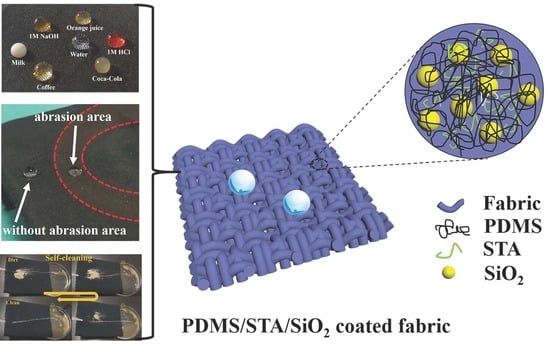
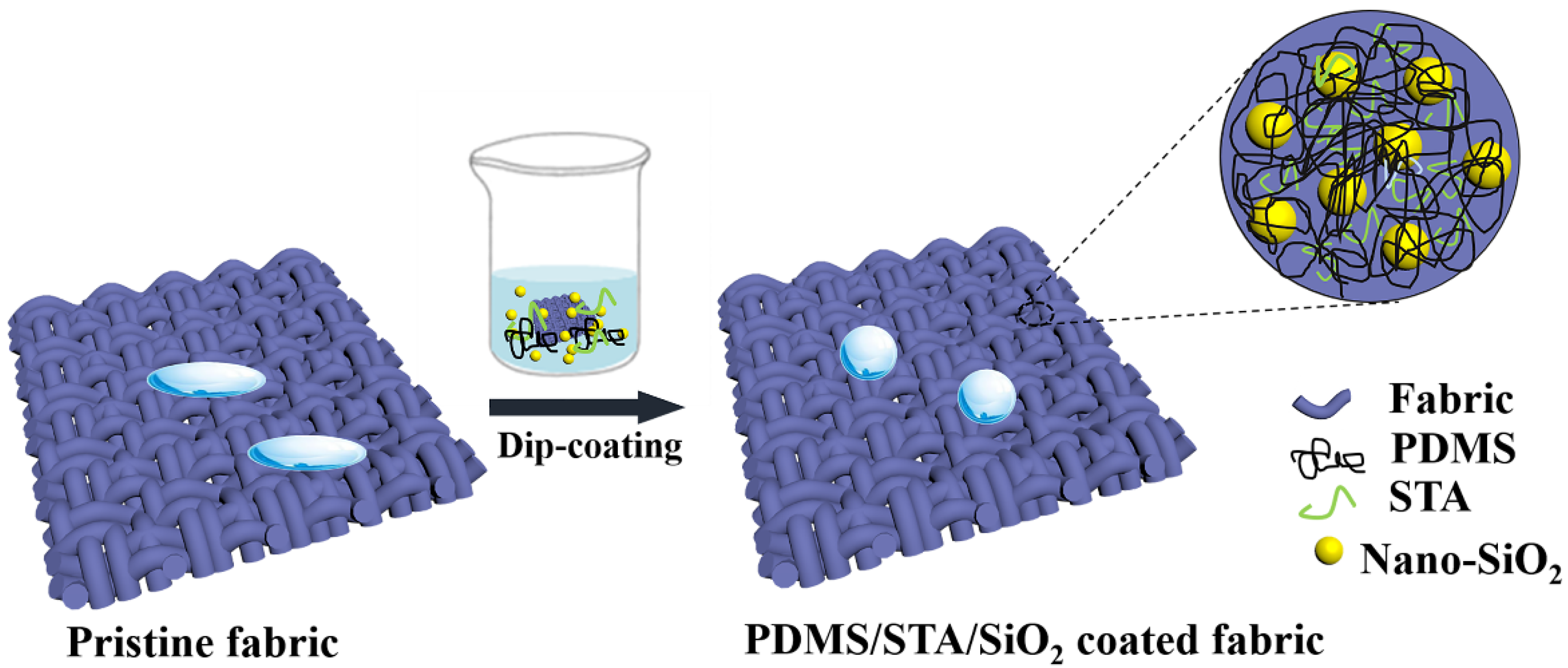
Dip-Coating Approach to Fabricate Durable PDMS/STA/SiO2 Superhydrophobic Polyester Fabrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Different Hydrophobic Fabrics
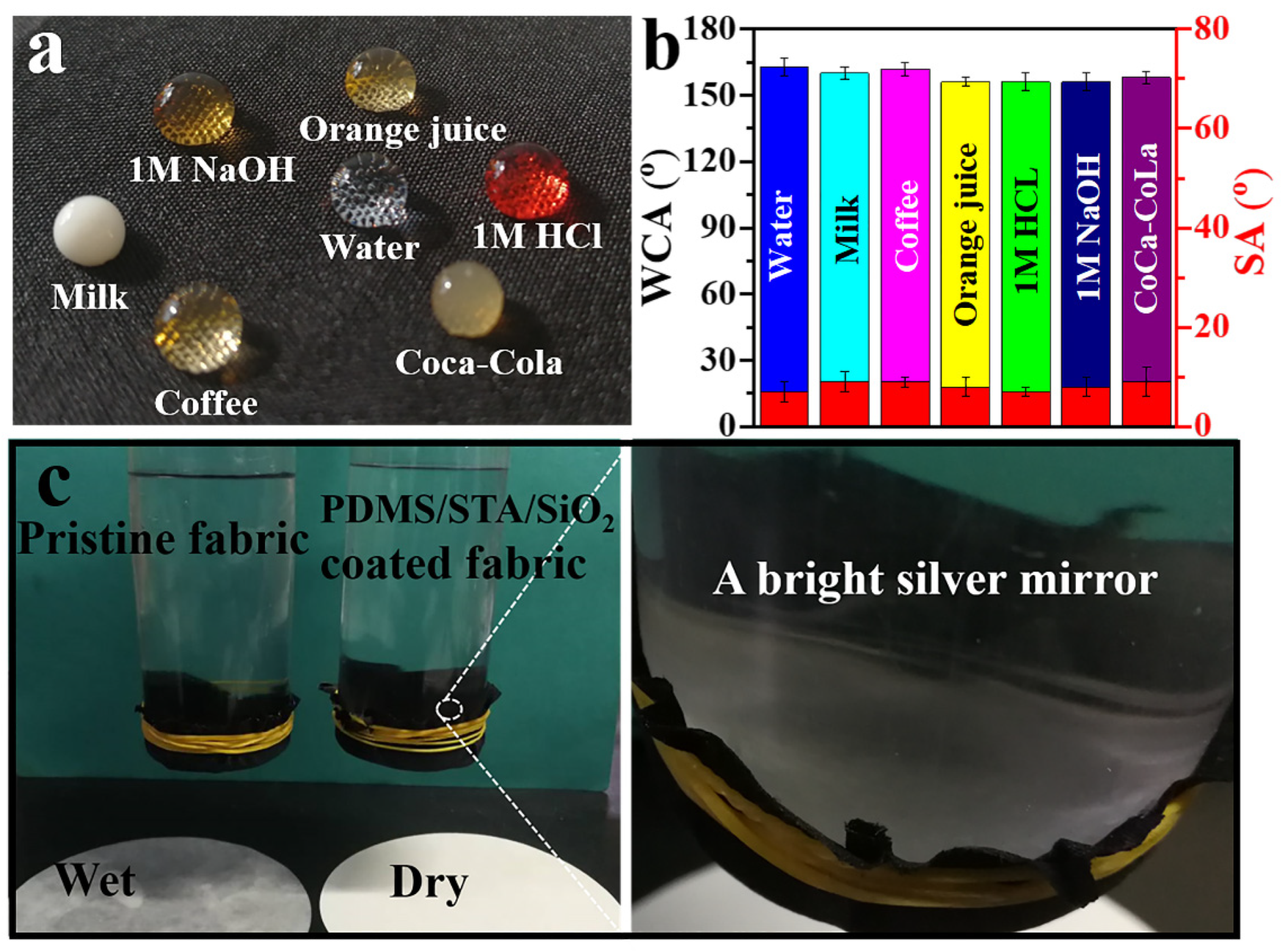
2.3. Tests on Different Liquid Resistance and Waterproof Properties
2.4. Self-Cleaning Property
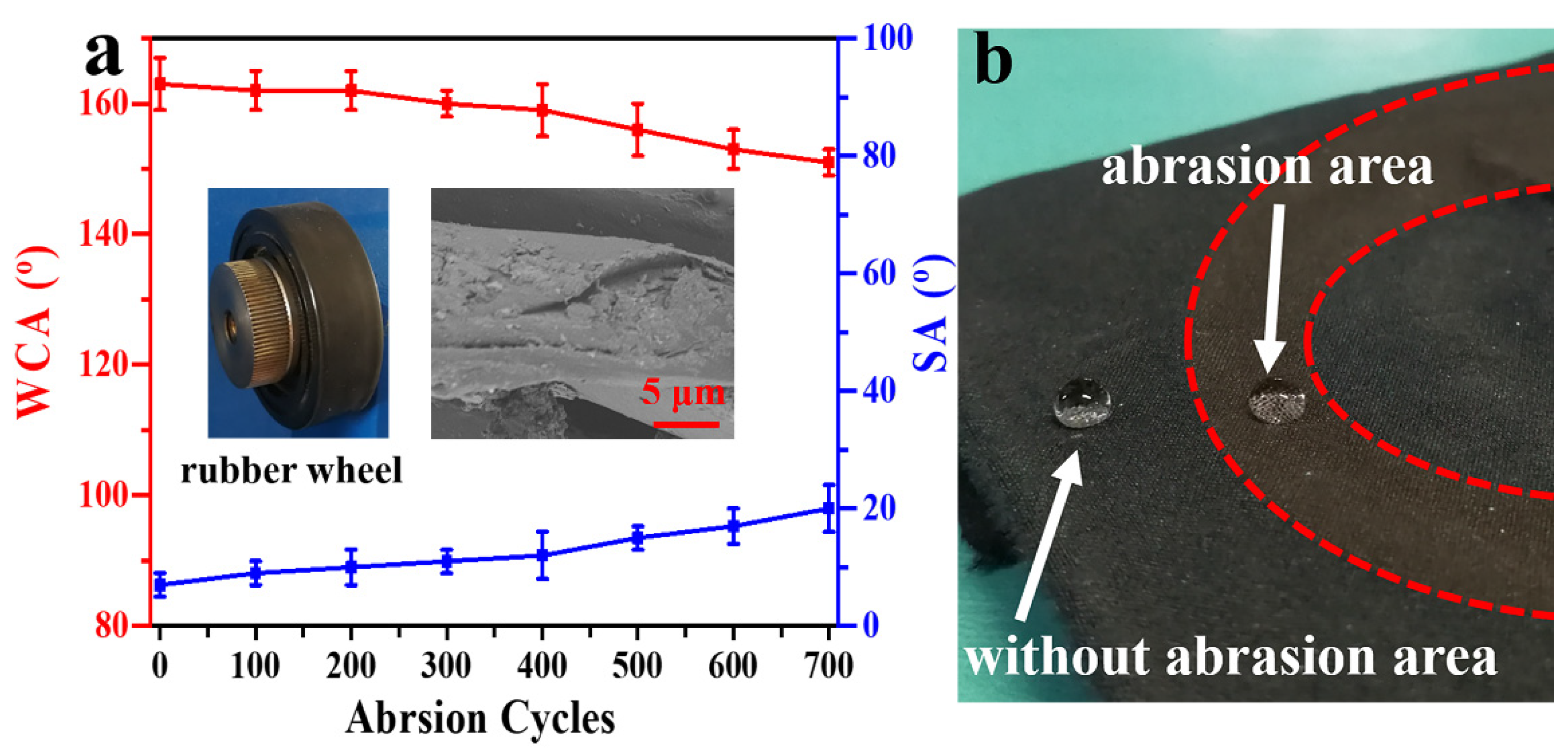
2.5. Abrasion Analysis and UV Resistance Test
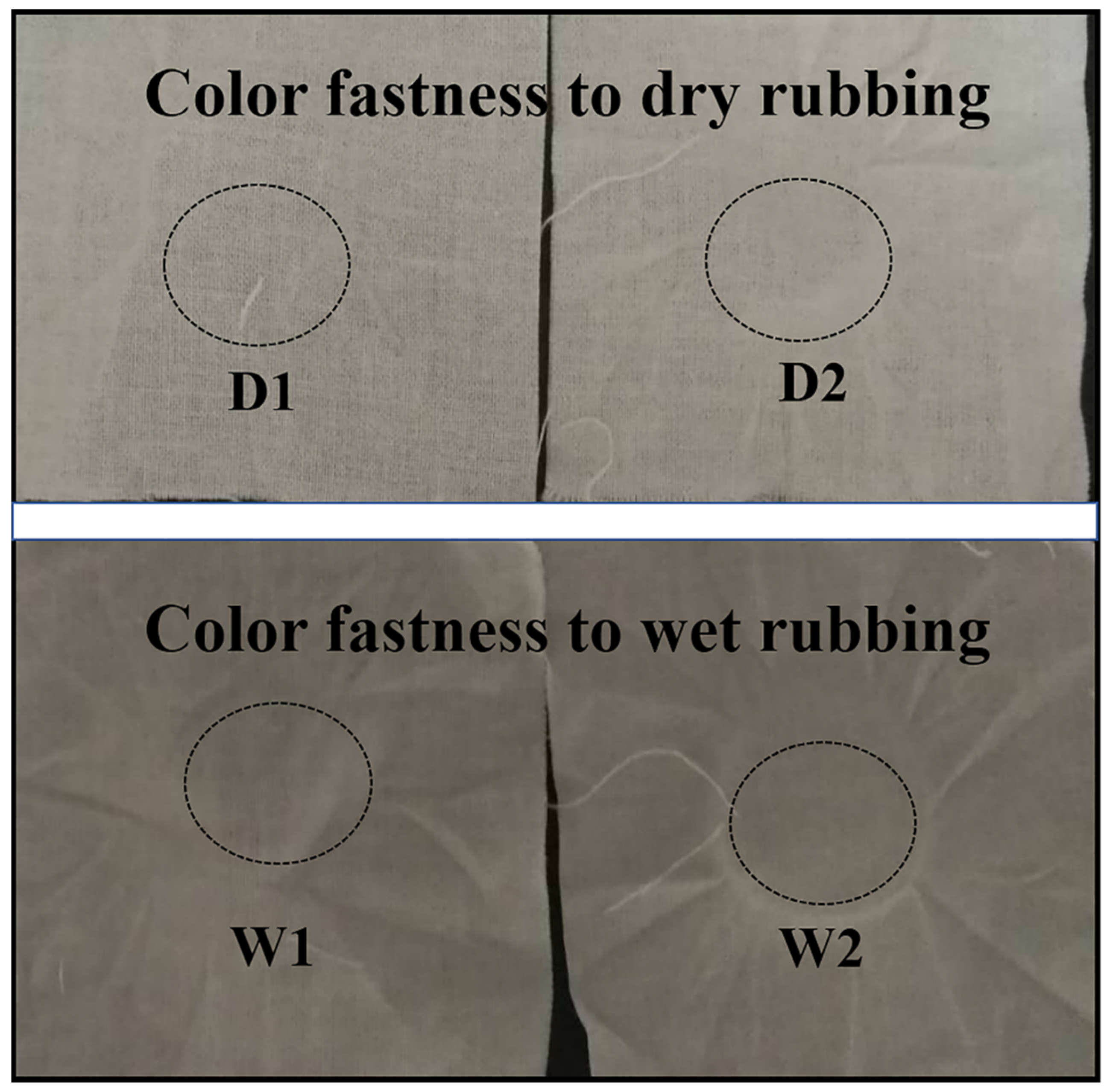
2.6. Effect of Coating on the Color Fastness of the Fabric
2.7. Characterization and Instruments
3. Results and Discussion
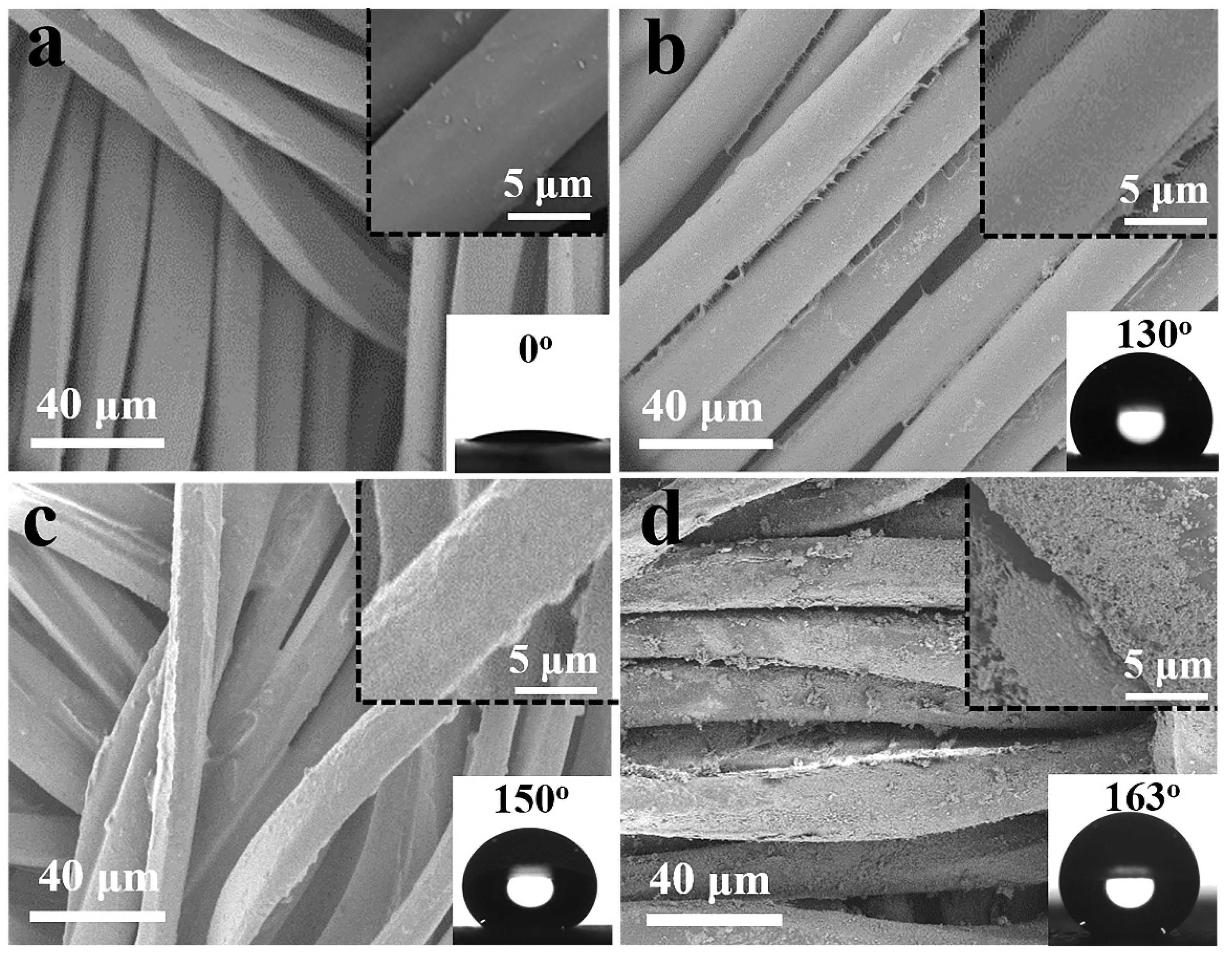
3.1. Fabrication and Characterization of Hydrophobic Fabrics
3.2. Superhydrophobicity of the PDMS/STA/SiO2-Coated Fabric
3.3. Self-Cleaning Property of the PDMS/STA/SiO2-Coated Fabric
3.4. UV Resistance of the PDMS/STA/SiO2-Coated Fabric
3.5. Effect of PDMS/STA/SiO2 Coating on the Color Fastness of the Fabric
3.6. Mechanical Stability of the PDMS/STA/SiO2-Coated Fabric
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laroche, A.; Ritzen, L.; Guillen, J.A.M.; Vercillo, V.; D’Acunzi, M.; Sharifi Aghili, A.; Hussong, J.; Vollmer, D.; Bonaccurso, E. Durability of Superamphiphobic Polyester Fabrics in Simulated Aerodynamic Icing Conditions. Coatings 2020, 10, 1058. [Google Scholar] [CrossRef]
- Lv, J.; Kong, X.; Zhu, C.; Zhang, J.; Feng, J. Robust, infrared-reflective, superhydrophobic and breathable coatings on polyester fabrics. Prog. Org. Coat. 2020, 147, 104664–104668. [Google Scholar] [CrossRef]
- Zimmermann, J.; Reifler, F.A.; Fortunato, G.; Gerhardt, L.-C.; Seeger, S. A Simple, One-Step Approach to Durable and Robust Superhydrophobic Textiles. Adv. Funct. Mater. 2008, 18, 3662–3669. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B.; Her, E.K. Fabrication of Superhydrophobic Surfaces with High and Low Adhesion Inspired from Rose Petal. Langmuir 2010, 26, 8207–8217. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zheng, Y.; Zhai, J.; Jiang, L. Bioinspired Super-antiwetting Interfaces with Special Liquid-Solid Adhesion. Acc. Chem. Res. 2010, 43, 368–377. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 234102. [Google Scholar] [CrossRef]
- Otten, A.; Herminghaus, S. How plants keep dry: A physicist’s point of view. Langmuir 2004, 20, 2405–2408. [Google Scholar] [CrossRef] [PubMed]
- Patankar, N.A. Mimicking the lotus effect: Influence of double roughness structures and slender pillars. Langmuir 2004, 20, 8209–8213. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zi, W.-W.; Xu, Z.-G.; Zhang, H.-L. Controlling the micro/nano structure of self-cleaning polymer coating. Appl. Surf. Sci. 2007, 253, 8830–8834. [Google Scholar] [CrossRef]
- Pugno, N.M. Towards a Spiderman suit: Large invisible cables and self-cleaning releasable superadhesive materials. J. Phys. Condens. Matter 2007, 19, 395001. [Google Scholar] [CrossRef]
- Shang, Y.; Si, Y.; Raza, A.; Yang, L.; Mao, X.; Ding, B.; Yu, J. An in situ polymerization approach for the synthesis of superhydrophobic and superoleophilic nanofibrous membranes for oil-water separation. Nanoscale 2012, 4, 7847–7854. [Google Scholar] [CrossRef]
- Tang, X.; Si, Y.; Ge, J.; Ding, B.; Liu, L.; Zheng, G.; Luo, W.; Yu, J. In situ polymerized superhydrophobic and superoleophilic nanofibrous membranes for gravity driven oil-water separation. Nanoscale 2013, 5, 11657–11664. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chu, Y.; Wang, Z.; Chen, N.; Lin, L.; Liu, F.; Pan, Q. Robust superhydrophobic polyurethane sponge as a highly reusable oil-absorption material. J. Mater. Chem. A 2013, 1, 5386–5393. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Qian, H.; Xu, D.; Du, C.; Zhang, D.; Li, X.; Huang, L.; Deng, L.; Tu, Y.; Mol, J.M.C.; Terryn, H.A. Dual-action smart coatings with a self-healing superhydrophobic surface and anti-corrosion properties. J. Mater. Chem. A 2017, 5, 2355–2364. [Google Scholar] [CrossRef] [Green Version]
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic materials and coatings: A review. Prog. Org. Coat. 2015, 78, 59344–59355. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xiong, D.; Deng, Y.; Shi, Y.; Wang, K. Mechanically Robust Superhydrophobic Steel Surface with Anti-Icing, UV-Durability, and Corrosion Resistance Properties. ACS Appl. Mater. Interfaces 2015, 7, 6260–6272. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.L.; Kim, C.-J.C.J. Turning a surface superrepellent even to completely wetting liquids. Science 2014, 346, 1096–1100. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Tian, X.; Verho, T.; Ras, R.H.A. Moving superhydrophobic surfaces toward real-world applications. Science 2016, 352, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Pan, M.; Yuan, J.; Liu, H.; Song, S.; Zhu, L. A waterborne coating for robust superamphiphobic surfaces. Prog. Org. Coat. 2020, 138, 105368. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Li, B.; Zhang, J.; Wang, A. Magnetic, Durable, and Superhydrophobic Polyurethane@Fe3O4@SiO2@Fluoropolymer Sponges for Selective Oil Absorption and Oil/Water Separation. ACS Appl. Mater. Interfaces 2015, 7, 4936–4946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Seeger, S. Polyester Materials with Superwetting Silicone Nanofilaments for Oil/Water Separation and Selective Oil Absorption. Adv. Funct. Mater. 2011, 21, 4699–4704. [Google Scholar] [CrossRef]
- He, T.; Liu, X.; Wang, Y.; Wu, D.; Liu, Y.; Liu, X. Fabrication of durable hierarchical superhydrophobic fabrics with Sichuan pepper-like structures via graft precipitation polymerization. Appl. Surf. Sci. 2020, 529, 36755–36757. [Google Scholar] [CrossRef]
- Kim, S.; Oh, J.-H.; Park, C.H. Development of Energy-Efficient Superhydrophobic Polypropylene Fabric by Oxygen Plasma Etching and Thermal Aging. Polymers 2020, 12, 2756. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zeng, X.; Li, H.; Lai, X.; Wu, T. One-pot fabrication of superhydrophobic and flame-retardant coatings on cotton fabrics via sol-gel reaction. J. Colloid Interface Sci. 2019, 533, 198–206. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, Z.; Liu, W.; Zhang, D.; Zhao, H.; Wang, L.; Li, X. Superhydrophobic oligoaniline-containing electroactive silica coating as pre-process coating for corrosion protection of carbon steel. Chem. Eng. J. (Loughb. Engl.) 2018, 348, 940–951. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, T.; Guo, Q.; Qiu, F.; Yang, D.; Ou, Z. Recyclable biomass Carbon@SiO2@MnO2 aerogel with hierarchical structures for fast and selective oil-water separation. Chem. Eng. J. (Loughb. Engl.) 2018, 351, 622–630. [Google Scholar] [CrossRef]
- Gao, J.; Li, B.; Huang, X.; Wang, L.; Lin, L.; Wang, H.; Xue, H. Electrically conductive and fluorine free superhydrophobic strain sensors based on SiO2/graphene-decorated electrospun nanofibers for human motion monitoring. Chem. Eng. J. (Loughb. Engl.) 2019, 373, 298–306. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, L.; Fu, F.; Liu, X. Fabrication of fluorine-free superhydrophobic cotton fabric using fumed silica and diblock copolymer via mist modification. Prog. Org. Coat. 2020, 148, 156678. [Google Scholar] [CrossRef]
- Fu, S.; Zhou, H.; Wang, H.; Ding, J.; Liu, S.; Zhao, Y.; Niu, H.; Rutledge, G.C.; Lin, T. Magnet-responsive, superhydrophobic fabrics from waterborne, fluoride-free coatings. RSC Adv. 2018, 8, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, R.; Majhy, B.; Sen, A.K. Facile Fabrication and Characterization of a PDMS-Derived Candle Soot Coated Stable Biocompatible Superhydrophobic and Superhemophobic Surface. ACS Appl. Mater. Interfaces 2017, 9, 31170–31180. [Google Scholar] [CrossRef]
- Milionis, A.; Languasco, J.; Loth, E.; Bayer, I.S. Analysis of wear abrasion resistance of superhydrophobic acrylonitrile butadiene styrene rubber (ABS) nanocomposites. Chem. Eng. J. (Loughb. Engl.) 2015, 281, 730–738. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Guo, R.; Bjornmalm, M.; Richardson, J.J.; Li, L.; Peng, C.; Bertleff-Zieschang, N.; Xu, W.; Jiang, J.; Caruso, F. Coatings super-repellent to ultralow surface tension liquids. Nat. Mater. 2018, 17, 1040–1054. [Google Scholar] [CrossRef]
- Su, X.; Li, H.; Lai, X.; Zhang, L.; Wang, J.; Liao, X.; Zeng, X. Vapor-Liquid Sol-Gel Approach to Fabricating Highly Durable and Robust Superhydrophobic Polydimethylsiloxane@Silica Surface on Polyester Textile for Oil-Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 28089–28099. [Google Scholar] [CrossRef]
- Pan, G.; Xiao, X.; Ye, Z. Fabrication of stable superhydrophobic coating on fabric with mechanical durability, UV resistance and high oil-water separation efficiency. Surf. Coat. Technol. 2019, 360, 318–328. [Google Scholar] [CrossRef]
- Tran, V.-H.T.; Lee, B.-K. Novel fabrication of a robust superhydrophobic PU@ZnO@Fe3O4@SA sponge and its application in oil-water separations. Sci. Rep. 2017, 7, 17520–17528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.L.; Zhang, F.; Song, L.; Zeng, R.C.; Li, S.Q.; Han, E.H. Corrosion resistance of a superhydrophobic surface on micro-arc oxidation coated Mg-Li-Ca alloy. J. Alloys Compd. 2017, 728, 815–826. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Y.; Zhang, Q.; Wang, K.; Carmalt, C.J.; Parkin, I.P.; Zhang, Z.; Zhang, X. Durable fire retardant, superhydrophobic, abrasive resistant and air/UV stable coatings. J. Colloid Interface Sci. 2021, 582, 301–311. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, H.; Qiu, J. Fabrication of biomimetic robust self-cleaning superhydrophobic wood with canna-leaf-like micro/nanostructure through morphgenetic method improved water-, UV-, and corrosion resistance properties. J. Mol. Struct. 2020, 1219, 128616. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Tenjimbayashi, M.; Samitsu, S.; Naito, M. Durable and Flexible Superhydrophobic Materials: Abrasion/Scratching/Slicing/Droplet Impacting/Bending/Twisting-Tolerant Composite with Porcupinefish-Like Structure. ACS Appl. Mater. Interfaces 2019, 11, 32381–32389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Y.; Tan, J.; Fan, X.; Liu, Y.; Gu, J.; Zhang, B.; Zhang, H.; Zhang, Q. Robust, self-healing, superhydrophobic coatings highlighted by a novel branched thiol-ene fluorinated siloxane nanocomposites. Compos. Sci. Technol. 2016, 137, 78–86. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Gu, Y.; Mi, T.; Wang, X.; Zhang, X. Dip-Coating Approach to Fabricate Durable PDMS/STA/SiO2 Superhydrophobic Polyester Fabrics. Coatings 2021, 11, 326. https://doi.org/10.3390/coatings11030326
Liu X, Gu Y, Mi T, Wang X, Zhang X. Dip-Coating Approach to Fabricate Durable PDMS/STA/SiO2 Superhydrophobic Polyester Fabrics. Coatings. 2021; 11(3):326. https://doi.org/10.3390/coatings11030326
Chicago/Turabian StyleLiu, Xiaoli, Youcai Gu, Tengfei Mi, Xiaomei Wang, and Xu Zhang. 2021. "Dip-Coating Approach to Fabricate Durable PDMS/STA/SiO2 Superhydrophobic Polyester Fabrics" Coatings 11, no. 3: 326. https://doi.org/10.3390/coatings11030326