High-Temperature Corrosion Behavior of Al-Coated Ni-Base Alloys in Lithium Molten Salt for Electroreduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Specimen
2.2. Coating Formulation
2.3. High-Temperature Corrosion Tests
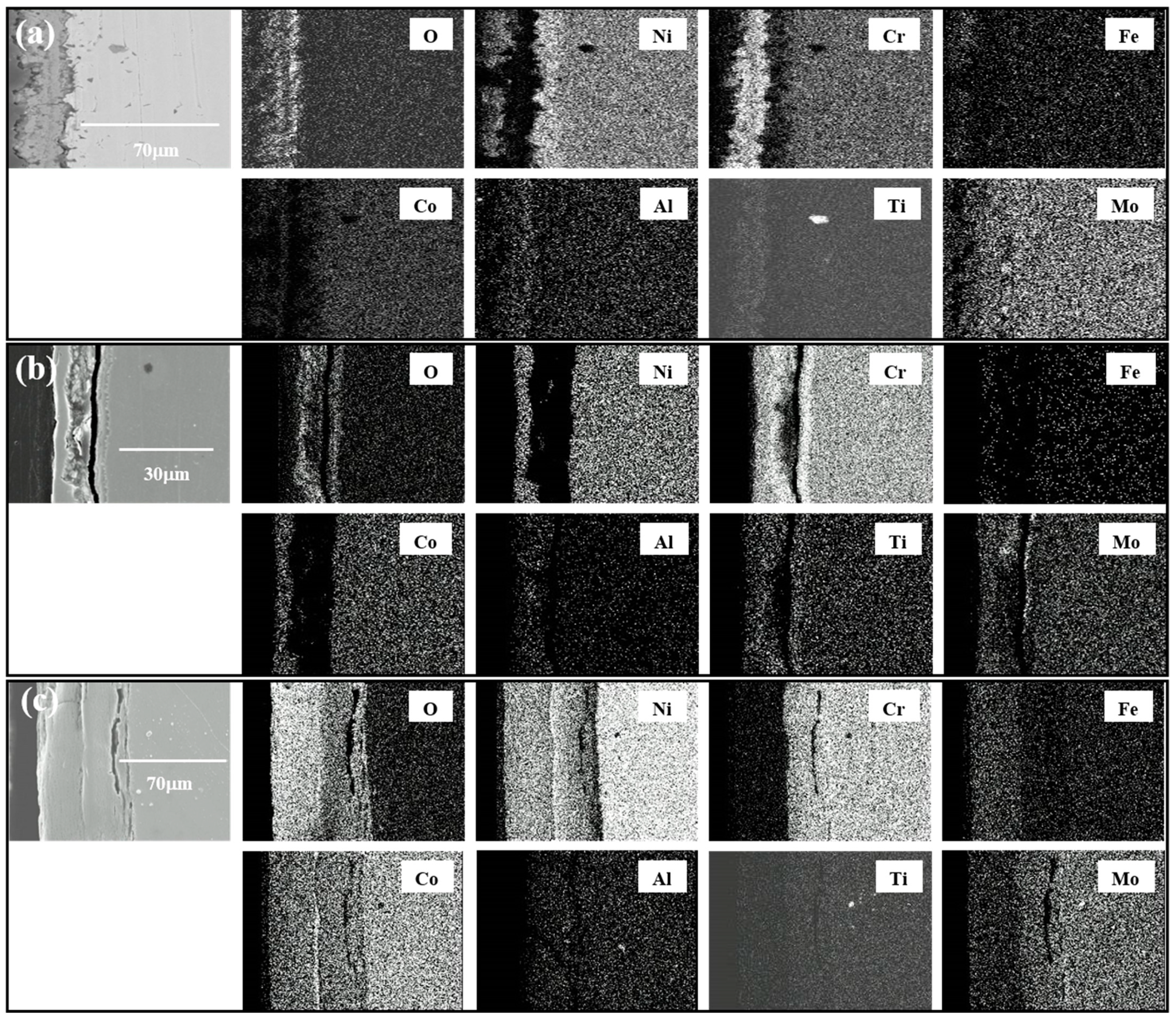
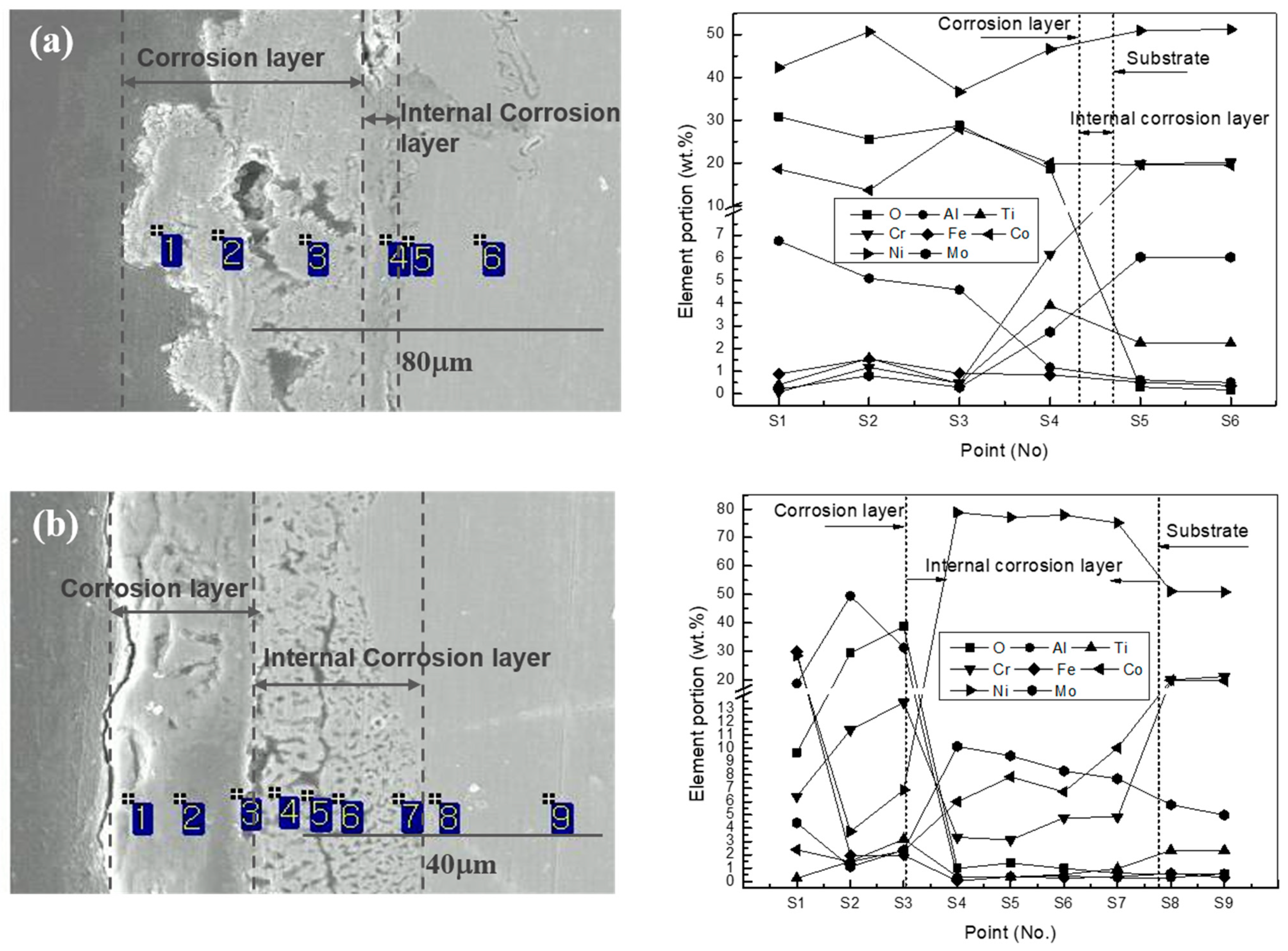
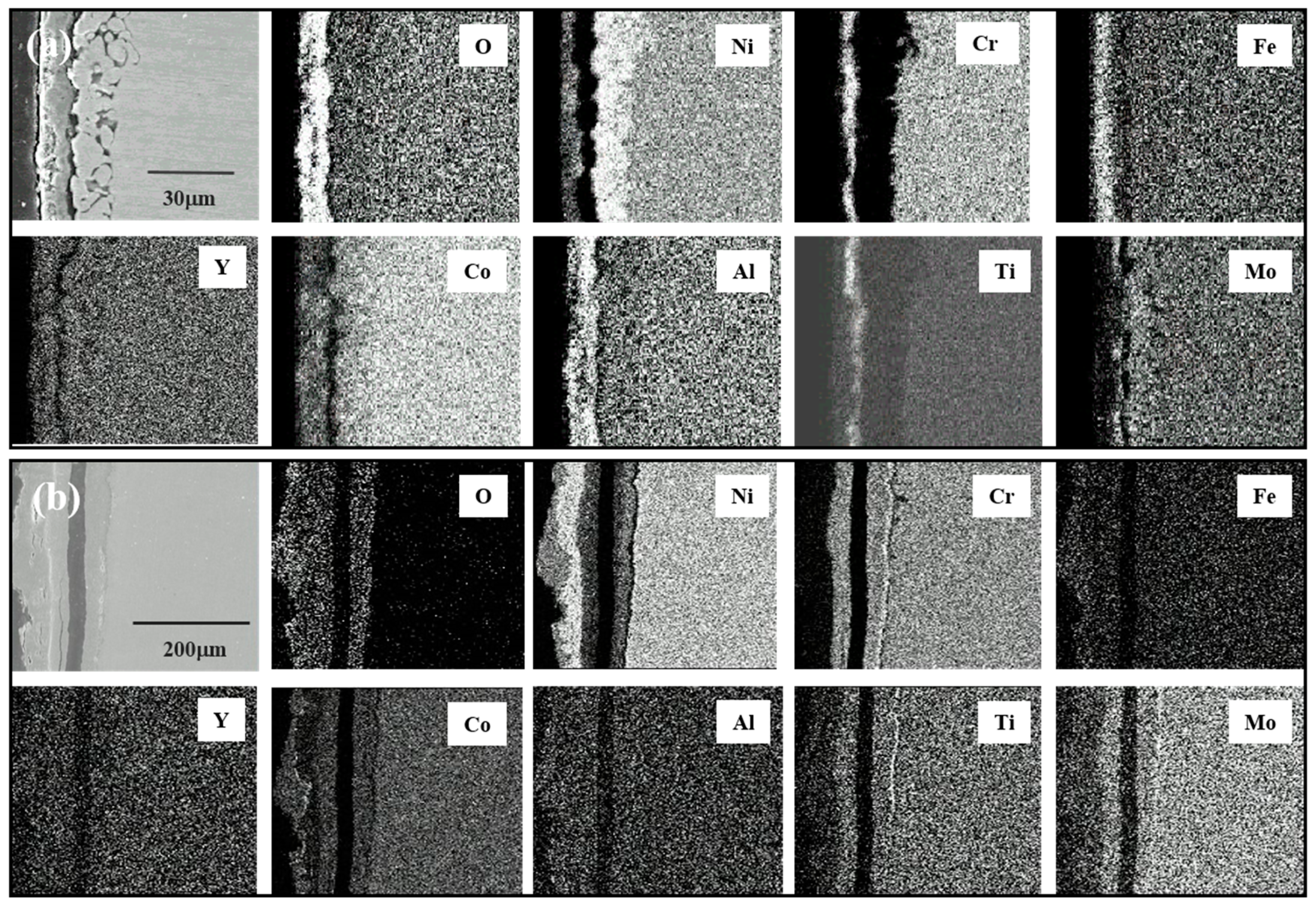
2.4. Characterization
3. Results and Discussion
3.1. Corrosion Kinetics
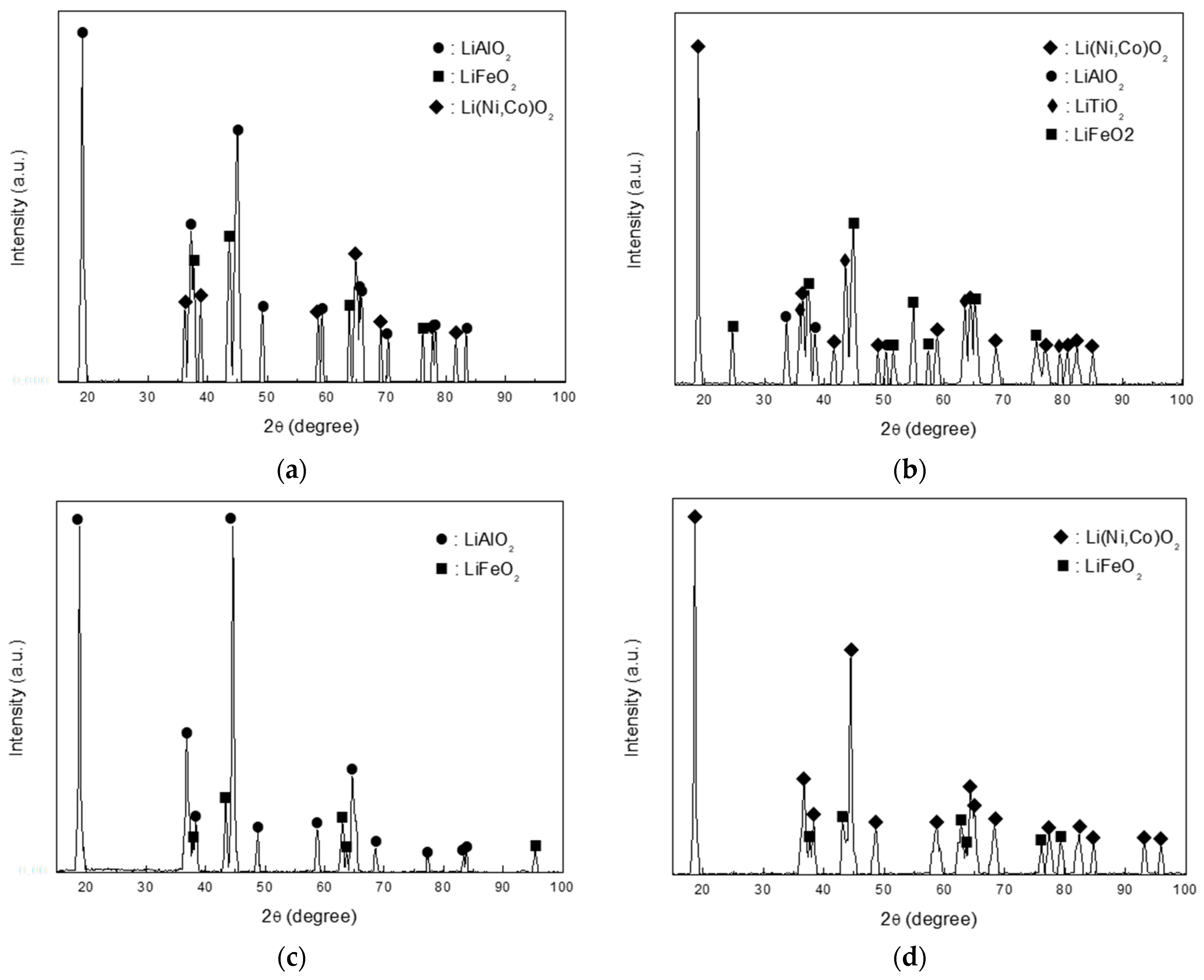
3.2. Phase of Corrosion Products
3.3. Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, K.; Smith, N.D.; Lichtenstein, T.; Kim, H.J. Electrochemical Studies of Molten Sulfates in LiCl-KCl-Na2SO4 at 700 °C. Corros. Sci. 2018, 133, 17–24. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, J.G.; Haro, S.; Martinez-Villafañe, A.; Salinas-Bravo, V.M.; Porcayo-Calderon, J. Corrosion Performance of Heat Resistant Alloys in Na2SO4-V2O5 Molten Salts. Mater. Sci. Eng. A 2006, 435–436, 258–265. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, S.; Xu, D.; Li, W.; Yan, J. Evolution of Microstructure and Hot Corrosion Performance of La2Hf2O7 Ceramic in Contact with Molten Sulfate-Vanadate Salt. Ceram. Int. 2018, 44, 2048–2057. [Google Scholar] [CrossRef]
- Garip, Y.; Garip, Z.; Ozdemir, O. Prediction modeling of Type-I hot corrosion performance of Ti-Al-Mo-X (X=Cr, Mn) alloys in (Na, K)2SO4 molten salt mixture environment at 900 °C. J. Alloys Compd. 2020, 843, 156010. [Google Scholar] [CrossRef]
- Garip, Y.; Ozdemir, O. Corrosion Behavior of the Resistance Sintered TiAl Based Intermetallics Induced by Two Different Molten Salt Mixture. Corros. Sci. 2020, 174, 108819. [Google Scholar] [CrossRef]
- Song, S.A.; Kim, H.T.; Kim, K.Y.; Lim, S.N.; Yoon, S.P.; Jang, S.C. Effect of LiNO2-Coated Cathode on Cell Performance in Molten Carbonate Fuel Cell. Int. J. Hydr. Energy 2019, 44, 12085–12093. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, C.W.; Ham, H.C.; Han, J.H.; Yoon, S.P.; Lee, K.B. Mechanical Strength Improvement of Aluminum Foam-Reinforced Matrix for Molten Carbonate Fuel Cells. Int. J. Hydr. Energy 2017, 42, 16235–16243. [Google Scholar] [CrossRef]
- Sher, F.; Al-Shara, N.K.; Iqbal, S.Z.; Jahan, Z.; Chen, G.Z. Enhancing Hydrogen Production From Steam Electrolysis in Molten Hydroxides via Selection of Non-Precious Metal Electrodes. Int. J. Hydr. Energy 2020, 45, 28260–28271. [Google Scholar] [CrossRef]
- Rehman, A.; Bidabadi, M.H.S.; Liang, Y.; Yu, Z.; Zhang, C.; Chen, H.; Yang, Z.-G. Hot Corrosion Behaviour of Yttrium and Aluminium Modified Wear Resistance Coating Alloy in Mixed Sulphate at 900 °C. Corros. Sci. 2020, 165, 108369. [Google Scholar] [CrossRef]
- Grosu, Y.; Anagnostopoulos, A.; Navarro, M.E.; Ding, Y.; Faik, A. Inhibiting Hot Corrosion of Molten Li2CO3-Na2CO3-K2CO3 Salt through Graphitization of Construction Materials for Concentrated Solar Powder. Sol. Energy Mater. Sol. Cells 2020, 215, 110650. [Google Scholar] [CrossRef]
- Fernandez, A.G.; Cabeza, L.F. Corrosion Evaluation of Eutectic Chloride Molten Salt for New Generation of CSP Plants. Part 2: Materials Screening Performance. J. Energy Storage 2020, 29, 101381. [Google Scholar] [CrossRef]
- Indacochea, J.E.; Smith, J.L.; Litko, K.R.; Karell, E.J.; Rarez, A.G. High-Temperature Oxidation and Corrosion of Structural Materials in Molten Chlorides. Oxid. Met. 2001, 55, 1–16. [Google Scholar] [CrossRef]
- Chen, G.Z.; Fray, D.J.; Farthing, T.W. Direct Electrochemical Reduction of Titanium Dioxide to Titanium in Molten Calcium Chloride. Nature 2000, 407, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Ruh, A.; Spiegel, M. Thermodynamic and Kinetic Consideration on the Corrosion of Fe, Ni and Cr Beneath a Molten KCl-ZnCl2 Mixture. Corros. Sci. 2006, 48, 679–695. [Google Scholar] [CrossRef]
- Fang, Y.; Cui, X.; Jin, G.; Liu, E.; Zhang, D.; Wen, X.; Mi, Q. Microstructural Evolution and Hot Corrosion Behavior of La0.8Ba0.2Ti3-δ-YSZ Double-Layer Thermal Barrier Coatings in Na2SO4 + V2O5 Molten Salt at 900 °C. Surf. Coat. Technol. 2020, 399, 126175. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Jiang, Z.; Zhang, B.; Li, Z.; Wu, J.; Feng, H.; Zhu, H.; Duan, F. Chloride- and Sulphate-Induced Hot Corrosion Mechanism of Super Austenitic Stainless Steel S31254 Under Dry Gas Environment. Corros. Sci. 2020, 163, 108295. [Google Scholar] [CrossRef]
- Zhuang, X.; Liu, W.; Xu, X. Hot Corrosion of Different Alloys in Chloride and Carbonate Molten-Salt Mixture Under Argon Atmosphere. Sol. Energy 2019, 189, 254–267. [Google Scholar] [CrossRef]
- Zhu, B.; Lindbergh, G. Corrosion Behavior of High-Chromium Ferritic Steels in Molten Carbonate in Cathode Environment. Electrochim. Acta 2001, 46, 2593–2604. [Google Scholar] [CrossRef]
- Lei, G.; Li, C.; Jiang, Z.; Huang, H. Irradiation Accelerated Fluoride Molten Salt Corrosion of Nickel-Based UNS N10003 Alloy Revealed by X-Ray Absorption Fine Structure. Corros. Sci. 2020, 165, 108408. [Google Scholar] [CrossRef]
- Wu, Y.; Leng, B.; Li, X.; Jiang, L.; Ye, X.; Chen, Y.; Yang, X.; Li, Z.; Zhou, X. Corrosion Behavior of a Wear Resistant Co-Mo-Cr-Si Alloy in Molten Fluoride Salts. J. Nucl. Mater. 2020, 542, 152529. [Google Scholar] [CrossRef]
- Doniger, W.H.; Falconer, C.; Elbakhshwan, M.; Britsch, K.; Couet, A.; Sridharan, K. Investigation of Impurity Driven Corrosion Behavior in Molten 2LiF-BeF2 Salt. Corros. Sci. 2020, 174, 108823. [Google Scholar] [CrossRef]
- McAlpine, S.W.; Skowronski, N.C.; Zhou, W.; Zheng, G.; Short, M.P. Corrosion of Commercial Alloys in FLiNaK Molten Salt Containing EuF3 and Simulant Fission Product Additives. J. Nucl. Mater. 2020, 532, 151994. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Liu, W.; Yin, H.; Tang, Z.; Qian, Y. Ni-Mo-Cr Alloy Corrosion in Molten NaCl-KCl-MgCl2 Salt and Vapour. Corros. Sci. 2021, 23, 180. [Google Scholar] [PubMed]
- Li, X.L.; He, S.M.; Zhou, X.T.; Huai, P.; Li, Z.J.; Li, A.G.; Yu, X.H. High-Temperature Corrosion Behavior of Ni-16Mo-7Cr-4Fe Superalloy Containing Yttrium in Molten LiF-NaF-KF Salt. J. Nucl. Mater. 2015, 464, 342–345. [Google Scholar] [CrossRef]
- Sidhu, T.S.; Prakash, S.; Agrawal, R.D. Evaluation of Hot Corrosion Resistance of HVOF Coatings on a Ni-Based Superalloy in Molten Salt Environment. Mater. Sci. Eng. A 2006, 430, 64–78. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Zou, B.; Zhou, X.; Dong, S.; Li, L.; Jiang, J.; Deng, L.; Cao, X. Hot Corrosion Behavior of Nanostructured Zirconia in Molten NaVO3 Salt. Ceram. Int. 2017, 43, 10415–10427. [Google Scholar] [CrossRef]
- Mohammadi Zahrani, E.; Alfantazi, A.M. High Temperature Corrosion and Electrochemical Behavior of INCONEL 625 Weld Overlay in PbSO4-Pb3O4-PbCl2-CdO-ZnO Molten Salt Medium. Corros. Sci. 2014, 85, 60–76. [Google Scholar] [CrossRef]
- Ai, H.; Ye, X.-X.; Jiang, L.; Leng, B.; Shen, M.; Li, Z.; Jia, Y.; Wang, J.-Q.; Zhou, X.; Xie, Y.; et al. On the Possibility of Severe Corrosion of a Ni-W-Cr Alloy in Fluoride Molten Salts at High Temperature. Corros. Sci. 2019, 149, 218–225. [Google Scholar] [CrossRef]
- Wang, X.; Xin, L.; Wang, F.; Zhu, S.; Wei, H.; Wang, X. Influence of Sputtered Nanocrystalline Coating on Oxidation and Hot Corrosion of a Nickel-Based Superalloy M951. J. Mater. Sci. Technol. 2014, 30, 867–877. [Google Scholar] [CrossRef]
- Mahini, S.; Asl, S.K.; Rabizadeh, T.; Aghajani, H. Effects of the Pack Al Content on the Microstructure and Hot Corrosion Behavior of Aluminide Coatings Applied on Inconel-600. Surf. Coat. Technol. 2020, 397, 125949. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Lee, S.K.; Kim, D.Y.; Lee, J.H.; Hur, J.M. Effects of Alloying Elements of Nickel-Based Alloys on the Hot-Corrosion Behavior in an Electrolytic Reduction Process. J. Alloys Compd. 2017, 695, 2878–2885. [Google Scholar] [CrossRef]
- Du, K.; Yu, R.; Gao, M.; Chen, Z.; Mao, X.; Zhu, H.; Wang, D. Durability of Platinum Coating Anode in Molten Carbonate Electrolysis Cell. Corros. Sci. 2019, 153, 12–18. [Google Scholar] [CrossRef]
- Kim, M.J.; Youn, J.Y.; Lim, J.H.; Eom, K.S.; Cho, E.A.; Kwon, H.S. Corrosion-Resistant Coating for Cathode Current Collector and Wet-Seal Area of Molten Carbonate Fuel Cells. Int. J. Hydr. Energy 2018, 43, 11363–11371. [Google Scholar] [CrossRef]
- Bradshaw, A.; Simms, N.J.; Nicholls, J.R. Development of Hot Corrosion Resistant Coatings for Gas Turbines Burning Biomass and Waste Derived Fuel Gases. Surf. Coat. Technol. 2013, 216, 8–22. [Google Scholar] [CrossRef]
- Bradshaw, A.; Simms, N.J.; Nicholls, J.R. Hot Corrosion Tests on Corrosion Resistant Coatings Developed for Gas Turbines Burning Biomass and Waste Derived Fuel Gases. Surf. Coat. Technol. 2013, 228, 248–257. [Google Scholar] [CrossRef]
- Vakilifard, H.; Ghasemi, R.; Rahimipour, M. Hot Corrosion Behavior of Plasma-Sprayed Functionally Graded Thermal Barrier Coatings in the Presence of Na2SO4 + V2O5 Molten Salt. Surf. Coat. Technol. 2017, 326, 238–246. [Google Scholar] [CrossRef]
- Gurrappa, I.; Sambasiva Rao, A. Thermal Barrier Coatings for Enhanced Efficiency of Gas Turbine Engines. Surf. Coat. Technol. 2006, 201, 3016–3029. [Google Scholar] [CrossRef]
- Cong, D.; Li, Z.; He, Q.; Chen, H.; Zhao, Z.; Zhang, L.; Wu, H. Wear Behavior of Corroded Al-Al2O3 Composite Coatings Prepared by Cold Spray. Surf. Coat. Technol. 2017, 326, 247–254. [Google Scholar] [CrossRef]
- Strangman, T.; Raybould, D.; Jameel, A.; Baker, W. Damage Mechanisms, Life Prediction, and Development of EB-PVD Thermal Barrier Coatings for Turbine Airfoils. Surf. Coat. Technol. 2007, 202, 658–664. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Yu, Y.; Wen, B.; Liu, Y.; Qiu, M. Tunable Electromagnetic Interference Shielding Ability in a One-Dimensional Bagasse Fiber/Polyaniline Heterostructure. ACS Appl. Polym. Mater. 2019, 1, 737–745. [Google Scholar] [CrossRef]
- Saini, P.; Choudhary, V.; Vijayan, N.; Kotnala, R.K. Improved Electromagnetic Interference Shielding Response of Poly(Aniline)-Coated Fabrics Containing Dielectric and Magnetic Nanoparticles. J. Phys. Chem. C 2012, 116, 13403–13412. [Google Scholar] [CrossRef]
- Allanore, A.; Yin, L.; Sadoway, D.R. A New Anode Material for Oxygen Evolution in Molten Oxide Electrolysis. Nature 2013, 497, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Adam, D. Aluminium Producers Promise a Cleaner Smelting Pot. Nature 2018, 557, 280. [Google Scholar]
- Barthelemy, C.; Marmottant, A.; Laurent, V.; Bouvet, S.; Stabrowski, V.U.S. Cermet electrode material. Patent No. 0073109 A1, 15 March 2018. [Google Scholar]
- Fernández, A.G.; Cabeza, L.F. Anodic Protection Assessment Using Alumina-Forming Alloys in Chloride Molten Salt for CSP, Plants. Coatings 2020, 10, 138. [Google Scholar] [CrossRef] [Green Version]
- Jubeen, F.; Liaqat, A.; Sultan, M.; Iqbal, S.Z.; Sajid, I.; Sher, F. Green Synthesis and Biological Evaluation of novel 5-Fluorouracil Derivatives as Potent Anticancer Agents. Saudi Pharm. J. 2019, 27, 1164–1173. [Google Scholar] [CrossRef]
- Shera, F.; Iqbal, S.Z.; Albazzaz, S.; Ali, U.; Mortari, D.A.; Rashid, T. Development of Biomass Derived Highly Porous Fast Adsorbents for Postcombustion CO2 Capture. Fuel 2020, 282, 118506. [Google Scholar] [CrossRef]
- Goebel, J.A.; Pettit, F.S.; Goward, G.W. Mechanism for the Hot Corrosion of Nickel-Base Alloys. Met. Translator 1971, 2, 2875–2883. [Google Scholar] [CrossRef]
- Rapp, R.A. Hot Corrosion of Materials: A Fluxing Mechanism? Corros. Sci. 2002, 44, 209–221. [Google Scholar] [CrossRef]
- Zeng, S.; Zhao, A.; Jiang, H.; Fan, X.; Duan, X.; Yan, X. Cyclic Oxidation Behavior of the Ti-6Al-4V Alloy. Oxid. Met. 2014, 81, 467–476. [Google Scholar] [CrossRef]
- Harada, Y. High Temperature Oxidation and High Temperature Corrosion of Metallic Materials. I. High Temperature Oxidation. J. Jpn. Therm. Spray. Soc. 1996, 33, 128–134. [Google Scholar]
- Izuta, H.; Komura, Y. High-Temperature Oxidation Behavior of Mechanically Alloyed Ni-Base Superalloys. J. Jpn. Inst. Met. 1994, 58, 1196–1205. [Google Scholar] [CrossRef] [Green Version]
- Turkdogan, E.T. Physical Chemistry of High Temperature Technology; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Cho, S.H.; Oh, S.C.; Park, S.B.; Ku, K.M.; Lee, J.H.; Hur, J.M.; Lee, H.S. High Temperature Corrosion Behavior of Ni-Based Alloys. Met. Mater. Int. 2012, 18, 939–949. [Google Scholar] [CrossRef]
- Tawancy, H.M. High-Temperature Oxidation Behavior of a Wrought Ni-Cr-W-Mn-Si-La Alloy. Oxid. Met. 1996, 45, 323–348. [Google Scholar] [CrossRef]
- Kim, I.H.; Jung, Y.I.; Choi, B.K.; Kim, H.G.; Jang, J.I. Corrosion and Oxidation Resistance Behaviors of Ta-Containing Low Alloying Zirconium. Met. Mater. Int. 2020. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.-B.; Choi, W.-S.; Lim, K.-S.; Cho, S.-H.; Lee, J.-H. High-Temperature Corrosion Behavior of Al-Coated Ni-Base Alloys in Lithium Molten Salt for Electroreduction. Coatings 2021, 11, 328. https://doi.org/10.3390/coatings11030328
Kim W-B, Choi W-S, Lim K-S, Cho S-H, Lee J-H. High-Temperature Corrosion Behavior of Al-Coated Ni-Base Alloys in Lithium Molten Salt for Electroreduction. Coatings. 2021; 11(3):328. https://doi.org/10.3390/coatings11030328
Chicago/Turabian StyleKim, Wan-Bae, Woo-Seok Choi, Kyu-Seok Lim, Soo-Haeng Cho, and Jong-Hyeon Lee. 2021. "High-Temperature Corrosion Behavior of Al-Coated Ni-Base Alloys in Lithium Molten Salt for Electroreduction" Coatings 11, no. 3: 328. https://doi.org/10.3390/coatings11030328





