High Entropy Alloy Coatings and Technology
Abstract
:1. Introduction
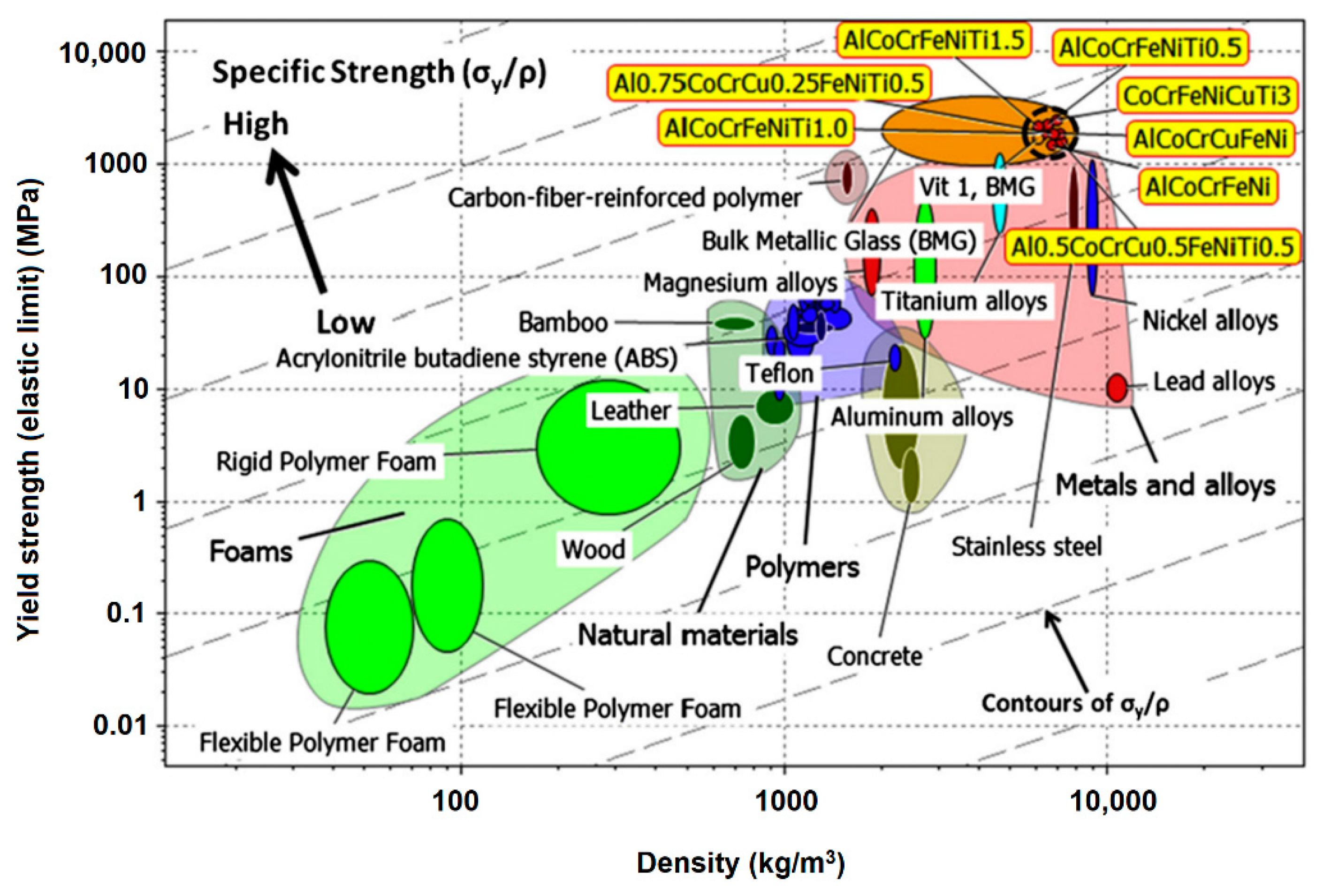
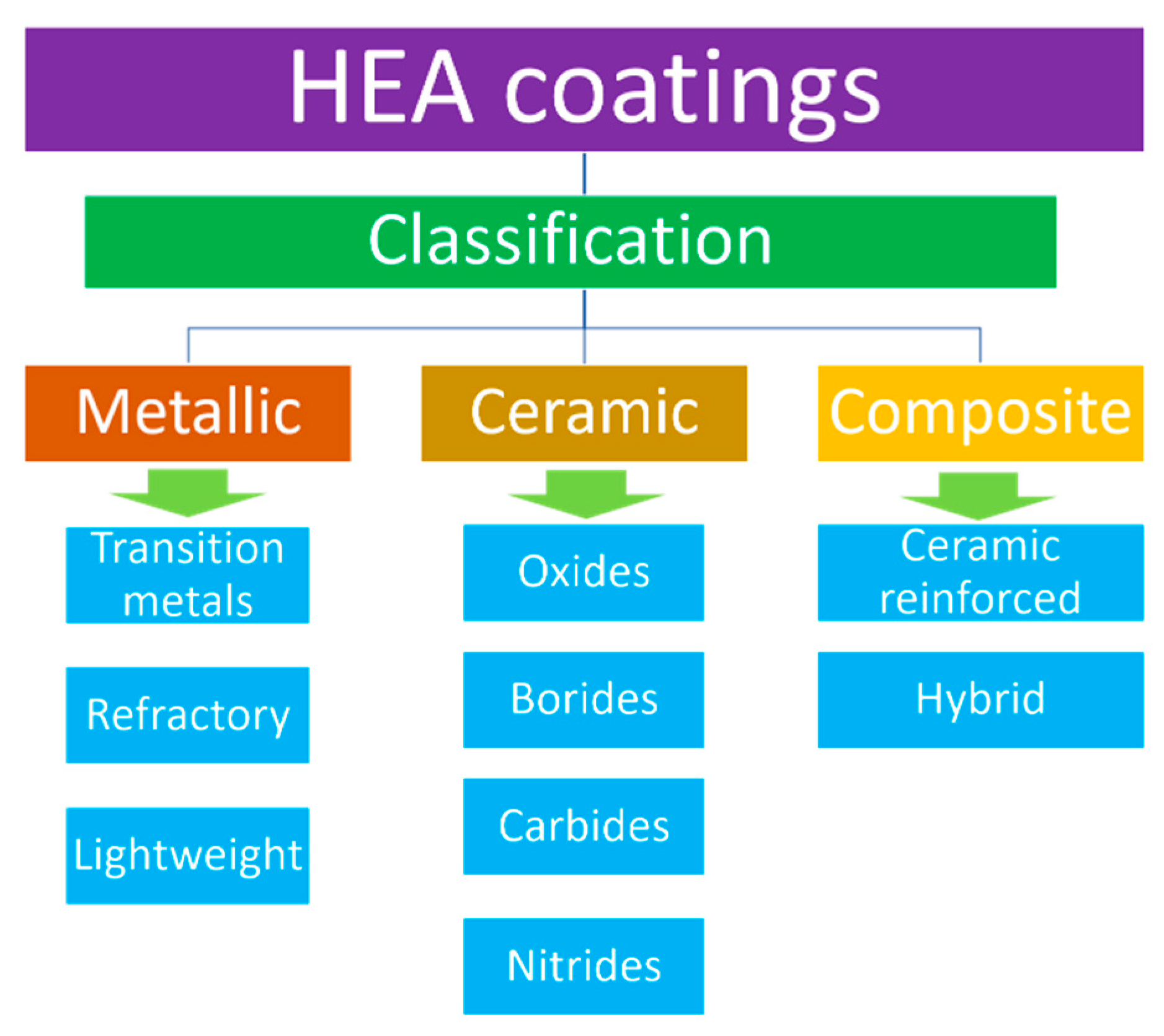
2. High Entropy Alloy Based Coatings
3. Research Trends on HEA Coatings and Technology
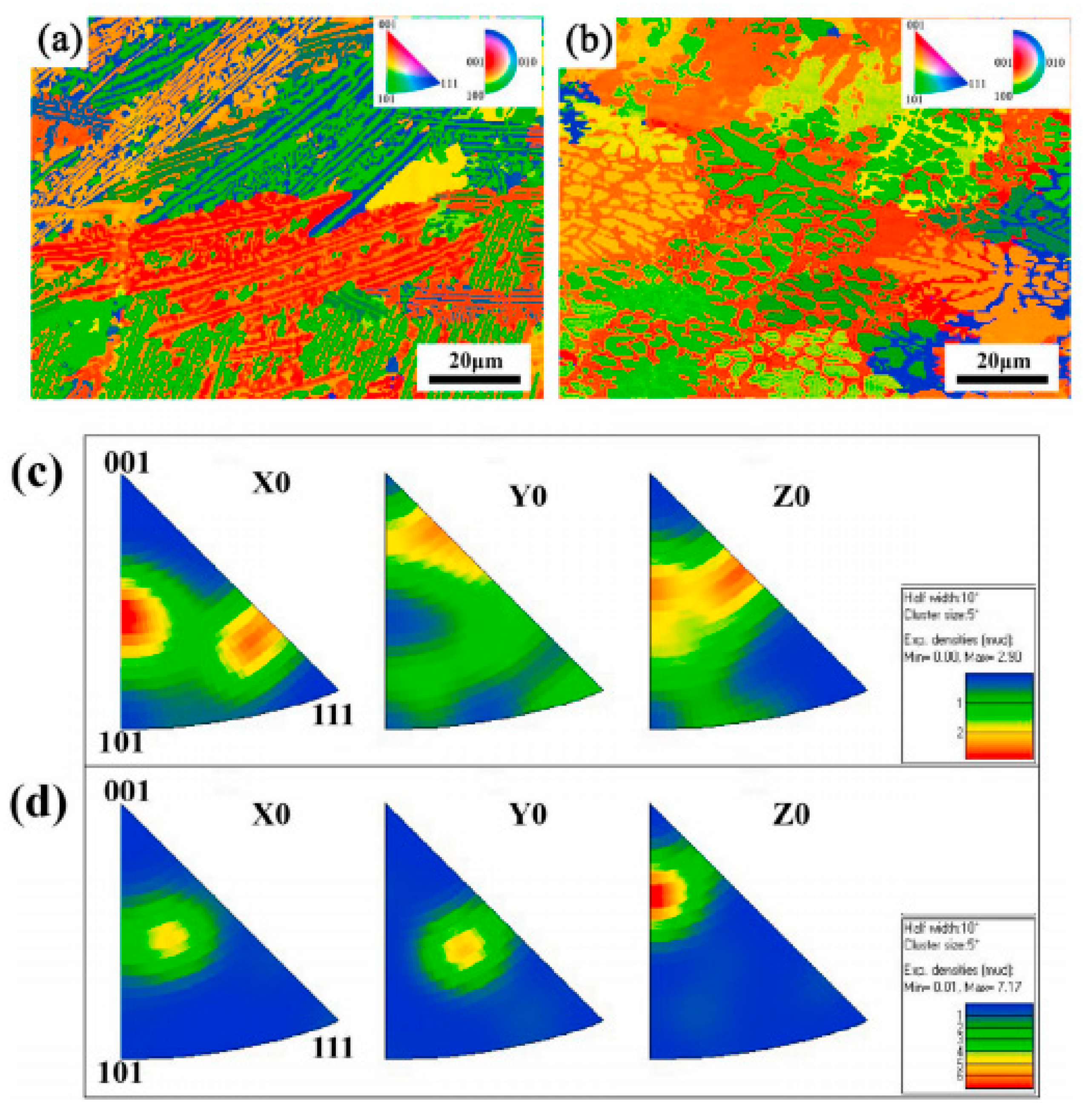
3.1. Metallic Coatings
3.2. Ceramic Coatings
3.3. Composite Coatings
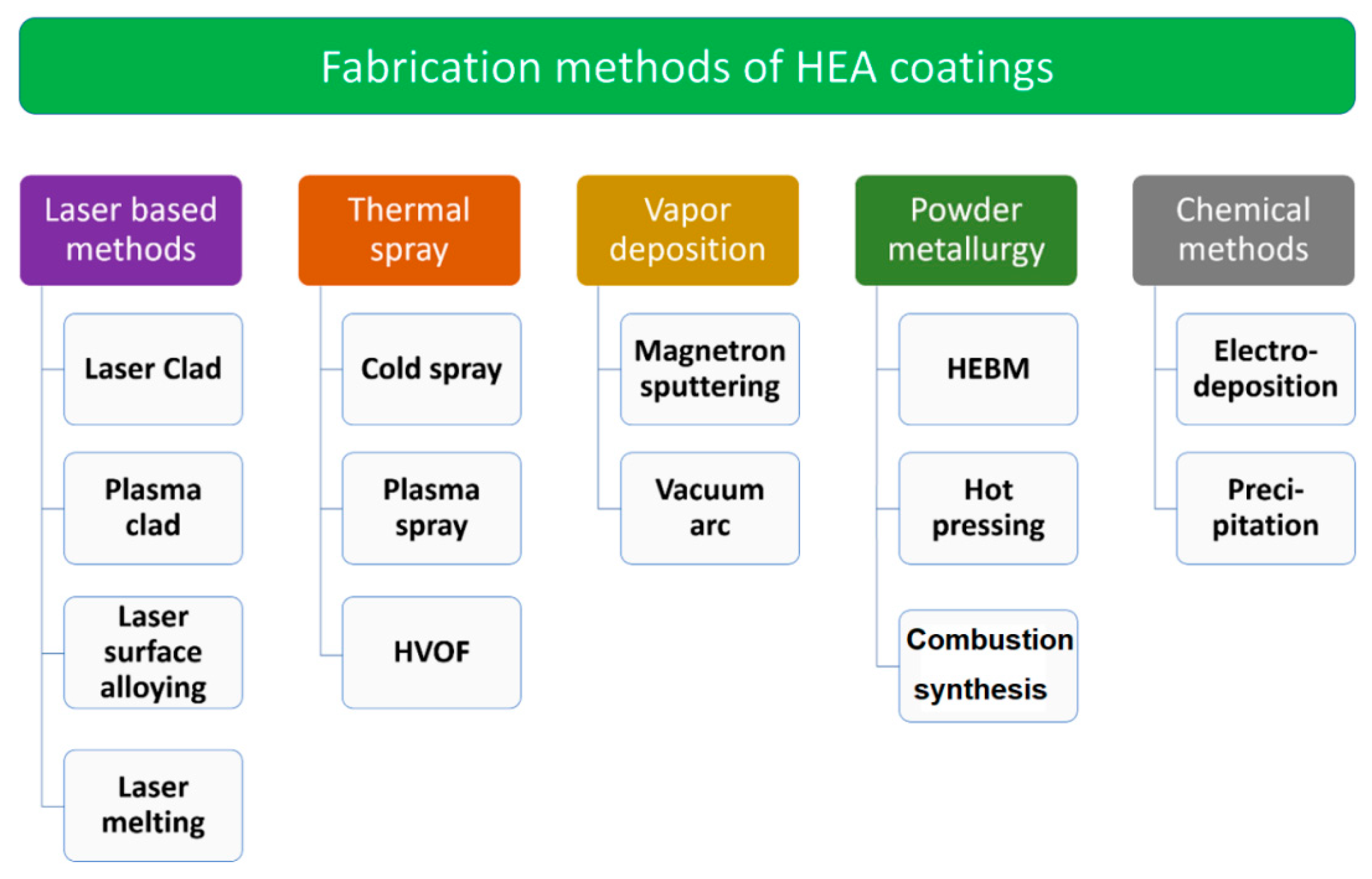
4. Methods of Fabrication of HEA Coatings
5. Post-Processing of HEA Coatings
6. Summary, Concluding Remarks, and Future Scope
- The multi-component HEA, and related coatings, form an attractive class of materials that display a wide potential for competitive applications.
- The full potential of these HEA coatings can be only realized by attempting enough research activities, a combination of new strategies, and data-driven methodology.
- In this article, the background and state of art in HEA-related coatings, their fabrication types, and applications according to their classification based on the previous literature are covered. However, there are several open questions such as stability of the phase, homogeneity of coating concerning metal to N, O, or C ratio, order-disordering in HEA coatings, etc.
- The rapid solidification processes involved in such post modification techniques, especially laser-based methods, need to be further investigated to control the porosity and residual stresses developed due to the high energy density laser beam.
- Despite few good reports on electroplating, chemical-based routes have to be attempted due to a scarcity of such HEAs coatings which can be an economical option when compared with laser and magnetron-based techniques.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, C.J.; Bryan, J.B. Structured, textured or engineered surfaces. Ann. CIRP 1999, 48, 541–556. [Google Scholar] [CrossRef]
- Stout, K.J. Engineered surfaces—A philosophy of manufacture. Proc. Inst. Mech. Eng. 1998, 212, 169–174. [Google Scholar] [CrossRef]
- Khodakarami, S.; Zhao, H.; Rabbi, K.F.; Miljkovic, N. Scalable Corrosion-Resistant Coatings for Thermal Applications. ACS Appl. Mater. Interfaces 2021, 13, 4519–4534. [Google Scholar] [CrossRef]
- Yan, M.; Sun, C.; Xu, J.; Ke, W. Anoxic Corrosion Behavior of Pipeline Steel in Acidic Soils. Ind. Eng. Chem. Res. 2014, 53, 17615–17624. [Google Scholar] [CrossRef]
- Koerner, C.M.; Hopkinson, D.P.; Ziomek-Moroz, M.E.; Rodriguez, A.; Xiang, F. Environmentally friendly tannic acid multilayer coating for reducing corrosion of carbon steel. Ind. Eng. Chem. Res. 2021, 60, 243–250. [Google Scholar] [CrossRef]
- Abadias, G.; Chason, E.; Keckes, J.; Marco, S.; Thompson, G.B.; Barthel, E.; Doll, G.L.; Murray, C.E.; Stoessel, C.H.; Martinu, L. Stress in thin films and coatings: Current status, challenges, and prospects. J. Vac. Sci. Technol. A 2018, 36, 020801. [Google Scholar] [CrossRef] [Green Version]
- Chinnusamy, S.; Ramasamy, V.; Venkatajalapathy, S.; Kaliyannan, G.V.; Palaniappan, S.K. Experimental investigation on the effect of ceramic coating on the wear resistance of Al6061 substrate. J. Mater. Res. Technol. 2019, 8, 6125–6133. [Google Scholar] [CrossRef]
- Ramazan, K.; Levent, U.; Ali, C.; Serdar, S.; Fehim, F. Three types of ceramic coating applicability in automotive industry for wear resistance purpose. Ind. Lubr. Tribol. 2005, 57, 140–144. [Google Scholar]
- Kulu, P.; Tarbe, R.; Vallikivi, A. Abrasive wear of powder materials and coatings. Mater. Sci. 2005, 11, 230–234. [Google Scholar]
- Borkar, T.; Harimkar, S. Microstructure and wear behaviour of pulse electrodeposited Ni–CNT composite coatings. Surf. Eng. 2011, 27, 524–530. [Google Scholar] [CrossRef]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.-Y.; Edgeworth, J.; Li, X.; Magnuson, C.W.; Velamakanni, A.; Piner, R.D.; et al. Oxidation Resistance of Graphene-Coated Cu and Cu/Ni Alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Aal, H.A. Functional surfaces for tribological applications: Inspiration and design. Surf. Topogr. Metrol. Prop. 2016, 4, 043001. [Google Scholar] [CrossRef]
- Qin, D.; Xu, G.; Yang, Y.; Chen, S. Multiphase ceramic coatings with high hardness and wear resistance on 5052 aluminum alloy by a microarc oxidation method. ACS Sustain. Chem. Eng. 2018, 6, 2431–2437. [Google Scholar] [CrossRef]
- Chen, X.; Han, Z.; Lu, K. Friction and Wear Reduction in Copper with a Gradient Nano-grained Surface Layer. ACS Appl. Mater. Interfaces 2018, 10, 13829–13838. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Y.; Chen, X.; Fu, X.; Chen, D.; Chen, S.; Gu, L.; Wei, F.; Bei, H.; Gao, Y.; et al. Tuning element distribution, structure and properties by composition in high-entropy alloys. Nat. Cell Biol. 2019, 574, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Varvenne, C.; Luque, A.; Curtin, W.A. Theory of strengthening in fcc high entropy alloys. Acta Mater. 2016, 118, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Kuzminova, Y.O.; Firsov, D.G.; Dagesyan, S.A.; Konev, S.D.; Sergeev, S.N.; Zhilyaev, A.P.; Kawasaki, M.; Akhatov, I.S.; Evlashin, S.A. Fatigue behavior of additive manufactured CrFeCoNi medium-entropy alloy. J. Alloys Compd. 2021, 863, 158609. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y. Functional properties and promising applications of high entropy alloys. Scr. Mater. 2020, 187, 188–193. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Sharma, A.; Oh, M.C.; Ahn, B. Microstructural evolution and mechanical properties of non-Cantor AlCuSiZnFe lightweight high entropy alloy processed by advanced powder metallurgy. Mater. Sci. Eng. A 2020, 797, 140066. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Yeh, J.W. Phases, microstructure and mechanical properties of AlxCoCrFeNi high-entropy alloys at elevated temperatures. J. Alloys Compd. 2014, 589, 143–152. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Todai, M.; Nagase, T.; Hori, T.; Matsugaki, A.; Sekita, A.; Nakano, T. Novel TiNbTaZrMo high-entropy alloys for metallic biomaterials. Scr. Mater. 2017, 129, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.D.; Liu, P.; Hirata, A.; Song, S.X.; Nieh, T.G.; Chen, M.W. Microstructural origins for a strong and ductile Al0.1CoCrFeNi high-entropy alloy with ultrafine grains. Materialia 2018, 4, 395–405. [Google Scholar] [CrossRef]
- Xu, X.D.; Chen, S.Y.; Ren, Y.; Hirata, A.; Fujita, T.; Liaw, P.K.; Chen, M.W. Temperature-dependent compression behavior of an Al0.5CoCrCuFeNi high-entropy alloy. Materialia 2019, 5, 100243. [Google Scholar] [CrossRef]
- Tucker, R.C., Jr. Thermal spray coatings. In ASM Handbook Vol. 5: Surface Engineering; Cotell, C.M., Sprague, J.A., Smidt, F.A., Jr., Eds.; ASM International: Materials Park, OH, USA, 1994; pp. 497–509. [Google Scholar]
- Lee, S.Y.; Byeon, S.; Kim, H.S.; Jin, H.; Lee, S. Deep learning-based phase prediction of high-entropy alloys: Optimization, generation, and explanation. Mater. Des. 2021, 197, 109260. [Google Scholar] [CrossRef]
- Fu, X.; Schuh, C.A.; Olivetti, E.A. Materials selection considerations for high entropy alloys. Scr. Mater. 2017, 138, 145–150. [Google Scholar] [CrossRef]
- Guo, Y.; Shang, X.; Liu, Q. Microstructure and properties of in situ TiN reinforced laser cladding CoCr2FeNiTix high-entropy alloy composite coatings. Surf. Coat. Technol. 2018, 344, 353–358. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Liu, B.; Yi, D.; Yang, X.; Zhang, W.; Chen, G.; Liu, Y.; Bai, P. Influence of NbC particles on microstructure and mechanical properties of AlCoCrFeNi high-entropy alloy coatings prepared by laser cladding. J. Alloys Compd. 2019, 788, 485–494. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, D.; Liang, X.; Chen, Y. Evolution of microstructure and mechanical properties of in situ synthesized TiC–TiB2/CoCrCuFeNi high entropy alloy coatings. Surf. Coat. Technol. 2015, 281, 109–116. [Google Scholar] [CrossRef]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A novel low-density, high-hardness, high entropy alloy with close-packed single-phase nanocrystalline structures. Mater. Res. Lett. 2015, 3, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Chae, M.J.; Sharma, A.; Oh, M.C.; Ahn, B. Lightweight AlCuFeMnMgTi High Entropy Alloy with High Strength-to-Density Ratio Processed by Powder Metallurgy. Met. Mater. Int. 2020, 1–10. [Google Scholar] [CrossRef]
- Juan, C.C.; Yeh, J.W.; Chin, T.S. A novel light high-entropy alloy Al20Be20Fe10Si15Ti35. In Proceedings of the E-MRS Fall Meeting, Symposium I, Warsaw, Poland, 14–18 September 2009. [Google Scholar]
- Pogrebnjak, A.D.; Beresnev, V.M.; Smyrnova, K.V.; Kravchenko, Y.O.; Zukowski, P.V.; Bondarenko, G.G. The influence of nitrogen pressure on the fabrication of the two-phase superhard nanocomposite (TiZrNbAlYCr)N coatings. Mater. Lett. 2018, 211, 316–318. [Google Scholar] [CrossRef] [Green Version]
- Bagdasaryan, A.A.; Pshyk, A.V.; Coy, L.E.; Konarski, P.; Misnik, M.; Ivashchenko, V.I.; Kempinski, M.; Mediukh, N.R.; Pogrebnjak, A.D.; Beresnev, V.M.; et al. A new type of (TiZrNbTaHf)N/MoN nanocomposite coating: Microstructure and properties depending on energy of incident ions. Compos. Part B-Eng. 2018, 146, 132–144. [Google Scholar] [CrossRef]
- Chang, S.Y.; Lin, S.Y.; Huang, Y.C.; Wu, C.L. Mechanical properties, deformation behaviors and interface adhesion of (AlCrTaTiZr)Nx multi-component coatings. Surf. Coat. Technol. 2010, 204, 3307–3314. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chang, S.Y.; Huang, Y.C.; Shieu, F.S.; Yeh, J.W. Mechanical performance and nanoindenting deformation of (AlCrTaTiZr)NCy multi-component coatings co-sputtered with bias. Surf. Coat. Technol. 2012, 206, 5096–5102. [Google Scholar] [CrossRef]
- Wan, H.; Song, D.; Shi, X.; Cai, Y.; Li, T.; Chen, C. Corrosion behavior of Al0.4CoCu0.6NiSi0.2Ti0.25 high-entropy alloy coating via 3D printing laser cladding in a sulphur environment. J. Mater. Sci. Technol. 2021, 60, 197–205. [Google Scholar] [CrossRef]
- Barron, P.J.; Carruthers, A.W.; Fellowes, J.W.; Jones, N.G.; Dawson, H.; Pickering, E.J. Towards V-based high-entropy alloys for nuclear fusion applications. Scr. Mater. 2020, 176, 12–16. [Google Scholar] [CrossRef]
- Tian, L.; Feng, Z.; Xiong, W. Microstructure, microhardness, and wear resistance of AlCoCrFeNiTi/Ni60 coating by plasma spraying. Coatings 2018, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Tsai, D.C.; Chang, Z.C.; Kuo, L.Y.; Lin, T.J.; Lin, T.N.; Shieu, F.S. Solid solution coating of (TiVCrZrHf)N with unusual structural evolution. Surf. Coat. Technol. 2013, 217, 84–87. [Google Scholar] [CrossRef]
- Chang, S.Y.; Li, C.E.; Chiang, S.C.; Huang, Y.C. 4-nm thick multilayer structure of multi-component (AlCrRuTaTiZr)Nx as robust diffusion barrier for Cu interconnects. J. Alloys Compd. 2012, 515, 4–7. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Liu, Q. A novel biomedical high-entropy alloy and its laser-clad coating designed by a cluster-plus-glue-atom model. Mater. Des. 2020, 196, 109085. [Google Scholar] [CrossRef]
- Nam, S.; Kim, C.; Kim, Y.M. Recent studies of the laser cladding of high entropy alloys. JWJ 2017, 35, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Ni, C.; Shi, Y.; Liu, J.; Huang, G. Characterization of Al0.5FeCu0.7NiCoCr high-entropy alloy coating on aluminum alloy by laser cladding. G. Opt. Laser Technol. 2018, 105, 257–263. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, W.; He, Y.; Li, M.; Guo, S. Formation of core–shell structure in high entropy alloy coating by laser cladding. Appl. Surf. Sci. 2016, 363, 543–547. [Google Scholar] [CrossRef]
- Qiu, X.W.; Zhang, Y.P.; Liu, C.G. Effect of Ti content on structure and properties of Al2CrFeNiCoCuTix high-entropy alloy coatings. J. Alloys Compd. 2014, 585, 282–286. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.Z. Synthesis and characterization of FeCoNiCrCu highentropy alloy coating by laser cladding. Mater. Des. 2011, 32, 1910–1915. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, Y.; Cui, X.; Jin, G.; Li, Y.; Liu, Z.; Dong, M. Design and Microstructure Characterization of FeCoNiAlCu High-Entropy Alloy Coating by Plasma Cladding: In Comparison with Thermodynamic Calculation. Surf. Coat. Technol. 2017, 330, 163–169. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, Y.; Li, J.; Liu, Y.; Liu, Y. Microstructure and mechanical properties of FeCoCrNiNbX high-entropy alloy coatings. Physica B 2018, 550, 112–116. [Google Scholar] [CrossRef]
- Meng, G.H.; Lin, X.; Xie, H.; Yue, T.M.; Ding, X.; Sun, L.; Qi, M. The effect of Cu rejection in laser forming of AlCoCrCuFeNi/Mg composite coating. Mater. Des. 2016, 108, 157–167. [Google Scholar] [CrossRef]
- Shu, F.; Yang, B.; Dong, S.; Zhao, H.; Xu, B.; Xu, F.; Liu, B.; He, P.; Feng, J. Effects of Fe-to-Co ratio on microstructure and mechanical properties of laser cladded FeCoCrBNiSi high-entropy alloy coatings. J. Appl. Surf. Sci. 2018, 450, 538–544. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Yi, J.Z.; Zhang, C.H. Synthesis and characterization of FeCoCrAlCu high-entropy alloy coating by laser surface alloying. Surf. Coat. Technol. 2015, 262, 64–69. [Google Scholar] [CrossRef]
- Ye, Q.; Feng, K.; Li, Z.; Lu, F.; Li, R.; Huang, J.; Wu, Y. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
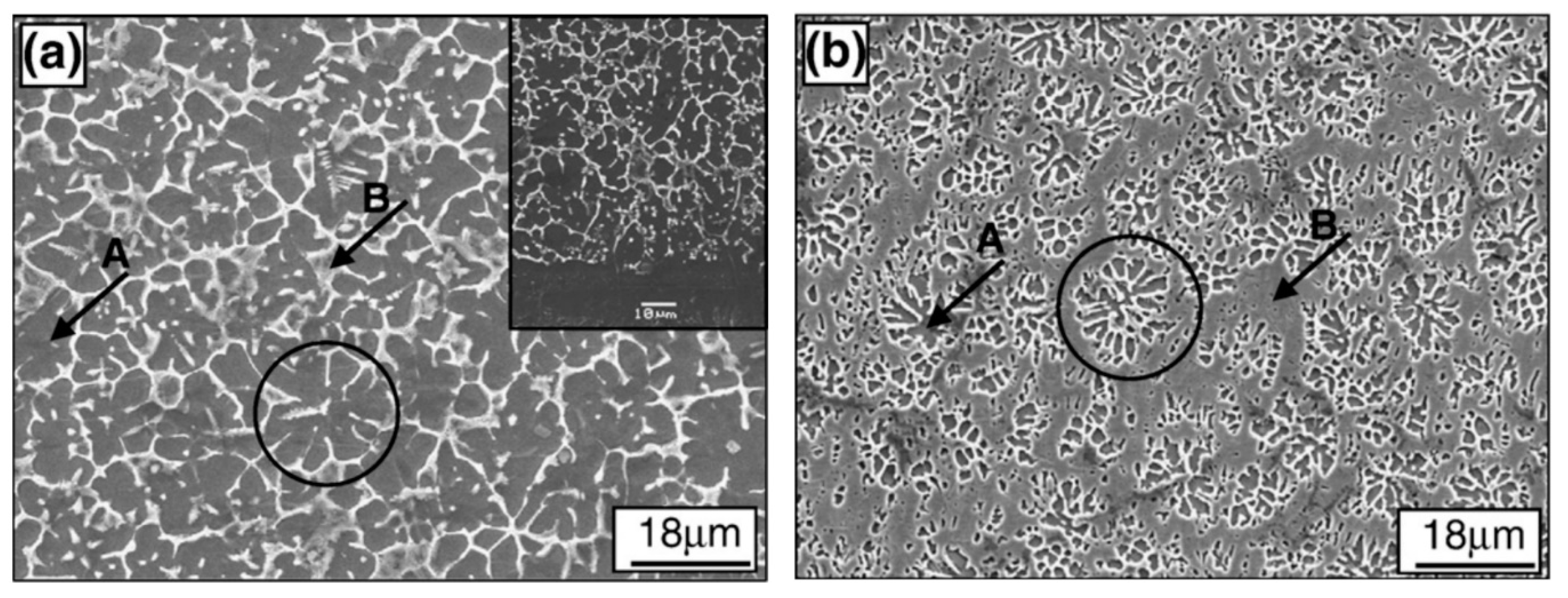
- Cai, Z.; Cui, X.; Jin, G.; Lu, B.; Zhang, D.; Zhang, Z. In situ TEM tensile testing on high-entropy alloy coating by laser surface alloying. J. Alloys Compd. 2017, 708, 380–384. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Zhang, C.H. Phase evolution characteristics of FeCoCrAlCuVxNi high entropy alloy coatings by laser high-entropy alloying. Mater. Lett. 2015, 141, 7–9. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, S.; Zhang, C.H.; Zhang, H.; Dong, S.Y. Phase evolution and properties in laser surface alloying of FeCoCrAlCuNix high-entropy alloy on copper substrate. Surf. Coat. Technol. 2017, 315, 368–376. [Google Scholar] [CrossRef]
- Yin, S.; Li, W.; Song, B.; Yan, X.; Kuang, M.; Xu, Y.; Wen, K.; Lupoi, R. Deposition of FeCoNiCrMn high entropy alloy (HEA) coating via cold spraying. J. Mater. Sci. Technol. 2019, 35, 1003–1007. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Mehner, T.; Thomas, L. Microstructure and wear resistance of AlCoCrFeNiTi high-entropy alloy coatings produced by HVOF. Coatings 2017, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Yue, T.M.; Xie, H.; Lin, X.; Yang, H.; Meng, G. Microstructure of laser re-melted AlCoCrCuFeNi high entropy alloy coatings produced by plasma spraying. Entropy 2013, 15, 2833–2845. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Wei, X.; Liu, C.; Lu, S.; Fu, M. Microstructure and wear behavior of atmospheric plasma-sprayed AlCoCrFeNiTi high-entropy alloy coating. J. Mater. Eng. Perform. 2016, 25, 5513–5521. [Google Scholar] [CrossRef]
- Tüten, N.; Canadinc, D.; Motallebzadeh, A.; Bal, B. Microstructure and tribological properties of TiTaHfNbZr high entropy alloy coatings deposited on Ti-6Al-4V substrates. Intermetallics 2019, 105, 99–106. [Google Scholar] [CrossRef]
- Lin, C.H.; Duh, J.G. Corrosion behavior of (Ti–Al–Cr–Si–V)xNy coatings on mild steels derived from RF magnetron sputtering. Surf. Coat. Technol. 2008, 203, 558–561. [Google Scholar] [CrossRef]
- Braic, V.; Vladescu, A.; Balaceanu, M.; Luculescu, C.R.; Braic, M. Nanostructured multi-element (TiZrNbHfTa)N and (TiZrNbHfTa)C hard coatings. Surf. Coat. Technol. 2012, 211, 117–121. [Google Scholar] [CrossRef]
- Shang, C.; Axinte, E.; Ge, W.; Zhang, Z.; Wang, Y. High-entropy alloy coatings with excellent mechanical, corrosion resistance and magnetic properties prepared by mechanical alloying and hot pressing sintering. Surf. Interface 2017, 9, 36–43. [Google Scholar] [CrossRef]
- Tian, Y.; Shen, Y.; Lu, C.; Feng, X. Microstructures and oxidation behavior of Al-CrMnFeCoMoW composite coatings on Ti-6Al-4V alloy substrate via high-energy mechanical alloying method. J. Alloys Compd. 2019, 779, 456–465. [Google Scholar] [CrossRef]
- Aliyu, A.; Srivastava, C. Microstructure-corrosion property correlation in electrodeposited AlCrFeCoNiCu high entropy alloys-graphene oxide composite coatings. Thin Solid Films 2019, 686, 137434. [Google Scholar] [CrossRef]
- Soare, V.; Burada, M.; Constantin, I.; Mitrica, D.; Badilit, V.; Caragea, A.; Târcolea, M. Electrochemical deposition and microstructural characterization of AlCrFeMnNi and AlCrCuFeMnNi high entropy alloy thin films. Appl. Surf. Sci. 2015, 358, 533–539. [Google Scholar] [CrossRef]
- Huang, P.K.; Yeh, J.W.; Shun, T.T.; Chen, S.K. Multi-principal-element alloys with improved oxidation and wear resistance for thermal spray coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Wang, L.M.; Chen, C.C.; Yeh, J.W.; Ke, S.T. The microstructure and strengthening mechanism of thermal spray coating NixCo0.6Fe0.2CrySizAlTi0.2 high-entropy alloys. Mater. Chem. Phys. 2011, 126, 880–885. [Google Scholar] [CrossRef]
- Hushchyk, D.V.; Yurkova, A.I.; Cherniavsky, V.V.; Bilyk, L.I.; Nakonechnyy, S.O. Nanostructured AlNiCoFeCrTi high-entropy coating performed by cold spray. Appl. Nanosci. 2020, 10, 4879–4890. [Google Scholar] [CrossRef]
- Ajdelsztajn, L.; Zúñiga, A.; Jodoin, B.; Lavernia, E.J. Cold gas dynamic spraying of a high temperature Al alloy. Surf. Coat. Technol. 2006, 201, 2109–2116. [Google Scholar] [CrossRef]
- Cai, Z.; Cui, X.; Jin, G.; Liu, Z.; Li, Y.; Dong, M. TEM observation on phase separation and interfaces of laser surface alloyed high-entropy alloy coating. Micron 2017, 103, 84–89. [Google Scholar] [CrossRef]
- Lu, J.; Wang, B.; Qiu, X.; Peng, Z.; Ma, M. Microstructure evolution and properties of CrCuFexNiTi high-entropy alloy coating by plasma cladding on Q235. Surf. Coat. Technol. 2017, 328, 313–318. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.; Pan, Y.; He, Y.; Shin, K. Synthesis and characterization of NiCoFeCrAl3 high entropy alloy coating by laser cladding. Adv. Mater. Res. 2010, 97-101, 1408–1411. [Google Scholar] [CrossRef]
- Lai, C.H.; Lin, S.J.; Yeh, J.W.; Chang, S.Y. Preparation and characterization of AlCrTaTiZr multi-element nitride coatings. Surf. Coat. Technol. 2006, 201, 3275–3280. [Google Scholar] [CrossRef]
- Chen, T.K.; Shun, T.T.; Yeh, J.W.; Wong, M.S. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2004, 188–189, 193–200. [Google Scholar] [CrossRef]
- Ren, B.; Shen, Z.; Liu, Z. Structure and mechanical properties of multi-element (AlCrMnMoNiZr)Nx coatings by reactive magnetron sputtering. J. Alloys Compd. 2013, 560, 171–176. [Google Scholar] [CrossRef]
- Tsai, D.C.; Deng, M.J.; Chang, Z.C.; Kuo, B.H.; Chen, E.C.; Chang, S.Y.; Shieu, F.S. Oxidation resistance and characterization of (AlCrMoTaTi)-Six-N coating deposited via magnetron sputtering. J. Alloys Compd. 2015, 647, 179–188. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, R.; Yang, Z.B.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Preparation, structure, and properties of high-entropy alloy multilayer coatings for nuclear fuel cladding: A case study of AlCrMoNbZr/(AlCrMoNbZr)N. J. Nucl. Mater. 2018, 512, 15–24. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, S.; Das, S.; Fecht, H.J.; Das, K. Development of lead free pulse electrodeposited tin based composite solder coating reinforced with ex situ cerium oxide nanoparticles. J. Alloys Compd. 2013, 574, 609–616. [Google Scholar] [CrossRef]
- Sharma, A.; Ahn, B. Dry sliding wear behavior of Sn and NiSn overlays on Cu connectors. Trib. Lett. 2018, 66, 136. [Google Scholar] [CrossRef]
- Chen, C.; Feng, X.; Shen, Y. Oxidation behavior of a high Si content Al–Si composite coating fabricated on Ti–6Al–4V substrate by mechanical alloying method. J. Alloys Compd. 2017, 701, 27–36. [Google Scholar] [CrossRef]
- Zhang, H.X.; Dai, J.J.; Sun, C.X.; Li, S.Y. Microstructure and wear resistance of TiAlNiSiV high-entropy laser cladding coating on Ti-6Al-4V. J. Mater. Proc. Technol. 2020, 282, 116671. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.Z. Grain refinement and boundary misorientation transition by annealing in the laser rapid solidified 6FeNiCoCrAlTiSi multicomponent ferrous alloy coating. Surf. Coat. Technol. 2011, 205, 4068–4072. [Google Scholar] [CrossRef]
- Tian, L.; Fu, M.; Xiong, W. Microstructural evolution of AlCoCrFeNiSi high-entropy alloy powder during mechanical alloying and its coating performance. Materials 2018, 11, 320. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Das, S.; Das, K. Pulse electrodeposition of lead-free tin-based composites for microelectronic packaging. In Electrodeposition of Composite Materials; Mohamed, A.M.A., Golden, T.D., Eds.; IntechOpen: Rijeka, Croatia, 2016; pp. 253–274. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Das, S.; Das, K. Pulse electroplating of ultrafine grained tin coating. In Electroplating of Nanostructures; Aliofkhazraei, M., Ed.; IntechOpen: Rijeka, Croatia, 2015; pp. 105–129. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Bhattacharya, S.; Das, S.; Das, K. Fabrication of Sn nanostructures by template assisted pulse electrodeposition. Surf. Eng. 2016, 32, 378–384. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, S.; Das, S.; Das, S. A study on the effect of pulse electrodeposition parameters on the morphology of pure tin coatings. Metall. Mater. Trans. A 2014, 45, 4610–4622. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Y.; Hu, J.; Zhao, Y.; Yu, L. Preparation of high entropy alloy thin film fenicobimn by electroplating deposition method. MSAIJ 2014, 11, 344–348. [Google Scholar]
- Braic, V.; Balaceanu, M.; Braic, M.; Vladescu, A.; Panseri, S.; Russo, A. Characterization of multi-principal-element (TiZrNbHfTa)N and (TiZrNbHfTa)C coatings for biomedical applications. J. Mech. Behav. Biomed. Mater. 2012, 10, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Yoosefan, F.; Ashrafi, A.; Vaghef, S.M.M.; Constantin, I. Synthesis of CoCrFeMnNi high entropy alloy thin films by pulse electrodeposition: Part 1: Effect of pulse electrodeposition parameters. Met. Mater. Int. 2020, 26, 1262–1269. [Google Scholar] [CrossRef]
- Yao, C.Z.; Zhang, P.; Liu, M.; Li, G.R.; Ye, J.Q.; Liu, P. Electrochemical preparation and magnetic study of Bi–Fe–Co–Ni–Mn high entropy alloy. Electrochim. Acta 2008, 53, 8359–8365. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sharma, A.; Das, S.; Das, K. Synthesis and properties of pulse electrodeposited lead-free tin-based Sn/ZrSiO4 nanocomposite coatings. Metall. Mater. Trans. A 2016, 47, 1292–1312. [Google Scholar] [CrossRef]
- Moskovskikh, D.; Vorotilo, S.; Buinevich, V.; Sedegov, A.; Kuskov, K.; Khort, A.; Shuck, C.; Zhukovskyi, M.; Mukasyan, A. Extremely hard and tough high entropy nitride ceramics. Sci. Rep. 2020, 10, 19874. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, Q.; Wang, H.; Shu, F.; Zhao, H.; He, W.; Yu, Z. Microstructure and mechanical properties of Co-based alloy coatings fabricated by laser cladding and plasma arc spray welding. J. Alloys Compd. 2019, 785, 846–854. [Google Scholar] [CrossRef]
- Lin, Y.; Cho, Y. Elucidating the microstructure and wear behavior for multicomponent alloy clad layers by in situ synthesis. Surf. Coat. Technol. 2008, 202, 4666–4672. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Hong, U.T.; Yeh, J.W.; Shih, H.C. Selected corrosion behaviors of a Cu0.5NiAlCoCrFeSi bulk glassy alloy in 288 °C high-purity water. Scr. Mater. 2006, 54, 1997–2001. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.; Jiao, H. Microstructure and properties of 6FeNiCoSiCrAlTi high-entropy alloy coating prepared by laser cladding. Appl. Surf. Sci. 2011, 257, 2259–2263. [Google Scholar] [CrossRef]
- Sharma, A.; Oh, M.C.; Kim, J.T.; Srivastava, A.K.; Ahn, B. Investigation of electrochemical corrosion behavior of additive manufactured Ti–6Al–4V alloy for medical implants in different electrolytes. J. Alloys Compd. 2020, 830, 154620. [Google Scholar] [CrossRef]
- Li, R.; Wang, M.; Yuan, T.; Song, B.; Shi, Y. Microstructural Modification of Laser-Deposited High-Entropy CrFeCoNiMoWC Alloy by Friction Stir Processing: Nanograin Formation and Deformation Mechanism. Metall. Mater. Trans. A 2017, 48, 841–854. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Meng, X.; Wan, L.; Cao, J.; Zhou, L.; Feng, J. Dynamic recrystallization and mechanical properties of friction stir processed Mg-Zn-Y-Zr alloys. J. Mater. Process. Technol. 2017, 249, 331. [Google Scholar] [CrossRef]
- Li, R.; Jin, Y.; Li, Z.; Zhu, Y.; Wu, M. Effect of the remelting scanning speed on the amorphous forming ability of Ni-based alloy using laser cladding plus a laser remelting process. Surf. Coat. Technol. 2014, 259, 725–731. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Akhtar, S.S. Laser re-melting of HVOF coating with WC blend: Thermal stress analysis. J. Mater. Process. Technol. 2012, 212, 2569–2577. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, N.; Guan, S.; Zhang, Y.; Li, D. Microstructure and properties of laser re-melting FeCoCrNiAl0.5Six high-entropy alloy coatings. Surf. Coat. Technol. 2018, 349, 867–873. [Google Scholar] [CrossRef]
- Cai, Z.; Cui, X.; Liu, Z.; Li, Y.; Dong, M.; Jin, G. Microstructure and wear resistance of laser cladded Ni-Cr-Co-Ti-V high-entropy alloy coating after laser remelting processing. Opt. Laser Technol. 2018, 99, 276–281. [Google Scholar] [CrossRef]
- Jiang, P.F.; Zhang, C.H.; Zhang, S.; Zhang, J.B.; Chen, J.; Liu, Y. Fabrication and wear behavior of TiC reinforced FeCoCrAlCu-based high entropy alloy coatings by laser surface alloying. Mater. Chem. Phys. 2020, 255, 123571. [Google Scholar] [CrossRef]


| Base Metal | HEA Coating | Coating Method | Phase | Microstructure | Approx. Thickness | Reference |
|---|---|---|---|---|---|---|
| AA5083 | Al0.5FeCu0.7NiCoCr | Laser cladding | FCC + BCC | Equiaxed and columnar grain microstructure | 600 µm | Ref. [50] |
| AZ31Mg | AlCoCrCuFeNiSi0.5/Y2O3 | Laser cladding | FCC + BCC | Core–shell microstructure | 1500 µm | Ref. [51] |
| Q235 steel | AlCoCrFeNi/NbC | Laser cladding | FCC + BCC + NbC | Rod-like and blocky particles with equiaxed fine structure | 1200 µm | Ref. [34] |
| Q235 steel | Al2CrFeNiCoCuTix | Laser cladding | FCC + BCC | Equiaxed grains and columnar crystals | 600 µm | Ref. [52] |
| Q235 steel | FeCoNiCrCu+Si,Mn,Mo | Laser cladding | FCC | Columnar and equiaxed grains | 2000 µm | Ref. [53] |
| AISI 1045 | FeCoNiAlCu | Plasma cladding | FCC + BCC | Columnar and equiaxed grains | 1000 µm | Ref. [54] |
| Pure Ti | FeCoCrNiNbx | Plasma cladding | FCC + BCC | Dendritic microstructure | 1500 µm | Ref. [55] |
| AZ91D Mg | AlCoCrCuFeNi | Laser melt injection | BCC + CuM2g | Dendritic eutectic structure | 400 µm | Ref. [56] |
| H13 steel | FeCoCrBNiSi | Laser cladding | FCC + Amorphous | Equiaxed microstructure | 200 µm | Ref. [57] |
| Q235 steel | FeCoCrAlCu | Laser surface alloying | BCC | Dendritic microstructure | 800 µm | Ref. [58] |
| A36 steel | FeCoNiCrMn | Laser surface alloying | FCC | Dendritic microstructure | 2000 µm | Ref. [59] |
| Ti64 | AlCoCrNiTiV | Laser surface alloying | BCC + (Co, Ni)Ti2 | Dendritic microstructure | 800 µm | Ref. [60] |
| Ni201 | FeCoCrAlCuVxNi | Laser surface alloying | FCC + BCC | Columnar and equiaxed grains | 400 µm | Ref. [61] |
| Pure Cu | FeCoCrAlCuNix | Laser surface alloying | FCC + BCC | Dendritic microstructure | 500 µm | Ref. [62] |
| Al6082 | FeCoNiCrMn | Cold spray | FCC | Dislocations and coarse grained. | 1500 µm | Ref. [63] |
| S235 steel | AlCoCrFeNiTi | HVOF | BCC | Lamellar structure | 210 µm | Ref. [64] |
| Pure Mg | AlCoCrCuFeNi | Plasma spray | BCC + FCC | Lamellar structure | 275 µm | Ref. [65] |
| 316 stainless steel | AlCoCrFeNiTi | Atmospheric plasma spray | BCC + FCC | Lamellar structure | 240 µm | Ref. [66] |
| Ti64 | TiTaHfNbZr | Magnetron sputtering | Amorphous | Couliflower shaped microstructure | 0.8 µm | Ref. [67] |
| Mild steel | (TiAlCrSiV)xNy | Magnetron sputtering | Amorphous + FCC | Columnar microstructure | 1.7 µm | Ref. [68] |
| C45 steel | (TiZrNbHfTa)N | Magnetron sputtering | FCC | Glassy dense and fine grained | 2 µm | Ref. [69] |
| Stainless steel | (TiZrNbAlYCr)N | Vacuum arc evaporation | FCC + BCC | Fine grained droplet type structure | 7 µm | Ref. [39] |
| Q235 steel | CoCrFeNi | Hot press sintering | FCC | Nanoscale grains | 700 µm | Ref. [70] |
| Q235 steel | CoCrFeNiCu | Hot press sintering | FCC | Nanoscale grains | 700 µm | Ref. [70] |
| Ti64 | CrMnFeCoMoW/Al | HEBM | FCC + BCC | Lamellar microstructure | 210 µm | Ref. [71] |
| Mild steel | AlCrFeCoNiCu/GO | Electrodeposition | FCC + BCC | Granular microstructure | 4 µm | Ref. [72] |
| Pure Cu | AlCrFeMnNi | Electrodeposition | Amorphous + BCC | Spherical and flaky | 350 µm | Ref. [73] |
| Pure Cu | AlCrCuFeMnNi | Electrodeposition | Amorphous + BCC | Spherical and flaky | 400 µm | Ref. [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A. High Entropy Alloy Coatings and Technology. Coatings 2021, 11, 372. https://doi.org/10.3390/coatings11040372
Sharma A. High Entropy Alloy Coatings and Technology. Coatings. 2021; 11(4):372. https://doi.org/10.3390/coatings11040372
Chicago/Turabian StyleSharma, Ashutosh. 2021. "High Entropy Alloy Coatings and Technology" Coatings 11, no. 4: 372. https://doi.org/10.3390/coatings11040372
APA StyleSharma, A. (2021). High Entropy Alloy Coatings and Technology. Coatings, 11(4), 372. https://doi.org/10.3390/coatings11040372






