 2.7 |
| 16%-CuO/γ-Al2O3 | 4.1 |
| 20%-CuO/γ-Al2O3 | 3.8 |
| Ni/Pt [55] | 1.2 |
| Ni/Ni(OH)2 [16] | 2.8 |
| Ionic liquids [16] | 0.1 |
| Catalyst | E(ons)oxi (V) | E(ons)red (V) | EHOMO (eV) | ELUMO (eV) | Eg (eV) |
|---|---|---|---|---|---|
| 4%-CuO/γ-Al2O3 | −0.64 | −0.25 | −3.16 | −4.14 | 0.98 |
| 8%-CuO/γ-Al2O3 | −0.43 | −0.13 | −3.56 | −4.31 | 0.75 |
| 12%-CuO/γ-Al2O3 | −0.53 | −0.21 | −3.98 | −4.69 | 0.71 |
| 16%-CuO/γ-Al2O3 | −0.41 | −0.18 | −3.90 | −4.20 | 0.22 |
| 20%-CuO/γ-Al2O3 | −0.32 | −0.06 | −3.89 | −4.30 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Shah, S.S.; Anjum, M.A.R.; Khan, M.R.; Janjua, N.K. Electro-Oxidation of Ammonia over Copper Oxide Impregnated γ-Al2O3 Nanocatalysts. Coatings 2021, 11, 313. https://doi.org/10.3390/coatings11030313
Khan S, Shah SS, Anjum MAR, Khan MR, Janjua NK. Electro-Oxidation of Ammonia over Copper Oxide Impregnated γ-Al2O3 Nanocatalysts. Coatings. 2021; 11(3):313. https://doi.org/10.3390/coatings11030313
Chicago/Turabian StyleKhan, Safia, Syed Sakhawat Shah, Mohsin Ali Raza Anjum, Mohammad Rizwan Khan, and Naveed Kausar Janjua. 2021. "Electro-Oxidation of Ammonia over Copper Oxide Impregnated γ-Al2O3 Nanocatalysts" Coatings 11, no. 3: 313. https://doi.org/10.3390/coatings11030313
APA StyleKhan, S., Shah, S. S., Anjum, M. A. R., Khan, M. R., & Janjua, N. K. (2021). Electro-Oxidation of Ammonia over Copper Oxide Impregnated γ-Al2O3 Nanocatalysts. Coatings, 11(3), 313. https://doi.org/10.3390/coatings11030313







