Plasma-Enhanced Atomic Layer Deposition of Zirconium Oxide Thin Films and Its Application to Solid Oxide Fuel Cells
Abstract
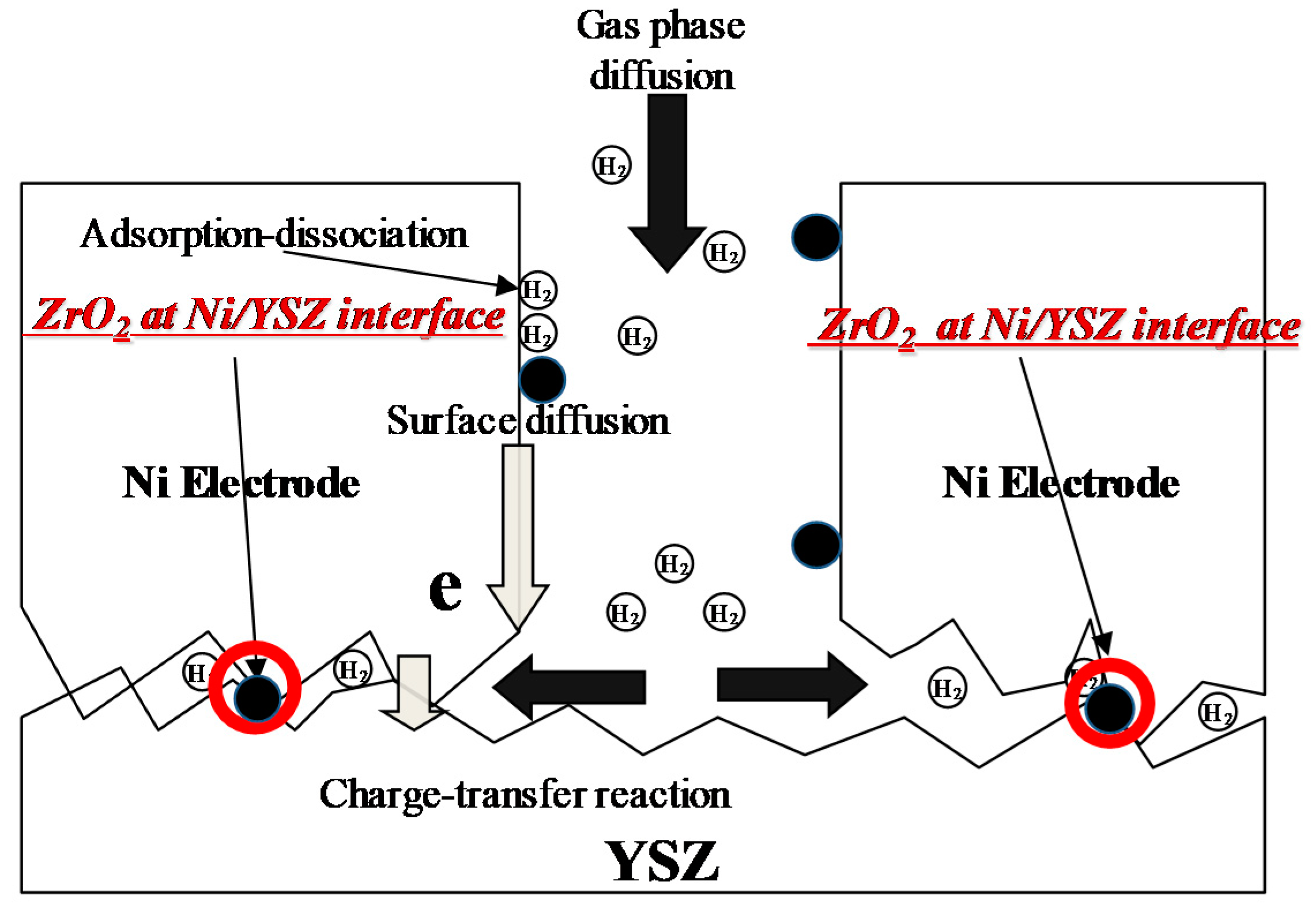
1. Introduction
2. Experimental
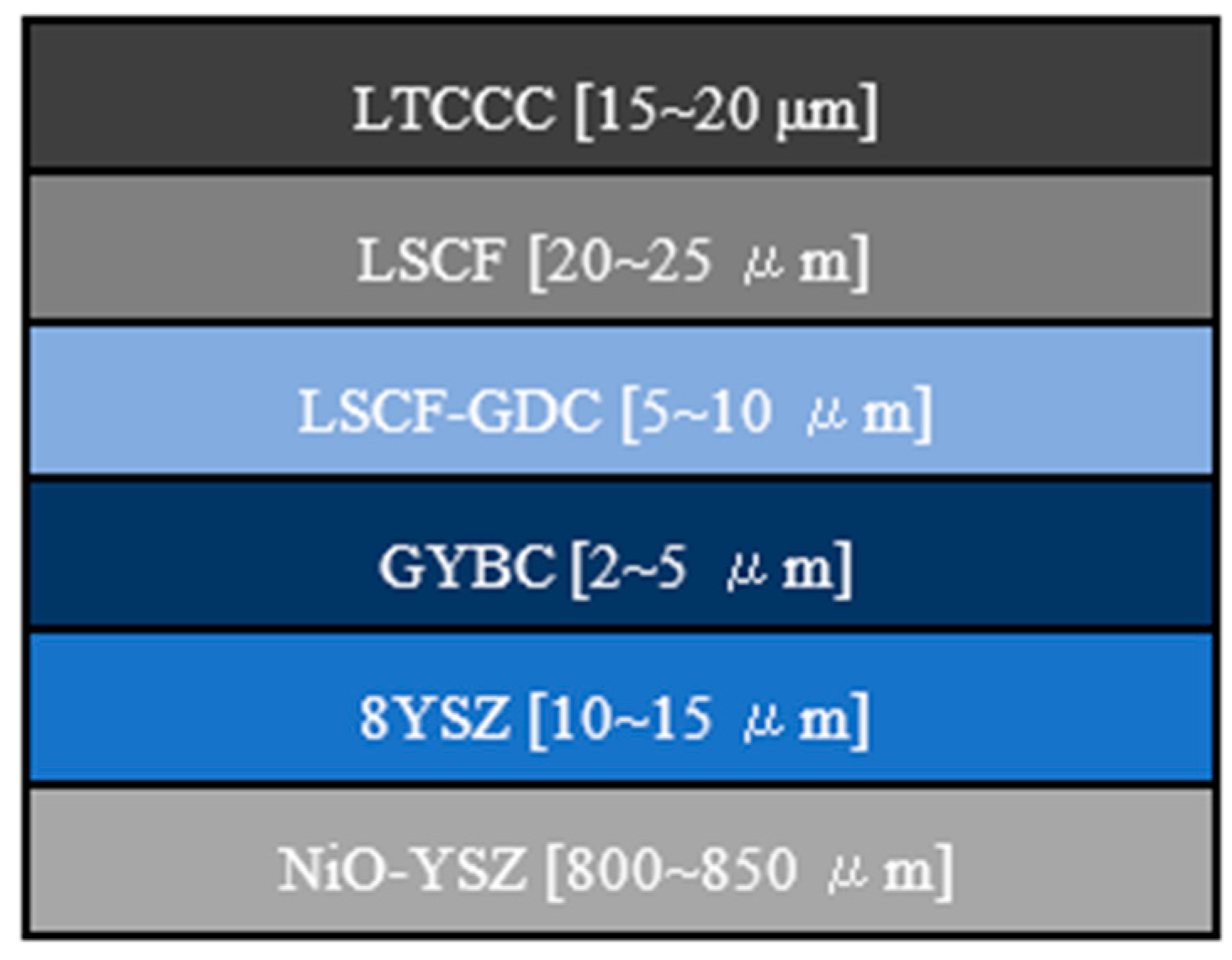
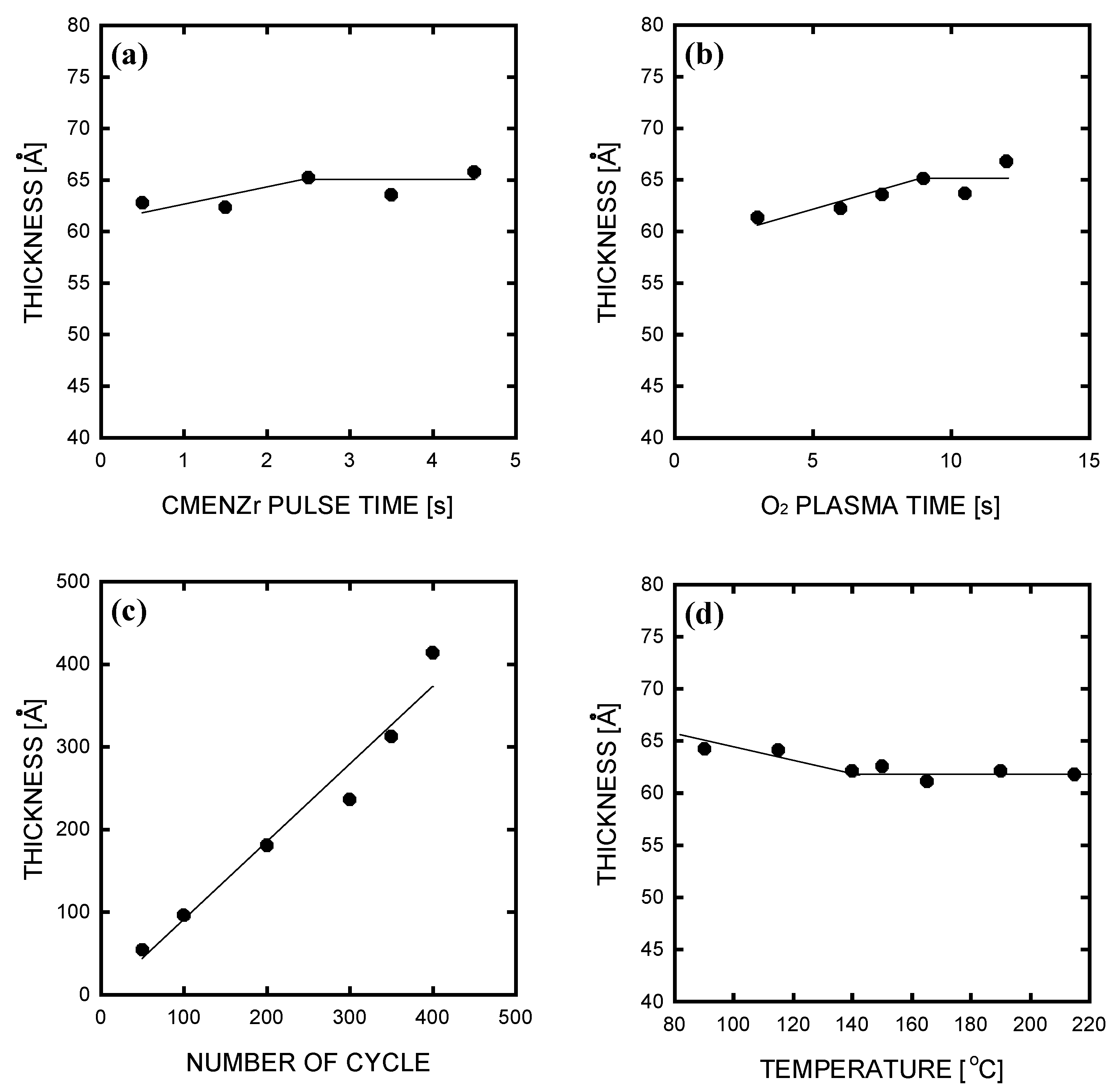
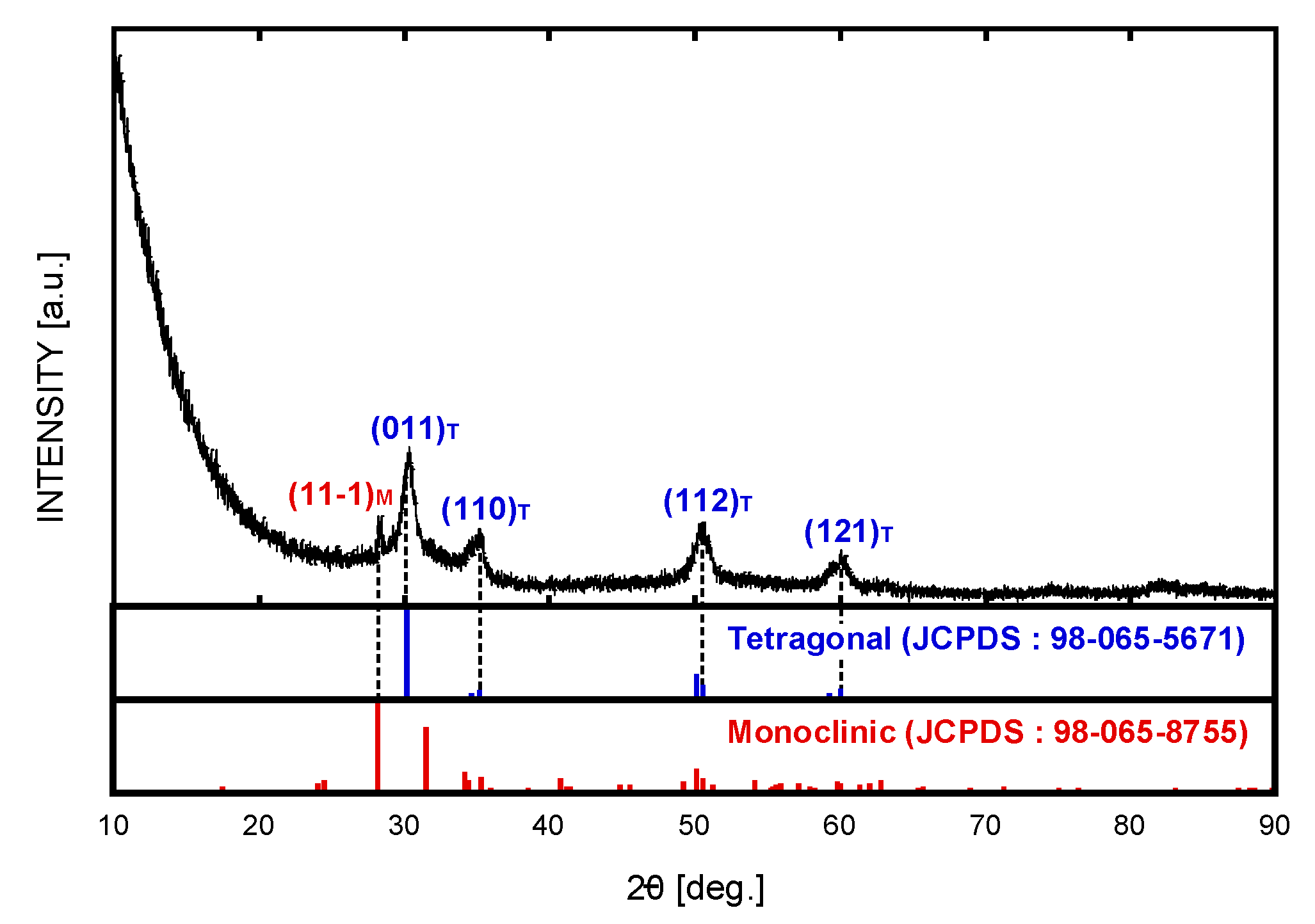
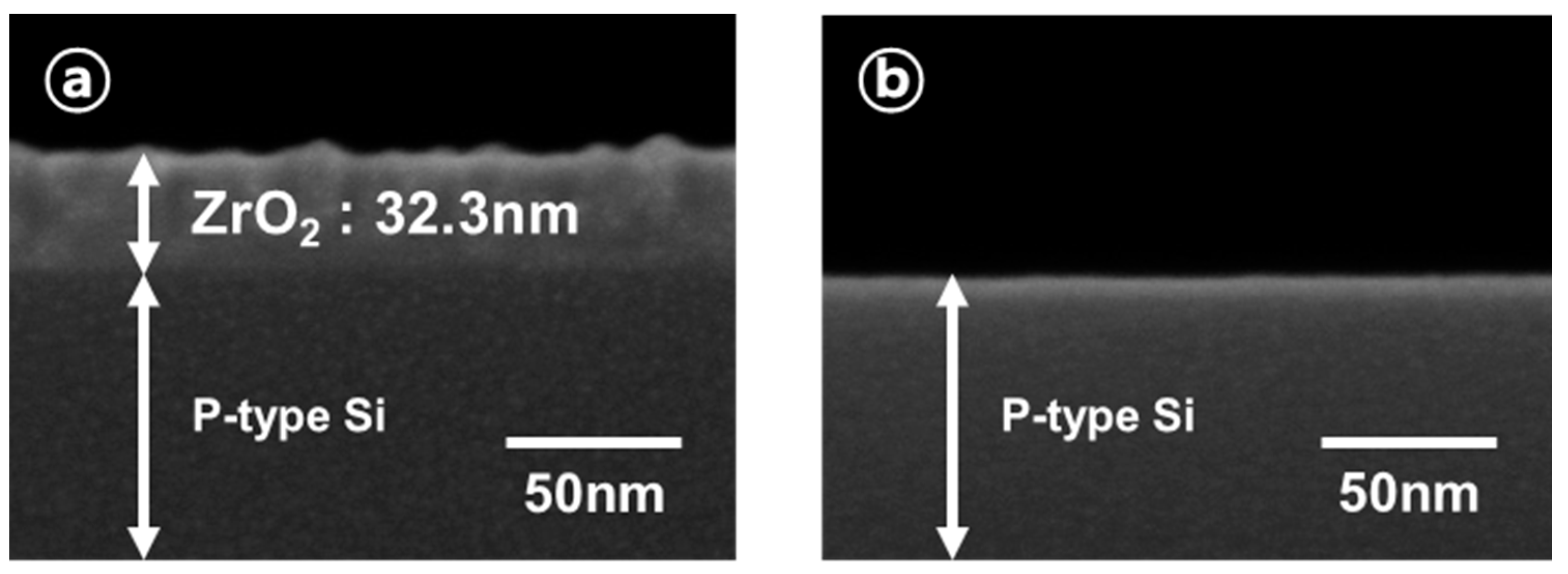
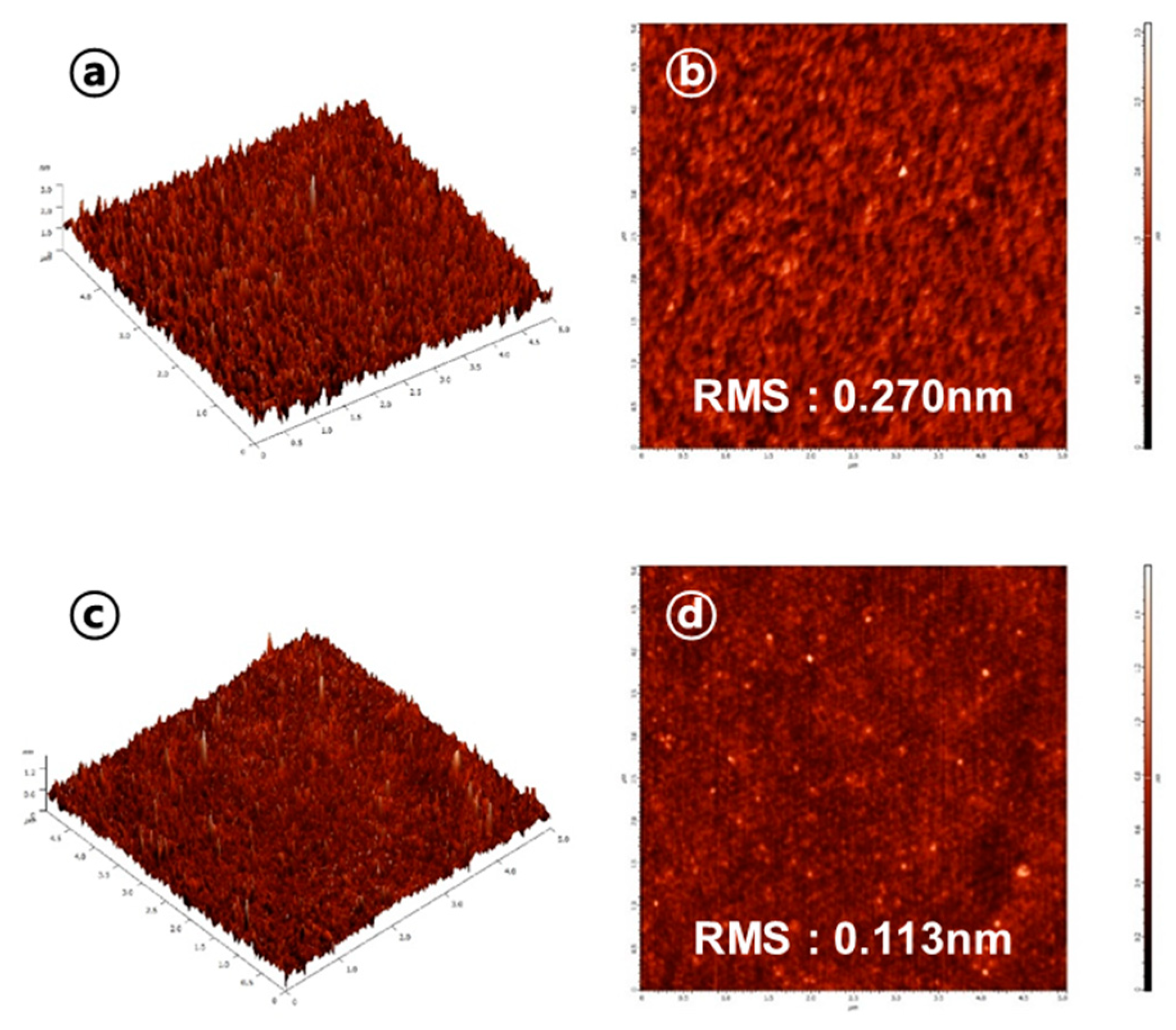
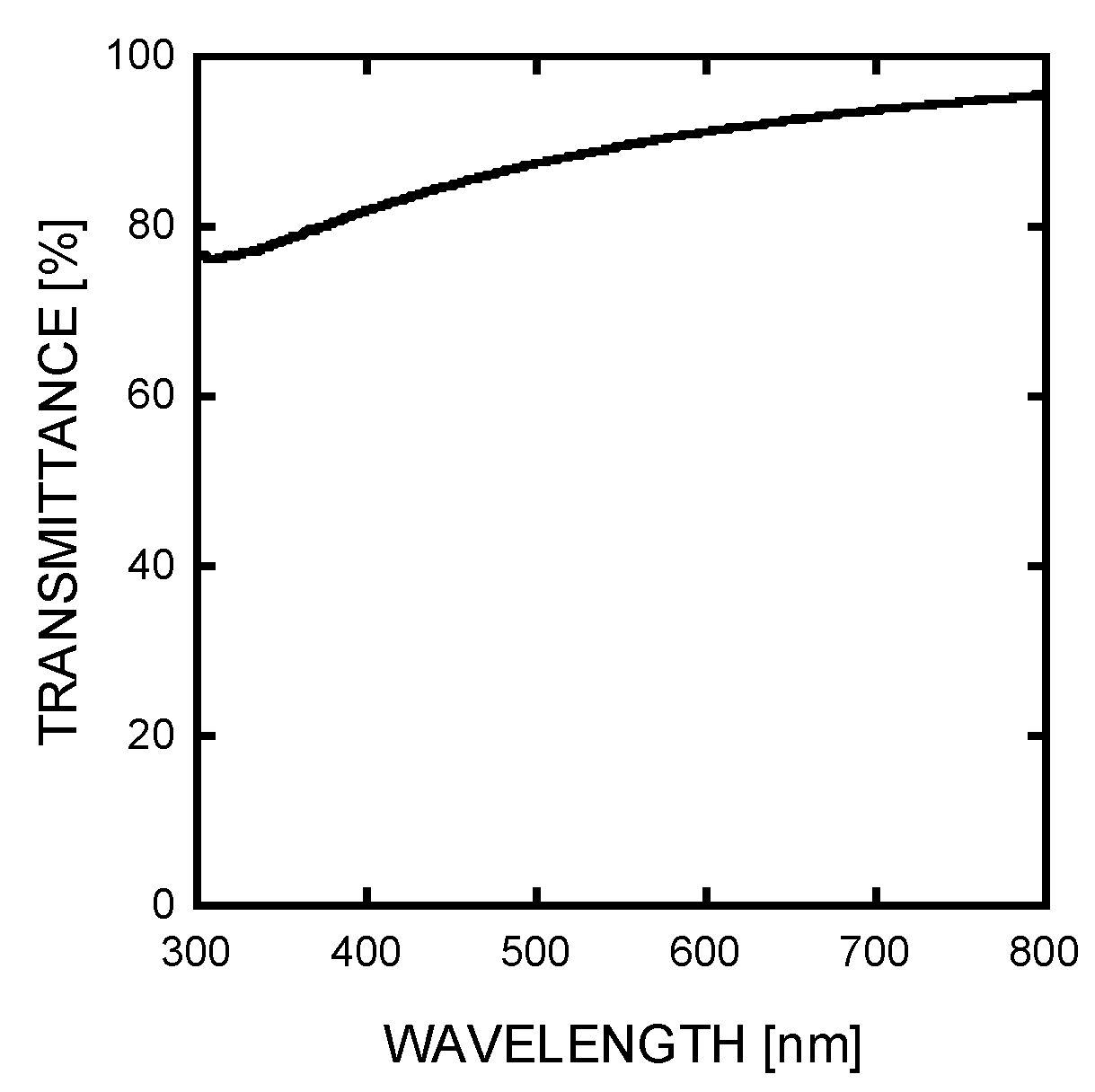
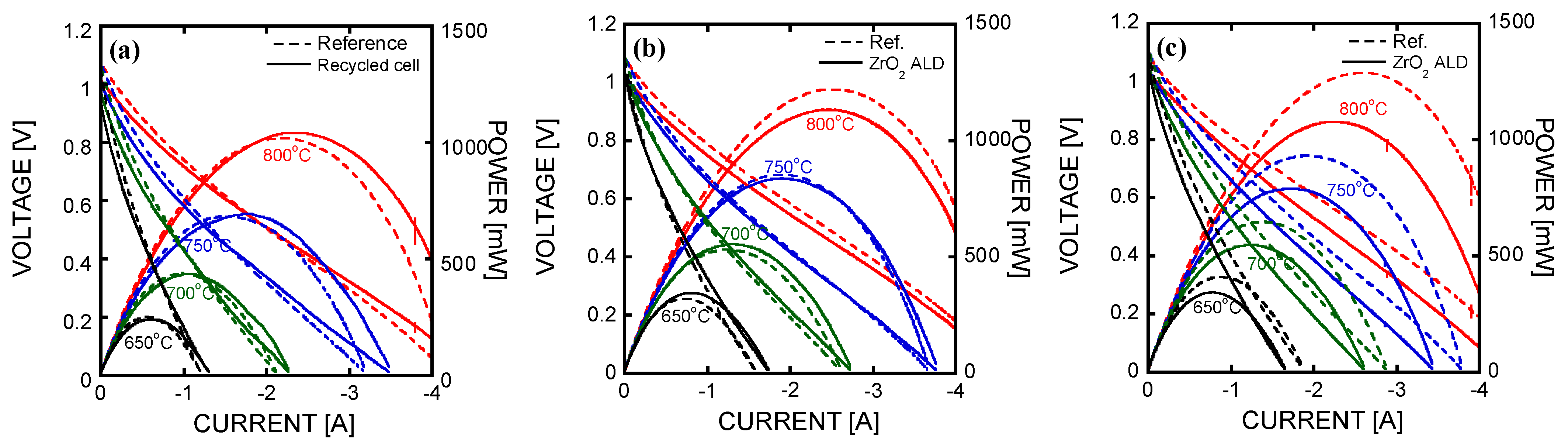
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ormerod, R.M. Solid oxide fuel cells. Chem. Soc. Rev. 2003, 32, 17–28. [Google Scholar] [CrossRef]
- Minh, N.Q. Solid oxide fuel cell technology-features and applications. Solid State Ion. 2004, 174, 271–277. [Google Scholar] [CrossRef]
- Jacobson, A.J. Materials for solid oxide fuel cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Yamamoto, O. Solid oxide fuel cells: Fundamental aspects prospects. Electrochim. Acta 2000, 45, 2423–2435. [Google Scholar] [CrossRef]
- Zhu, W.; Ding, D.; Xia, C. Enhancement in three-phase boundary of SOFC electrodes by and ion impregnation method: A modeling comparison. Electrochem. Solid-State Lett. 2008, 11, B83–B86. [Google Scholar] [CrossRef]
- Li, C.; Lee, E.K.; Kim, Y.T.; Lee, D. Enhancing triple-phase boundary at fuel electrode of direct carbon fuel cell using a fuel-filled ceria-coated porous anode. Int. J. Hydrog. Energy 2014, 39, 17314–17321. [Google Scholar] [CrossRef]
- Dusastre, V.; Kilner, J.A. Optimisation of composite cathodes for intermediate temperature SOFC applications. Solid State Ion. 1999, 126, 163–174. [Google Scholar] [CrossRef]
- Suntola, T. Atomic layer epitaxy. Mater. Sci. Rep. 1989, 4, 261–312. [Google Scholar] [CrossRef]
- Zaera, F. The surface chemistry of thin film atomic layer deposition (ALD) processes for electronic device manufacturing. J. Mater. Chem. 2008, 18, 3521–3526. [Google Scholar] [CrossRef]
- Berthelot, A.; Caillat, C.; Huard, V.; Barnola, S.; Boeck, B.; Del-Puppo, H.; Emonet, N.; Lalanne, F. Highly reliable TiN/ZrO2/TiN 3D stacked capacitors for 45 nm embedded DRAM technologies. In Proceedings of the ESSDERC 2006—Proceedings of the 36th European Solid-State Device Research Conference, Montreux, Switzerland, 19–21 September 2006; pp. 343–346. [Google Scholar]
- Wang, Z.; Zhu, W.G.; Du, A.Y.; Wu, L.; Fang, Z.; Tran, X.A.; Liu, W.J.; Zhang, K.L.; Yu, H.Y. Highly uniform, self-compliance, and forming-free ALD HfO2-based RRAM with Ge doping. IEEE Trans. Electron Devices 2012, 59, 1203–1208. [Google Scholar] [CrossRef]
- Xuan, Y.; Wu, Y.Q.; Shen, T.; Yang, T.; Ye, P.D. High performance submicron inversion-type enhancement-mode InGaAs MOSFETs with ALD Al2O3, HfO2 and HfAlO as gate dielectrics. In Proceedings of the Technical Digest—International Electron Devices Meeting, IEDM, Washington, DC, USA, 10–12 December 2007; pp. 637–640. [Google Scholar]
- Boyadjiev, S.; Georgieva, V.; Vergov, L.; Baji, Z.; Gáber, F.; Szilágyi, I.M. Gas sensing properties of very thin TiO2 films prepared by atomic layer deposition (ALD). Proc. J. Phys. Conf. Ser. 2014, 559, 012013. [Google Scholar] [CrossRef]
- Seo, S.; Jeong, S.; Park, H.; Shin, H.; Park, N.G. Atomic layer deposition for efficient and stable perovskite solar cells. Chem. Commun. 2019, 55, 2403–2416. [Google Scholar] [CrossRef]
- Pickrahn, K.L.; Gorlin, Y.; Seitz, L.C.; Garg, A.; Nordlund, D.; Jaramillo, T.F.; Bent, S.F. Applications of ALD MnO to electrochemical water splitting. Phys. Chem. Chem. Phys. 2015, 17, 14003–14011. [Google Scholar] [CrossRef]
- Pavlenko, M.; Siuzdak, K.; Coy, E.; Załęski, K.; Jancelewicz, M.; Iatsunskyi, I. Enhanced solar-driven water splitting of 1D core-shell Si/TiO2/ZnO nanopillars. Int. J. Hydrogen Energy 2020, 45, 26426–26433. [Google Scholar] [CrossRef]
- Neudeck, S.; Mazilkin, A.; Reitz, C.; Hartmann, P.; Janek, J.; Brezesinski, T. Effect of low-temperature Al2O3 ALD coating on Ni-rich layered oxide composite cathode on the long-term cycling performance of lithium-ion batteries. Sci. Rep. 2019, 9, 5328. [Google Scholar] [CrossRef] [PubMed]
- Cassir, M.; Goubin, F.; Bernay, C.; Vernoux, P.; Lincot, D. Synthesis of ZrO2 thin films by atomic layer deposition: Growth kinetics, structural and electrical properties. Appl. Surf. Sci. 2002, 193, 120–128. [Google Scholar] [CrossRef]
- Kukli, K.; Forsgren, K.; Ritala, M.; Leskelä, M.; Aarik, J.; Hårsta, A. Dielectric properties of zirconium oxide grown by atomic layer deposition from iodide precursor. J. Electrochem. Soc. 2001, 148, F227–F232. [Google Scholar] [CrossRef]
- Lao, S.X.; Martin, R.M.; Chang, J.P. Plasma enhanced atomic layer deposition of HfO2 and ZrO2 high-k thin films. J. Vac. Sci. Technol. A Vac. Surf. Film. 2005, 23, 488–496. [Google Scholar] [CrossRef]
- Hausmann, D.M.; Kim, E.; Becker, J.; Gordon, R.G. Atomic layer deposition of hafnium and zirconium oxides using metal amide precursors. Chem. Mater. 2002, 14, 4350–4358. [Google Scholar] [CrossRef]
- Yun, S.J.; Lim, J.W.; Lee, J.H. PEALD of zirconium oxide using tetrakis(ethylmethylamino) zirconium and oxygen. Electrochem. Solid-State Lett. 2004, 7, F81–F84. [Google Scholar] [CrossRef]
- Blanquart, T.; Niinistö, J.; Aslam, N.; Banerjee, M.; Tomczak, Y.; Gavagnin, M.; Longo, V.; Puukilainen, E.; Wanzenboeck, H.D.; Kessels, W.M.M.; et al. Zr(NEtMe)2(guan-NEtMe2)2] as a novel atomic layer deposition precursor: ZrO2 film growth and mechanistic studies. Chem. Mater. 2013, 25, 3088–3095. [Google Scholar] [CrossRef]
- Jung, J.S.; Lee, S.K.; Hong, C.S.; Shin, J.H.; Kim, J.M.; Kang, J.G. Atomic layer deposition of ZrO2 thin film on Si(100) using {η5:η1-Cp(CH2)3NMe}Zr(NMe2)2/O3 as precursors. Thin Solid Film. 2015, 589, 831–837. [Google Scholar] [CrossRef]
- Kanomata, K.; Tokoro, K.; Imai, T.; Pansila, P.; Miura, M.; Ahmmad, B.; Kubota, S.; Hirahara, K.; Hirose, F. Room-temperature atomic layer deposition of ZrO2 using tetrakis(ethylmethylamino) zirconium and plasma-excited humidified argon. Appl. Surf. Sci. 2016, 387, 497–502. [Google Scholar] [CrossRef]
- Roy, P.C.; Jeong, H.S.; Doh, W.H.; Kim, C.M. Atomic layer deposition (ALD) of ZrO2 in ultrahigh vacuum (UHV). Bull. Korean Chem. Soc. 2013, 34, 1221–1224. [Google Scholar] [CrossRef]
- Weinreich, W.; Tauchnitz, T.; Polakowski, P.; Drescher, M.; Riedel, S.; Sundqvist, J.; Seidel, K.; Shirazi, M.; Elliott, S.D.; Ohsiek, S.; et al. TEMAZ/O3 atomic layer deposition process with doubled growth rate and optimized interface properties in metal–insulator–metal capacitors. J. Vac. Sci. Technol. A Vac. Surf. Film. 2013, 31, 01A123. [Google Scholar] [CrossRef]
- Dey, S.K.; Wang, C.G.; Tang, D.; Kim, M.J.; Carpenter, R.W.; Werkhoven, C.; Shero, E. Atomic layer chemical vapor deposition of ZrO2-based dielectric films: Nanostructure and nanochemistry. J. Appl. Phys. 2003, 93, 4144–4157. [Google Scholar] [CrossRef]
- Wiemer, C.; Debernardi, A.; Lamperti, A.; Molle, A.; Salicio, O.; Lamagna, L.; Fanciulli, M. Influence of lattice parameters on the dielectric constant of tetragonal ZrO2 and La-doped ZrO2 crystals in thin films deposited by atomic layer deposition on Ge (001). Appl. Phys. Lett. 2011, 99, 232907. [Google Scholar] [CrossRef]
- Putkonen, M.; Niinist, J.; Kukli, K.; Sajavaara, T.; Karppinen, M.; Yamauchi, H.; Niinist, L. ZrO2 thin films grown on silicon substrates by atomic layer deposition with Cp2Zr(CH3)2 and water as precursors. Chem. Vap. Depos. 2003, 9, 207–212. [Google Scholar] [CrossRef]
- Lee, B.; Choi, K.J.; Hande, A.; Kim, M.J.; Wallace, R.M.; Kim, J.; Suydam, J. A novel thermally-stable zirconium amidinate ALD precursor for ZrO2 thin films. Microelectron. Eng. 2009, 86, 272–276. [Google Scholar] [CrossRef]
- Niinist, J.; Kukli, K.; Kariniemi, M.; Ritala, M.; Leskel, M.; Blasco, N.; Dussarrat, C. Novel mixed alkylamido-cyclopentadienyl precursors for ALD of ZrO2 thin films. J. Mater. Chem. 2008, 18, 5243–5247. [Google Scholar] [CrossRef]
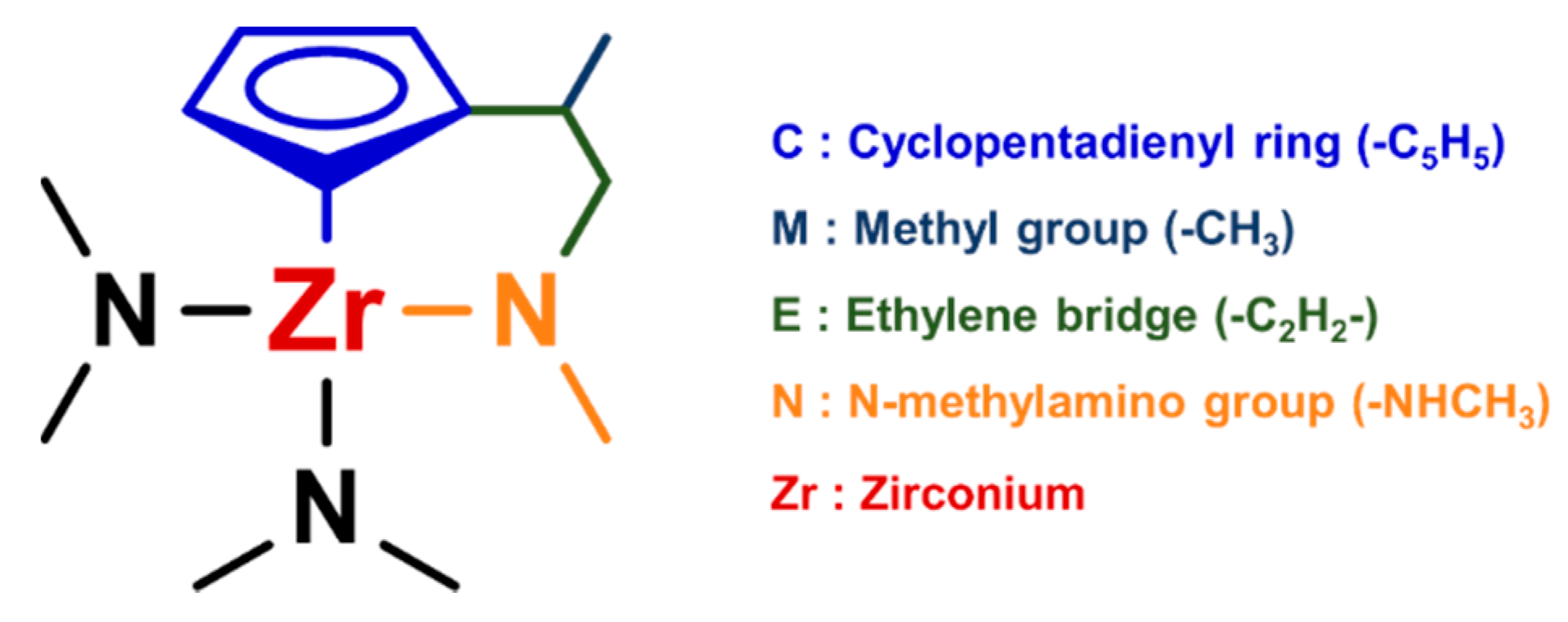




| ZrI4 | ZTB | Zr(MEA)2 (guan-MEA)2 | Cp2Zr(CH3)2 | Zr-AMD | ZAC | CMEN-Zr | |
|---|---|---|---|---|---|---|---|
| ALD type | Thermal ALD | PEALD | Thermal ALD | Thermal ALD | Thermal ALD | Thermal ALD | PEALD |
| Substrate | P-type Si | - | (100) Si | (100) Si | P-type Si | (100) Si | P-type Si |
| Thickness [nm] | 42 | 7 | 6~11 | 3.3~19 | 4.3 | 5.9~6.5 | 31.2~33.1 |
| Annealing Condition | - | 250 °C, Air (1 h) | - | - | 450 °C, N2 + H2 (30 min) | - | 600 °C, N2 (2 h) |
| Leakage Current Density [A/cm2] | ~107~108 (at 1 MV/cm) | 2 × 10−1 (at −1.5 V) | 8 × 10−3 (at 1 V) | 10−6 (at 1 MV/cm) | 2 × 10−3 (at −1 V) | 10−7 (at 1 V) | 7.8 × 10−8 (at −1 MV/cm) |
| Breakdown Electric Field [MV/cm] | 2~2.5 | - | - | 1.9~9.5 | - | - | −3.9~−4.7 |
| Dielectric Constant | 18–24 | 22 | 12~18 | 12.5 | 14 | 26–28 | 20–25 |
| ΔVFB [V] | - | 0.03 | - | 0.25 | - | - | 0.6~2 |
| Reference | [18] | [19] | [23] | [30] | [31] | [32] | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; Seo, G.; Kim, J.; Bae, S.; Park, J.-W.; Hwang, J.-H. Plasma-Enhanced Atomic Layer Deposition of Zirconium Oxide Thin Films and Its Application to Solid Oxide Fuel Cells. Coatings 2021, 11, 362. https://doi.org/10.3390/coatings11030362
Oh J, Seo G, Kim J, Bae S, Park J-W, Hwang J-H. Plasma-Enhanced Atomic Layer Deposition of Zirconium Oxide Thin Films and Its Application to Solid Oxide Fuel Cells. Coatings. 2021; 11(3):362. https://doi.org/10.3390/coatings11030362
Chicago/Turabian StyleOh, Jiwon, Giwon Seo, Jaehwan Kim, Seungmuk Bae, Jeong-Woo Park, and Jin-Ha Hwang. 2021. "Plasma-Enhanced Atomic Layer Deposition of Zirconium Oxide Thin Films and Its Application to Solid Oxide Fuel Cells" Coatings 11, no. 3: 362. https://doi.org/10.3390/coatings11030362
APA StyleOh, J., Seo, G., Kim, J., Bae, S., Park, J.-W., & Hwang, J.-H. (2021). Plasma-Enhanced Atomic Layer Deposition of Zirconium Oxide Thin Films and Its Application to Solid Oxide Fuel Cells. Coatings, 11(3), 362. https://doi.org/10.3390/coatings11030362





